Mitochondrial Retrograde Signaling Contributes to Metabolic Differentiation in Yeast Colonies
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Proteomic Analysis of Colony Cell Subpopulations
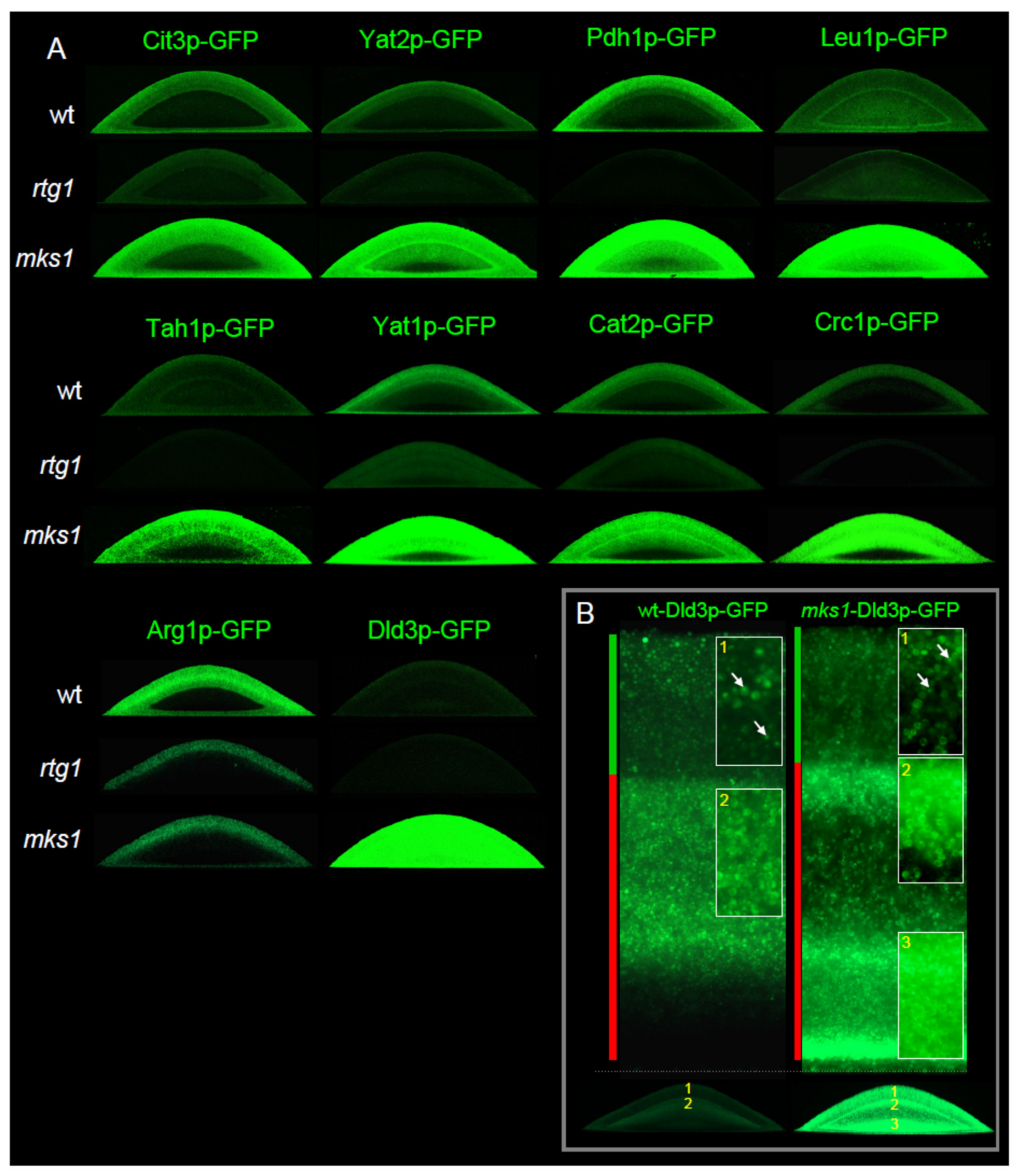
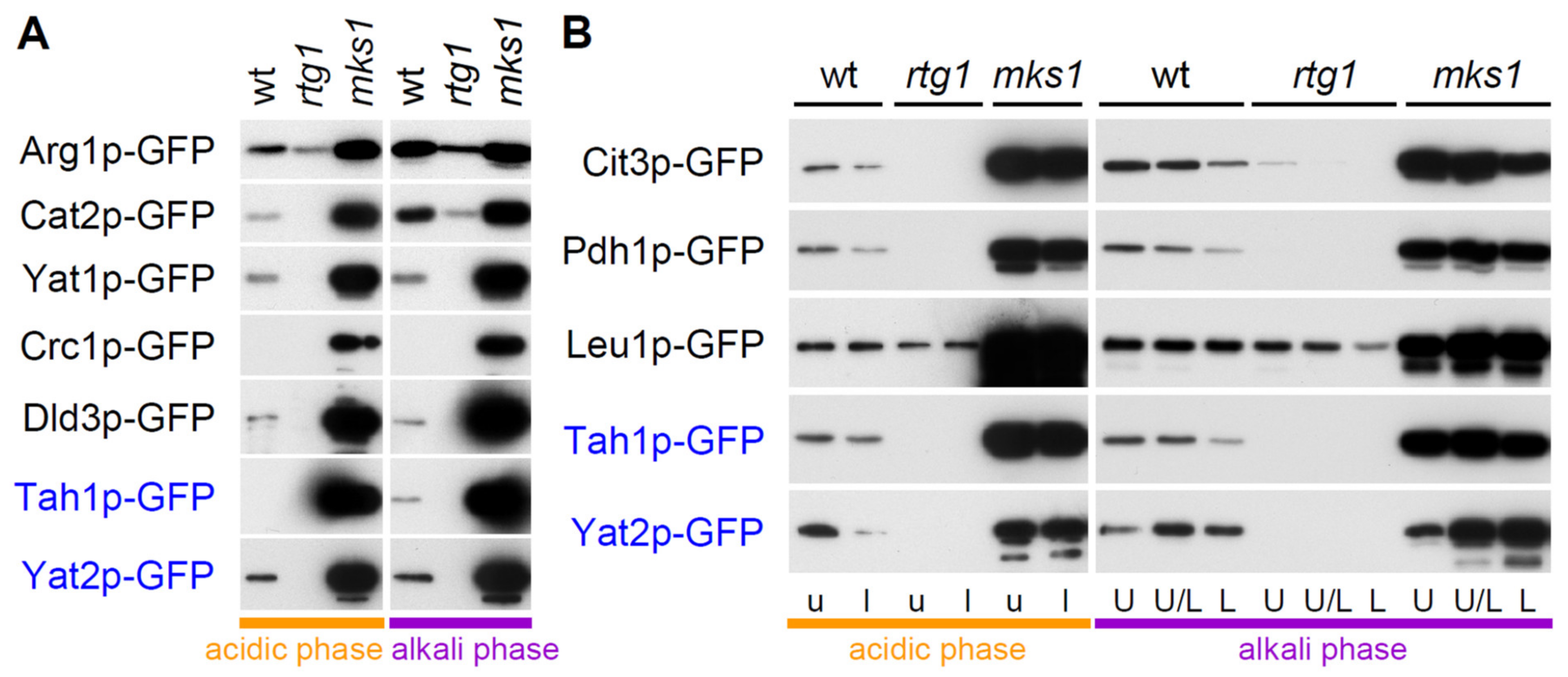
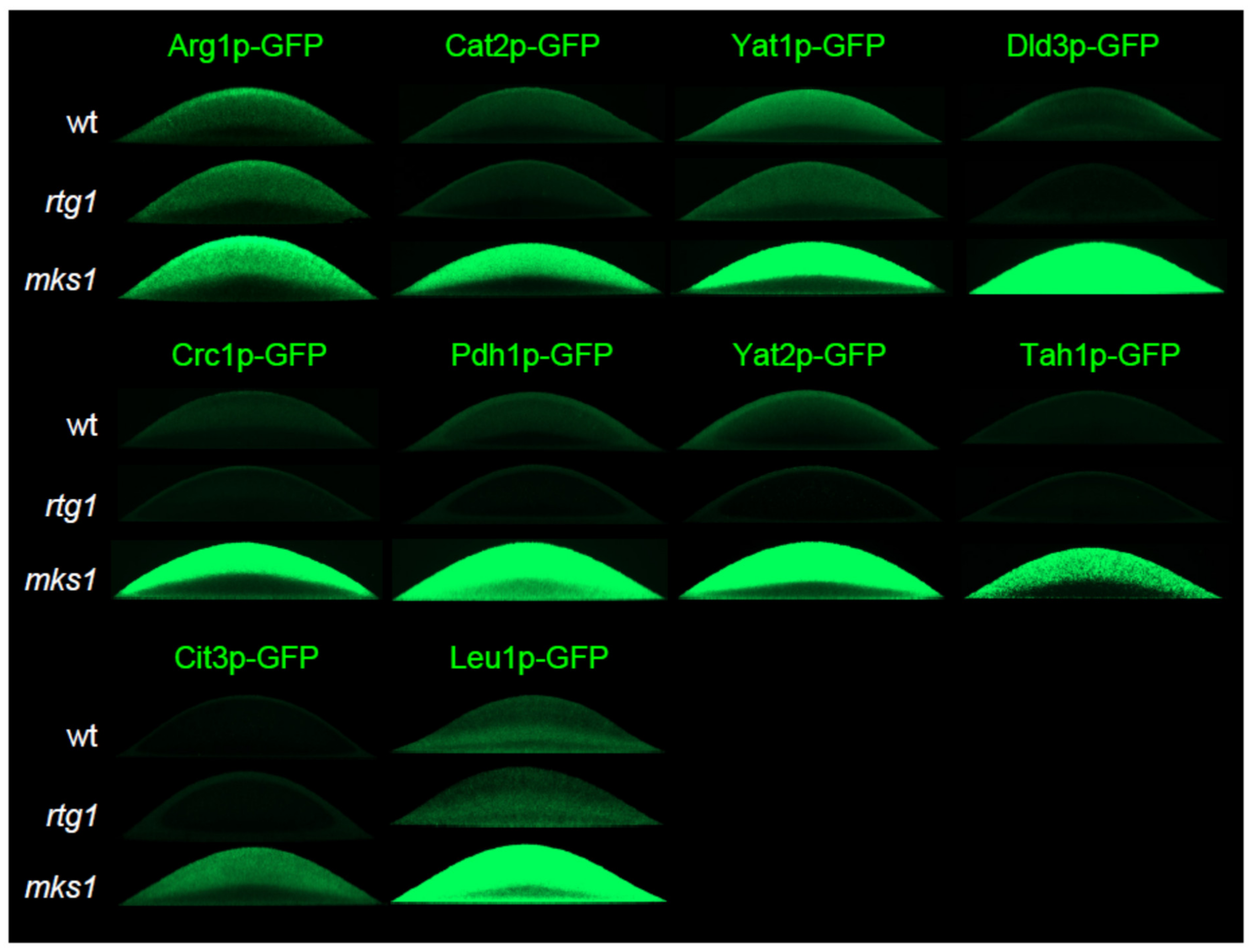
2.2. Group 1 Proteins Are Regulated by the RTG Pathway in a Standard Way
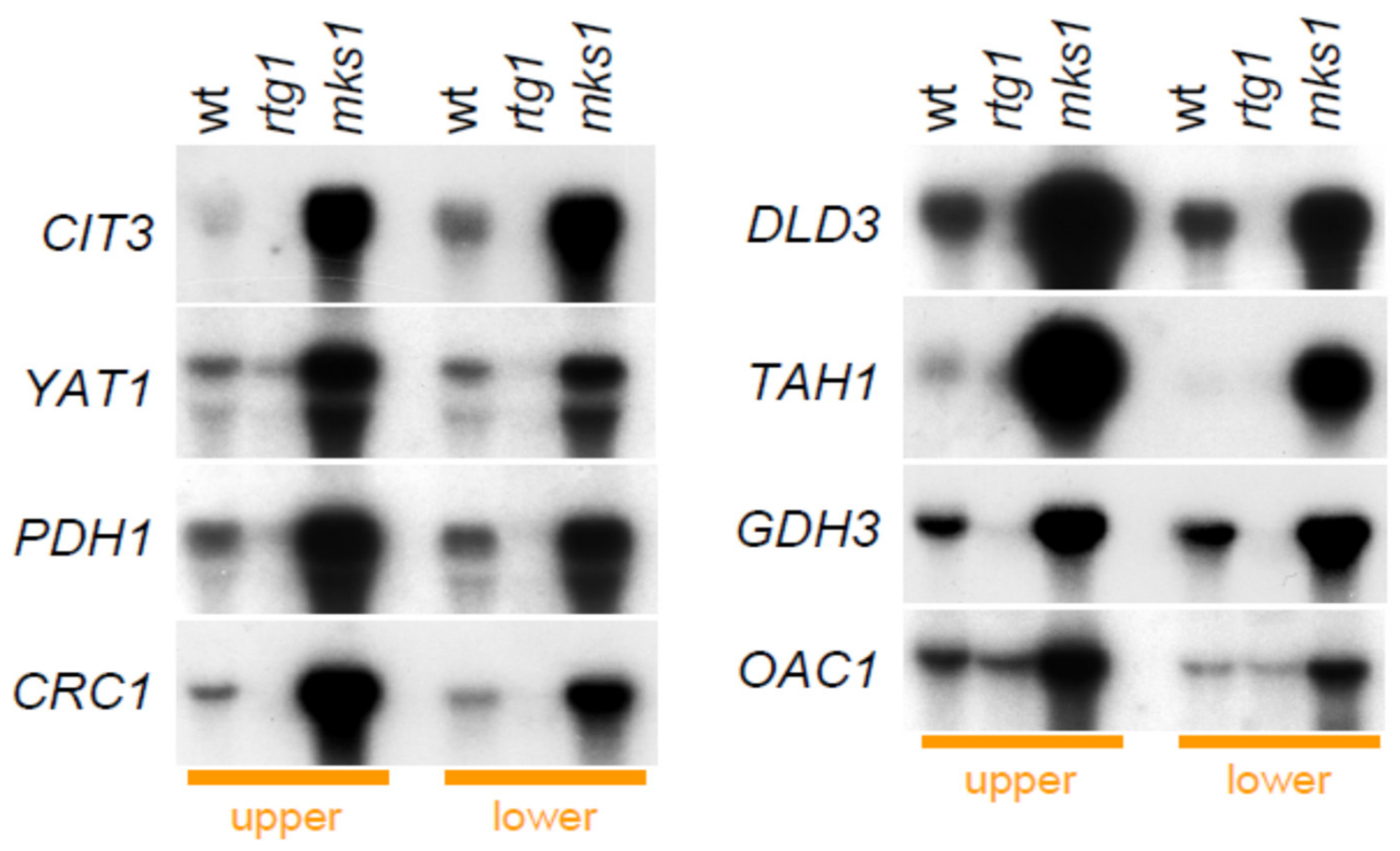
2.3. Group 1 Proteins Are Regulated by the RTG Pathway at the Level of mRNA Expression
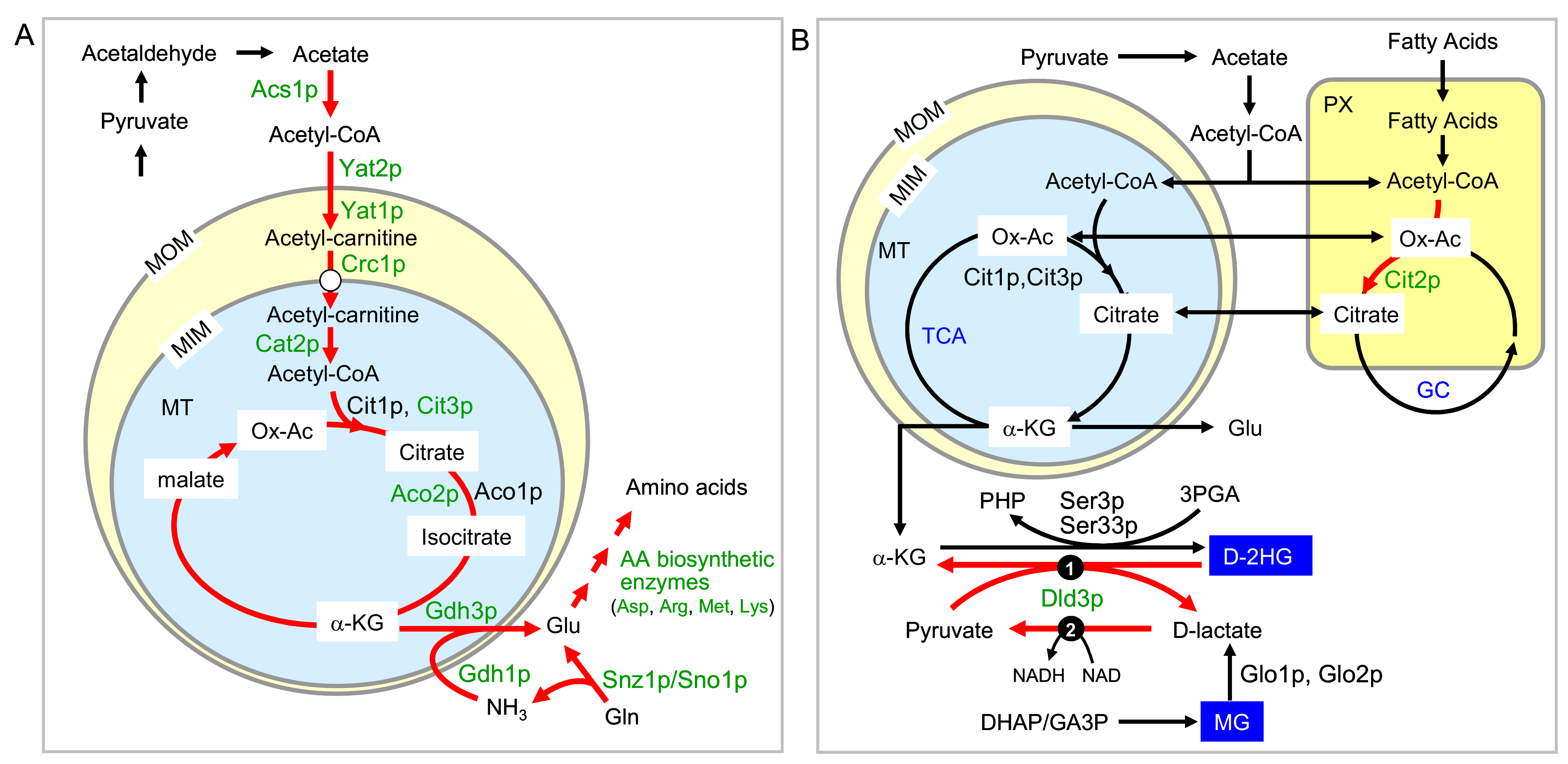
2.4. Two Other Groups of Proteins Are Regulated by Rtg Proteins Independently of Mks1p Repression
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Cultivation
4.2. Colony Imaging Microscopy
4.3. Cell Biomass Harvesting
4.4. Proteomics Analysis
4.5. Determination of the Amount of GFP-Labeled Proteins by Western Blot
4.6. RNA Isolation and Northern Blotting
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guha, M.; Avadhani, N.G. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion 2013, 13, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Jazwinski, S.M. The retrograde response: A conserved compensatory reaction to damage from within and from without. Prog. Mol. Biol. Transl. Sci. 2014, 127, 133–154. [Google Scholar] [PubMed]
- Liu, Z.; Butow, R.A. Mitochondrial retrograde signaling. Annu. Rev. Genet. 2006, 40, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Junior, J.R.; Spirek, M.; Liu, Z.; Butow, R.A. Interaction between Rtg2p and Mks1p in the regulation of the RTG pathway of Saccharomyces cerevisiae. Gene 2005, 354, 2–8. [Google Scholar] [CrossRef]
- Liu, Z.; Sekito, T.; Spirek, M.; Thornton, J.; Butow, R.A. Retrograde signaling is regulated by the dynamic interaction between Rtg2p and Mks1p. Mol. Cell 2003, 12, 401–411. [Google Scholar] [CrossRef]
- Dilova, I.; Aronova, S.; Chen, J.C.; Powers, T. Tor signaling and nutrient-based signals converge on Mks1p phosphorylation to regulate expression of Rtg1.Rtg3p-dependent target genes. J. Biol. Chem. 2004, 279, 46527–46535. [Google Scholar] [CrossRef]
- Dilova, I.; Chen, C.Y.; Powers, T. Mks1 in concert with TOR signaling negatively regulates RTG target gene expression in S. cerevisiae. Curr. Biol. 2002, 12, 389–395. [Google Scholar] [CrossRef]
- Chelstowska, A.; Butow, R.A. RTG genes in yeast that function in communication between mitochondria and the nucleus are also required for expression of genes encoding peroxisomal proteins. J. Biol. Chem. 1995, 270, 18141–18146. [Google Scholar] [CrossRef]
- Epstein, C.B.; Waddle, J.A.; Hale IV, W.; Dave, V.; Thornton, J.; Macatee, T.L.; Garner, H.R.; Butow, R.A. Genome-wide responses to mitochondrial dysfunction. Mol. Biol. Cell 2001, 12, 297–308. [Google Scholar] [CrossRef]
- Guaragnella, N.; Coyne, L.P.; Chen, X.J.; Giannattasio, S. Mitochondria-cytosol-nucleus crosstalk: Learning from Saccharomyces cerevisiae. FEMS Yeast Res. 2018, 18, foy088. [Google Scholar] [CrossRef]
- Palkova, Z.; Vachova, L. Yeast cell differentiation: Lessons from pathogenic and non-pathogenic yeasts. Semin. Cell Dev. Biol. 2016, 57, 110–119. [Google Scholar] [CrossRef]
- Vachova, L.; Palkova, Z. How structured yeast multicellular communities live, age and die? FEMS Yeast Res. 2018, 18, foy033. [Google Scholar] [CrossRef]
- Podholová, K.; Plocek, V.; Rešetárová, S.; Kučerová, H.; Hlaváček, O.; Váchová, L.; Palková, Z. Divergent branches of mitochondrial signaling regulate specific genes and the viability of specialized cell types of differentiated yeast colonies. Oncotarget 2016, 7, 15299–15314. [Google Scholar] [CrossRef]
- Cap, M.; Stepanek, L.; Harant, K.; Vachova, L.; Palkova, Z. Cell differentiation within a yeast colony: Metabolic and regulatory parallels with a tumor-affected organism. Mol. Cell 2012, 46, 436–448. [Google Scholar] [CrossRef]
- Palkova, Z.; Devaux, F.; Ricicova, M.; Minarikova, L.; Le Crom, S.; Jacq, C. Ammonia pulses and metabolic oscillations guide yeast colony development. Mol. Biol. Cell 2002, 13, 3901–3914. [Google Scholar] [CrossRef]
- Palkova, Z.; Janderova, B.; Gabriel, J.; Zikanova, B.; Pospisek, M.; Forstova, J. Ammonia mediates communication between yeast colonies. Nature 1997, 390, 532–536. [Google Scholar] [CrossRef]
- Vachova, L.; Hatakova, L.; Cap, M.; Pokorna, M.; Palkova, Z. Rapidly developing yeast microcolonies differentiate in a similar way to aging giant colonies. Oxid. Med. Cell Longev. 2013, 2013, 102485. [Google Scholar] [CrossRef]
- Cap, M.; Vachova, L.; Palkova, Z. Longevity of U cells of differentiated yeast colonies grown on respiratory medium depends on active glycolysis. Cell Cycle 2015, 14, 3488–3497. [Google Scholar] [CrossRef]
- Franken, J.; Kroppenstedt, S.; Swiegers, J.H.; Bauer, F.F. Carnitine and carnitine acetyltransferases in the yeast Saccharomyces cerevisiae: A role for carnitine in stress protection. Curr. Genet. 2008, 53, 347–360. [Google Scholar] [CrossRef]
- Van Rossum, H.M.; Kozak, B.U.; Niemeijer, M.S.; Duine, H.J.; Luttik, M.A.; Boer, V.M.; Kotter, P.; Daran, J.M.; van Maris, A.J.; Pronk, J.T. Alternative reactions at the interface of glycolysis and citric acid cycle in Saccharomyces cerevisiae. FEMS Yeast Res. 2016, 16, fow017. [Google Scholar] [CrossRef]
- Xiberras, J.; Klein, M.; Nevoigt, E. Glycerol as a substrate for Saccharomyces cerevisiae based bioprocesses—Knowledge gaps regarding the central carbon catabolism of this ‘non-fermentable’ carbon source. Biotechnol. Adv. 2019, 37, 107378. [Google Scholar] [CrossRef] [PubMed]
- Xiberras, J.; Klein, M.; Prosch, C.; Malubhoy, Z.; Nevoigt, E. Anaplerotic reactions active during growth of Saccharomyces cerevisiae on glycerol. FEMS Yeast Res. 2020, 20, foz086. [Google Scholar] [CrossRef] [PubMed]
- Graybill, E.R.; Rouhier, M.F.; Kirby, C.E.; Hawes, J.W. Functional comparison of citrate synthase isoforms from S. cerevisiae. Arch. Biochem. Biophys. 2007, 465, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, K.; Nishimura, T.; Byrne, S.D.; Okamoto, M.; Takahashi-Nakaguchi, A.; Chibana, H.; Okumura, F.; Kamura, T. The ubiquitin ligase SCF(Ucc1) acts as a metabolic switch for the glyoxylate cycle. Mol. Cell 2015, 59, 22–34. [Google Scholar] [CrossRef]
- Liu, Z.; Butow, R.A. A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol. Cell Biol. 1999, 19, 6720–6728. [Google Scholar] [CrossRef]
- Schuller, H.J. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 139–160. [Google Scholar] [CrossRef]
- Becker-Kettern, J.; Paczia, N.; Conrotte, J.F.; Kay, D.P.; Guignard, C.; Jung, P.P.; Linster, C.L. Saccharomyces cerevisiae forms D-2-Hydroxyglutarate and couples its degradation to D-lactate formation via a cytosolic transhydrogenase. J. Biol. Chem. 2016, 291, 6036–6058. [Google Scholar] [CrossRef]
- Pallotta, M.L. Mitochondrial involvement to methylglyoxal detoxification: D-Lactate/Malate antiporter in Saccharomyces cerevisiae. Antonie Van Leeuwenhoek 2012, 102, 163–175. [Google Scholar] [CrossRef]
- De Bari, L.; Atlante, A.; Armeni, T.; Kalapos, M.P. Synthesis and metabolism of methylglyoxal, S-D-lactoylglutathione and D-lactate in cancer and Alzheimer’s disease. Exploring the crossroad of eternal youth and premature aging. Ageing Res. Rev. 2019, 53, 100915. [Google Scholar] [CrossRef]
- Stewart, B.J.; Navid, A.; Kulp, K.S.; Knaack, J.L.; Bench, G. D-Lactate production as a function of glucose metabolism in Saccharomyces cerevisiae. Yeast 2013, 30, 81–91. [Google Scholar] [CrossRef]
- Passarella, S.; Schurr, A. l-Lactate transport and metabolism in mitochondria of Hep G2 cells-The Cori cycle revisited. Front. Oncol. 2018, 8, 120. [Google Scholar] [CrossRef]
- Dilova, I.; Powers, T. Accounting for strain-specific differences during RTG target gene regulation in Saccharomyces cerevisiae. FEMS Yeast Res. 2006, 6, 112–119. [Google Scholar] [CrossRef]
- Tate, J.J.; Cox, K.H.; Rai, R.; Cooper, T.G. Mks1p is required for negative regulation of retrograde gene expression in Saccharomyces cerevisiae but does not affect nitrogen catabolite repression-sensitive gene expression. J. Biol. Chem. 2002, 277, 20477–20482. [Google Scholar] [CrossRef]
- Giannattasio, S.; Liu, Z.; Thornton, J.; Butow, R.A. Retrograde response to mitochondrial dysfunction is separable from TOR1/2 regulation of retrograde gene expression. J. Biol. Chem. 2005, 280, 42528–42535. [Google Scholar] [CrossRef]
- Sekito, T.; Thornton, J.; Butow, R.A. Mitochondria-to-nuclear signaling is regulated by the subcellular localization of the transcription factors Rtg1p and Rtg3p. Mol. Biol. Cell 2000, 11, 2103–2115. [Google Scholar] [CrossRef]
- Palkova, Z.; Wilkinson, D.; Vachova, L. Aging and differentiation in yeast populations: Elders with different properties and functions. FEMS Yeast Res. 2014, 14, 96–108. [Google Scholar] [CrossRef]
- Wilkinson, D.; Marsikova, J.; Hlavacek, O.; Gilfillan, G.D.; Jezkova, E.; Aaløkken, R.; Vachova, L.; Palkova, Z. Transcriptome remodeling of differentiated cells during chronological ageing of yeast colonies: New insights into metabolic differentiation. Oxid. Med. Cell Longev. 2018, 2018, 4932905. [Google Scholar] [CrossRef]
- Ihmels, J.; Friedlander, G.; Bergmann, S.; Sarig, O.; Ziv, Y.; Barkai, N. Revealing modular organization in the yeast transcriptional network. Nat. Genet. 2002, 31, 370–377. [Google Scholar] [CrossRef]
- Graack, H.R.; Wittmann-Liebold, B. Mitochondrial ribosomal proteins (MRPs) of yeast. Biochem. J. 1998, 329, 433–448. [Google Scholar] [CrossRef]
- Lai, L.C.; Kosorukoff, A.L.; Burke, P.V.; Kwast, K.E. Metabolic-state-dependent remodeling of the transcriptome in response to anoxia and subsequent reoxygenation in Saccharomyces cerevisiae. Eukaryot Cell 2006, 5, 1468–1489. [Google Scholar] [CrossRef]
- Kim, H.J.; Maiti, P.; Barrientos, A. Mitochondrial ribosomes in cancer. Semin. Cancer Biol. 2017, 47, 67–81. [Google Scholar] [CrossRef]
- Sheff, M.A.; Thorn, K.S. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 2004, 21, 661–670. [Google Scholar] [CrossRef]
- Guldener, U.; Heck, S.; Fielder, T.; Beinhauer, J.; Hegemann, J.H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996, 24, 2519–2524. [Google Scholar] [CrossRef]
- Gietz, R.D.; Woods, R.A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002, 350, 87–96. [Google Scholar]
- Vachova, L.; Chernyavskiy, O.; Strachotova, D.; Bianchini, P.; Burdikova, Z.; Fercikova, I.; Kubinova, L.; Palkova, Z. Architecture of developing multicellular yeast colony: Spatio-temporal expression of Ato1p ammonium exporter. Environ. Microbiol. 2009, 11, 1866–1877. [Google Scholar] [CrossRef]
- Vachova, L.; Stovicek, V.; Hlavacek, O.; Chernyavskiy, O.; Stepanek, L.; Kubinova, L.; Palkova, Z. Flo11p, drug efflux pumps, and the extracellular matrix cooperate to form biofilm yeast colonies. J. Cell Biol. 2011, 194, 679–687. [Google Scholar] [CrossRef]
- Marsikova, J.; Pavlickova, M.; Wilkinson, D.; Vachova, L.; Hlavacek, O.; Hatakova, L.; Palkova, Z. The Whi2p-Psr1p/Psr2p complex regulates interference competition and expansion of cells with competitive advantage in yeast colonies. Proc. Natl. Acad. Sci. USA 2020, 117, 15123–15131. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Muller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Vachova, L.; Kucerova, H.; Devaux, F.; Ulehlova, M.; Palkova, Z. Metabolic diversification of cells during the development of yeast colonies. Environ. Microbiol. 2009, 11, 494–504. [Google Scholar] [CrossRef]

| Strain | Genotype | Source |
|---|---|---|
| BY4742 | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0 | Euroscarf |
| BY-rtg1 | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX | [13] |
| BY-mks1 | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX | [13] |
| BY-rtg1mks1 | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::hph, mks1::NatMX | [13] |
| BY-rtg2mks1 | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg2::hph, mks1::NatMX | [13] |
| BY-Arg1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, ARG1-yEGFP-KanMX | this study |
| BY-rtg1-Arg1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, ARG1-yEGFP-KanMX | this study |
| BY-mks1-Arg1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, ARG1-yEGFP-KanMX | this study |
| BY-Cat2p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, CAT2-yEGFP-KanMX | this study |
| BY-rtg1-Cat2p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, CAT2-yEGFP-KanMX | this study |
| BY-mks1-Cat2p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, CAT2-yEGFP-KanMX | this study |
| BY-Cit3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, CIT3-yEGFP-KanMX | this study |
| BY-rtg1-Cit3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, CIT3-yEGFP-KanMX | this study |
| BY-mks1-Cit3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, CIT3-yEGFP-KanMX | this study |
| BY-Crc1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, CRC1-yEGFP-KanMX | this study |
| BY-rtg1-Crc1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, CRC1-yEGFP-KanMX | this study |
| BY-mks1-Crc1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, CRC1-yEGFP-KanMX | this study |
| BY-Dld3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, DLD3-yEGFP-KanMX | this study |
| BY-rtg1-Dld3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, DLD3-yEGFP-KanMX | this study |
| BY-mks1-Dld3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, DLD3-yEGFP-KanMX | this study |
| BY-Gdh3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, GDH3-yEGFP-KanMX | this study |
| BY-rtg1-Gdh3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, GDH3-yEGFP-KanMX | this study |
| BY-mks1-Gdh3p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, GDH3-yEGFP-KanMX | this study |
| BY-Leu1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, LEU1-yEGFP-KanMX | this study |
| BY-rtg1-Leu1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, LEU1-yEGFP-KanMX | this study |
| BY-mks1-Leu1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, LEU1-yEGFP-KanMX | this study |
| BY-Oac1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, OAC1-yEGFP-KanMX | this study |
| BY-rtg1-Oac1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, OAC1-yEGFP-KanMX | this study |
| BY-mks1-Oac1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, OAC1-yEGFP-KanMX | this study |
| BY-Pdh1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, PDH1-yEGFP-KanMX | this study |
| BY-rtg1-Pdh1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, PDH1-yEGFP-KanMX | this study |
| BY-mks1-Pdh1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, PDH1-yEGFP-KanMX | this study |
| BY-Tah1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, TAH1-yEGFP-KanMX | this study |
| BY-rtg1-Tah1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, TAH1-yEGFP-KanMX | this study |
| BY-mks1-Tah1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, TAH1-yEGFP-KanMX | this study |
| BY-Yat1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, YAT1-yEGFP-KanMX | this study |
| BY-rtg1-Yat1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, YAT1-yEGFP-KanMX | this study |
| BY-mks1-Yat1p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, YAT1-yEGFP-KanMX | this study |
| BY-Yat2p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, YAT2-yEGFP-KanMX | this study |
| BY-rtg1-Yat2p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, rtg1::NatMX, YAT2-yEGFP-KanMX | this study |
| BY-mks1-Yat2p-GFP | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, mks1::NatMX, YAT2-yEGFP-KanMX | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plocek, V.; Fadrhonc, K.; Maršíková, J.; Váchová, L.; Pokorná, A.; Hlaváček, O.; Wilkinson, D.; Palková, Z. Mitochondrial Retrograde Signaling Contributes to Metabolic Differentiation in Yeast Colonies. Int. J. Mol. Sci. 2021, 22, 5597. https://doi.org/10.3390/ijms22115597
Plocek V, Fadrhonc K, Maršíková J, Váchová L, Pokorná A, Hlaváček O, Wilkinson D, Palková Z. Mitochondrial Retrograde Signaling Contributes to Metabolic Differentiation in Yeast Colonies. International Journal of Molecular Sciences. 2021; 22(11):5597. https://doi.org/10.3390/ijms22115597
Chicago/Turabian StylePlocek, Vítězslav, Kristýna Fadrhonc, Jana Maršíková, Libuše Váchová, Alexandra Pokorná, Otakar Hlaváček, Derek Wilkinson, and Zdena Palková. 2021. "Mitochondrial Retrograde Signaling Contributes to Metabolic Differentiation in Yeast Colonies" International Journal of Molecular Sciences 22, no. 11: 5597. https://doi.org/10.3390/ijms22115597
APA StylePlocek, V., Fadrhonc, K., Maršíková, J., Váchová, L., Pokorná, A., Hlaváček, O., Wilkinson, D., & Palková, Z. (2021). Mitochondrial Retrograde Signaling Contributes to Metabolic Differentiation in Yeast Colonies. International Journal of Molecular Sciences, 22(11), 5597. https://doi.org/10.3390/ijms22115597






