The Multifaceted Nature of Nucleobindin-2 in Carcinogenesis
Abstract
:1. Introduction
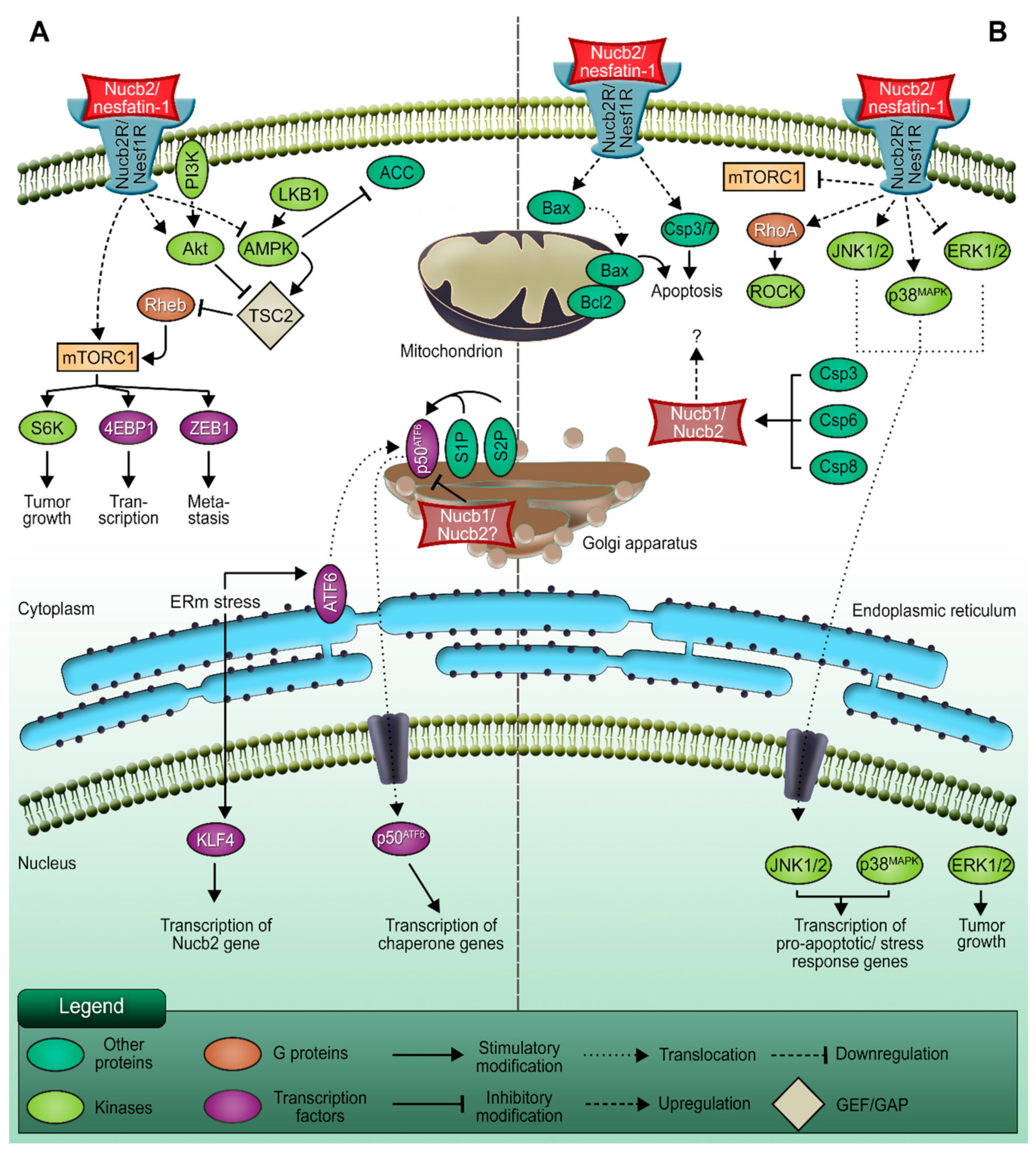
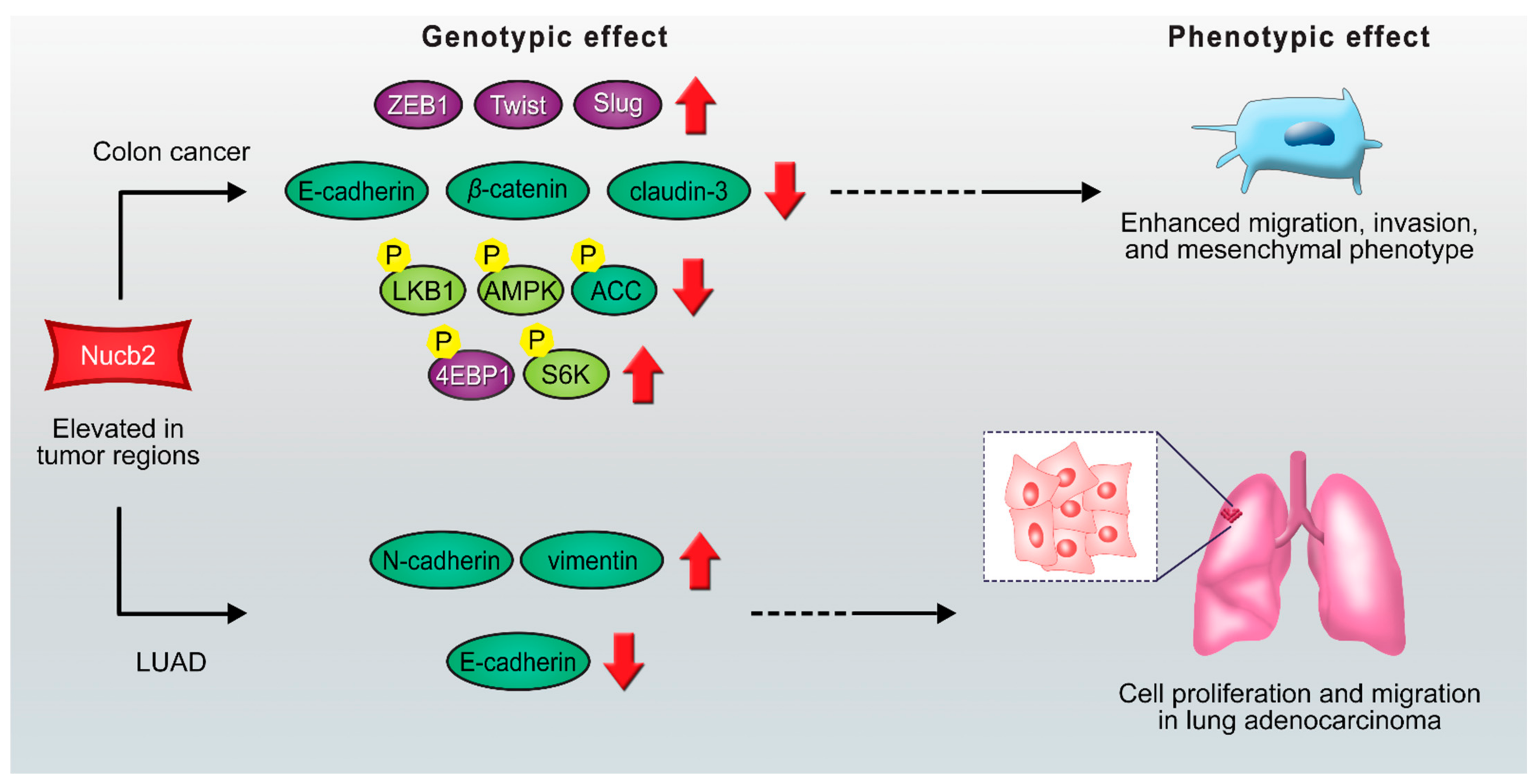
2. Nucb2 Involvement in Cancer Progression
2.1. The Action of Transcription Factors on the Migration and Invasion of Nucb2-Mediated Cancer Cells
2.2. The Potential Role of Nucb2 in Melanoma Metastasis under Endoplasmic Reticulum (ERm) Stress
2.3. Nucb2 Expression during Cancer-Associated Anorexia-Cachexia
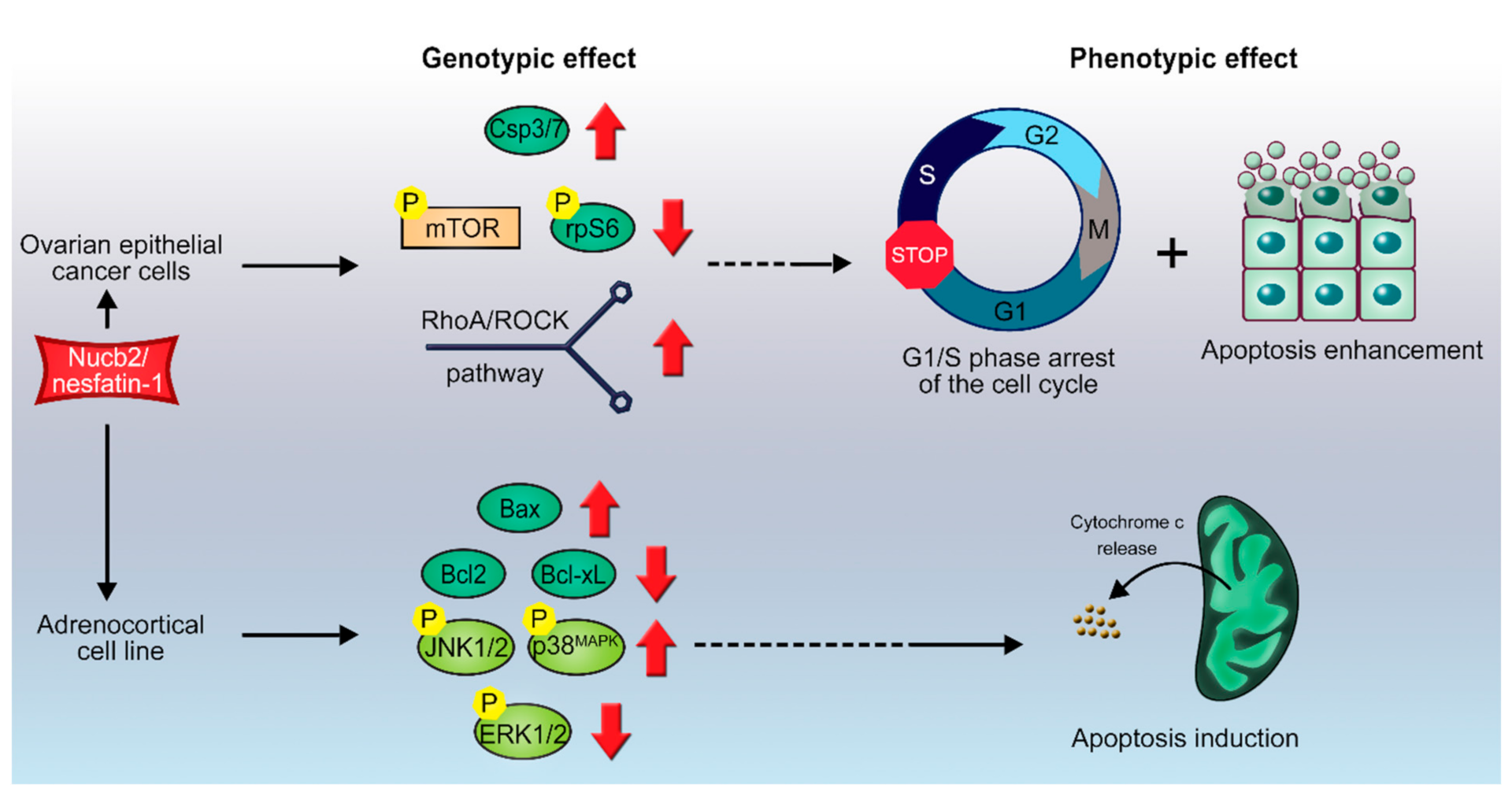
3. Apoptotic Potential of Nucb2
3.1. Nucb2/nesfatin-1-Induced Apoptosis in Ovarian Cancer
3.2. Nucb2/Nesfatin-1-Induced Cell Death in Adrenocortical Carcinoma
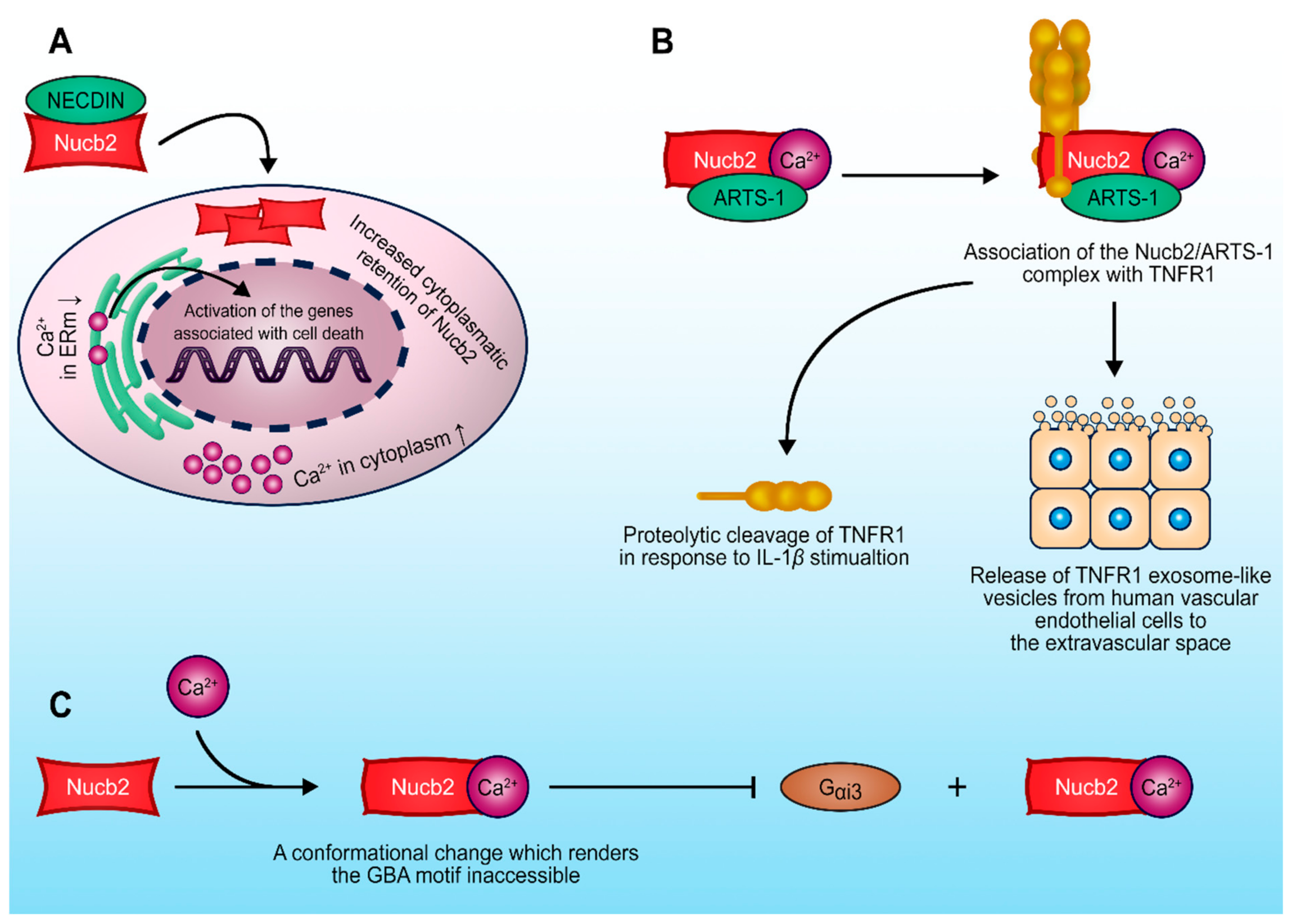
4. Nucb2-Protein Interactions in the Regulation of the Tumorigenesis Process
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4EBIP1 | eukaryotic initiation factor 4E-binding protein |
| ACC | acetyl-CoA carboxylase |
| ACCs | adrenocortical cell carcinomas |
| ACTs | adrenal cortex tumors |
| AMPK | 5’AMP-activated protein kinase |
| ARTS-1 | aminopeptidase regulator of TNFR1 shedding |
| ATF6 | activating transcription factor 6 |
| Bax | Bcl2 associated X protein |
| CACS | cancer anorexia-cachexia syndrome |
| ccRCC | clear-cell renal cell carcinoma |
| CTCs | circulating tumor cells |
| EMT | epithelial–mesenchymal transition |
| ER | estrogen receptor |
| ERm | endoplasmic reticulum |
| ERE | estrogen response element |
| GAPs | GTPase-activating proteins |
