SA-Mediated Regulation and Control of Abiotic Stress Tolerance in Rice
Abstract
:1. Introducing SA as a Mitigator of Abiotic Stress in Rice
Biosynthesis and Metabolism Salicylic Acid
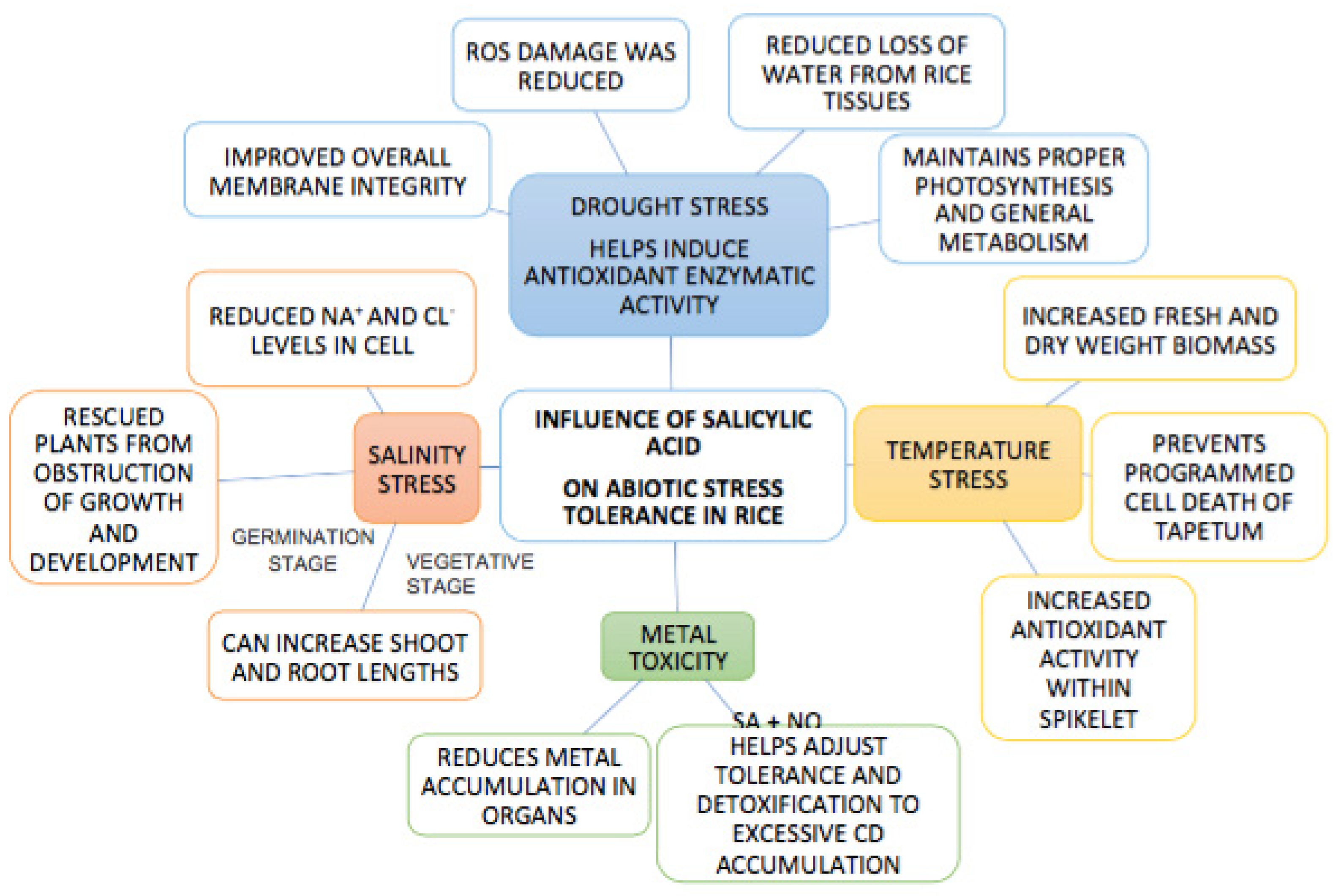
2. The Influence of Salicylic acid on Abiotic Stress Tolerance in Rice
2.1. SA’s Influence on Salinity Stress
2.2. SA’s Influence on Drought Stress
2.3. SA’s Effect on Temperature Stress
2.4. SA’s Effect on Metal Toxicity
2.5. SA’s Effect on Nutrient Deficiency
3. Mechanisms Regulating SA-Induced Stress-Tolerance
3.1. SA Interacts with Osmolytes
3.2. SA Facilitates Mineral Acquisition
3.3. SA Modulates ROS-Signaling and Antioxidant Activity
3.4. SA Influences Secondary Metabolites
3.5. SA and Hormones
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjum, N.A.; Gill, S.; Gill, R. Plant Adaptation to Environmental Change: Significance of Amino acids and Their Derivatives; Anjum, N.A., Gill, S.S., Gill, R., Eds.; CABI: Wallingford, UK, 2014. [Google Scholar]
- R Khan, M.I.; A Khan, N. Salicylic Acid and Jasmonates: Approaches in Abiotic Stress Tolerance. J. Plant Biochem. Physiol. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic Stress Responses in Plants: Present and Future. Abiotic Stress Responses Plants 2012. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Syeed, S.; Nazar, R.; Anjum, N.A. An Insight into the Role of Salicylic Acid and Jasmonic Acid in Salt Stress Tolerance. In Phytohormones and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2012; Volume 2, pp. 277–300. [Google Scholar]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Variation in Salt Tolerance of Wheat Cultivars: Role of Glycinebetaine and Ethylene. Pedosphere 2012, 22, 746–754. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.; Al Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Silverman, P.; Seskar, M.; Kanter, D.; Schweizer, P.; Metraux, J.P.; Raskin, I. Salicylic acid in rice. Biosynthesis, conjugation, and possible role. Plant Physiol. 1995, 108, 633–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Qi, M.; Mei, C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004, 40, 909–919. [Google Scholar] [CrossRef]
- Anwar, S.; Iqbal, M.; Raza, S.H.; Iqbal, N. Efficacy of seed preconditioning with salicylic and ascorbic acid in increasing vigor of rice (Oyza sativa L.) seedling. Pak. J. Bot. 2013, 45, 157–162. [Google Scholar]
- Naser Alavi, S.M.; Arvin, M.J.; Manoochehri Kalantari, K. Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings. J. Plant Interact. 2014, 9, 683–688. [Google Scholar] [CrossRef]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Horváth, E.; Pál, M.; Szalai, G.; Páldi, E.; Janda, T. Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol. Plant. 2007, 51, 480–487. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef] [Green Version]
