Preferent Diaphragmatic Involvement in TK2 Deficiency: An Autopsy Case Study
Abstract
:1. Introduction
2. Results
2.1. Clinical Summary
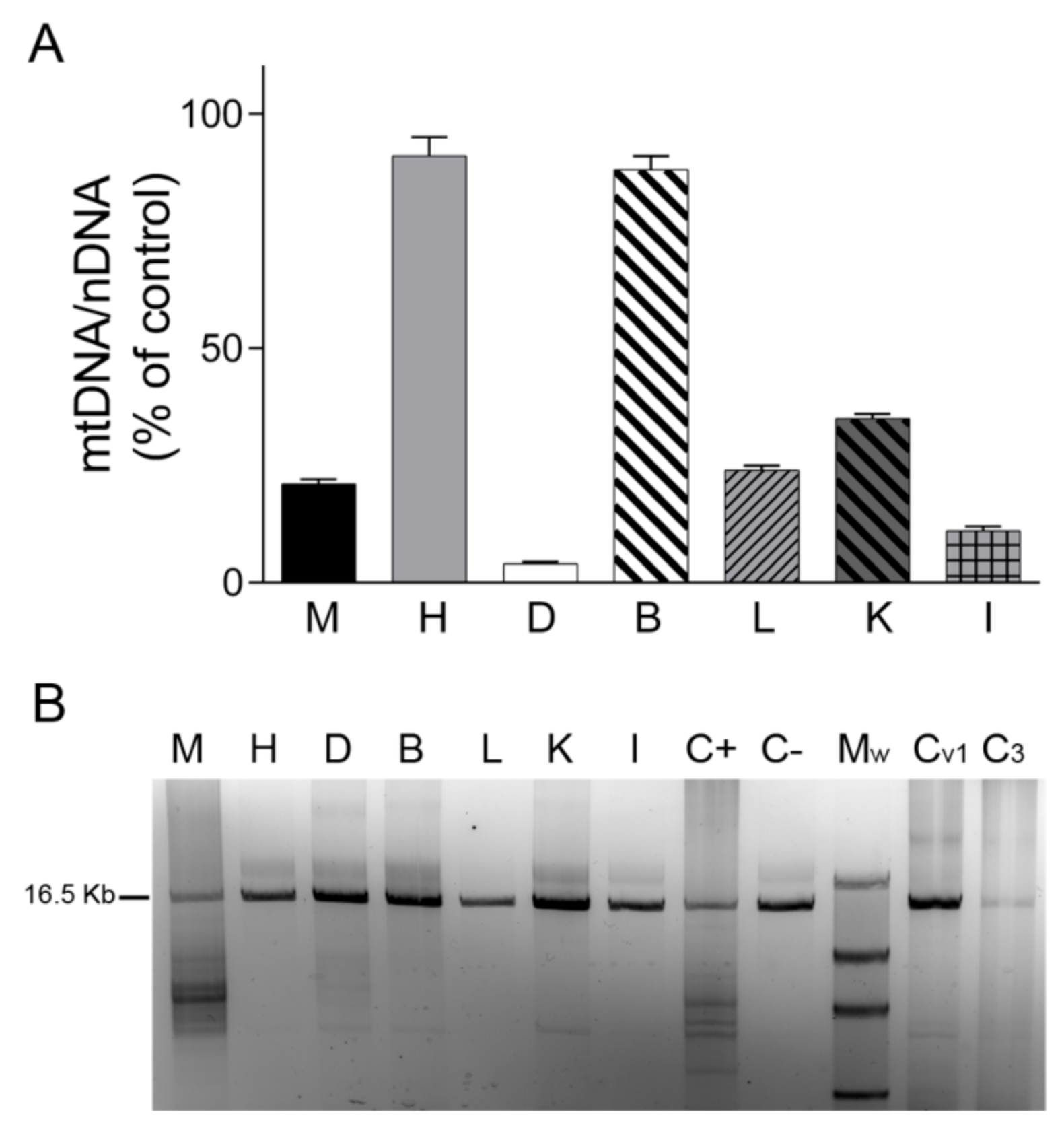
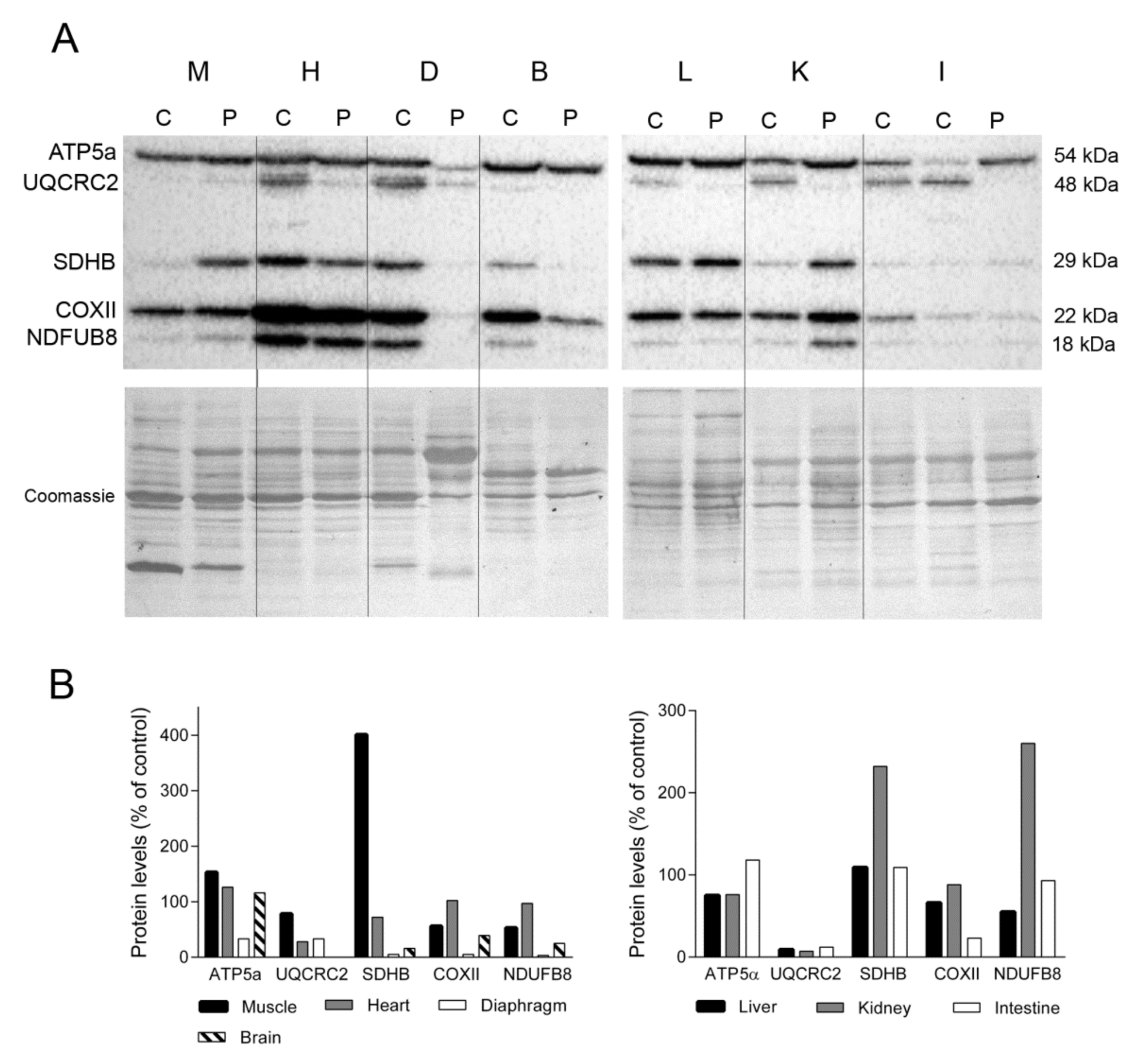
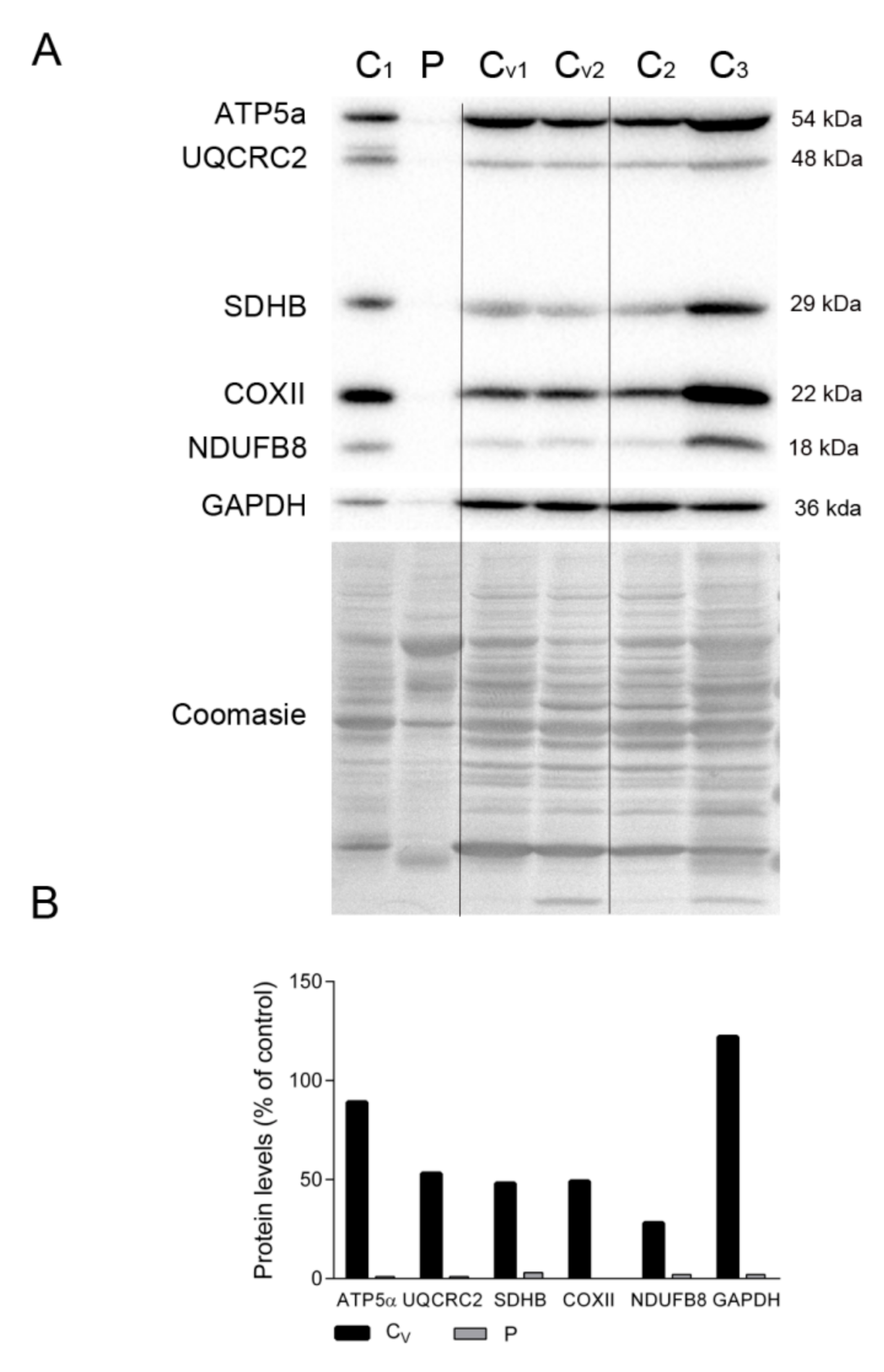
2.2. mtDNA and OXPHOS Proteins in TK2 Deficiency
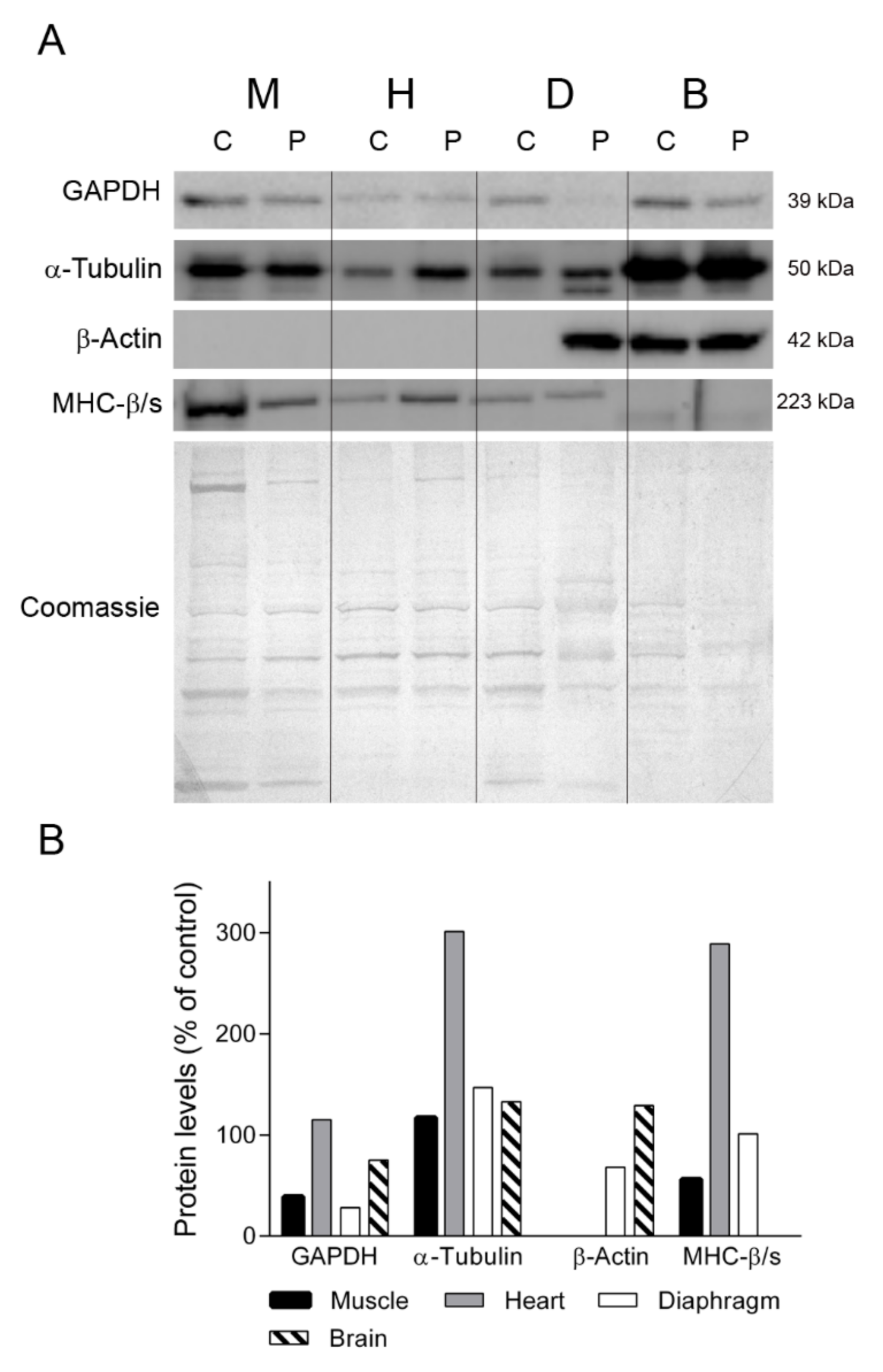
2.3. General Protein Profile in TK2 Deficiency
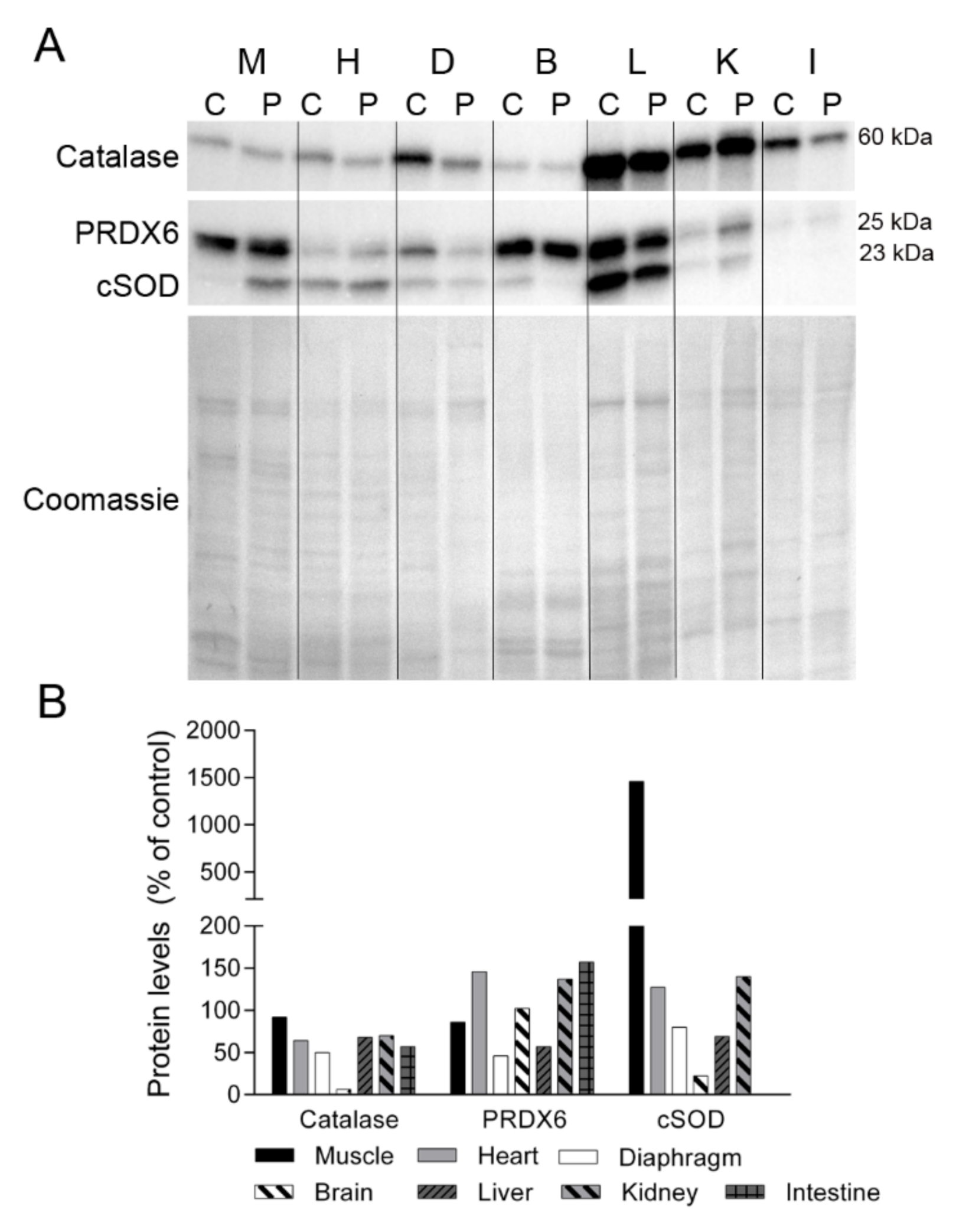
2.4. Oxidative Stress Markers in TK2 Deficiency
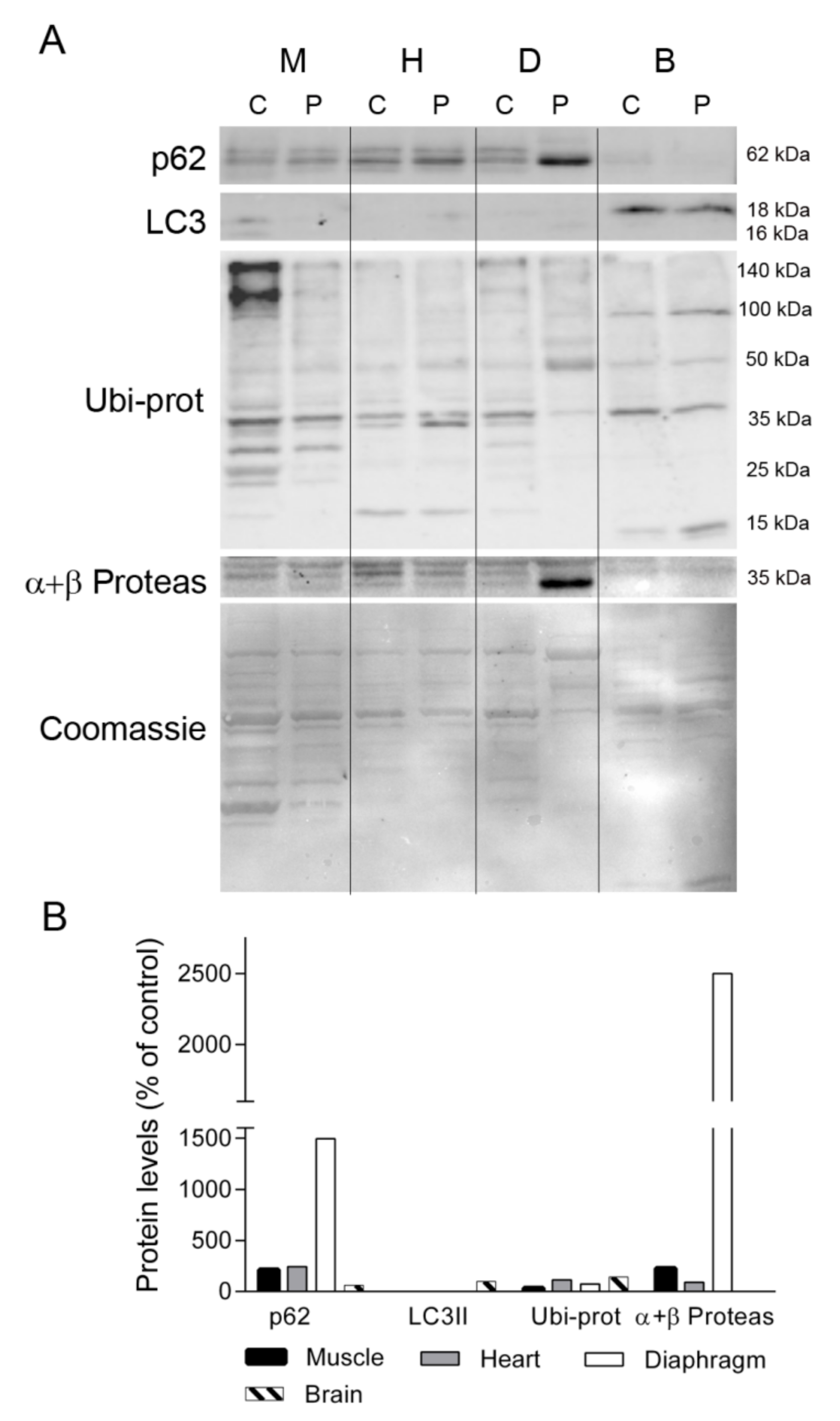
2.5. Protein Degradation Markers in TK2 Deficiency
2.6. TK1 Levels in TK2 Deficiency
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Tissue Processing
4.3. Western Blotting
4.4. Proteomic Analysis
4.5. Mitochondrial DNA Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saada, A.; Shaag, A.; Mandel, H.; Nevo, Y.; Eriksson, S.; Elpeleg, O. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat. Genet. 2001, 29, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Mitochondrial purine and pyrimidine metabolism and beyond. Nucleotides Nucleic Acids 2016, 35, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Garone, C.; Taylor, R.W.; Nascimento, A.; Poulton, J.; Fratter, C.; Domínguez-González, C.; Evans, J.C.; Loos, M.; Isohanni, P.; Suomalainen, A.; et al. Retrospective natural history of thymidine kinase 2 deficiency. J. Med. Genet. 2018, 55, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, E.; Dai, H.; Stefans, V.; Vogel, H.; Al Jasmi, F.; Schrier Vergano, S.A.; Castro, D.; Bernes, S.; Bhambhani, V.; et al. Clinical and molecular spectrum of thymidine kinase 2-related mtDNA maintenance defect. Mol. Genet. Metab. 2018, 124, 124–130. [Google Scholar] [CrossRef]
- Domínguez-González, C.; Hernández-Laín, A.; Rivas, E.; Hernández-Voth, A.; Sayas Catalán, J.; Fernández-Torrón, R.; Fuiza-Luces, C.; García García, J.; Morís, G.; Olivé, M.; et al. Late-onset thymidine kinase 2 deficiency: A review of 18 cases. Orphanet J. Rare Dis. 2019, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- de Fuenmayor-Fernández de la Hoz, C.P.; Morís, G.; Jiménez-Mallebrera, C.; Badosa, C.; Hernández-Laín, A.; Blázquez Encinar, A.; Martín, M.Á.; Domínguez-González, C. Recurrent rhabdomyolysis and exercise intolerance: A new phenotype of late-onset thymidine kinase 2 deficiency. Mol. Genet. Metab. Rep. 2021, 26. [Google Scholar] [CrossRef]