| GDIs | guanine nucleotide dissociation inhibitors |
| GEFs | guanine nucleotide-exchange factors |
| GIV/Girdin | Gα-interacting vesicle-associated protein |
| GPCR | G-protein-coupled receptors |
| IDPs | inherently disordered proteins |
| KLF4 | Krüppel-like factor 4 |
| LKB1 | liver kinase B1 |
| LUAD | lung adenocarcinoma |
| MAPKs | serine/threonine mitogen activated protein kinases |
| MMPs | matrix metalloproteinase |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mTOR complex 1 |
| Nucb2 | Nucleobindin-2 |
| ERK1/2 | extracellular signal-regulated kinases 1 and 2 |
| JNK-1/2 | c-Jun N-terminal kinases-1 and -2 |
| PSA | prostate specific antigen |
| PVN | paraventricular nucleus |
| RhoA | Ras homologue gene family member A |
| ROCKs | Rho-associated coiled-coil-containing kinases |
| Slug | snail family zinc finger 2 |
| S6K | S6 kinase |
| S1P | site-1 protease |
| TNF | tumor necrosis factor |
| TNFRs | tumor necrosis factor receptors |
| TSC2 | tuberos sclerosis complex 2 |
| Twist | twist family bHLH transcription factor 1 |
| UPR | unfolded protein response |
| ZEB1 | zinc finger E-box-binding homeobox transcription factor 1 |
References
- Barnikol-Watanabe, S.; Groß, N.A.; Götz, H.; Henkel, T.; Karabinos, A.; Kratzin, H.; Barnikol, H.U.; Hilschmann, N. Human Protein NEFA, a Novel DNA Binding/EF-Hand/Leucine Zipper Protein. Molecular Cloning and Sequence Analysis of the cDNA, Isolation and Characterization of the Protein. Biol. Chem. Hoppe Seyler. 1994, 375, 497–512. [Google Scholar] [CrossRef]
