Molecular Mechanisms of Fetal Tendon Regeneration Versus Adult Fibrous Repair
Abstract
1. Introduction
2. Results
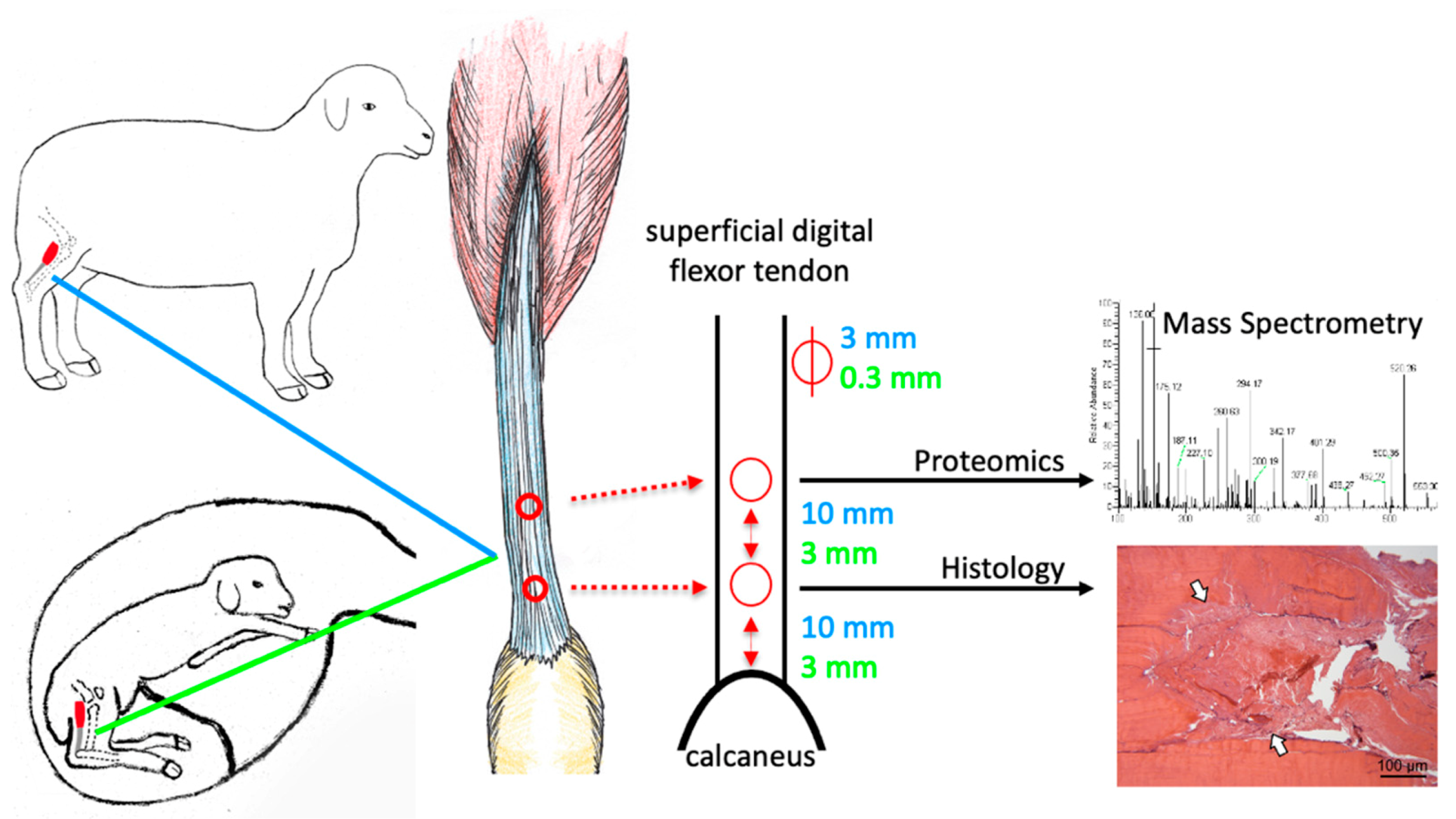
2.1. The Ovine Model Allows Studying of Fetal Tendon Regeneration
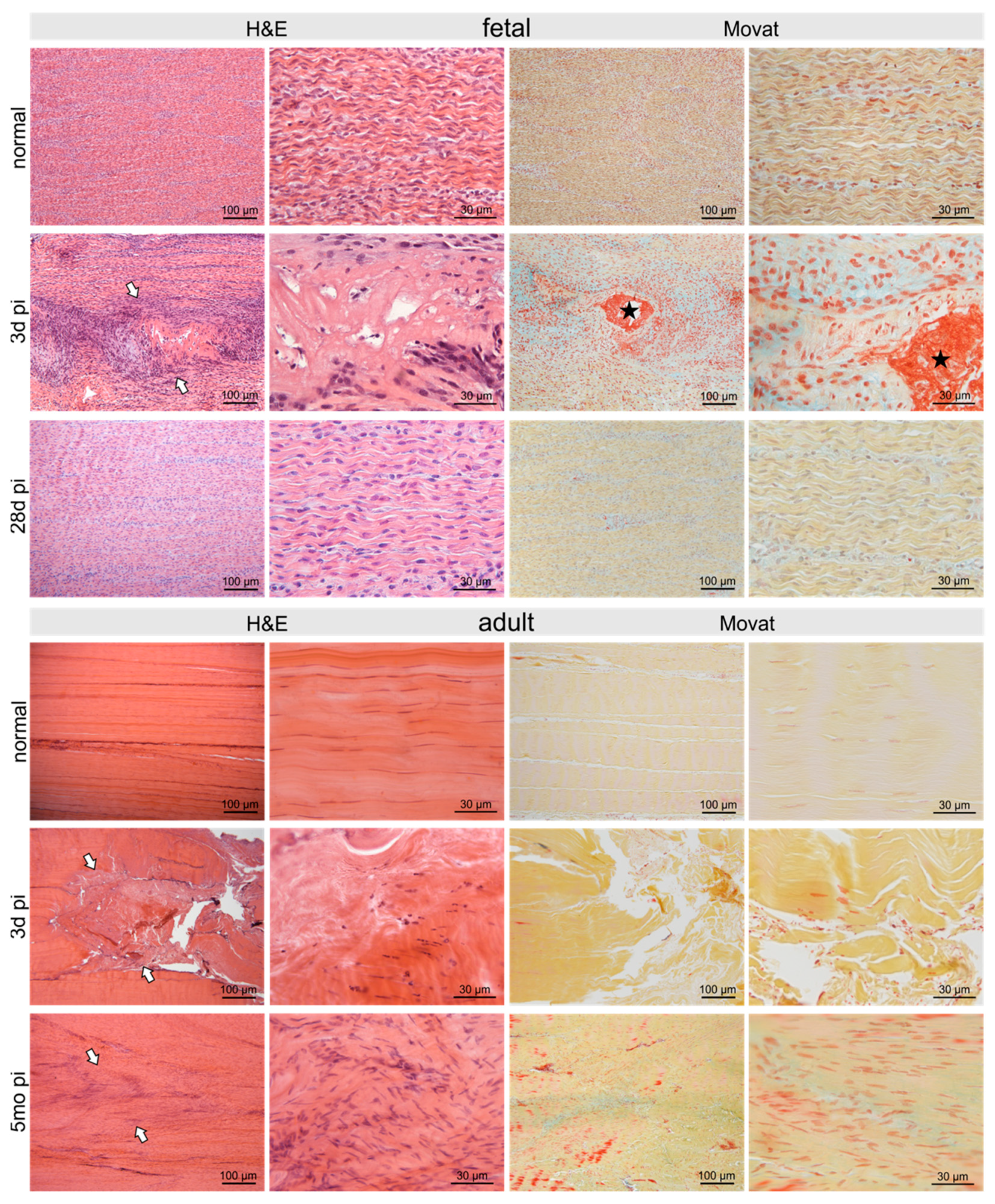
2.2. Histological Long-Term Evaluation of Tendon Healing Confirmed Fetal Regenerative Versus Adult Scarring Tendon Repair
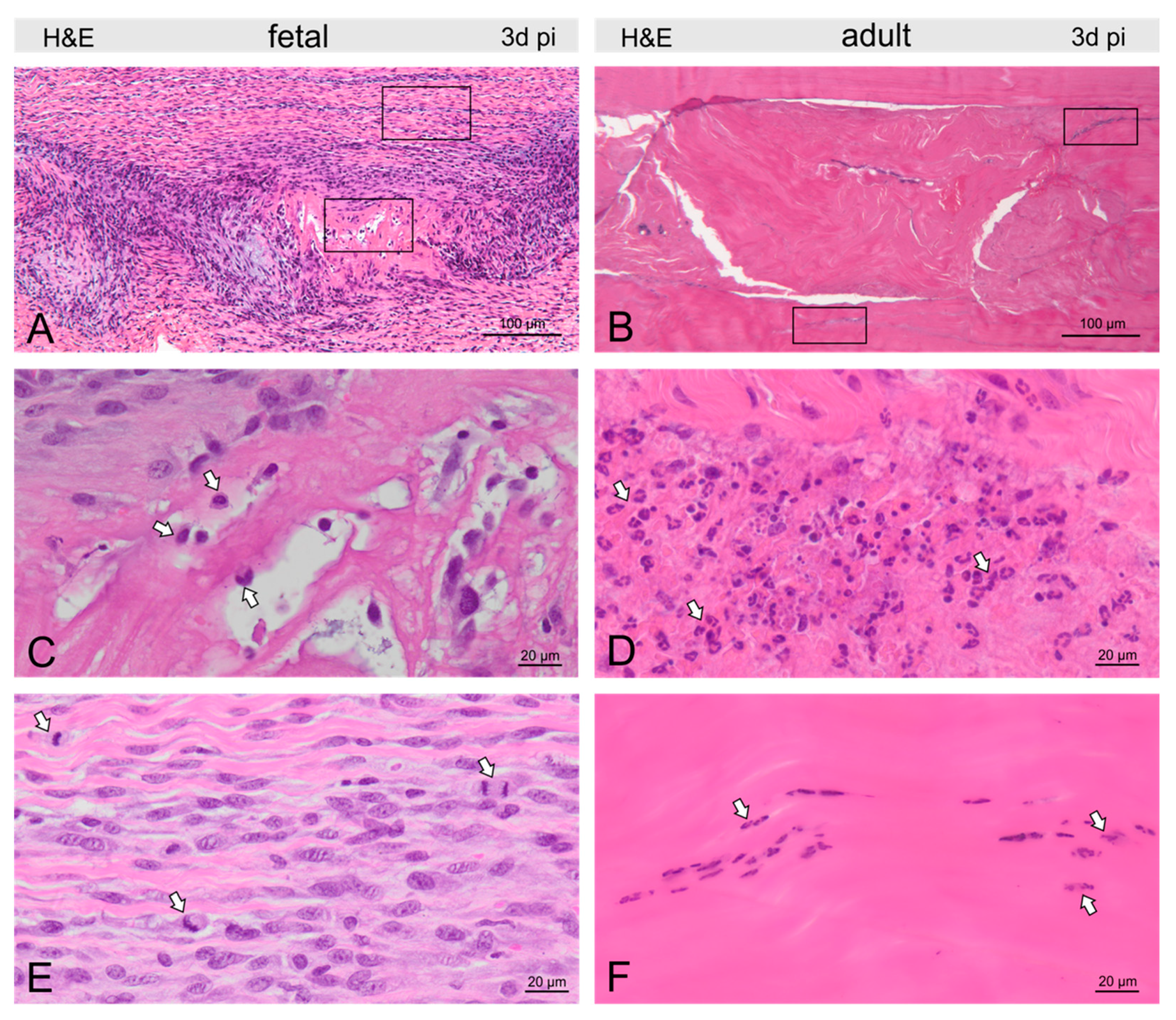
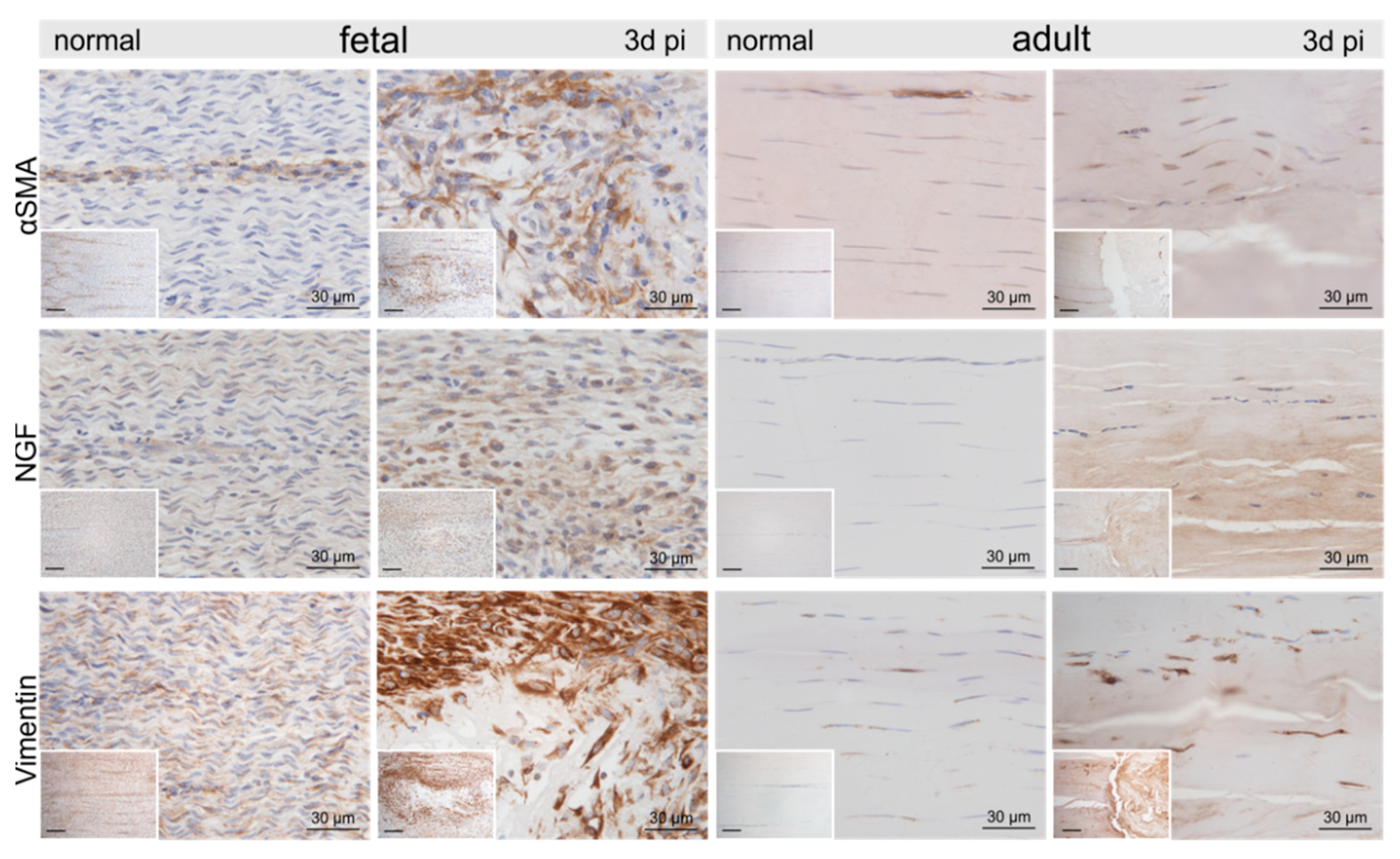
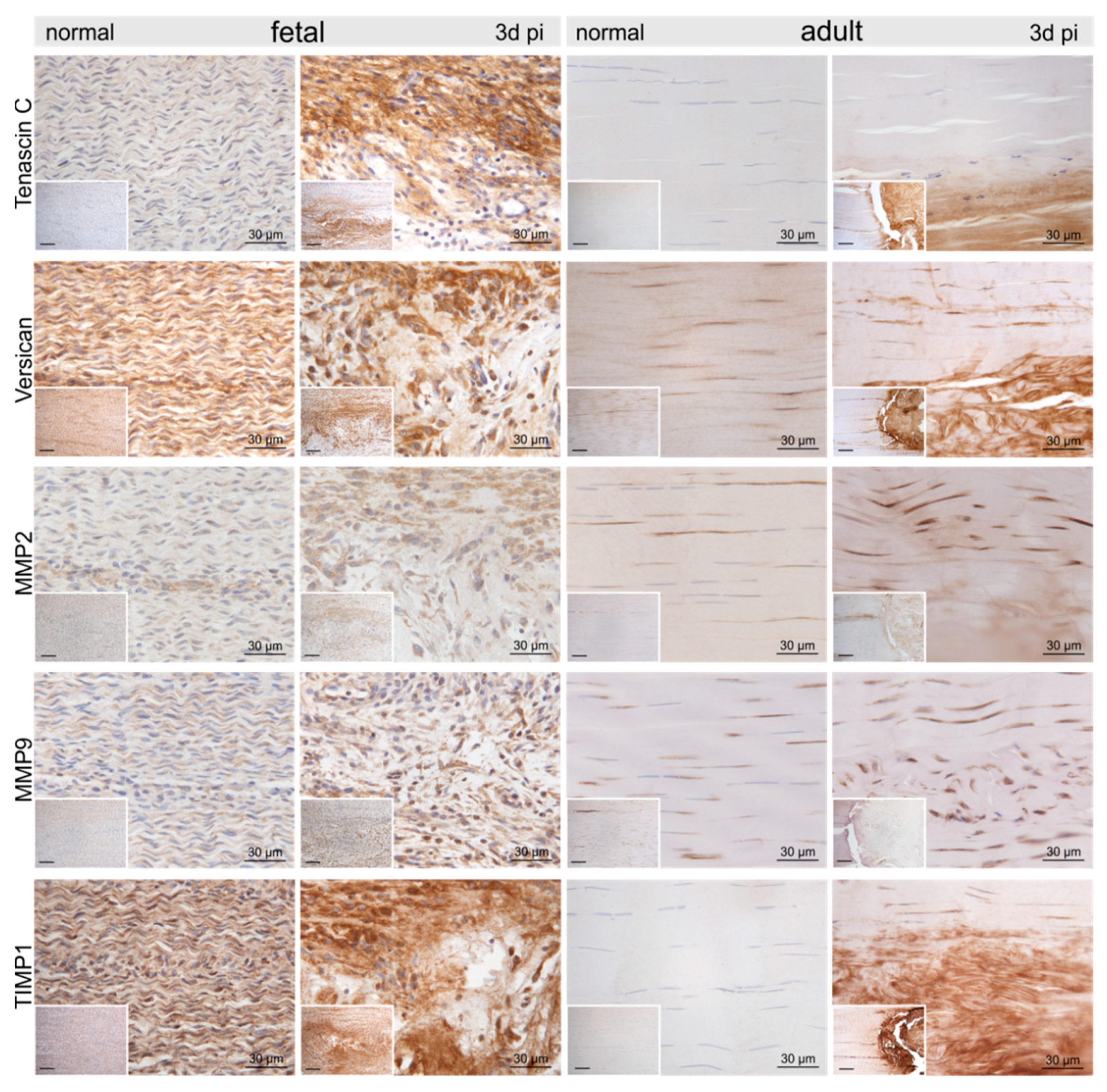
2.3. Injury- and Repair-Associated Histologic and Immunohistochemical Changes in Adult and Fetal Sheep Three Days Post-Injury
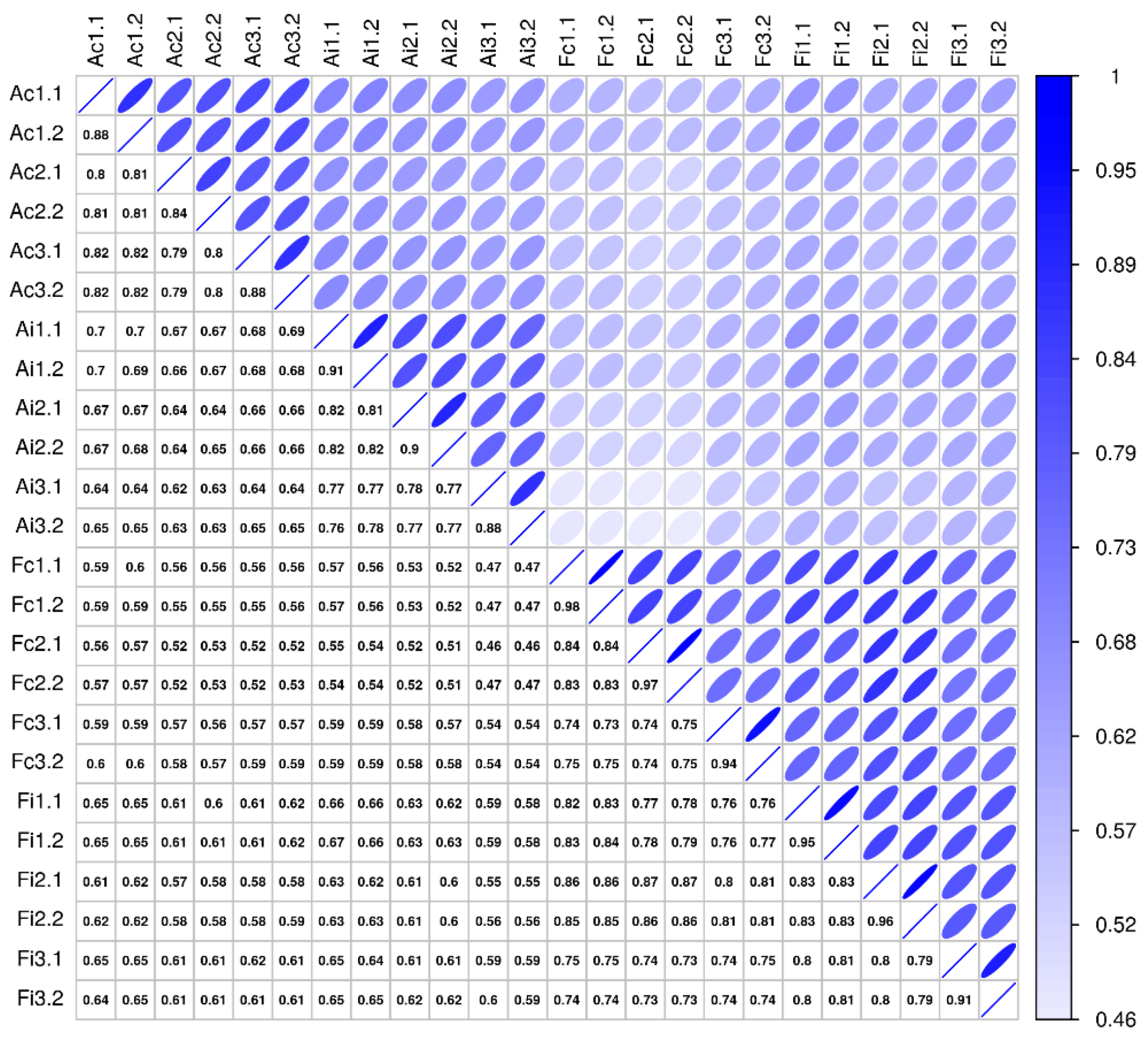
2.4. Molecular Secretome Profiling by High-Resolution Mass Spectrometry
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Tissue Harvest
4.3. Histology and Immunohistochemistry
4.4. Secretome Analysis by Mass Spectrometry
4.5. Data Analysis and Statistics of Mass Spectrometry Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Walden, G.; Liao, X.; Donell, S.; Raxworthy, M.J.; Riley, G.P.; Saeed, A. A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng. Part B Rev. 2017, 23, 44–58. [Google Scholar] [CrossRef]
- Gajhede-Knudsen, M.; Ekstrand, J.; Magnusson, H.; Maffulli, N. Recurrence of Achilles tendon injuries in elite male football players is more common after early return to play: An 11-year follow-up of the UEFA Champions League Injury Study. Br. J. Sports Med. 2013, 47, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P. Etiology and pathophysiology of chronic tendon disorders in sports. Scand. J. Med. Sci. Sports 1997, 7, 78–85. [Google Scholar] [CrossRef]
- Screen, H.R.C.; Birk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Curini, V.; Russo, V.; Mauro, A.; Giacinto, O.D.; Marchisio, M.; Alfonsi, M.; Mattioli, M. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS ONE 2012, 7, e30974. [Google Scholar] [CrossRef]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Iorio, A.D.; Amicis, D.D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Webbon, P.M. Harnessing the stem cell for the treatment of tendon injuries: Heralding a new dawn? Br. J. Sports Med. 2005, 39, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Hueber, A.J.; Reilly, J.H.; Xu, Y.; Fazzi, U.G.; Murrell, G.A.C.; McInnes, I.B. inflammation is present in early human tendinopathy. Am. J. Sports Med. 2010, 38, 2085–2091. [Google Scholar] [CrossRef]
- Rees, J.D.; Stride, M.; Scott, A. Tendons-time to revisit inflammation. Br. J. Sports Med. 2013, 48, 1553–1557. [Google Scholar] [CrossRef]
- Riley, G. Tendinopathy–From basic science to treatment. Nat. Clin. Pract. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J.; Kampen, J.; Kahrs, J.; Plitz, W. Achilles tendon rupture: Experimental results on spontaneous repair in a sheep-model. Knee Surg. Sports Traumatol. Arthrosc. 2000, 8, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Juncosa-Melvin, N.; Boivin, G.P.; Galloway, M.T.; Shearn, J.T.; Gooch, C.; Awad, H. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J. Orthop. Res. 2008, 26, 1–9. [Google Scholar] [CrossRef]
- Beredjiklian, P.K.; Favata, M.; Cartmell, J.S.; Flanagan, C.L.; Crombleholme, T.M.; Soslowsky, L.J. Regenerative versus reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Ann. Biomed. Eng. 2003, 31, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Favata, M.; Beredjiklian, P.K.; Zgonis, M.H.; Beason, D.P.; Crombleholme, T.M.; Jawad, A.F.; Soslowsky, L.J. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J. Orthop. Res. 2006, 24, 2124–2132. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Posnick, J.C.; Lin, K.Y.; Thorner, P. Fetal tendon healing: Development of an experimental model. Plast. Reconstr. Surg. 1993, 92, 1155. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Posnick, J.C.; Lin, K.Y. The in vivo response of foetal tendons to sutures. J. Hand Surg. 1995, 20, 314–318. [Google Scholar] [CrossRef]
- Herdrich, B.J.; Danzer, E.; Davey, M.G.; Bermudez, D.M.; Radu, A.; Zhang, L.; Zhang, Z.; Soslowsky, L.J.; Liechty, K.W. Fetal tendon wound size modulates wound gene expression and subsequent wound phenotype. Wound Repair Regen. 2010, 18, 543–549. [Google Scholar] [CrossRef]