- Hernandez-Voth, A.; Sayas Catalan, J.; Corral Blanco, M.; Castaño Mendez, A.; Martin, M.A.; De Fuenmayor Fernandez De La Hoz, C.; Villena Garrido, V.; Dominguez-Gonzalez, C. Deoxynucleoside therapy for respiratory involvement in adult patients with thymidine kinase 2-deficient myopathy. BMJ Open Respir. Res. 2020, 7. [Google Scholar] [CrossRef]
- Barcia, G.; Khirani, S.; Amaddeo, A.; Assouline, Z.; Pennisi, A.; Boddaert, N.; Romero, N.; Desguerre, I.; Schiff, M.; Rötig, A.; et al. Evidence of diaphragmatic dysfunction with severe alveolar hypoventilation syndrome in mitochondrial respiratory chain deficiency. Neuromuscul. Disord. 2020, 30, 593–598. [Google Scholar] [CrossRef]
- Götz, A.; Isohanni, P.; Pihko, H.; Paetau, A.; Herva, R.; Saarenpää-Heikkilä, O.; Valanne, L.; Marjavaara, S.; Suomalainen, A. Thymidine kinase 2 defects can cause multi-tissue mtDNA depletion syndrome. Brain 2008, 131, 2841–2850. [Google Scholar] [CrossRef]
- Mazurova, S.; Magner, M.; Kucerova-Vidrova, V.; Vondrackova, A.; Stranecky, V.; Pristoupilova, A.; Zamecnik, J.; Hansikova, H.; Zeman, J.; Tesarova, M.; et al. Thymidine kinase 2 and alanyl-tRNA synthetase 2 deficiencies cause lethal mitochondrial cardiomyopathy: Case reports and review of the literature. Cardiol. Young 2017, 27, 936–944. [Google Scholar] [CrossRef]
- Lopez-Gomez, C.; Levy, R.J.; Sanchez-Quintero, M.J.; Juanola-Falgarona, M.; Barca, E.; Garcia-Diaz, B.; Tadesse, S.; Garone, C.; Hirano, M. Deoxycytidine and Deoxythymidine Treatment for Thymidine Kinase 2 Deficiency. Ann. Neurol. 2017, 81, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-González, C.; Madruga-Garrido, M.; Mavillard, F.; Garone, C.; Aguirre-Rodríguez, F.J.; Donati, M.A.; Kleinsteuber, K.; Martí, I.; Martín-Hernández, E.; Morealejo-Aycinena, J.P.; et al. Deoxynucleoside Therapy for Thymidine Kinase 2–Deficient Myopathy. Ann. Neurol. 2019, 86, 293–303. [Google Scholar] [CrossRef]