- Oh-I, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nat. Cell Biol. 2006, 443, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneya, M.; Chen, J.; Brown, J.E.; Tripathi, G.; Hallschmid, M.; Patel, S.; Kern, W.; Hillhouse, E.W.; Lehnert, H.; Tan, B.K.; et al. Identification of Nesfatin-1 in Human and Murine Adipose Tissue: A Novel Depot-Specific Adipokine with Increased Levels in Obesity. Endocrinology 2010, 151, 3169–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, R.; Tiwari, A.; Unniappan, S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem. Biophys. Res. Commun. 2009, 381, 643–648. [Google Scholar] [CrossRef] [PubMed]
- García-Galiano, D.; Pineda, R.; Ilhan, T.; Castellano, J.M.; Ruiz-Pino, F.; Sánchez-Garrido, M.A.; Vazquez, M.J.; Sangiao-Alvarellos, S.; Romero-Ruiz, A.; Pinilla, L.; et al. Cellular Distribution, Regulated Expression, and Functional Role of the Anorexigenic Peptide, NUCB2/Nesfatin-1, in the Testis. Endocrinology 2012, 153, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, J.; Tang, Y.; Bi, F.; Liu, J.-N. The novel function of nesfatin-1: Anti-hyperglycemia. Biochem. Biophys. Res. Commun. 2010, 391, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Adamik, B.; Hawari, F.I.; Ma, G.; Rouhani, F.N.; Zhang, J.; Levine, S.J. Extracellular TNFR1 Release Requires the Calcium-dependent Formation of a Nucleobindin 2-ARTS-1 Complex. J. Biol. Chem. 2006, 281, 