- Jumali, S.S.; Said, I.M.; Ismail, I.; Zainal, Z. Genes induced by high concentration of salicylic acid in Mitragyna speciosa. AJCS 2011, 5, 296–303. [Google Scholar]
- Chai, J.; Liu, J.; Zhou, J.; Xing, D. Mitogen-activated protein kinase 6 regulates NPR1 gene expression and activation during leaf senescence induced by salicylic acid. J. Exp. Bot. 2014, 65, 6513–6528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadarajah, K.; Kumar, I.S. Drought Response in Rice: The miRNA Story. Int. J. Mol. Sci. 2019, 20, 3766. [Google Scholar] [CrossRef] [Green Version]
- Sendon, P.M.; Seo, H.S.; Song, J.T. Salicylic acid signaling: Biosynthesis, metabolism, and crosstalk with jasmonic acid. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 501–506. [Google Scholar]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Catinot, J.; Buchala, A.; Abou-Mansour, E.; Métraux, J.-P. Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett. 2008, 582, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Heidari, M.A.; Kazemi, M.; Filinejad, A.R. Salicylic acid induced changes in some physiological parameters in chickpea (Cicer arietinum L.) under salt stress. J. Agric. Technol. 2013, 9, 311–316. [Google Scholar]
- Boukraâ, D.; Benabdelli, K.; Belabid, L.; Bennabi, F. Effect of salinity on chickpea seed germination pre-treated with salicylic acid. Sci. J. Biol. Sci. 2013, 2, 86–93. [Google Scholar]
- Lee, S.; Kim, S.G.; Park, C.M. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 2010, 188, 626–637. [Google Scholar] [CrossRef]
- Torabian, A. Effect of salicylic acid on germination and growth of alfalfa (Medicago sativa L.) seedlings under water potential loss at salinity stress. Plant Ecophysiol. 2010, 2, 151–155. [Google Scholar]
- Chandra, A.; Anand, A.; Dubey, A. Effect of salicylic acid on morphological and biochemical attributes in cowpea-PubMed. Eff. Salicylic Acid Morphol. Biochem. Attrib. Cowpea 2007, 28, 193–196. [Google Scholar]
- Souana, K.; Taïbi, K.; Ait Abderrahim, L.; Amirat, M.; Achir, M.; Boussaid, M.; Mulet, J.M. Salt-tolerance in Vicia faba L. is mitigated by the capacity of salicylic acid to improve photosynthesis and antioxidant response. Sci. Hortic. 2020, 273, 109641. [Google Scholar] [CrossRef]
- El-Tayeb, M.A. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Tammam, A.; Alhamd, M.F.; Hemeda, M. (PDF) Study of Salt Tolerance in Wheat (Triticum aestium L.) Cultivar Banysoif 1. Available online: https://www.researchgate.net/publication/26575940_Study_of_salt_tolerance_in_wheat_Triticum_aestium_L_cultivar_Banysoif_1 (accessed on 18 April 2021).