- Lopez-Gomez, C.; Hewan, H.; Sierra, C.; Akman, H.O.; Sanchez-Quintero, M.J.; Juanola-Falgarona, M.; Tadesse, S.; Tanji, K.; Konofagou, E.E.; Hirano, M. Bioavailability and cytosolic kinases modulate response to deoxynucleoside therapy in TK2 deficiency. EBioMedicine 2019, 46, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Blázquez-Bermejo, C.; Molina-Granada, D.; Vila-Julià, F.; Jiménez-Heis, D.; Zhou, X.; Torres-Torronteras, J.; Karlsson, A.; Martí, R.; Cámara, Y. Age-related metabolic changes limit efficacy of deoxynucleoside-based therapy in thymidine kinase 2-deficient mice. EBioMedicine 2019, 46, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Dorado, B.; Area, E.; Akman, H.O.; Hirano, M. Onset and organ specificity of Tk2 deficiency depends on Tk1 down-regulation and transcriptional compensation. Hum. Mol. Genet. 2011, 20, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, M.; Salviati, L.; Sacconi, S.; Otaegui, D.; Camaño, P.; Marina, A.; Bacman, S.; Moraes, C.T.; Carlo, J.R.; Garcia, M.; et al. Mitochondrial DNA depletion: Mutations in thymidine kinase gene with myopathy and SMA. Neurology 2002, 59, 1197–1202. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in Mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsucci, D.; Ienco, E.C.; Siciliano, G.; Mancuso, M. Mitochondrial disorders and drugs: What every physician should know. Drugs Context 2019, 8, 1–16. [Google Scholar] [CrossRef]
- Powers, S.K.; Ozdemir, M.; Hyatt, H. Redox Control of Proteolysis during Inactivity-Induced Skeletal Muscle Atrophy. Antioxid. Redox Signal. 2020, 33, 559–569. [Google Scholar] [CrossRef]
- Kramerova, I.; Kudryashova, E.; Wu, B.; Ottenheijm, C.; Granzier, H.; Spencer, M.J. Novel role of calpain-3 in the triad-associated protein complex regulating calcium release in skeletal muscle. Hum. Mol. Genet. 2008, 17, 3271–3280. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Gonzalez, C.; Badosa, C.; Madruga-Garrido, M.; Martí, I.; Paradas, C.; Ortez, C.; Kalko, S.G.; Blázquez-Bermejo, C.; Cámara, Y.; Martí, R.; et al. Growth Differentiation Factor 15 is a potential biomarker of therapeutic response for TK2 deficient myopathy. Sci. Rep. 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, R.; Eriksson, S. Basic biochemical characterization of cytosolic enzymes in thymidine nucleotide synthesis in adult rat tissues: Implications for tissue specific mitochondrial DNA depletion and deoxynucleoside-based therapy for TK2-deficiency. BMC Mol. Cell Biol. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, K.; Radell, P.; Eriksson, L.I.; Hultenby, K.; Rooyackers, O. Effect of prolonged mechanical ventilation on diaphragm muscle mitochondria in piglets. Acta Anaesthesiol. Scand. 2005, 49, 1101–1107. [Google Scholar] [CrossRef]
- Kavazis, A.N.; Talbert, E.E.; Smuder, A.J.; Hudson, M.B.; Nelson, W.B.; Powers, S.K. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic. Biol. Med. 2009, 46, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Hudson, M.B.; Nelson, W.B.; Talbert, E.E.; Min, K.; Szeto, H.H.; Kavazis, A.N.; Smuder, A.J. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care Med. 2011, 39, 1749–1759. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Jung, B.; Liang, F.; Azuelos, I.; Hussain, S.; Goldberg, P.; Godin, R.; Danialou, G.; Chaturvedi, R.; Rygiel, K.; et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am. J. Respir. Crit. Care Med. 2012, 186, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Azuelos, I.; Jung, B.; Giordano, C.; Matecki, S.; Hussain, S.; White, K.; Li, T.; Liang, F.; Benedetti, A.; et al. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J. Appl. Physiol. 2015, 118, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Hudson, M.B.; Smuder, A.J.; Nelson, W.B.; Wiggs, M.P.; Shimkus, K.L.; Fluckey, J.D.; Szeto, H.H.; Powers, S.K. Partial support ventilation and mitochondrial-targeted antioxidants protect against ventilator-induced decreases in diaphragm muscle protein synthesis. PLoS ONE 2015, 10, e0137693. [Google Scholar] [CrossRef]
- Sollanek, K.J.; Smuder, A.J.; Wiggs, M.P.; Morton, A.B.; Koch, L.G.; Britton, S.L.; Powers, S.K. Role of intrinsic aerobic capacity and ventilator-induced diaphragm dysfunction. J. Appl. Physiol. 2015, 118, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Smuder, A.J.; Sollanek, K.; Nelson, W.B.; Min, K.; Talbert, E.E.; Kavazis, A.N.; Hudson, M.B.; Sandri, M. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic. Biol. Med. 2018, 115, 179–190. [Google Scholar] [CrossRef]
- Tang, H.; Lee, M.; Budak, M.T.; Pietras, N.; Hittinger, S.; Vu, M.; Khuong, A.; Hoang, C.D.; Hussain, S.N.A.; Levine, S.; et al. Intrinsic apoptosis in mechanically ventilated human diaphragm: Linkage to a novel Fos/FoxO1/Stat3-Bim axis. FASEB J. 2011, 25, 2921–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duiverman, M.L.; Huberts, A.S.; Eykern, L.A.; Bladder, G.; Wijkstra, P.J. Respiratory muscle activity and patient–ventilator asynchrony during different settings of noninvasive ventilation in stable hypercapnic COPD: Does high inspiratory pressure lead to respiratory muscle unloading? Int. J. COPD 2017, 12, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, M.; Hooijman, P.E.; Beishuizen, A.; De Waard, M.C.; Paul, M.A.; Hartemink, K.J.; Van Hees, H.W.H.; Lawlor, M.W.; Brocca, L.; Bottinelli, R.; et al. Diaphragm atrophy and weakness in the absence of mitochondrial dysfunction in the critically Ill. Am. J. Respir. Crit. Care Med. 2017, 196, 1544–1558. [Google Scholar] [CrossRef]
- Drummond, M.C.; Friderici, K.H. A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression. PLoS Genet. 2013, 9, e1003743. [Google Scholar] [CrossRef] [Green Version]
- Wagatsuma, A.; Yamazaki, Y.; Mizuno, Y.; Yamada, S. Molecular properties and gene expression of albumin in the skeletal muscle following hindlimb immobilization in a shortened position. Acta Neuropathol. 2001, 101, 540–546. [Google Scholar] [CrossRef]
- Greaves, D.S.; Dufresne, M.J.; Fackrell, H.B. Age-related changes and tissue distribution of parvalbumin in normal and dystrophic mice of strain 129 Rej. Muscle Nerve 1991, 14, 543–552. [Google Scholar] [CrossRef]
- Dupont-Versteegden, E.E.; Kitten, A.M.; Katz, M.S.; McCarter, R.J. Elevated levels of albumin in soleus and diaphragm muscles of mdx mice. Proc. Soc. Exp. Biol. Med. 1996, 213, 281–286. [Google Scholar] [CrossRef]
- Heilig, A.; Pette, D. Albumin: Origin, distribution and regulation by contractile activity. Eur. J. Biochem. 1988, 508, 503–508. [Google Scholar] [CrossRef]
- Hussain, S.N.A.; Mofarrahi, M.; Sigala, I.; Kim, H.C.; Vassilakopoulos, T.; Maltais, F.; Bellenis, I.; Chaturvedi, R.; Gottfried, S.B.; Metrakos, P.; et al. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am. J. Respir. Crit. Care Med. 2010, 182, 1377–1386. [Google Scholar] [CrossRef]
- Corpeno, R.; Dworkin, B.; Cacciani, N.; Salah, H.; Bergman, H.M.; Ravara, B.; Vitadello, M.; Gorza, L.; Gustafson, A.M.; Hedström, Y.; et al. Time course analysis of mechanical ventilation-induced diaphragm contractile muscle dysfunction in the rat. J. Physiol. 2014, 592, 3859–3880. [Google Scholar] [CrossRef]
- Levine, S.; Nguyen, T.; Taylor, N.; Frischia, M.E.; Budak, M.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kayser, L.; et al. Rapid Disuse Atrophy of Diaphragm Fibers in Mechanically Ventilated Humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Duarte, J.A.; Nguyen, B. Endurance Exercise Protects Skeletal Muscle Against Both Doxorubicin-induced and Inactivity-induced Muscle Wasting. Pflug. Arch. 2019, 471, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Davidson, M.; Yen, H.C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 2007, 7, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.; Rivera, H.; Blázquez, A.; Merinero, B.; Ugalde, C.; Arenas, J.; Cuezva, J.M.; Martín, M.A. Mitochondrial bioenergetics and dynamics interplay in complex I-deficient fibroblasts. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 443–453. [Google Scholar] [CrossRef]
- Rabilloud, T.; Heller, M.; Gasnier, F.; Luche, S.; Rey, C.; Aebersold, R.; Benahmed, M.; Louisot, P.; Lunardi, J.L. Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J. Biol. Chem. 2002, 277, 19396–19401. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Eriksson, S.; Wang, L. The expression and activity of thymidine kinase 1 and deoxycytidine kinase are modulated by hydrogen peroxide and nucleoside analogs. Nucleotides Nucleic Acids 2020, 39, 1347–1358. [Google Scholar] [CrossRef]
- Levine, S.; Biswas, C.; Dierov, J.; Barsotti, R.; Shrager, J.B.; Nguyen, T.; Sonnad, S.; Kucharchzuk, J.C.; Kaiser, L.R.; Singhal, S.; et al. Increased proteolysis, myosin depletion, and atrophic AKT-FOXO signaling in human diaphragm disuse. Am. J. Respir. Crit. Care Med. 2011, 183, 483–490. [Google Scholar] [CrossRef]
- Shin, W.H.; Park, J.H.; Chung, K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson’s disease. BMB Rep. 2020, 53, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Azzu, V.; Brand, M.D. Degradation of an intramitochondrial protein by the cytosolic proteasome. J. Cell Sci. 2010, 123, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Lavie, J.; De Belvalet, H.; Sonon, S.; Ion, A.M.; Dumon, E.; Melser, S.; Lacombe, D.; Dupuy, J.W.; Lalou, C.; Bénard, G. Ubiquitin-Dependent Degradation of Mitochondrial Proteins Regulates Energy Metabolism. Cell Rep. 2018, 23, 2852–2863. [Google Scholar] [CrossRef]
- Ravanelli, S.; den Brave, F.; Hoppe, T. Mitochondrial Quality Control Governed by Ubiquitin. Front. Cell Dev. Biol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Miereles, C.; Bailey, J.L.; Debigare, R.; Zheng, B.; Price, S.R.; Mitch, W.E. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Investig. 2004, 113, 115–123. [Google Scholar] [CrossRef]
- Han, J.W.; Thieleczek, R.; Varsanyi, M.; Heilmeyer, L.M.J. Compartmentalized ATP synthesis in skeletal muscle triads. Biochemistry 1992, 31, 377–384. [Google Scholar] [CrossRef]
- Kockskämper, J.; Zima AV, B. LA Modulation of sarcoplasmic reticulum Ca2+ release by glycolysis in cat atrial myocytes. J. Physiol. 2005, 564, 697–714. [Google Scholar] [CrossRef]
- Seo, I.R.; Moh, S.H.; Lee, E.H.; Meissner, G. and Kim do, H. Aldolase potentiates DIDS activation of the ryanodine receptor in rabbit skeletal sarcoplasmic reticulum. Biochem. J. 2006, 399, 325–333. [Google Scholar] [CrossRef]
- Karlström, A.R.; Neumüller, M.; Gronowitz, J.S.; Källander, C.F.R. Molecular forms in human serum of enzymes synthesizing DNA precursors and DNA. Mol. Cell. Biochem. 1990, 92, 23–35. [Google Scholar] [CrossRef]
- Welinder, C.; Ekblad, L. Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 2011, 10, 1416–1419. [Google Scholar] [CrossRef]
- Neuhoff, V.; Arold, N.; Taube, D.E.W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–266. [Google Scholar] [CrossRef]
- Rivera, H.; Merinero, B.; Martinez-Pardo, M.; Arroyo, I.; Ruiz-Sala, P.; Bornstein, B.; Serra-Suhe, C.; Gallardo, E.; Marti, R.; Moran, M.J.; et al. Marked mitochondrial DNA depletion associated with a novel SUCLG1 gene mutation resulting in lethal neonatal acidosis, multi-organ failure, and interrupted aortic arch. Mitochondrion 2010, 10, 362–368. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laine-Menéndez, S.; Domínguez-González, C.; Blázquez, A.; Delmiro, A.; García-Consuegra, I.; Fernández-de la Torre, M.; Hernández-Laín, A.; Sayas, J.; Martín, M.Á.; Morán, M. Preferent Diaphragmatic Involvement in TK2 Deficiency: An Autopsy Case Study. Int. J. Mol. Sci. 2021, 22, 5598. https://doi.org/10.3390/ijms22115598
Laine-Menéndez S, Domínguez-González C, Blázquez A, Delmiro A, García-Consuegra I, Fernández-de la Torre M, Hernández-Laín A, Sayas J, Martín MÁ, Morán M. Preferent Diaphragmatic Involvement in TK2 Deficiency: An Autopsy Case Study. International Journal of Molecular Sciences. 2021; 22(11):5598. https://doi.org/10.3390/ijms22115598
Chicago/Turabian StyleLaine-Menéndez, Sara, Cristina Domínguez-González, Alberto Blázquez, Aitor Delmiro, Inés García-Consuegra, Miguel Fernández-de la Torre, Aurelio Hernández-Laín, Javier Sayas, Miguel Ángel Martín, and María Morán. 2021. "Preferent Diaphragmatic Involvement in TK2 Deficiency: An Autopsy Case Study" International Journal of Molecular Sciences 22, no. 11: 5598. https://doi.org/10.3390/ijms22115598
APA StyleLaine-Menéndez, S., Domínguez-González, C., Blázquez, A., Delmiro, A., García-Consuegra, I., Fernández-de la Torre, M., Hernández-Laín, A., Sayas, J., Martín, M. Á., & Morán, M. (2021). Preferent Diaphragmatic Involvement in TK2 Deficiency: An Autopsy Case Study. International Journal of Molecular Sciences, 22(11), 5598. https://doi.org/10.3390/ijms22115598