6860–6873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, N.; Taniura, H.; Niinobe, M.; Takayama, C.; Tominaga-Yoshino, K.; Ogura, A.; Yoshikawa, K. The Postmitotic Growth Suppressor Necdin Interacts with a Calcium-binding Protein (NEFA) in Neuronal Cytoplasm. J. Biol. Chem. 2000, 275, 31674–31681. [Google Scholar] [CrossRef] [Green Version]
- Skorupska, A.; Bystranowska, D.; Dąbrowska, K.; Ożyhar, A. Calcium ions modulate the structure of the intrinsically disordered Nucleobindin-2 protein. Int. J. Biol. Macromol. 2020, 154, 1091–1104. [Google Scholar] [CrossRef]
- Martinelli, A.H.S.; Lopes, F.C.; John, E.B.O.; Carlini, C.R.; Ligabue-Braun, R. Modulation of Disordered Proteins with a Focus on Neurodegenerative Diseases and Other Pathologies. Int. J. Mol. Sci. 2019, 20, 1322. [Google Scholar] [CrossRef] [Green Version]
- Santofimia-Castaño, P.; Rizzuti, B.; Xia, Y.; Abian, O.; Peng, L.; Velázquez-Campoy, A.; Neira, J.L.; Iovanna, J. Targeting intrinsically disordered proteins involved in cancer. Cell. Mol. Life Sci. 2020, 77, 1695–1707. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Qi, C.; Li, L.; Luo, F.; Xu, Y. Clinical significance of NUCB2 mRNA expression in prostate cancer. J. Exp. Clin. Cancer Res. 2013, 32, 56. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Takagi, K.; Miki, Y.; Onodera, Y.; Akahira, J.-I.; Ebata, A.; Ishida, T.; Watanabe, M.; Sasano, H.; Suzuki, T. Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2011, 103, 136–143. [Google Scholar] [CrossRef]