- Tufail, A.; Arfan, M.; Gurmani, A.R.; Khan, A.; Bano, A. Salicylic acid induced salinity tolerance in maize (Zea mays). Pak. J. Bot. 2013, 45, 75–82. [Google Scholar]
- Simaei, M.; Khavari-Nejad, R.A.; Bernard, F. Exogenous Application of Salicylic Acid and Nitric Oxide on the Ionic Contents and Enzymatic Activities in NaCl-Stressed Soybean Plants. Am. J. Plant Sci. 2012, 3, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Ramírez, A.; Rodríguez, D.; Reyes, D.; Jiménez, J.A.; Nicolás, G.; López-Climent, M.; Gómez-Cadenas, A.; Nicolás, C. Cross-talk between gibberellins and salicylic acid in early stress responses in Arabidopsis thaliana seeds. Plant Signal. Behav. 2009, 4, 750–751. [Google Scholar] [CrossRef] [Green Version]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Syeed, S.; Masood, A.; Nazar, R.; Iqbal, N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010, 1, e1. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Physiological Mechanism of Salicylic Acid for Alleviation of Salt Stress in Rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Szalai, G.; Páldi, E.; Janda, T. Effect of salt stress on the endogenous salicylic acid content in maize (Zea mays L.) plants. Acta Biol. Szeged. 2005, 49, 47–48. [Google Scholar]
- Saeidnejad, A.; Mardani, H.; Naghibolghora, M. Protective effects of salicyclic acid on physiological parameters and antioxidants response in maize seedlings under salinity stress. J. Appl. Environ. Biol. Sci. 2012, 2, 364–373. [Google Scholar]
- Noreen, S.; Ashraf, M. Alleviation of adverse effects of salt stress on Sunflower (Helianthus annuus L.) by exogenous application of salicylic acid: Growth and photosynthesis. Pak. J. Bot. 2008, 40, 1657–1663. [Google Scholar]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csiszár, J.; Horváth, E.; Váry, Z.; Gallé, Á.; Bela, K.; Brunner, S.; Tari, I. Glutathione transferase supergene family in tomato: Salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol. Biochem. 2014, 78, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Peng, X.; Wei, L.; Kang, G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene 2013, 529, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Cheah, B.H.; Nadarajah, K.; Divate, M.D.; Wickneswari, R. Identification of four functionally important microRNA families with contrasting differential expression profiles between drought-tolerant and susceptible rice leaf at vegetative stage. BMC Genom. 2015, 16, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, B.H.; Jadhao, S.; Vasudevan, M.; Wickneswari, R.; Nadarajah, K. Identification of functionally important microRNAs from rice inflorescence at heading stage of a qDTY4.1-QTL bearing Near Isogenic Line under drought conditions. PLoS ONE 2017, 12, e0186382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Usha, K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 2003, 39, 137–141. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.C.; Zhu, Y.G.; Zhao, F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Pál, M.; Kovács, V.; Szalai, G.; Soós, V.; Ma, X.; Liu, H.; Mei, H.; Janda, T. Salicylic Acid and Abiotic Stress Responses in Rice. J. Agron. Crop Sci. 2014, 200, 1–11. [Google Scholar] [CrossRef]
- Dhawan, G.; Kumar, A.; Dwivedi, P.; Gopala Krishnan, S.; Pal, M.; Vinod, K.K.; Nagarajan, M.; Bhowmick, P.K.; Bollinedi, H.; Ellur, R.K.; et al. Introgression of qDTY1.1 Governing Reproductive Stage Drought Tolerance into an Elite Basmati Rice Variety “Pusa Basmati 1” through Marker Assisted Backcross Breeding. Agronomy 2021, 11, 202. [Google Scholar] [CrossRef]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Szalai, G.; Antunovics, Z.; Horvath, E. Effect of benzoic acid and aspirin on chilling tolerance and photosynthesis in young maize plants [Zea mays L.]. Maydica 2000, 45, 29–33. [Google Scholar]
- Janda, T.; Szalai, G.; Leskó, K.; Yordanova, R.; Apostol, S.; Popova, L.P. Factors contributing to enhanced freezing tolerance in wheat during frost hardening in the light. Phytochemistry 2007, 68, 1674–1682. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Qu, L.J.; Chen, J.; Liu, M.; Pan, N.; Okamoto, H.; Lin, Z.; Li, C.; Li, D.; Wang, J.; Zhu, G.; et al. Molecular Cloning and Functional Analysis of a Novel Type of Bowman-Birk Inhibitor Gene Family in Rice. Plant Physiol. 2003, 133, 560–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Habibi, G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 2012, 56, 57–63. [Google Scholar]
- Alam, M.M.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2013, 7, 1053–1063. [Google Scholar]
- Saruhan, N.; Saglam, A.; Kadioglu, A. Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol. Plant. 2012, 34, 97–106. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Xu, W.; Peng, X.; Han, Q.; Zhu, Y.; Guo, T. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J. Proteome Res. 2012, 11, 6066–6079. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Xu, Z.S.; Pan-Pan, L.; Hu, D.; Chen, M.; Li, L.C.; Ma, Y.Z. A wheat PI4K gene whose product possesses threonine autophophorylation activity confers tolerance to drought and salt in Arabidopsis. J. Exp. Bot. 2013, 64, 2915–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Nam, J.; Park, H.C.; Na, G.; Miura, K.; Jin, J.B.; Yoo, C.Y.; Baek, D.; Kim, D.H.; Jeong, J.C.; et al. Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J. 2007, 49, 79–90. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.; Huang, J.; Cui, K.; Nie, L.; Shah, T.; Chen, C.; Wang, K. Impact of high-temperature stress on rice plant and its traits related to tolerance. J. Agric. Sci. 2011, 149, 545–556. [Google Scholar] [CrossRef]
- Verma, R.K.; Kanwar, M.K. Assessment of Temperature Stress on Rice at Grain Filling Stage in Raipur District of Chattisgarh, India. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 253–259. [Google Scholar] [CrossRef]
- Akasha, A.; Ashraf, M.; Shereen, A. Mahboob Heat Tolerance Screening Studies and Evaluating Salicylic Acid Efficacy against High Temperature in Rice (Oryza sativa L.) Genotypes. J. Plant Biochem. Physiol. 2019, 7, 2. [Google Scholar]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic acid reverses pollen abortion of rice caused by heat stress. Bmc Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Pouramir-Dashtmian, F.; Khajeh-Hosseini, M.; Esfahani, M. Improving chilling tolerance of rice seedling by seed priming with salicylic acid. Arch. Agron. Soil Sci. 2014, 60, 1291–1302. [Google Scholar] [CrossRef]
- Satake, T.; Yoshida, S. High temperature-induced sterility in indica rices at flowering. Jpn. J. Crop Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.R.; Tarpley, L. Effects of Night Temperature, Spikelet Position and Salicylic Acid on Yield and Yield-Related Parameters of Rice (Oryza sativa L.) Plants. J. Agron. Crop Sci. 2011, 197, 40–49. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Zhang, X.F.; Tao, L.X.; Fu, G.F. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J. Plant Growth Regul. 2020, 40, 464–485. [Google Scholar] [CrossRef]
- Pathak, M.R.O.; da Silva, J.A.T.; Wani, S.H. Polyamines in response to abiotic stress tolerance through transgenic approaches. GM Crops Food 2014, 5, 87–96. [Google Scholar] [CrossRef]
- Bhuyan, M.H.M.B.; Parvin, K.; Mohsin, S.M.; Al Mahmud, J.; Hasanuzzaman, M.; Fujita, M. Modulation of cadmium tolerance in rice: Insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants 2020, 9, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Popova, L.P.; Maslenkova, L.T.; Ivanova, A.; Stoinova, Z. Role of salicylic acid in alleviating heavy metal stress. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 447–466. [Google Scholar]