- Bayer, M.L.; Yeung, C.-Y.C.; Kadler, K.E.; Qvortrup, K.; Baar, K.; Svensson, R.B.; Magnusson, S.P.; Krogsgaard, M.; Koch, M.; Kjaer, M. The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials 2010, 31, 4889–4897. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Ritter, S.Y.; Subbaiah, R.; Bebek, G.; Crish, J.; Scanzello, C.R.; Krastins, B.; Sarracino, D.; Lopez, M.F.; Crow, M.K.; Aigner, T.; et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: Comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013, 65, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Mufti, A.M.; Wani, G.M.; Wani, N.A.; Buchoo, B.A.; Khan, M.Z. Prenatal development of ovine fetus. Small Rumin. Res. 2000, 38, 87–89. [Google Scholar] [CrossRef]
- Poulos, R.C.; Hains, P.G.; Shah, R.; Lucas, N.; Xavier, D.; Manda, S.S.; Anees, A.; Koh, J.M.S.; Mahboob, S.; Wittman, M.; et al. Strategies to enable large-scale proteomics for reproducible research. Nat. Commun. 2020, 11, 3793. [Google Scholar] [CrossRef] [PubMed]
- Zwickl, H.; Traxler, E.; Staettner, S.; Parzefall, W.; Grasl-Kraupp, B.; Karner, J.; Schulte-Hermann, R.; Gerner, C. A novel technique to specifically analyze the secretome of cells and tissues. Electrophoresis 2005, 26, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.J.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 839–850. [Google Scholar] [CrossRef]
- Degen, K.E.; Gourdie, R.G. Embryonic wound healing: A primer for engineering novel therapies for tissue repair. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 258–270. [Google Scholar] [CrossRef]
- Colwell, A.S.; Longaker, M.T.; Lorenz, H.P. Identification of differentially regulated genes in fetal wounds during regenerative repair. Wound Repair Regen. 2008, 16, 450–459. [Google Scholar] [CrossRef]
- Russo, V.; Mauro, A.; Martelli, A.; Giacinto, O.D.; Marcantonio, L.D.; Nardinocchi, D.; Berardinelli, P.; Barboni, B. Cellular and molecular maturation in fetal and adult ovine calcaneal tendons. J. Anat. 2015, 226, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.W.; Allukian, M.; Herdrich, B.J.; Caskey, R.C.; Zgheib, C.; Xu, J.; Dorsett-Martin, W.; Mitchell, M.E.; Liechty, K.W. Modulation of the inflammatory response by increasing fetal wound size or interleukin-10 overexpression determines wound phenotype and scar formation. Wound Repair Regen. 2014, 22, 406–414. [Google Scholar] [CrossRef]
- Nitsos, I.; Rees, S.M.; Duncan, J.; Kramer, B.W.; Harding, R.; Newnham, J.P.; Moss, T.J.M. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J. Soc. Gynecol. Investig. 2006, 13, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Moss, T.J.M.; Knox, C.L.; Kallapur, S.G.; Nitsos, I.; Theodoropoulos, C.; Newnham, J.P.; Ikegami, M.; Jobe, A.H. Experimental amniotic fluid infection in sheep: Effects of ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am. J. Obstet. Gynecol. 2008, 198, 122.e1–122.e8. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Mayer, R.L.; Egerbacher, M.; Gabner, S.; Kańduła, M.M.; Rosser, J.; Haltmayer, E.; Auer, U.; Gültekin, S.; Huber, J.; et al. Fetal articular cartilage regeneration versus adult fibrocartilaginous repair: Secretome proteomics unravels molecular mechanisms in an ovine model. Dis. Models Mech. 2018, 11, dmm033092-247. [Google Scholar] [CrossRef] [PubMed]
- Jeanblanc, C.; Goodrich, A.D.; Colletti, E.; Mokhtari, S.; Porada, C.D.; Zanjani, E.D.; Almeida-Porada, G. Temporal definition of haematopoietic stem cell niches in a large animal model of in utero stem cell transplantation. Br. J. Haematol. 2014, 166, 268–278. [Google Scholar] [CrossRef]
- Almeida-Porada, G.; Porada, C.; Zanjani, E.D. Plasticity of human stem cells in the fetal sheep model of human stem cell transplantation. Int. J. Hematol. 2004, 79, 1–6. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Weber, B.; Wolint, P.; Frauenfelder, T.; Zeisberger, S.M.; Behr, L.; Sammut, S.; Scherman, J.; Brokopp, C.E.; Schwartländer, R.; et al. intramyocardial transplantation and tracking of human mesenchymal stem cells in a novel intra-uterine pre-immune fetal sheep myocardial infarction model: A proof of concept study. PLoS ONE 2013, 8, e57759. [Google Scholar] [CrossRef]
- Sawyer, M.; Moe, J.; Osburn, B.I. Ontogeny of immunity and leukocytes in the ovine fetus and elevation of immunoglobulins related to congenital infection. Am. J. Vet. Res. 1978, 39, 643–648. [Google Scholar]
- Dziegielewska, K.M.; Møller, J.E.; Potter, A.M.; Ek, J.; Lane, M.A.; Saunders, N.R. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 2000, 299, 335–345. [Google Scholar]
- Silverstein, A.M.; Lukes, R. Fetal response to antigenic stimulus. I. Plasmacellular and lymphoid reactions in the human fetus to intrauterine infection. Lab. Investig. 1962, 11, 918–932. [Google Scholar]
- Silverstein, A.M.; Uhr, J.W.; Kraner, K.; Lukes, R. Fetal response to antigenic stimulus. II. Antibody production by the fetal lamb. J. Exp. Med. 1963, 117, 799–812. [Google Scholar] [CrossRef]
- Silverstein, A.M.; Thorbecke, G.J.; Kraner, K.; Lukes, R. Fetal response to antigenic stimulus. III. Gamma-globulin production in normal and stimulated fetal lambs. J. Immunol. 1963, 91, 384–395. [Google Scholar] [PubMed]
- Silverstein, A.M.; Prendergast, R.; Kraner, K. Fetal response to antigenic stimulus. IV. Rejection of skin homografts by the fetal lamb. J. Exp. Med. 1964, 119, 955–964. [Google Scholar] [CrossRef]
- Silverstein, A.M. Ontogeny of the immune response. Science 1964, 144, 1423–1428. [Google Scholar] [CrossRef]
- Silverstein, A.M.; Parshall, C.J.; Uhr, J.W. Immunologic maturation in utero: Kinetics of the primary antibody response in the fetal lamb. Science 1966, 154, 1675–1677. [Google Scholar] [CrossRef]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, 2265. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Pérez-García, S.; Carrión, M.; Gutiérrez-Cañas, I.; Villanueva-Romero, R.; Castro, D.; Martínez, C.; González-Álvaro, I.; Blanco, F.J.; Juarranz, Y.; Gomariz, R.P. Profile of matrix-remodeling proteinases in osteoarthritis: Impact of fibronectin. Cells 2019, 9, 40. [Google Scholar] [CrossRef]
- Teodoro, W.R.; Queiroz, Z.A.d.J.; Santos, L.A.D.; Catanozi, S.; Filho, A.D.S.; Bueno, C.; Vendramini, M.B.G.; Fernezlian, S.d.M.; Eher, E.M.; Sampaio-Barros, P.D.; et al. Proposition of a novel animal model of systemic sclerosis induced by type V collagen in C57BL/6 mice that reproduces fibrosis, vasculopathy and autoimmunity. Arthritis Res. Ther. 2019, 21, 278. [Google Scholar] [CrossRef]
- Michael, M.; Vermeren, S. A neutrophil-centric view of chemotaxis. Essays Biochem. 2019, 63, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, O.; Parzefall, W.; Kainzbauer, E.; Wimmer, H.; Grasl-Kraupp, B.; Gerner, C.; Schulte-Hermann, R. Proteomics reveals acute pro-inflammatory and protective responses in rat Kupffer cells and hepatocytes after chemical initiation of liver cancer and after LPS and IL-6. Proteom. Clin. Appl. 2009, 3, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Muqaku, B.; Pils, D.; Mader, J.C.; Aust, S.; Mangold, A.; Muqaku, L.; Slany, A.; Favero, G.D.; Gerner, C. Neutrophil extracellular trap formation correlates with favorable overall survival in high grade ovarian cancer. Cancers 2020, 12, 505. [Google Scholar] [CrossRef]
- Reichl, B.; Niederstaetter, L.; Boegl, T.; Neuditschko, B.; Bileck, A.; Gojo, J.; Buchberger, W.; Peyrl, A.; Gerner, C. Determination of a tumor-promoting microenvironment in recurrent medulloblastoma: A multi-omics study of cerebrospinal fluid. Cancers 2020, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Hammerman, M.; Blomgran, P.; Dansac, A.; Eliasson, P.; Aspenberg, P. Different gene response to mechanical loading during early and late phases of rat achilles tendon healing. J. Appl. Physiol. 2017, 123, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.; Andersson, T.; Aspenberg, P. Achilles tendon healing in rats is improved by intermittent mechanical loading during the inflammatory phase. J. Orthopaed. Res. 2012, 30, 274–279. [Google Scholar] [CrossRef]
- Hammerman, M.; Dietrich-Zagonel, F.; Blomgran, P.; Eliasson, P.; Aspenberg, P. Different mechanisms activated by mild versus strong loading in rat Achilles tendon healing. PLoS ONE 2018, 13, e0201211. [Google Scholar] [CrossRef]
- Eliasson, P.; Andersson, T.; Hammerman, M.; Aspenberg, P. Primary gene response to mechanical loading in healing rat achilles tendons. J. Appl. Physiol. 2013, 114, 1519–1526. [Google Scholar] [CrossRef]
- Freedman, B.R.; Rodriguez, A.B.; Leiphart, R.J.; Newton, J.B.; Ban, E.; Sarver, J.J.; Mauck, R.L.; Shenoy, V.B.; Soslowsky, L.J. Dynamic loading and tendon healing affect multiscale tendon properties and ECM stress transmission. Sci. Rep. 2018, 8, 3153. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Aukland, K. Distribution volumes and macromolecular mobility in rat tail tendon interstitium. Am. J. Physiol. 1991, 260, H409–H419. [Google Scholar] [CrossRef]
- Aukland, K.; Wiig, H.; Tenstad, O.; Renkin, E.M. Interstitial exclusion of macromolecules studied by graded centrifugation of rat tail tendon. Am. J. Physiol. 1997, 273, H2794–H2803. [Google Scholar] [CrossRef] [PubMed]
- Aukland, K.; Reed, R.K.; Wiig, H. The problem of gaining access to interstitial fluid. An attempt to rationalize a wicked discussion on wicks. Lymphology 1997, 30, 111–115. [Google Scholar]
- Bileck, A.; Kreutz, D.; Muqaku, B.; Slany, A.; Gerner, C. Comprehensive assessment of proteins regulated by dexamethasone reveals novel effects in primary human peripheral blood mononuclear cells. J. Proteome Res. 2014, 13, 5989–6000. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Murdoch, D.J.; Chow, E.D. A graphical display of large correlation matrices. Am. Stat. 1996, 50, 178. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef]
- Reiner, A.; Yekutieli, D.; Benjamini, Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 2003, 19, 368–375. [Google Scholar] [CrossRef]
- Wu, D.; Smyth, G.K. Camera: A competitive gene set test accounting for inter-gene correlation. Nucleic Acids Res. 2012, 40, e133. [Google Scholar] [CrossRef] [PubMed]

| Group | a.q-Value | a.dir | f.q-Value | f.dir | fvsa.q-Value | fvsa.dir |
|---|---|---|---|---|---|---|
| Antimicrobial | 5.25 × 10−5 | + | 2.46 × 10−3 | + | 5.97 × 10−2 | - |
| Pro-inflammatory | 3.68 × 10−6 | + | 1.31 × 10−2 | + | 3.90 × 10−3 | - |
| Anti-inflammatory | 4.61 × 10−1 | - | 1.60 × 10−2 | + | 9.71 × 10−2 | + |
| Redox-regulating proteins | 2.27 × 10−1 | - | 8.24 × 10−1 | + | 1.87 × 10−1 | + |
| Protease/peptidase and regulators | 5.29 × 10−1 | + | 9.39 × 10−1 | + | 7.89 × 10−1 | - |
| ECM-related | 3.80 × 10−1 | + | 9.91 × 10−1 | + | 7.98 × 10−1 | - |
| Platelet-related | 4.85 × 10−1 | + | 1.95 × 10−1 | + | 9.97 × 10−1 | + |
| Acute phase-response | 9.94 × 10−1 | + | 6.06 × 10−3 | + | 3.89 × 10−1 | + |
| Erythrocyte-related | 7.42 × 10−1 | + | 9.12 × 10−1 | - | 6.57 × 10−1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribitsch, I.; Bileck, A.; Aldoshin, A.D.; Kańduła, M.M.; Mayer, R.L.; Egerbacher, M.; Gabner, S.; Auer, U.; Gültekin, S.; Huber, J.; et al. Molecular Mechanisms of Fetal Tendon Regeneration Versus Adult Fibrous Repair. Int. J. Mol. Sci. 2021, 22, 5619. https://doi.org/10.3390/ijms22115619
Ribitsch I, Bileck A, Aldoshin AD, Kańduła MM, Mayer RL, Egerbacher M, Gabner S, Auer U, Gültekin S, Huber J, et al. Molecular Mechanisms of Fetal Tendon Regeneration Versus Adult Fibrous Repair. International Journal of Molecular Sciences. 2021; 22(11):5619. https://doi.org/10.3390/ijms22115619
Chicago/Turabian StyleRibitsch, Iris, Andrea Bileck, Alexander D. Aldoshin, Maciej M. Kańduła, Rupert L. Mayer, Monika Egerbacher, Simone Gabner, Ulrike Auer, Sinan Gültekin, Johann Huber, and et al. 2021. "Molecular Mechanisms of Fetal Tendon Regeneration Versus Adult Fibrous Repair" International Journal of Molecular Sciences 22, no. 11: 5619. https://doi.org/10.3390/ijms22115619
APA StyleRibitsch, I., Bileck, A., Aldoshin, A. D., Kańduła, M. M., Mayer, R. L., Egerbacher, M., Gabner, S., Auer, U., Gültekin, S., Huber, J., Kreil, D. P., Gerner, C., & Jenner, F. (2021). Molecular Mechanisms of Fetal Tendon Regeneration Versus Adult Fibrous Repair. International Journal of Molecular Sciences, 22(11), 5619. https://doi.org/10.3390/ijms22115619