- Takagi, K.; Miki, Y.; Tanaka, S.; Hashimoto, C.; Watanabe, M.; Sasano, H.; Ito, K.; Suzuki, T. Nucleobindin 2 (NUCB2) in human endometrial carcinoma: A potent prognostic factor associated with cell proliferation and migration. Endocr. J. 2016, 63, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Pang, X.; Dong, M.; Wen, F.; Zhang, Y. Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation in vitro. Biochem. Biophys. Res. Commun. 2013, 440, 467–472. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [Green Version]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Kan, J.-Y.; Yen, M.-C.; Wang, J.-Y.; Wu, D.-C.; Chiu, Y.-J.; Ho, Y.-W.; Kuo, P.-L. Nesfatin-1/Nucleobindin-2 enhances cell migration, invasion, and epithelial-mesenchymal transition via LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget 2016, 7, 31336–31349. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yun, X.; Ruan, X.; Chi, J.; Yu, Y.; Yigong, L.; Zheng, X.; Gao, M. High expression of NUCB2 promotes papillary thyroid cancer cells proliferation and invasion. OncoTargets Ther. 2019, 12, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Li, W.; Qi, K.; Zhou, J.; Gu, M.; Wang, Z. A novel function of NUCB2 in promoting the development and invasion of renal cell carcinoma. Oncol. Lett. 2017, 15, 2425–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, C.; Ma, H.; Zhang, H.-T.; Gao, J.