Nature of Linear Spectral Properties and Fast Electronic Relaxations in Green Fluorescent Pyrrolo[3,4-c]Pyridine Derivative
Abstract
:1. Introduction
2. Results and Discussion
2.1. Linear Photophysical Properties and Photostability of HPPT
2.2. Femtosecond Transient Absorption Pump-Probe Spectroscopy of HPPT
2.3. Quantum Chemical Analysis of the Electronic Structure of HPPT
3. Experimental Section
3.1. Synthesis of HPPT and Linear Photophysical and Photochemical Measurements
3.2. Femtosecond Transient Absorption Spectral Measurements
3.3. Computational Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devaiah, C.T.; Hemavathi, B.; Ahipa, T.N. New blue emissive conjugated small molecules with low lying HOMO energy levels for optoelectronic applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 175, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, Y.; Zhao, H.; Zong, Q.; Fang, Y. Novel A-D-A type small molecules with b-alkynylated BODIPY flanks for bulk heterojunction solar cells. Org. Electron. 2017, 49, 321–333. [Google Scholar] [CrossRef]
- Sundaram, G.S.M.; Binz, K.; Sharma, V.; Yeung, M.; Sharma, V. Live-cell fluorescence imaging: Assessment of thioflavin T uptake in human epidermal carcinoma cells. Med. Chem. Commun. 2018, 9, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, J.; Xie, Z.; Shan, D.; Gerhard, E.; Qian, G.; Yang, J. Fluorescence imaging enabled poly(lactide-co-glycolide). Acta Biomater. 2016, 29, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.L.; Patel, P.S.; Klein, M. A fluorescein-containing, small-molecule, water-soluble receptor for cytosine free bases. Bioorg. Med. Chem. 2010, 18, 7034–7042. [Google Scholar] [CrossRef]
- Luo, T.; Li, Y.; Xu, Y.; Zhang, S.; Wang, Y.; Kou, X.; Xiao, D. Rapid synthesis of a hyperfluorescence 2-pyridone derivative as a fluorescent molecular sensor for picric acid. Sens. Actuators B Chem. 2017, 253, 231–238. [Google Scholar] [CrossRef]
- Ghiasuddin, M.; Akram, M.; Adeel, M.; Khalid, M.N.; Tahir, M.U.; Khan, M.A.; Asghar, M.A.; Ullah, M.; Iqbal, M. A combined experimental and computational study of 3-bromo-5-(2,5-difluorophenyl) pyridine and 3,5-bis(naphthalen-1-yl); pyridine: Insight into the synthesis, spectroscopic, single crystal XRD, electronic, nonlinear optical and biological properties. J. Mol. Struct. 2018, 1160, 129–141. [Google Scholar] [CrossRef]
- Adeniyi, A.A.; Ajibade, P.A. The spectroscopic and the QTAIM properties of pyridine and phenanthroline derivatives using experimental and computational methods. Spectrochim. Acta A 2014, 128, 540–551. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, L.; Zhang, X.; Ruan, B.; Hu, X.; Deng, X.; Cai, Q.; Yang, X. Synthesis and fluorescent property of biodegradable polyphosphazene targeting long-term in vivo tracking. Macromolecules 2016, 49, 8508–8519. [Google Scholar] [CrossRef]
- Ershov, O.V.; Fedoseev, S.V.; Ievlev, M.Y.; Belikov, M.Y. 2-Pyridone-based fluorophores: Synthesis and fluorescent properties of pyrrolo[3,4-c] pyridine derivatives. Dyes Pigm. 2016, 134, 459–464. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Fedorova, O.A.; Fedorov, Y.V. Fluorescent and colorimetric chemosensors for cations based on 1,8-naphthalimide derivatives: Design principles and optical signalling mechanisms. Russ. Chem. Rev. 2014, 83, 155–182. [Google Scholar] [CrossRef]
- Kravchenko, D.V.; Kuzovkova, Y.A.; Kysil, V.M.; Tkachenko, S.E.; Maliarchouk, S.; Okun, I.M.; Balakin, K.V.; Ivachtchenko, A.V. Synthesis and structure-activity relationship of 4-substituted 2-(2-acetyloxyethyl)-8-(morpholine-4-sulfonyl) pyrrolo [3,4-c]quinoline-1,3-diones as potent caspase-3 inhibitors. J. Med. Chem. 2005, 48, 3680–3683. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence phenomena of carbon dots derived from citric acid and urea—A molecular insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef]
- Vasil’ev, A.N.; Lyshchikov, A.N.; Nasakin, O.E.; Paramonov, S.A.; Kayukov, Y.S. Alkyl 2-amino-5,6-dialkyl-3-cyanopyridine-4-carboxylates in reactions with electrophilic reagents. Russ. J. Org. Chem. 2007, 43, 1537–1544. [Google Scholar] [CrossRef]
- Sun, M.; Qu, S.; Hao, Z.; Ji, W.; Jing, P.; Zhang, H.; Zhang, L.; Zhao, J.; Shen, D. Towards efficient solid-state photoluminescence based on carbon-nanodots and starch composites. Nanoscale 2014, 6, 13076–13081. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Kluwer: New York, NY, USA, 1999. [Google Scholar]
- Hales, J.M.; Matichak, J.; Barlow, S.; Ohira, S.; Yesudas, K.; Brédas, J.L.; Perry, J.W.; Marder, S.R. Design of polymethine dyes with large third-order optical nonlinearities and loss figures of merit. Science 2010, 327, 1485–1488. [Google Scholar] [CrossRef]
- Strickler, S.J.; Berg, R.A. Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Ollis, D.; Mills, A.; Lawrie, K. Kinetics of methylene blue (MB) photocatalyzed reduction and dark regeneration in a colorimetric oxygen sensor. Appl. Catal. B 2016, 184, 201–207. [Google Scholar] [CrossRef]
- Corredor, C.C.; Belfield, K.D.; Bondar, M.V.; Przhonska, O.V.; Yao, S. One- and two-photon photochemical stability of linear and branched fluorene derivatives. J. Photochem. Photobiol. A 2006, 184, 105–112. [Google Scholar] [CrossRef]
- Azim, S.A.; Al-Hazmy, S.M.; Ebeid, E.M.; El-Daly, S.A. El-Daly, A new coumarin laser dye 3-(benzothiazol-2-yl)-7-hydroxycoumarin. Opt. Laser Technol. 2005, 37, 245–249. [Google Scholar] [CrossRef]
- El-Daly, S.A.; El-Azim, S.A.; Elmekawey, F.M.; Elbaradei, B.Y.; Shama, S.A.; Asiri, A.M. Photophysical parameters, excitation energy transfer, and photoreactivity of 1,4-bis (5-phenyl-2-oxazolyl) benzene (POPOP) laser dye. Int. J. Photoenergy 2012, 2012, 458126. [Google Scholar] [CrossRef] [Green Version]
- Fita, P.; Fedoseeva, M.; Vauthey, E. Ultrafast excited-state dynamics of eosin B: A potential probe of the hydrogen-bonding properties of the environment. J. Phys. Chem. A 2011, 115, 2465–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golibrzuch, K.; Ehlers, F.; Scholz, M.; Oswald, R.; Lenzer, T.; Oum, K.; Kim, H.; Koo, S. Ultrafast excited state dynamics and spectroscopy of 13,13′-diphenyl-b-carotene. Phys. Chem. Chem. Phys. 2011, 13, 6340–6351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.S.; Selvaraju, C.; Padma Malar, E.J.; Natarajan, P. Existence of a new emitting singlet state of Proflavine: Femtosecond dynamics of the excited state processes and quantum chemical studies in different solvents. J. Phys. Chem. A 2012, 116, 37–45. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Tang, L.; Oscar, B.; Han, F.; Fang, C. Early time excited-state structural evolution of pyranine in methanol revealed by femtosecond stimulated Raman spectroscopy. J. Phys. Chem. A 2013, 117, 6024–6042. [Google Scholar] [CrossRef]
- Sajadi, M.; Obernhuber, T.; Kovalenko, S.A.; Mosquera, M.; Dick, B.; Ernsting, N.P. Dynamic polar solvation is reported by fluorescing 4-aminophthalimide faithfully despite H-bonding. J. Phys. Chem. A 2009, 113, 44–55. [Google Scholar] [CrossRef]
- Eom, I.; Joo, T. Polar solvation dynamics of coumarin 153 by ultrafast time-resolved fluorescence. J. Chem. Phys. 2009, 131, 244501–244507. [Google Scholar]
- Pérez-Lustres, J.L.; Rodriguez-Prieto, F.; Mosquera, M.; Senyushkina, T.A.; Ernsting, N.P.; Kovalenko, S.A. Ultrafast proton transfer to solvent: Molecularity and intermediates from solvation- and diffusion-controlled regimes. J. Am. Chem. Soc. 2007, 129, 5408–5418. [Google Scholar] [CrossRef]
- Chapman, C.F.; Fee, R.S.; Maroncelli, M. Measurements of the solute dependence of solvation dynamics in 1-propanol: The role of specific hydrogen-bonding interactions. J. Phys. Chem. 1995, 99, 4811–4819. [Google Scholar] [CrossRef]
- Cao, S.; Li, H.; Liu, Y.; Zhang, M.; Wang, M.; Zhou, Z.; Chen, J.; Zhang, S.; Xu, J.; Knutson, J.R. Femtosecond fluorescence spectra of NADH in solution: Ultrafast solvation dynamics. J. Phys. Chem. B 2020, 124, 771–776. [Google Scholar] [CrossRef]
- Sui, B.; Bondar, M.V.; Anderson, D.; Rivera-Jacquez, H.J.; Masunov, A.E.; Belfield, K.D. New two-photon absorbing BODIPY-based fluorescent probe: Linear photophysics, stimulated emission, and ultrafast spectroscopy. J. Phys. Chem. C 2016, 120, 14317–14329. [Google Scholar] [CrossRef]
- Liu, T.; Bondar, M.V.; Belfield, K.D.; Anderson, D.; Masunov, A.E.; Hagan, D.J.; Van Stryland, E.W. Linear photophysics and femtosecond nonlinear spectroscopy of a star-shaped squaraine derivative with efficient two-photon absorption. J. Phys. Chem. C 2016, 120, 11099–11110. [Google Scholar] [CrossRef]
- Shafer, F.P. Dye Lasers; Springer: New York, NY, USA, 1973. [Google Scholar]
- Kasprzyk, W.; Świergosz, T.; Koper, F. Fluorescence assay for the determination of d-panthenol based on novel ring-fused 2-pyridone derivative. Int. J. Mol. Sci. 2020, 21, 8386. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Bednarz, S.; Bogdał, D. Luminescence phenomena of biodegradable photoluminescent poly (diol citrates). Chem. Commun. 2013, 49, 6445–6447. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Koper, F.; Flis, A.; Szreder, D.; Pamuła, E.; Bogdał, D.; Wybraniec, S.; Ortyl, J.; Świergosz, T. Fluorescence assay for the determination of glutathione based on a ring-fused 2-pyridone derivative in dietary supplements. Analyst 2021, 146, 1897–1906. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Bednarz, S.; Zmudzki, P.; Galica, M.; Bogdał, D. Novel efficient fluorophores synthesized from citric acid. RSC Adv. 2015, 5, 34795–34799. [Google Scholar] [CrossRef]
- Miyasaka, H.; Murakami, M.; Okada, T.; Nagata, Y.; Itaya, A.; Kobatake, S.; Irie, M. Picosecond and femtosecond laser photolysis studies of a photochromic diarylethene derivative: Multiphoton gated reaction. Chem. Phys. Lett. 2003, 371, 40–48. [Google Scholar] [CrossRef]
- Bashmakova, N.V.; Shaydyuk, Y.O.; Levchenko, S.M.; Masunov, A.E.; Przhonska, O.V.; Bricks, J.L.; Kachkovsky, O.D.; Slominsky, Y.L.; Piryatinski, Y.P.; Belfield, K.D.; et al. Design and electronic structure of new styryl dye bases: Steady-state and time-resolved spectroscopic studies. J. Phys. Chem. A 2014, 118, 4502–4509. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
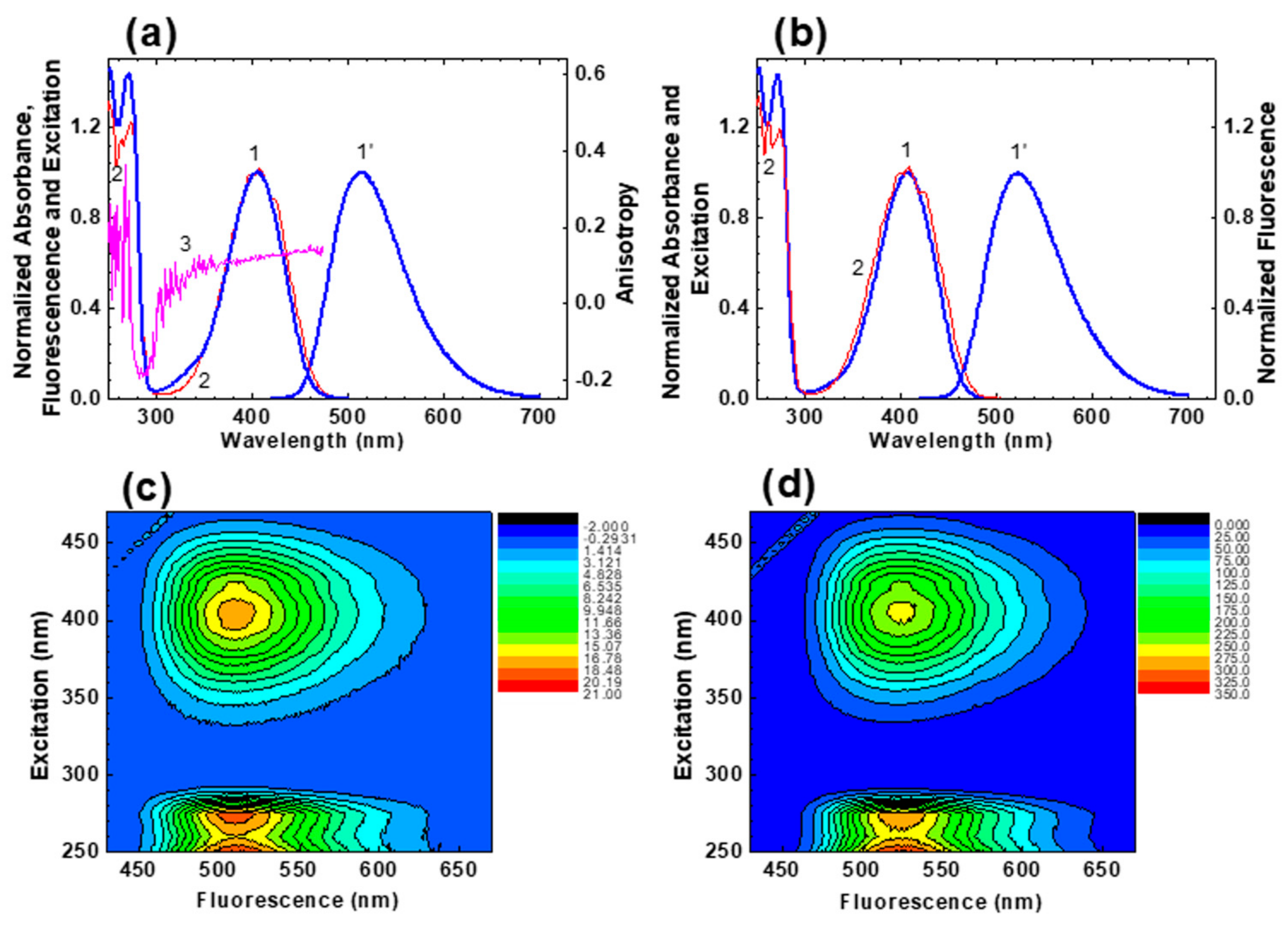
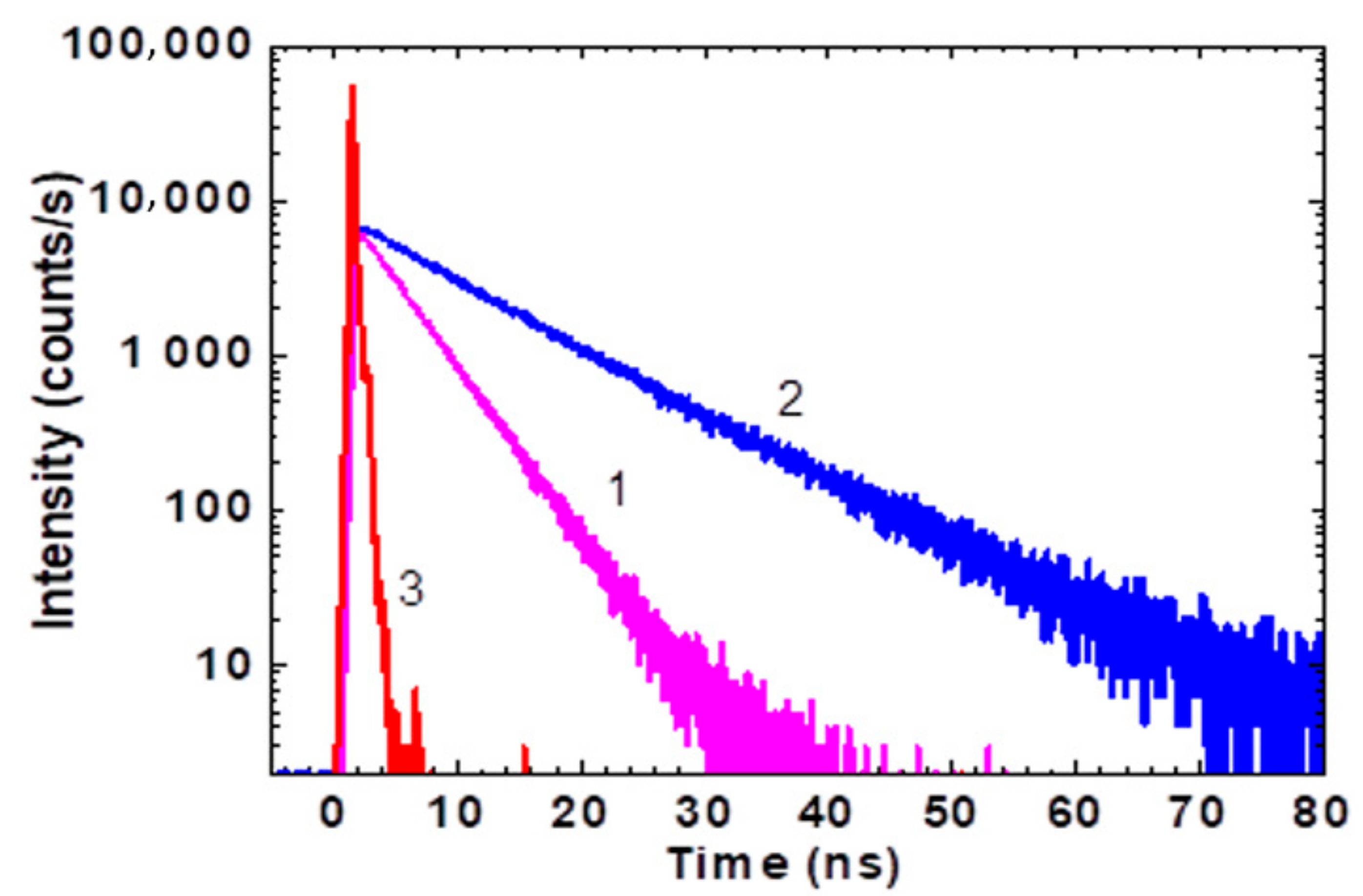
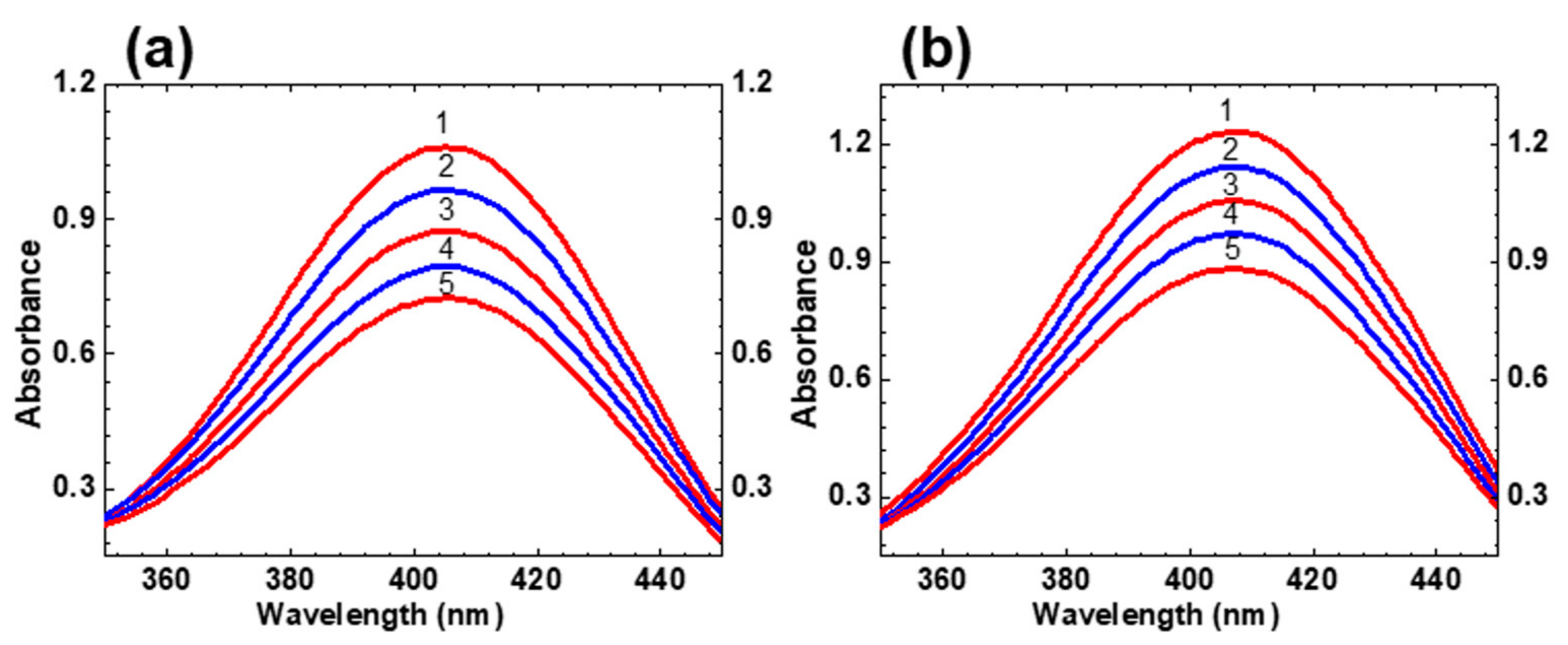

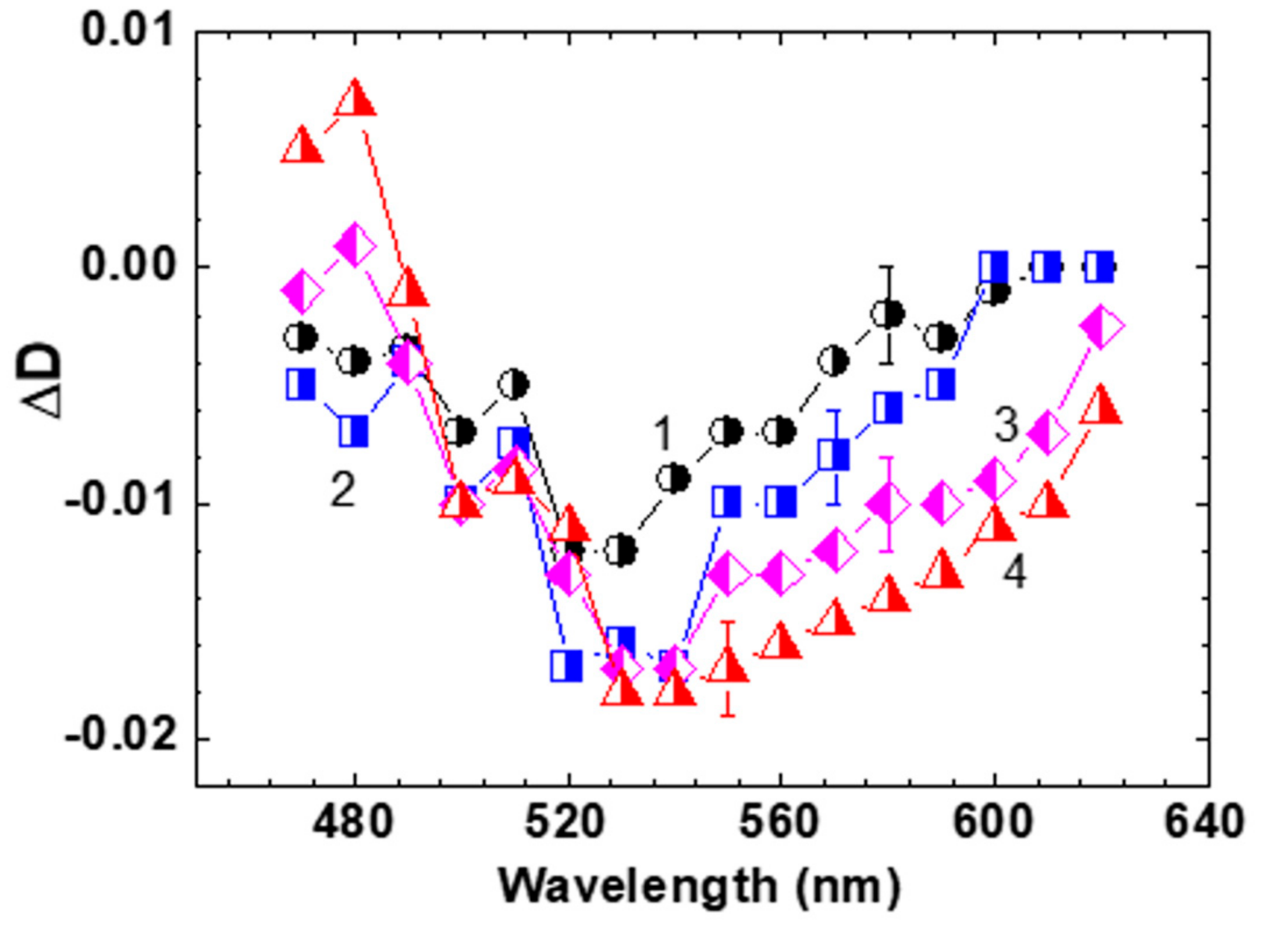
| Solvent | Stokes Shift, cm−1 (nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Methanol | 405 ± 1 | 514 ± 1 | 5240 (109) | 4.5 ± 0.5 | 2.8 | 29 ± 2 | 10.7 ± 2 | 9.7 ± 1 | 6 × 10−4 |
| Water | 406 ± 1 | 523 ± 1 | 5510 (117) | 4.0 ± 0.5 | 2.6 | 9 ± 1 | 3.9 ± 1 | 4.0 ± 0.5 | 5 × 10−4 |