- Bai, X.; Dong, Y.; Kong, J.; Xu, L.; Liu, S. Effects of application of salicylic acid alleviates cadmium toxicity in perennial ryegrass. Plant Growth Regul. 2015, 75, 695–706. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Wang, F.; Ye, Y.; Zhu, C. Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep. 2016, 35, 719–731. [Google Scholar] [CrossRef]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Cruz, D.S.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. BioMetals 2012, 25, 1155–1165. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Fujita, M.; Tran, L.-S.P. Interactive Effects of Salicylic Acid and Nitric Oxide in Enhancing Rice Tolerance to Cadmium Stress. Int. J. Mol. Sci. 2019, 20, 5798. [Google Scholar] [CrossRef] [Green Version]
- Gondor, O.K.; Pál, M.; Darkó, É.; Janda, T.; Szalai, G. Salicylic Acid and Sodium Salicylate Alleviate Cadmium Toxicity to Different Extents in Maize (Zea mays L.). PLoS ONE 2016, 11, e0160157. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Trivedi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uraguchi, S.; Kamiya, T.; Clemens, S.; Fujiwara, T. Characterization of OsLCT1, a cadmium transporter from indica rice (Oryza sativa). Physiol. Plant. 2014, 151, 339–347. [Google Scholar] [CrossRef]
- Wang, F.; Tan, H.; Huang, L.; Cai, C.; Ding, Y.; Bao, H.; Chen, Z.X.; Zhu, C. Application of exogenous salicylic acid reduces Cd toxicity and Cd accumulation in rice. Ecotoxicol. Environ. Saf. 2021, 207, 111198. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deus, A.C.F.; de Mello Prado, R.; de Cássia Félix Alvarez, R.; de Oliveira, R.L.L.; Felisberto, G. Role of Silicon and Salicylic Acid in the Mitigation of Nitrogen Deficiency Stress in Rice Plants. Silicon 2020, 12, 997–1005. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Sanders, G.; Arndt, S. Plant Response to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought stress responses and signal transduction in plants. In Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives; Springer: New York, NY, USA, 2015; Volume 2, pp. 195–225. [Google Scholar]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef]
- Gururani, M.; Mohanta, T.; Bae, H. Current Understanding of the Interplay between Phytohormones and Photosynthesis under Environmental Stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef] [Green Version]
- Kohli, A.; Sreenivasulu, N.; Lakshmanan, P.; Kumar, P.P. The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep. 2013, 32, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential Mechanisms of Abiotic Stress Tolerance in Crop Plants Induced by Thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [Green Version]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive Oxygen Species and Root Hairs in Arabidopsis Root Response to Nitrogen, Phosphorus and Potassium Deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. BioMetals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Peñuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2003, 217, 758–766. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Janda, T.; Szalai, G.; Rios-Gonzalez, K.; Veisz, O.; Páldi, E. Comparative study of frost tolerance and antioxidant activity in cereals. Plant Sci. 2003, 164, 301–306. [Google Scholar] [CrossRef]
- Shakirova, F.M. Role of hormonal system in the manifestation of growth promoting and antistress action of salicylic acid. In Salicylic Acid: A Plant Hormone; Springer: Dordrecht, The Netherlands, 2007; pp. 69–89. [Google Scholar]
- Iwamoto, M.; Higo, H.; Higo, K. Differential diurnal expression of rice catalase genes: The 5′-flanking region of CatA is not sufficient for circadian control. Plant Sci. 2000, 151, 39–46. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions between Heat Shock and Elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyomasu, T.; Goda, C.; Sakai, A.; Miyamoto, K.; Shenton, M.R.; Tomiyama, S.; Mitsuhashi, W.; Yamane, H.; Kurata, N.; Okada, K. Characterization of diterpene synthase genes in the wild rice species Oryza brachyatha provides evolutionary insight into rice phytoalexin biosynthesis. Biochem. Biophys. Res. Commun. 2018, 503, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Courot, E.; Clément, C.; Ricord, S.; Crouzet, J.; Aziz, A.; Cordelier, S. Molecular Engineering of Phytoalexins in Plants: Benefits and Limitations for Food and Agriculture. J. Agric. Food Chem. 2017, 65, 2643–2644. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.-W.; Okada, K.; Koga, J.; Jikumaru, Y.; Nojiri, H.; Yamane, H. Effects of cytokinin on production of diterpenoid phytoalexins in rice. J. Pestic. Sci. 2010, 35, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Irving, H.R. Developing a model of plant hormone interactions. Plant Signal. Behav. 2011, 6, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Dong, Y.; Xu, L.; Liu, S.; Bai, X. Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachis hypogaea L. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, M.J.; Terrile, M.C.; Casalongué, C.A. Auxin and salicylic acid signalings counteract the regulation of adaptive responses to stress. Plant Signal. Behav. 2011, 6, 452–454. [Google Scholar] [CrossRef]
- Ghanta, S.; Datta, R.; Bhattacharyya, D.; Sinha, R.; Kumar, D.; Hazra, S.; Mazumdar, A.B.; Chattopadhyay, S. Multistep involvement of glutathione with salicylic acid and ethylene to combat environmental stress. J. Plant Physiol. 2014, 171, 940–950. [Google Scholar] [CrossRef]
- Hao, F.; Zhao, S.; Dong, H.; Zhang, H.; Sun, L.; Miao, C. Nia1 and Nia2 are Involved in Exogenous Salicylic Acid-induced Nitric Oxide Generation and Stomatal Closure in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Castelló, M.J.; Medina-Puche, L.; Lamilla, J.; Tornero, P. NPR1 paralogs of Arabidopsis and their role in salicylic acid perception. PLoS ONE 2018, 13, e0209835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Naidoo, S.; van den Berg, N. The nonexpressor of pathogenesis-related genes 1 (NPR1) and related family: Mechanistic insights in plant disease resistance. Front. Plant Sci. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, C.; Yee, T.; Nadarajah, K. OsNPR1 gene expression in response to salusilic acid in rice. J. Adv. Biol. 2014, 4, 528–538. [Google Scholar]
- Ueno, Y.; Yoshida, R.; Kishi-Kaboshi, M.; Matsushita, A.; Jiang, C.-J.; Goto, S.; Takahashi, A.; Hirochika, H.; Takatsuji, H. MAP kinases phosphorylate rice WRKY45. Plant Signal. Behav. 2013, 8, e24510. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, A.; Fukushima, S.; Goto, S.; Matsushita, A.; Shimono, M.; Sugano, S.; Jiang, C.J.; Akagi, A.; Yamazaki, M.; Inoue, H.; et al. Genome-wide identification of WRKY45-regulated genes that mediate benzothiadiazole-induced defense responses in rice. Bmc Plant Biol. 2013, 13, 150. [Google Scholar] [CrossRef] [Green Version]
- De Vleesschauwer, D.; Xu, J.; Höfte, M. Making sense of hormone-mediated defense networking: From rice to Arabidopsis. Front. Plant Sci. 2014, 5, 1–15. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadarajah, K.; Abdul Hamid, N.W.; Abdul Rahman, N.S.N. SA-Mediated Regulation and Control of Abiotic Stress Tolerance in Rice. Int. J. Mol. Sci. 2021, 22, 5591. https://doi.org/10.3390/ijms22115591
Nadarajah K, Abdul Hamid NW, Abdul Rahman NSN. SA-Mediated Regulation and Control of Abiotic Stress Tolerance in Rice. International Journal of Molecular Sciences. 2021; 22(11):5591. https://doi.org/10.3390/ijms22115591
Chicago/Turabian StyleNadarajah, Kalaivani, Nur Wahida Abdul Hamid, and Nur Sabrina Natasha Abdul Rahman. 2021. "SA-Mediated Regulation and Control of Abiotic Stress Tolerance in Rice" International Journal of Molecular Sciences 22, no. 11: 5591. https://doi.org/10.3390/ijms22115591