-D.; Xu, Y. Nucleobindin 2 expression is an independent prognostic factor for clear cell renal cell carcinoma. Histopathology 2014, 66, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, C.; Wang, A.; Yao, B.; Li, L.; Wang, Y.; Xu, Y. Prognostication of prostate cancer based on NUCB2 protein assessment: NUCB2 in prostate cancer. J. Exp. Clin. Cancer Res. 2013, 32, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.-J.; Lv, J.-X.; Liu, J.; Zhang, X.-B.; Wang, L.-B. Nucleobindin-2 Promotes the Growth and Invasion of Glioblastoma. Cancer Biotherapy Radiopharm. 2019, 34, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Chen, L.; Chen, W. High NUCB2 expression level is associated with metastasis and may promote tumor progression in colorectal cancer. Oncol. Lett. 2018, 15, 9188–9194. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-M.; Xu, Z.-Q.; Ma, H.-S. Nesfatin-1/Nucleobindin-2 Is a Potent Prognostic Marker and Enhances Cell Proliferation, Migration, and Invasion in Bladder Cancer. Dis. Markers 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Belachew, E.B.; Sewasew, D.T. Molecular Mechanisms of Endocrine Resistance in Estrogen-Receptor-Positive Breast Cancer. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Thomas, C.; Gustafsson, J.-Å. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef]
- Lim, E.; Tarulli, G.; Portman, N.; Hickey, T.E.; Tilley, W.D.; Palmieri, C. Pushing estrogen receptor around in breast cancer. Endocr. Relat. Cancer 2016, 23, T227–T241. [Google Scholar] [CrossRef]
- Ellis, M.J.; Ding, L.; