Spatio-Temporal Distribution of Cell Wall Components in the Placentas, Ovules and Female Gametophytes of Utricularia during Pollination
Abstract
1. Introduction
2. Results
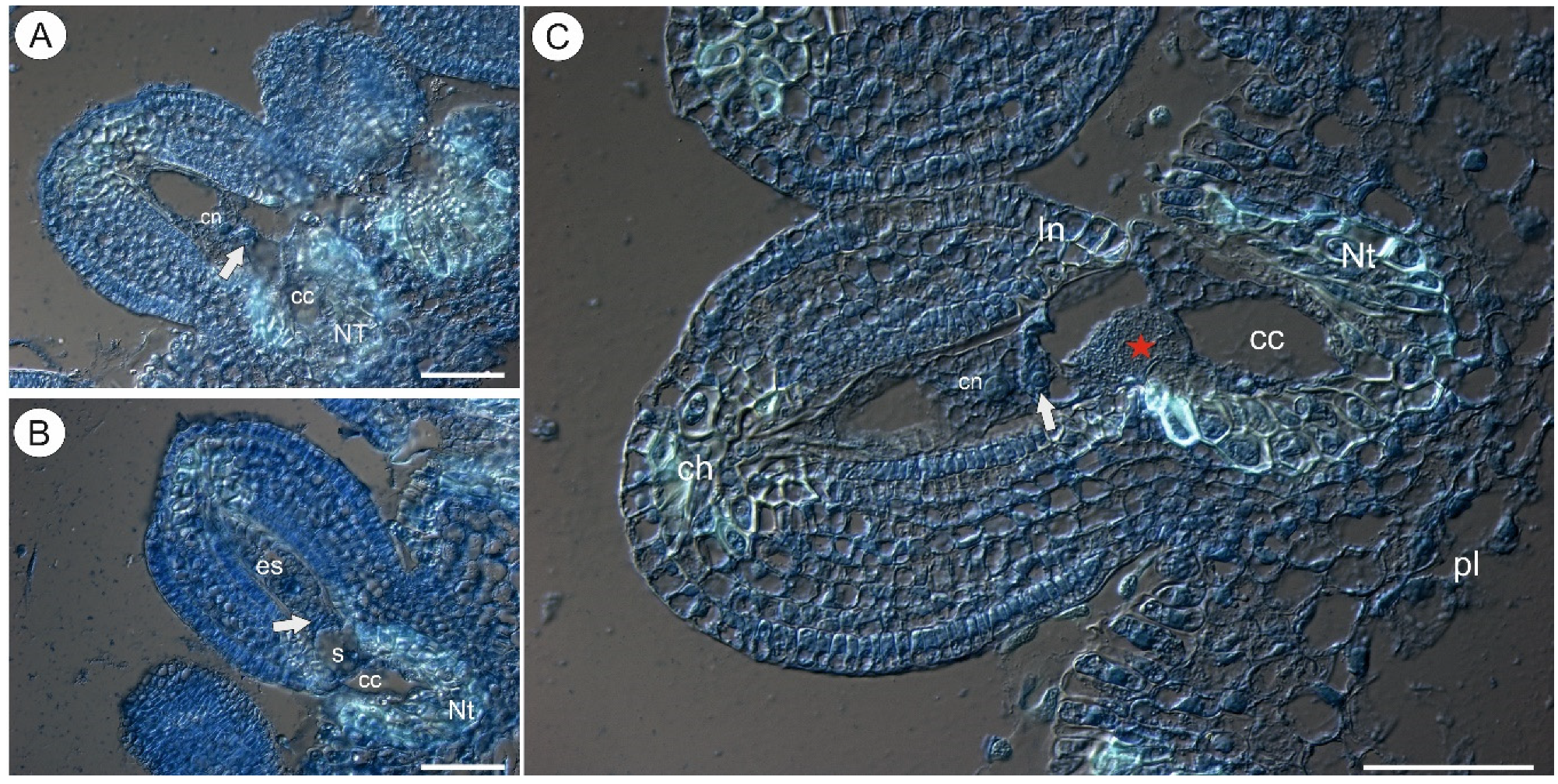
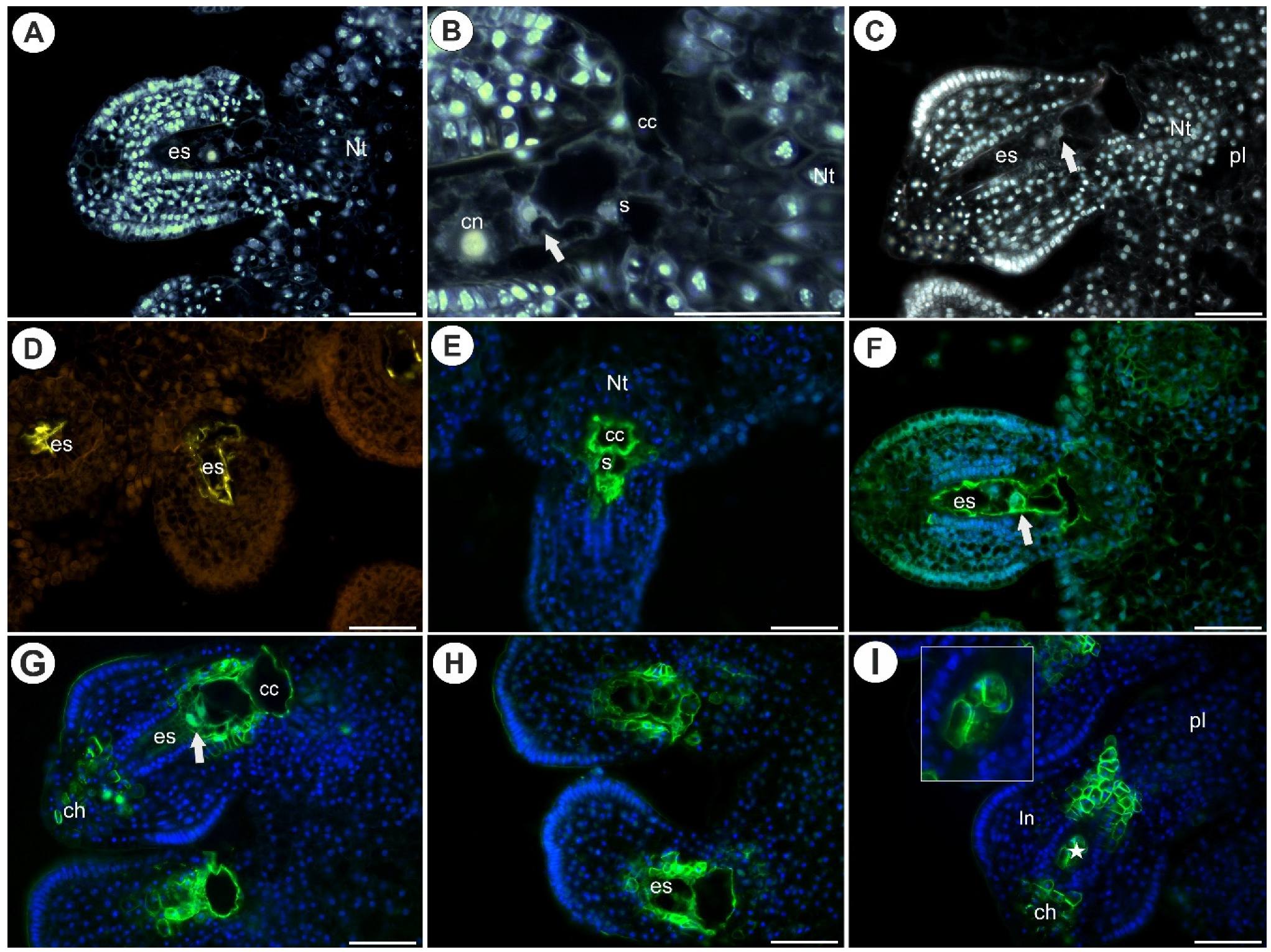
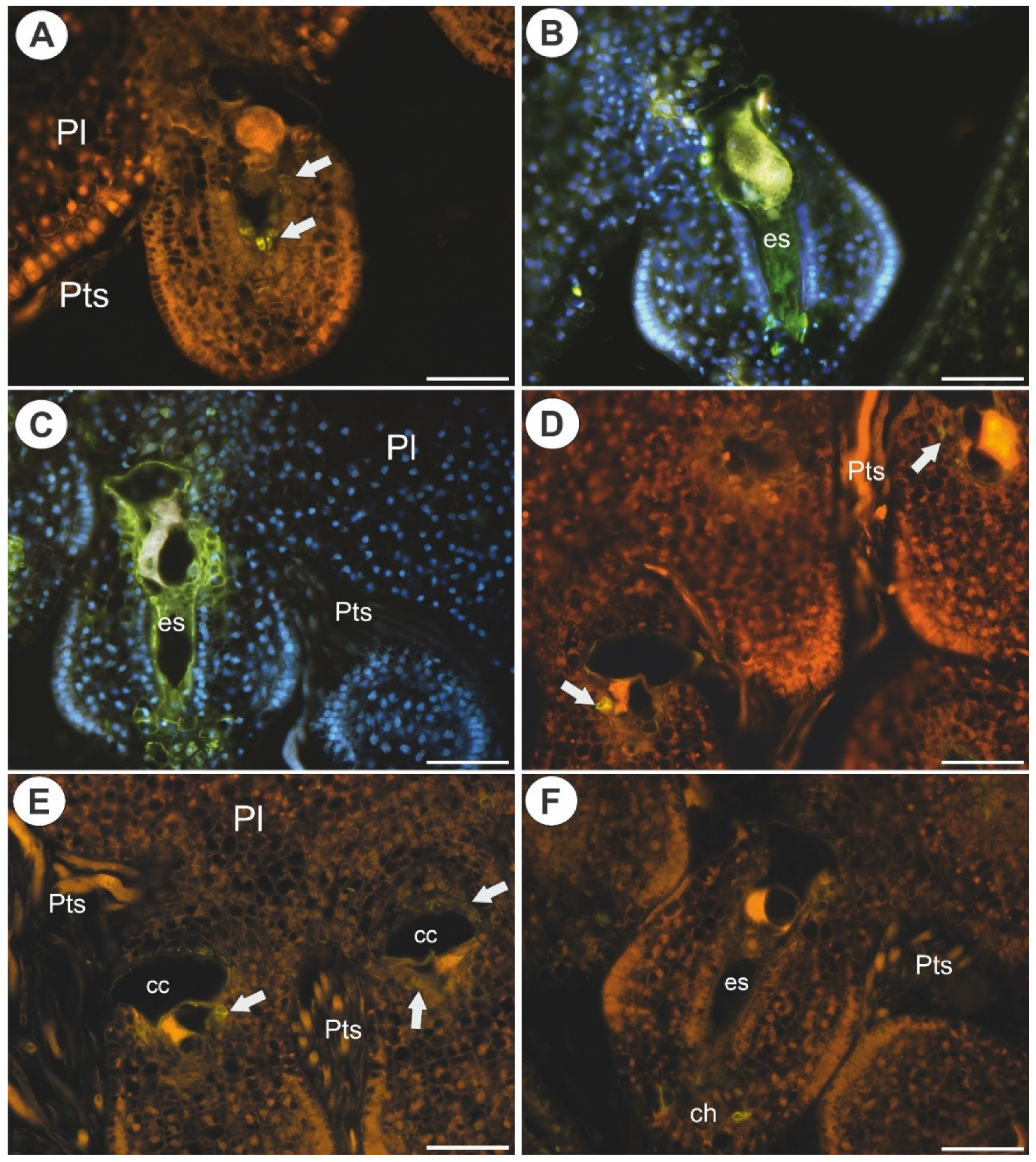
2.1. Arabinogalactan Proteins in the Placenta and Ovules before Pollination
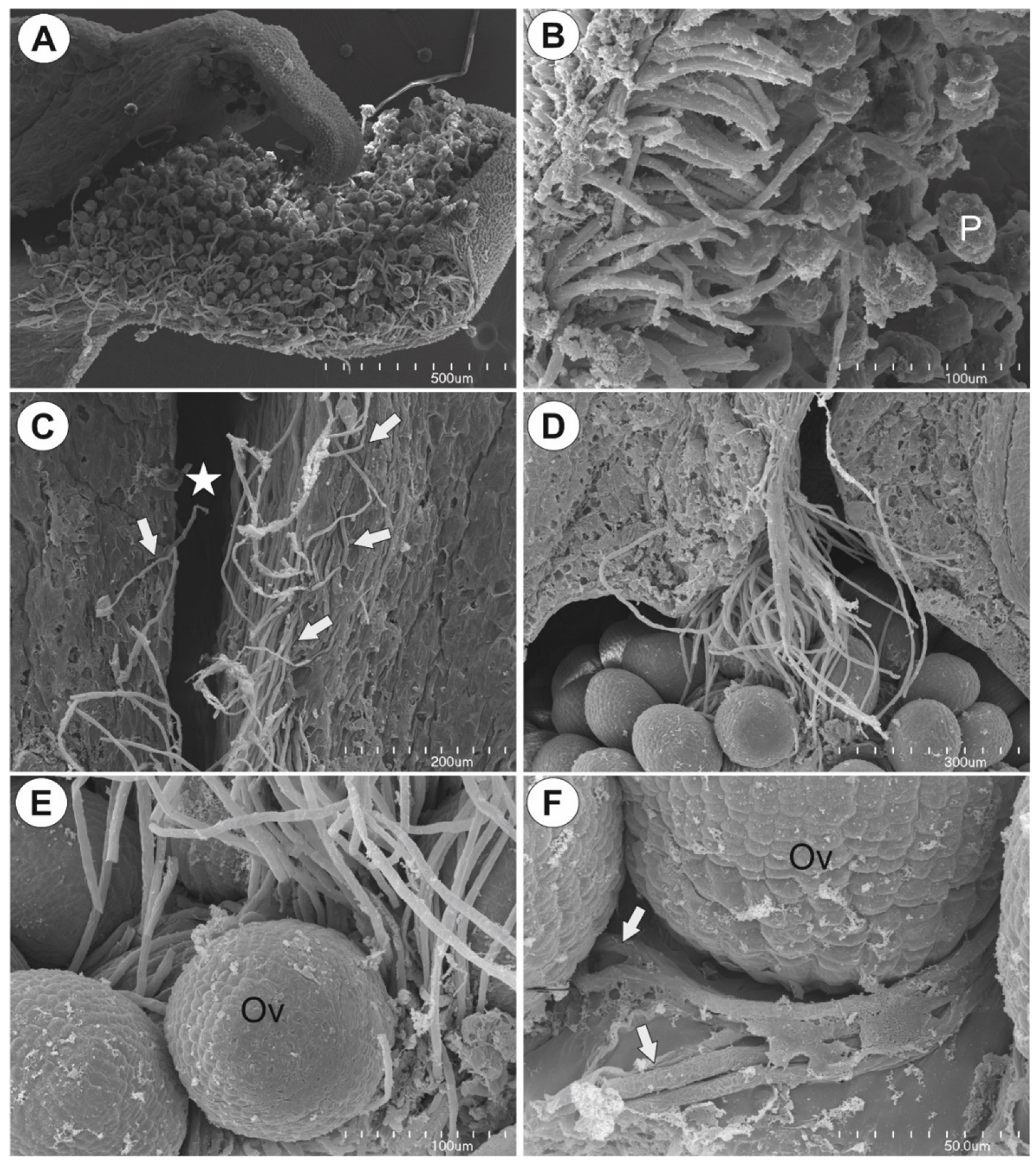
2.2. Observation of Pollen Tube Growth
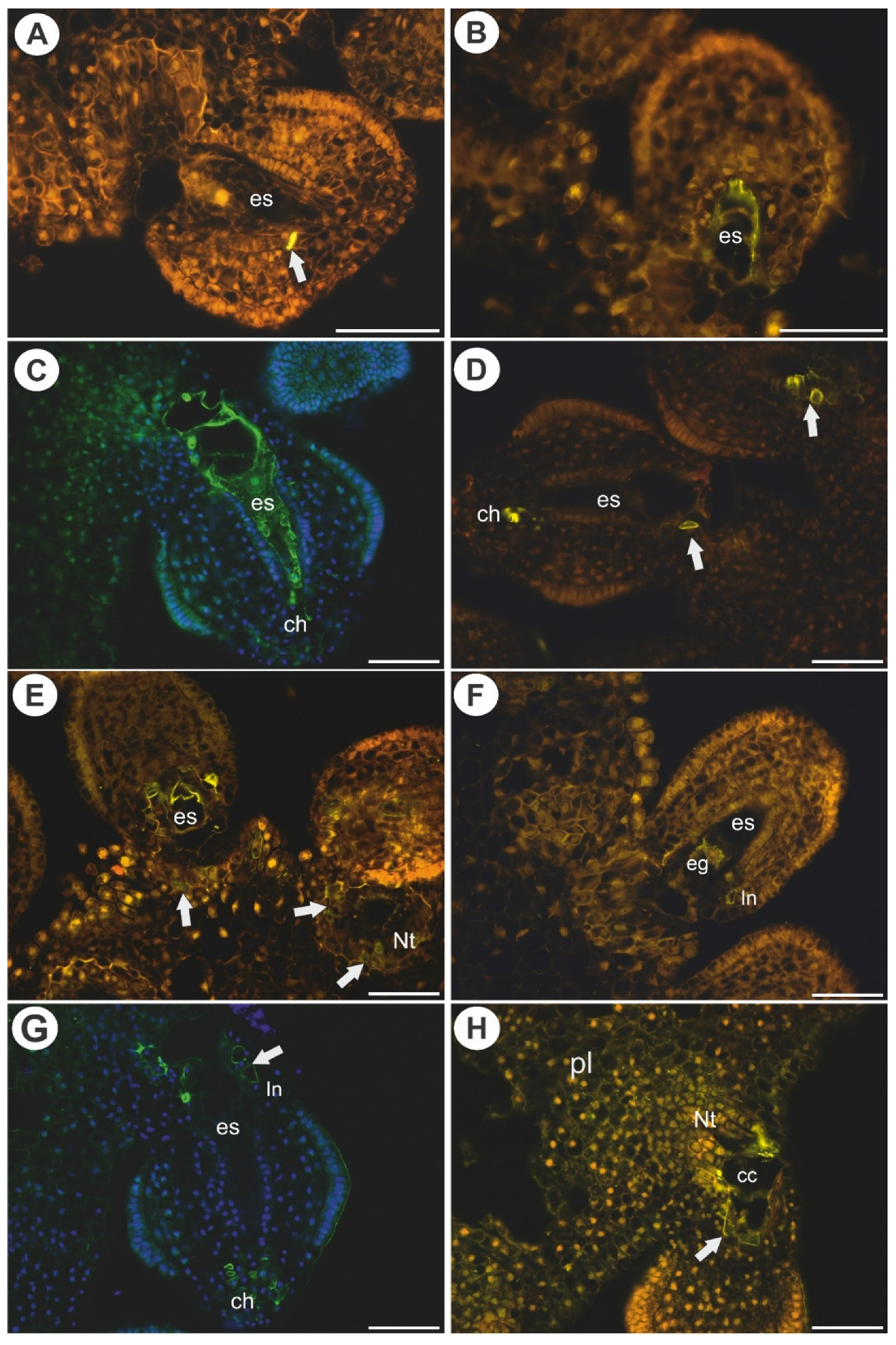
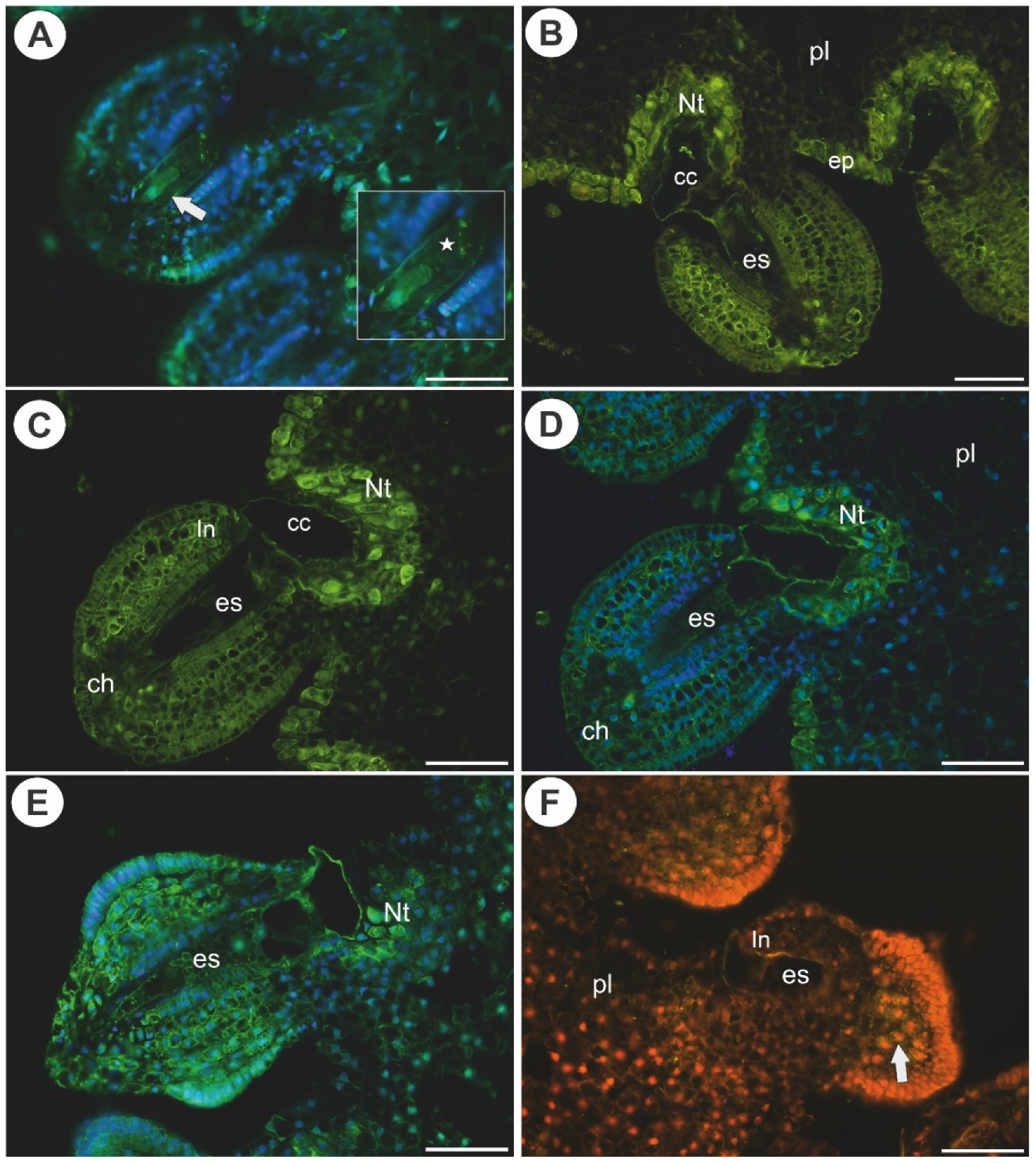
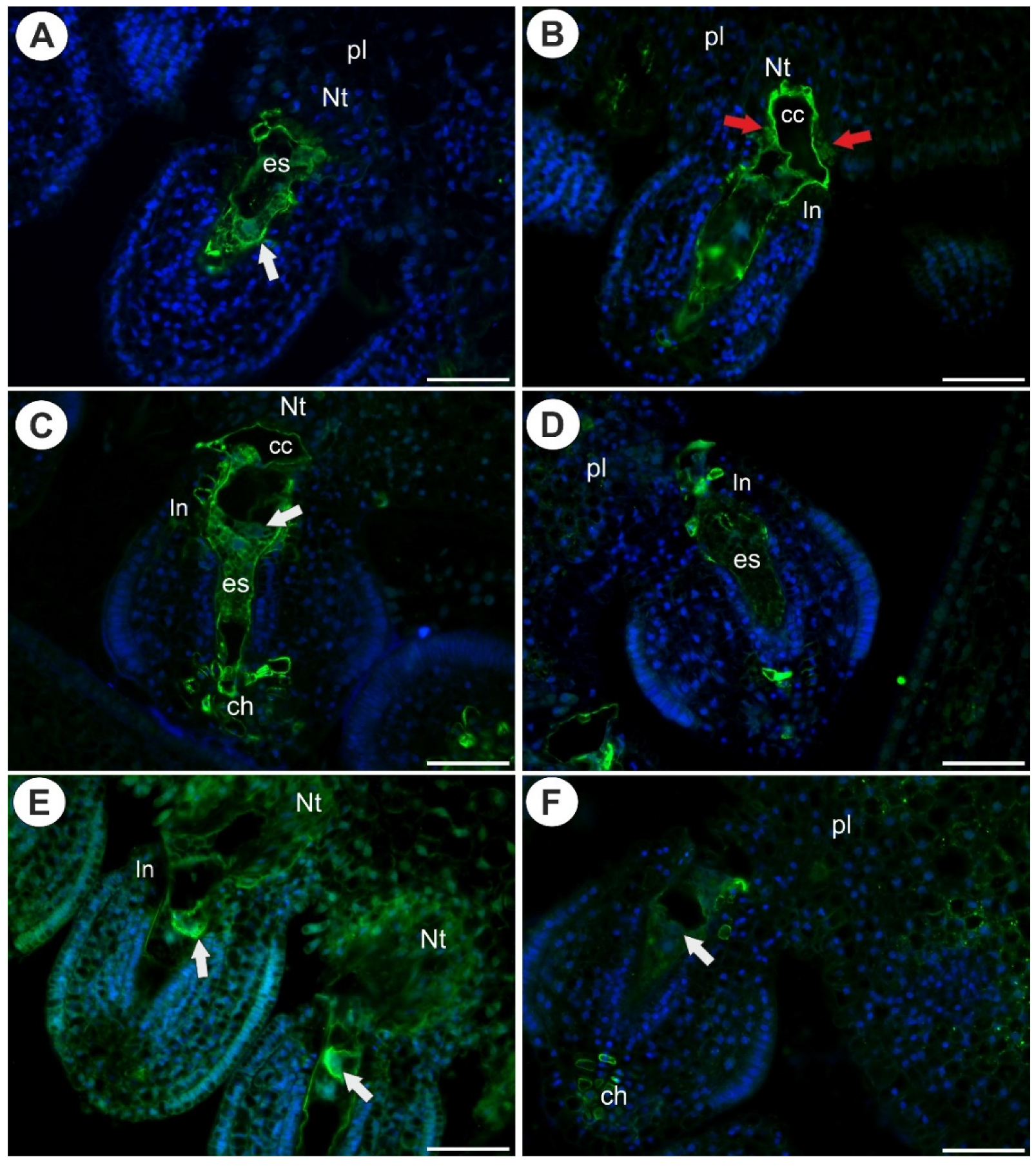
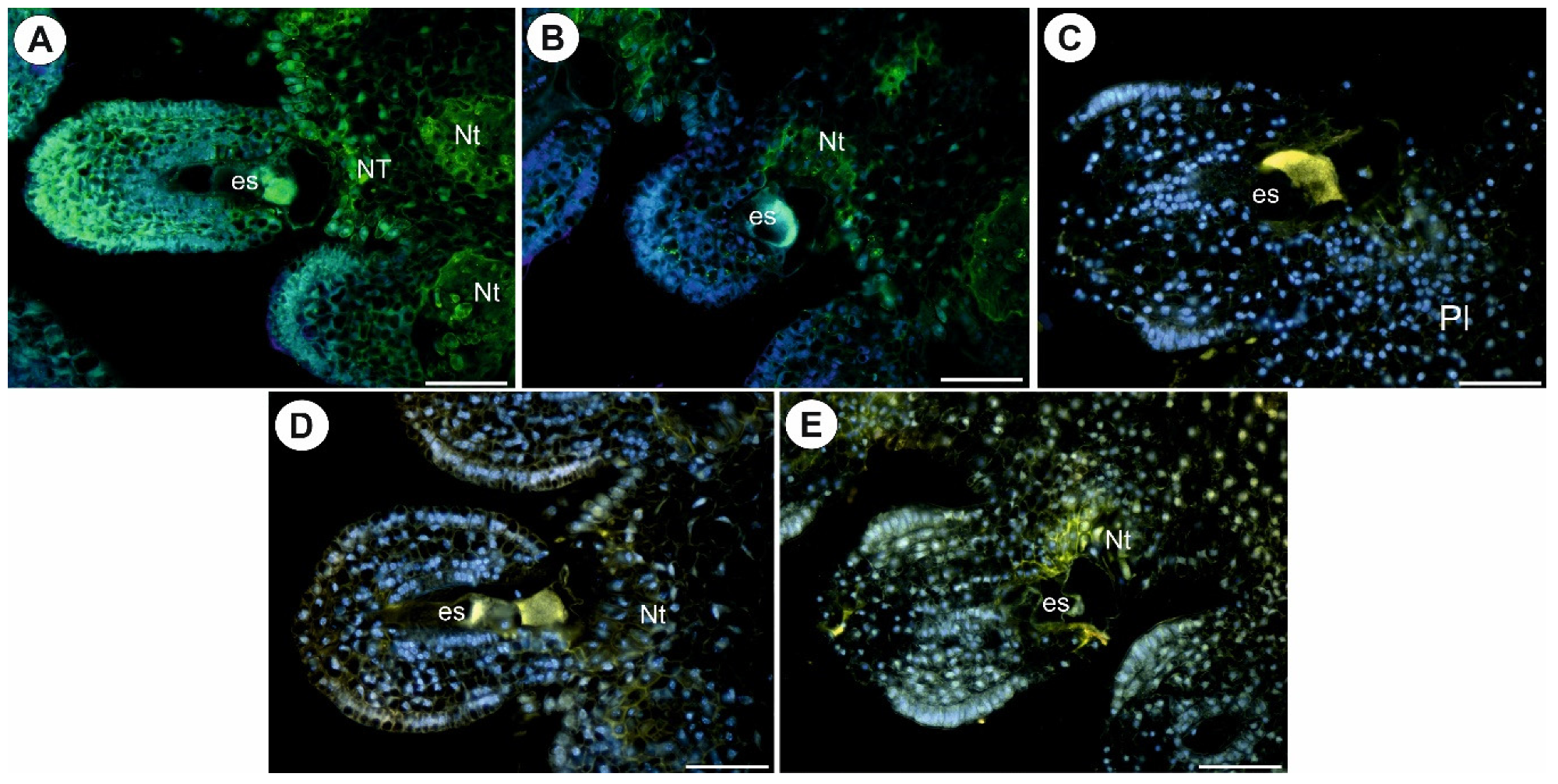
2.3. Arabinogalactan Proteins in the Placentas and Ovules after Pollination
3. Discussion
3.1. Occurrence of AGPs before Pollination
3.2. AGPs Occurrence after Pollination and Fertilization
3.3. Placental Epidermis and Nutritive Tissue as a Transmission Track for Pollen Tubes
4. Materials and Methods
Sample Preparation
5. Conclusions
- In both of the examined species, arabinogalactan proteins (AGPs) were observed in the placenta, ovule (integument, chalaza) and female gametophyte of both pollinated and unpollinated flowers, thus the production of AGPs was not dependent on pollination; however, the production of some AGPs was lower after fertilization.
- The production of some AGPs in the ovular tissues (nucellus, integument) was not dependent on the presence of a mature embryo sac. Thus, gametophytic control of the sporophytic tissues may differ from species to species.
- The occurrence of AGPs in the placenta epidermis and nutritive tissue of Utricularia supports the hypothesis that they play an active role as a transmission track for pollen tubes.
- We plan to study cell wall components in ovules and placentas of other Utricularia species that have differently developed nutrient tissues and form special syncytia.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouman, F. The Ovule. In Embryology of Angiosperms; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1984; pp. 123–157. [Google Scholar]
- Endress, P.K. Angiosperm ovules: Diversity, development, evolution. Ann. Bot. 2011, 107, 1465–1489. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M. Changes in the Ovary Related to Pollen Tube Guidance. Ann. Bot. 2000, 85, 79–85. [Google Scholar] [CrossRef]
- Herrero, M. Ovary signals for directional pollen tube growth. Sex. Plant. Reprod. 2001, 14, 3–7. [Google Scholar] [CrossRef]
- Li, H.J.; Meng, J.G.; Yang, W.C. Multilayered signaling pathways for pollen tube growth and guidance. Plant. Reprod. 2018, 31, 31–41. [Google Scholar] [CrossRef]
- Losada, J.M.; Herrero, M. Arabinogalactan proteins mediate intercellular crosstalk in the ovule of apple flowers. Plant. Reprod. 2019, 32, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.; Laux, T.; Hormaza, J.I. The role of the integuments in pollen tube guidance in flowering plants. New Phytol. 2018, 221, 1074–1089. [Google Scholar] [CrossRef]
- Leslie, A.B.; Boyce, C.K. Ovule Function and the Evolution of Angiosperm Reproductive Innovations. Int. J. Plant. Sci. 2012, 173, 640–648. [Google Scholar] [CrossRef]
- Lora, J.; Hormaza, J.I.; Herrero, M. The Diversity of the Pollen Tube Pathway in Plants: Toward an Increasing Control by the Sporophyte. Front. Plant. Sci. 2016, 7, 107. [Google Scholar] [CrossRef]
- Gotelli, M.M.; Lattar, E.C.; Zini, L.M.; Galati, B.G. Style morphology and pollen tube pathway. Plant. Reprod. 2017, 30, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.; Herrero, M.; Tucker, M.R.; Hormaza, J.I. The transition from somatic to germline identity shows conserved and specialized features during angiosperm evolution. New Phytol. 2017, 216, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Bencivenga, S.; Colombo, L.; Masiero, S. Cross talk between the sporophyte and the megagametophyte during ovule development. Sex. Plant. Reprod. 2011, 24. [Google Scholar] [CrossRef]
- Bedinger, P.A.; Broz, A.K.; Tovar-Mendez, A.; McClure, B. Pollen-Pistil Interactions and Their Role in Mate Selection. Plant. Physiol. 2017, 173, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Q.; Russell, S.D.; Strout, G.W.; Mao, L.J. Organization of isolated embryo sacs and eggs of Plumbago zeylanica (Plumbaginaceae) before and after fertilization. Am. J. Bot. 1990, 77, 401–1410. [Google Scholar] [CrossRef]
- Chute, H.M.; Maheshwari, P. An Introduction to the Embryology of Angiosperms. Bull. Torrey Bot. Club 1951, 78, 272. [Google Scholar] [CrossRef]
- Khan, R. A contribution to the embryology of Utricularia flexuosa Vahl. Phytomorphology 1954, 4, 80–117. [Google Scholar]
- Higashiyama, T.; Inatsugi, R.; Sakamoto, S.; Sasaki, N.; Mori, T.; Kuroiwa, H.; Nakada, T.; Nozaki, H.; Kuroiwa, T.; Nakano, A. Species Preferentiality of the Pollen Tube Attractant Derived from the Synergid Cell of Torenia fournieri. Plant. Physiol. 2006, 142, 481–491. [Google Scholar] [CrossRef]
- Płachno, B.J. Female germ unit in Genlisea and Utricularia, with remarks about the evolution of the extra-ovular female gametophyte in members of Lentibulariaceae. Protoplasma 2011, 248, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Kuroiwa, H.; Kawano, S.; Kuroiwa, T. Kinetics of double fertilization in Torenia fournieri based on direct observations of the naked embryo sac. Planta 1997, 203, 101–110. [Google Scholar] [CrossRef]
- Higashiyama, T.; Yang, W.-C. Gametophytic Pollen Tube Guidance: Attractant Peptides, Gametic Controls, and Receptors. Plant. Physiol. 2017, 173, 112–121. [Google Scholar] [CrossRef]
- Higashiyama, T.; Kuroiwa, H.; Kawano, S.; Kuroiwa, T. Guidance in Vitro of the Pollen Tube to the Naked Embryo Sac of Torenia fournieri. Plant. Cell 1998, 10, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Yabe, S.; Sasaki, N.; Nishimura, Y.; Miyagishima, S.; Kuroiwa, H.; Kuroiwa, T. Pollen tube attraction by the synergid cell. Science 2001, 293, 1480–1483. [Google Scholar] [CrossRef]
- Higashiyama, T.; Kuroiwa, H.; Kuroiwa, T. Pollen-tube guidance: Beacons from the female gametophyte. Curr. Opin. Plant. Biol. 2003, 6, 36–41. [Google Scholar] [CrossRef]
- Okuda, S.; Tsutsui, H.; Shiina, K.; Sprunck, S.; Takeuchi, H.; Yui, R.; Kasahara, R.D.; Hamamura, Y.; Mizukami, A.; Susaki, D.; et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nat. Cell Biol. 2009, 458, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, A.G.; Inatsugi, R.; Jiao, J.; Kotake, T.; Kuwata, K.; Ootani, K.; Okuda, S.; Sankaranarayanan, S.; Sato, Y.; Maruyama, D.; et al. The AMOR Arabinogalactan Sugar Chain Induces Pollen-Tube Competency to Respond to Ovular Guidance. Curr. Biol. 2016, 26, 1091–1097. [Google Scholar] [CrossRef]
- Pereira, A.M.; Lopes, A.L.; Coimbra, S. Arabinogalactan Proteins as Interactors along the Crosstalk between the Pollen Tube and the Female Tissues. Front. Plant. Sci. 2016, 7, 1895. [Google Scholar] [CrossRef]
- Su, S.; Higashiyama, T. Arabinogalactan proteins and their sugar chains: Functions in plant reproduction, research methods, and biosynthesis. Plant. Reprod. 2018, 31, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Szczuka, E.; Zdunek, A. Arabinogalactan proteins: Distribution during the development of male and female gametophytes. Plant. Physiol. Biochem. 2019, 135, 9–18. [Google Scholar] [CrossRef]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef]
- Coimbra, S.; Duarte, C. Arabinogalactan proteins may facilitate the movement of pollen tubes from the stigma to the ovules in Actinidia deliciosa and Amaranthus hypochondriacus. Euphytica 2003, 133, 171–178. [Google Scholar] [CrossRef]
- Coimbra, S.; Almeida, J.; Junqueira, V.; Costa, M.L.; Pereira, L.G. Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. J. Exp. Bot. 2007, 58, 4027–4035. [Google Scholar] [CrossRef]
- Gawecki, R.; Sala, K.; Kurczyńska, E.U.; Świątek, P.; Płachno, B.J. Immunodetection of some pectic, arabinogalactan proteins and hemicellulose epitopes in the micropylar transmitting tissue of apomictic dandelions (Taraxacum, Asteraceae, Lactuceae). Protoplasma 2017, 254, 657–668. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Świątek, P.; Stolarczyk, P.; Kocki, J. Immunodetection of Pectic Epitopes, Arabinogalactan Proteins, and Extensins in Mucilage Cells from the Ovules of Pilosella officinarum Vaill. and Taraxacum officinale agg. (Asteraceae). Int. J. Mol. Sci. 2020, 21, 9642. [Google Scholar] [CrossRef]
- Chudzik, B.; Koscinska-Pajak, M.; Snieżko, R. Immunodetection of arabinogalactan proteins (AGPs) in apomictic ovules of Chondrilla juncea L. Acta Biol. Crac. Ser. Bot. 2005, 47, 35. [Google Scholar]
- Płachno, B.J.; Świątek, P. Actin cytoskeleton in the extra-ovular embryo sac of Utricularia nelumbifolia (Lentibulariaceae). Protoplasma 2012, 249, 663–670. [Google Scholar] [CrossRef][Green Version]
- Khan, R. Lentibulariaceae. Bull. Indian Nat. Sci. Acad. 1970, 41, 290–297. [Google Scholar]
- Lora, J.; Perez, V.; Herrero, M.; Hormaza, J.I. Ovary Signals for Pollen Tube Guidance in Chalazogamous Mangifera indica L. Front. Plant. Sci. 2021, 11, 601706. [Google Scholar] [CrossRef]
- Płachno, B.J.; Świątek, P.; Jobson, R.W.; Małota, K.; Brutkowski, W. Serial block face SEM visualization of unusual plant nuclear tubular extensions in a carnivorous plant (Utricularia, Lentibulariaceae). Ann. Bot. 2017, 120, 673–680. [Google Scholar] [CrossRef]
- Suárez, C.; Zienkiewicz, A.; Castro, A.J.; Zienkiewicz, K.; Majewska-Sawka, A.; Rodríguez-García, M.I. Cellular localization and levels of pectins and arabinogalactan proteins in olive (Olea europaea L.) pistil tissues during development: Implications for pollen-pistil interaction. Planta 2013, 237, 305–319. [Google Scholar] [CrossRef]
- da Costa, M.L.; Lopes, A.L.; Amorim, M.I.; Coimbra, S. Immunolocalization of AGPs and Pectins in Quercus suber Gametophytic Structures. Methods Mol. Biol. 2017, 1669, 117–137. [Google Scholar]
- Leszczuk, A.; Szczuka, E. Arabinogalactan proteins: Immunolocalization in the developing ovary of a facultative apomict Fragaria x ananassa (Duch.). Plant. Physiol. Biochem. 2018, 123, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.C. The Hypostase in Torenia fournieri Lind.: A Histochemical Study of the Cell Walls. Ann. Bot. 1983, 51, 17–26. [Google Scholar] [CrossRef]
- Pereira, A.M.; Masiero, S.; Nobre, M.S.; Costa, M.L.; Solís, M.-T.; Testillano, P.S.; Sprunck, S.; Coimbra, S. Differential ex-pression patterns of arabinogalactan proteins in Arabidopsis thaliana reproductive tissues. J. Exp. Bot. 2014, 65, 5459–5471. [Google Scholar] [CrossRef]
- Chudzik, B.; Burlik, M.; Zarzyka, B.; Szczuka, E. Localization of arabinogalactan proteins during development of gametophytes in Bellis perennis L. Acta Biol. Cracov. Bot. 2010, 52, 52. [Google Scholar]
- Mendes, S.P.; Mastroberti, A.A.; Mariath, J.E.; Vieira, R.C.; De Toni, K.L. Ovule and female gametophyte development in the Bromeliaceae: An embryological study of Pitcairnia encholirioides. Bots 2014, 92, 883–894. [Google Scholar] [CrossRef]
- Lopes, A.; Costa, M.; Sobral, R.; Costa, M.M.; Amorim, M.I.; Coimbra, S. Arabinogalactan proteins and pectin distribution during female gametogenesis in Quercus suber L. Ann. Bot. 2016, 117, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.G.; Marulanda, N.F.; Silva, S.R.; Płachno, B.J.; Adamec, L.; Miranda, V.F.O. Phylogeny of the ‘orchid-like’ bladderworts (gen. Utricularia sect. Orchidioides and Iperua: Lentibulariaceae) with remarks on the stolon–tuber system. Ann. Bot. 2017, 120, 709–723. [Google Scholar] [CrossRef][Green Version]
- Lora, J.; Hormaza, J.I. Pollen wall development in mango (Mangifera indica L.; Anacardiaceae). Plant. Reprod. 2018, 31, 385–397. [Google Scholar] [CrossRef]
- Coimbra, S.; Jones, B.; Pereira, L.G. Arabinogalactan proteins (AGPs) related to pollen tube guidance into the embryo sac in Arabidopsis. Plant. Signal. Behav. 2008, 3, 455–456. [Google Scholar] [CrossRef]
- Losada, J.M.; Herrero, M. Pollen tube access to the ovule is mediated by glycoprotein secretion on the obturator of apple (Malus x domestica, Borkh). Ann. Bot. 2017, 119, 989–1000. [Google Scholar] [CrossRef]
- Merz, M. Untersuchungen über die Samenentwicklung der Utricularien. Flora 1897, 84, 69–87. [Google Scholar]
- Merl, E.H. Beiträge zur Kenntnis der Utricularien und Genlisen. Flora 1915, 108, 127–200. [Google Scholar]
- Płachno, B.J.; Świątek, P. Cytoarchitecture of Utricularia nutritive tissue. Protoplasma 2008, 234, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M. Studies in the Lentibulariaceae the embryology of U. stellaris Linn. f. var. inflexa. Part II. Microsporangium, male gametophyte, fertilization, endosperm, embryo and seed. Proc. Natl. Inst. Sci. India 1964, 30, 280–290. [Google Scholar]
- Siddiqui, S.A. Studies in the Lentibulariaceae Pollination, fertilisation, endosperm, embryo and seed in U. dichotoma Labill. Bot. Jahrb. Syst. 1978, 100, 237–245. [Google Scholar]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant. Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Yates, E.A.; Valdor, J.-F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, J. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Renner, T.; Ibarra-Laclette, E.; Farr, K.M.; Chang, T.-H.; Cervantes-Pérez, S.A.; Zheng, C.; Sankoff, D.; Tang, H.; Purbojati, R.W.; et al. Long-read sequencing uncovers the adaptive topography of a carnivorous plant genome. Proc. Natl. Acad. Sci. USA 2017, 114, E4435–E4441. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.R.; Pinheiro, D.G.; Penha, H.A.; Płachno, B.J.; Michael, T.P.; Meer, E.J.; Miranda, V.F.O.; Varani, A.M. Intraspecific Variation within the Utricularia amethystina Species Morphotypes Based on Chloroplast Genomes. Int. J. Mol. Sci. 2019, 20, 6130. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.R.; Moraes, A.P.; Penha, H.A.; Julião, M.H.M.; Domingues, D.S.; Michael, T.P.; Miranda, V.F.O.; Varani, A.M. The Terrestrial Carnivorous Plant Utricularia reniformis Sheds Light on Environmental and Life-Form Genome Plasticity. Int. J. Mol. Sci. 2019, 21, 3. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Lyons, E.; Hernández-Guzmán, G.; Pérez-Torres, C.A.; Carretero-Paulet, L.; Chang, T.-H.; Lan, T.; Welch, A.J.; Juárez, M.J.A.; Simpson, J.; et al. Architecture and evolution of a minute plant genome. Nature 2013, 498, 94–98. [Google Scholar] [CrossRef]
- Poppinga, S.; Weisskopf, C.; Westermeier, A.S.; Masselter, T.; Speck, T. Fastest predators in the plant kingdom: Functional morphology and biomechanics of suction traps found in the largest genus of carnivorous plants. AoB Plants 2016, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Świątek, P.; Miranda, V.F.O.; Stolarczyk, P. The Structure and Occurrence of a Velum in Utricularia Traps (Lentibulariaceae). Front. Plant. Sci. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Świątek, P.; Adamec, L.; Carvalho, S.; Miranda, V.F.O. The Trap Architecture of Utricularia multifida and Utricularia westonii (subg. Polypompholyx). Front. Plant. Sci. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.I.; Bushell, C.; Koide, Y.; Fozard, J.A.; Piao, C.; Yu, M.; Newman, J.; Whitewoods, C.; Avondo, J.; Kennaway, R.; et al. Shaping of a three-dimensional carnivorous trap through modulation of a planar growth mechanism. PLoS Biol. 2019, 17, e3000427. [Google Scholar] [CrossRef] [PubMed]
- Whitewoods, C.D.; Gonçalves, B.; Cheng, J.; Cui, M.; Kennaway, R.; Lee, K.; Bushell, C.; Yu, M.; Piao, C.; Coen, E. Evolution of carnivorous traps from planar leaves through simple shifts in gene expression. Science 2020, 367, 91–96. [Google Scholar] [CrossRef]
- Whitewoods, C.D.; Coen, E. Growth and Development of Three-Dimensional Plant Form. Curr. Biol. 2017, 27, R910–R918. [Google Scholar] [CrossRef]
- Rutishauser, R. EvoDevo: Past and Future of Continuum and Process Plant Morphology. Philosophy 2020, 5, 41. [Google Scholar] [CrossRef]
- Reut, M.S.; Płachno, B.J. Unusual developmental morphology and anatomy of vegetative organs in Utricularia dichotoma—Leaf, shoot and root dynamics. Protoplasma 2020, 257, 371–390. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Epitope | References |
|---|---|---|
| JIM8 | Arabinogalactan | [56] |
| JIM13 | Arabinogalactan/arabinogalactan protein | [57,58] |
| JIM14 | Arabinogalactan/arabinogalactan protein | [57,58] |
| LM2 | Arabinogalactan/arabinogalactan protein | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachno, B.J.; Kapusta, M.; Świątek, P.; Banaś, K.; Miranda, V.F.O.; Bogucka-Kocka, A. Spatio-Temporal Distribution of Cell Wall Components in the Placentas, Ovules and Female Gametophytes of Utricularia during Pollination. Int. J. Mol. Sci. 2021, 22, 5622. https://doi.org/10.3390/ijms22115622
Płachno BJ, Kapusta M, Świątek P, Banaś K, Miranda VFO, Bogucka-Kocka A. Spatio-Temporal Distribution of Cell Wall Components in the Placentas, Ovules and Female Gametophytes of Utricularia during Pollination. International Journal of Molecular Sciences. 2021; 22(11):5622. https://doi.org/10.3390/ijms22115622
Chicago/Turabian StylePłachno, Bartosz Jan, Małgorzata Kapusta, Piotr Świątek, Krzysztof Banaś, Vitor F. O. Miranda, and Anna Bogucka-Kocka. 2021. "Spatio-Temporal Distribution of Cell Wall Components in the Placentas, Ovules and Female Gametophytes of Utricularia during Pollination" International Journal of Molecular Sciences 22, no. 11: 5622. https://doi.org/10.3390/ijms22115622
APA StylePłachno, B. J., Kapusta, M., Świątek, P., Banaś, K., Miranda, V. F. O., & Bogucka-Kocka, A. (2021). Spatio-Temporal Distribution of Cell Wall Components in the Placentas, Ovules and Female Gametophytes of Utricularia during Pollination. International Journal of Molecular Sciences, 22(11), 5622. https://doi.org/10.3390/ijms22115622