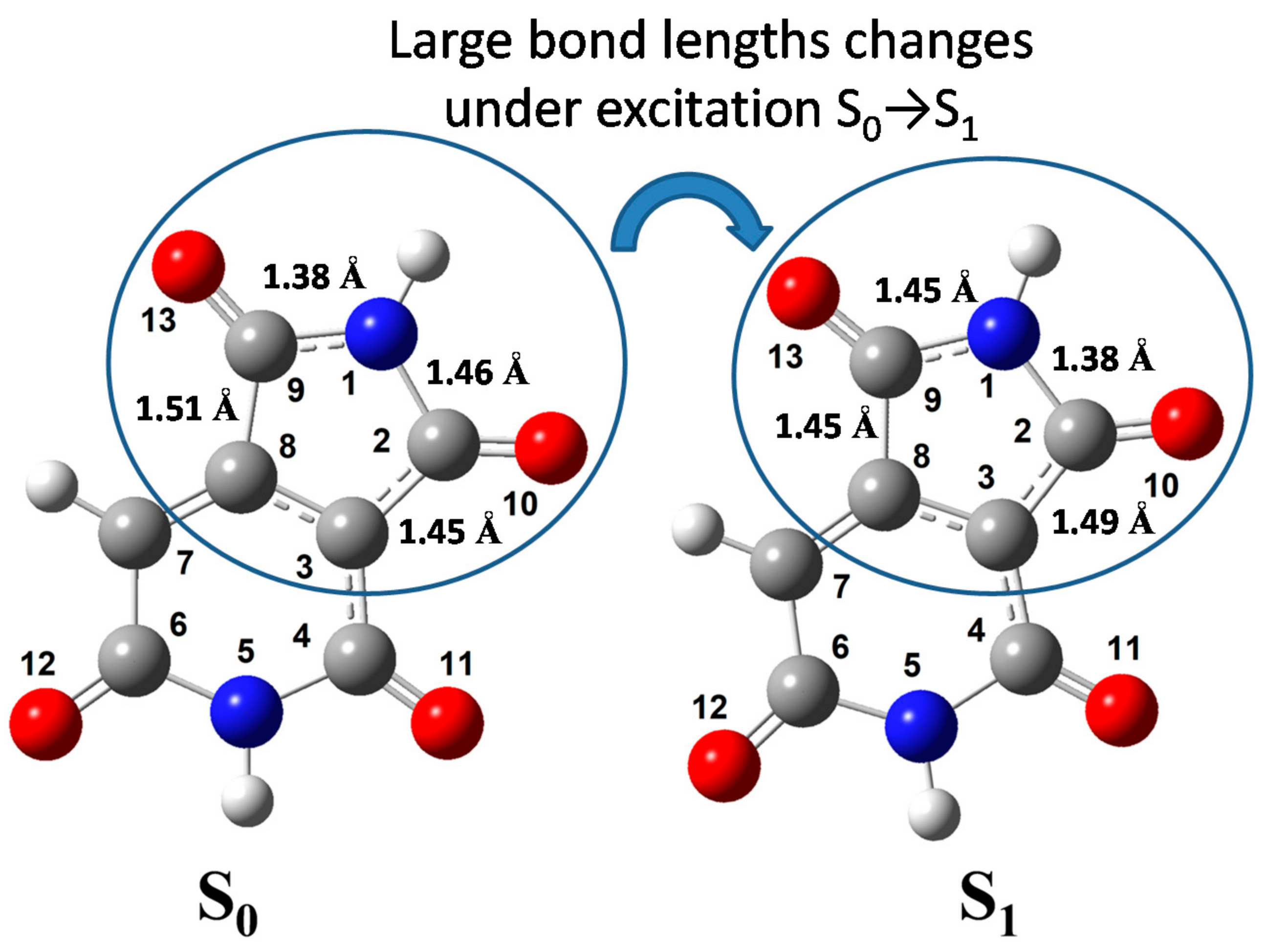
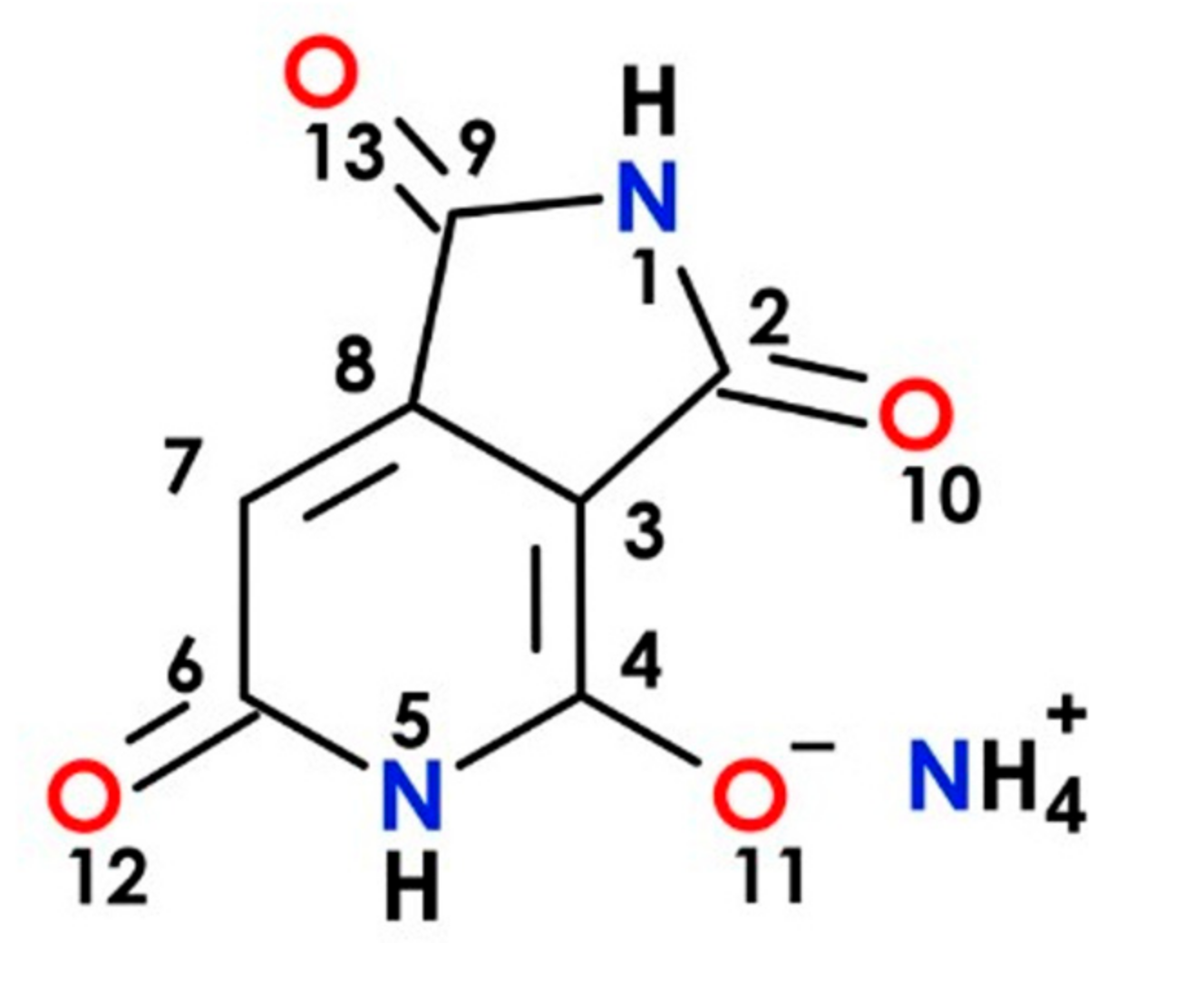
| Atomic Charges and Charge Distribution upon Excitation | Bond Lengths and Their Changes upon Excitation | ||||||
|---|---|---|---|---|---|---|---|
| Atom Number | q0 | q* | Δq = q* − q0 | Bond | ι0, Å | ι*, Å | Δι = ι* − ι0, Å |
| 1—N | −0.603 | −0.583 | 0.020 | 1.2 N-C | 1.456 | 1.3798 | −0.0762 |
| 2—C | +0.509 | +0.533 | 0.024 | 1.9 N-C | 1.3781 | 1.4478 | 0.0697 |
| 3—C | −0.065 | −0.092 | −0.027 | 2.3 C-C | 1.4461 | 1.4888 | 0.0427 |
| 4—C | +0.516 | +0.533 | 0.017 | 2.10 C-O | 1.2228 | 1.2325 | 0.0097 |
| 5—N | −0.605 | −0.604 | 0.001 | 3.4 C-C | 1.4367 | 1.4352 | −0.0015 |
| 6—C | +0.584 | +0.573 | −0.011 | 3.8 C-C | 1.4161 | 1.4547 | 0.0386 |
| 7—C | −0.265 | −0.249 | 0.016 | 4.5 C-N | 1.4212 | 1.3972 | −0.0240 |
| 8—C | +0.065 | +0.132 | 0.067 | 4.11 C-O | 1.2335 | 1.2435 | 0.0100 |
| 9—C | +0.555 | +0.424 | −0.131 | 5.6 N-C | 1.4049 | 1.4129 | 0.0080 |
| 10—O | −0.527 | −0.550 | −0.023 | 6.7 C-C | 1.4505 | 1.4431 | −0.0074 |
| 11—O | −0.563 | −0.509 | 0.054 | 6.12 C-O | 1.2381 | 1.2397 | 0.0016 |
| 12—O | −0.590 | −0.579 | 0.011 | 7.8 C-C | 1.366 | 1.3800 | 0.0140 |
| 13—O | −0.529 | −0.591 | −0.062 | 8.9 C-C | 1.5062 | 1.4468 | −0.0594 |
| 9.13 C-O | 1.2241 | 1.2512 | 0.0271 | ||||
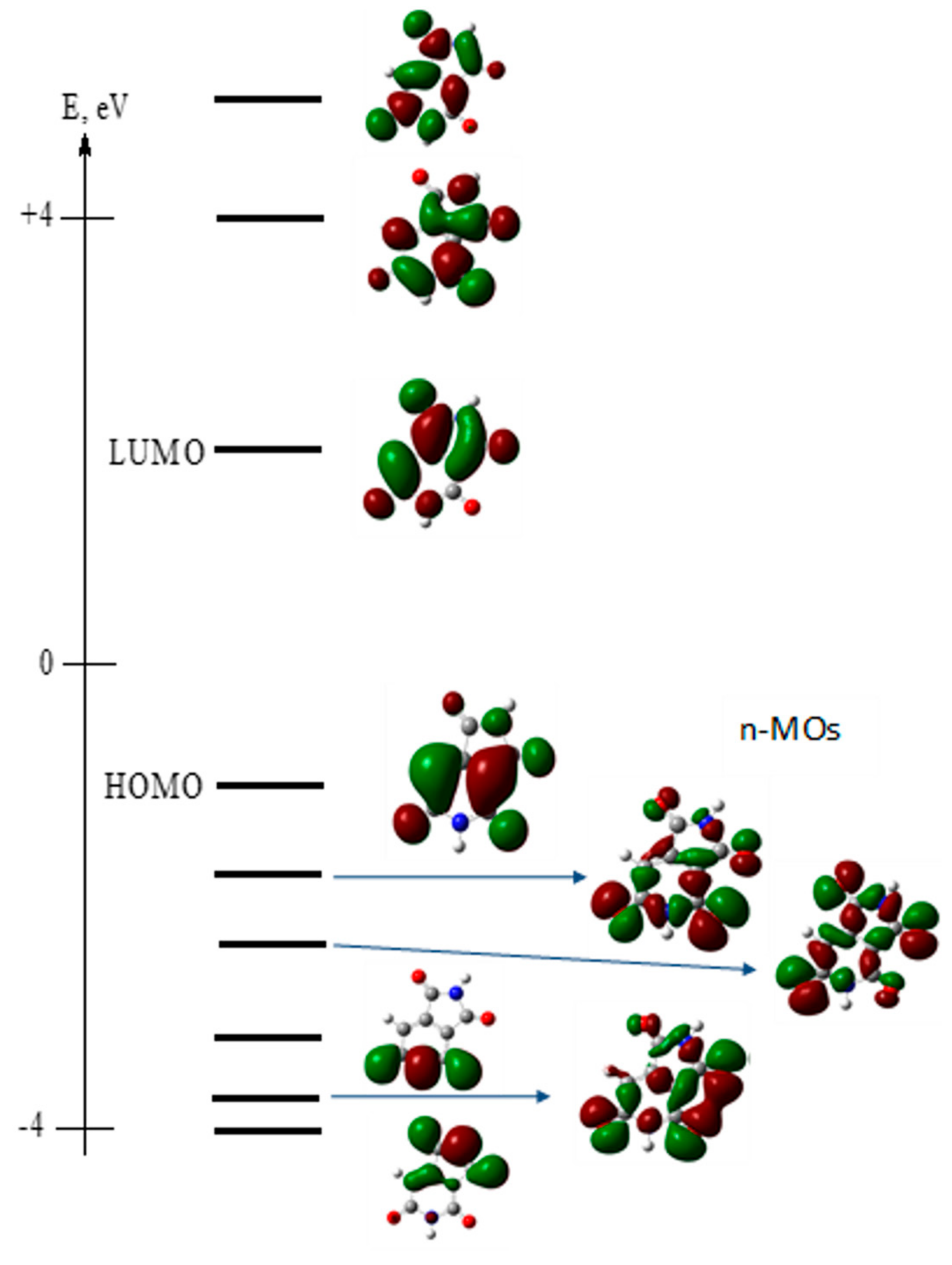
| Transition | λ, nm | f | , D | Transition Type | Main Configuration | |||
|---|---|---|---|---|---|---|---|---|
| S0 → S1 Absorption | 396 | 0.1219 | 3.2024 | 2.8835 | 1.3931 | 0.0000 | π → π* | 0.69 HOMO → LUMO> |
| S0 → S2 | 343 | 0.0001 | 0.0853 | −0.0049 | −0.0010 | 0.0852 | n → π* | 0.69 HOMO-1 → LUMO> |
| S0 → S3 | 319 | 0.0000 | 0.0110 | 0.0001 | 0.0006 | −0.0110 | n → π* | 0.69 HOMO-2 → LUMO> |
| S0 → S4 | 278 | 0.0001 | 0.1002 | −0.0004 | 0.0004 | 0.1002 | n → π* | 0.67 HOMO-4 → LUMO> |
| S0 → S5 | 270 | 0.0098 | 0.7497 | −0.4872 | −0.5699 | 0.0000 | π → π* | 0.69 HOMO-3 → LUMO> |
| S1 → S0 Fluorescence | 513 | 0.0717 | 2.7954 | −2.3859 | 1.4565 | 0.0000 | π → π* | 0.70 HOMO → LUMO> |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashmakova, N.V.; Shaydyuk, Y.O.; Dmytruk, A.M.; Świergosz, T.; Kachkovsky, O.D.; Belfield, K.D.; Bondar, M.V.; Kasprzyk, W. Nature of Linear Spectral Properties and Fast Electronic Relaxations in Green Fluorescent Pyrrolo[3,4-c]Pyridine Derivative. Int. J. Mol. Sci. 2021, 22, 5592. https://doi.org/10.3390/ijms22115592
Bashmakova NV, Shaydyuk YO, Dmytruk AM, Świergosz T, Kachkovsky OD, Belfield KD, Bondar MV, Kasprzyk W. Nature of Linear Spectral Properties and Fast Electronic Relaxations in Green Fluorescent Pyrrolo[3,4-c]Pyridine Derivative. International Journal of Molecular Sciences. 2021; 22(11):5592. https://doi.org/10.3390/ijms22115592
Chicago/Turabian StyleBashmakova, Nataliia V., Yevgeniy O. Shaydyuk, Andriy M. Dmytruk, Tomasz Świergosz, Olexiy D. Kachkovsky, Kevin D. Belfield, Mykhailo V. Bondar, and Wiktor Kasprzyk. 2021. "Nature of Linear Spectral Properties and Fast Electronic Relaxations in Green Fluorescent Pyrrolo[3,4-c]Pyridine Derivative" International Journal of Molecular Sciences 22, no. 11: 5592. https://doi.org/10.3390/ijms22115592
APA StyleBashmakova, N. V., Shaydyuk, Y. O., Dmytruk, A. M., Świergosz, T., Kachkovsky, O. D., Belfield, K. D., Bondar, M. V., & Kasprzyk, W. (2021). Nature of Linear Spectral Properties and Fast Electronic Relaxations in Green Fluorescent Pyrrolo[3,4-c]Pyridine Derivative. International Journal of Molecular Sciences, 22(11), 5592. https://doi.org/10.3390/ijms22115592







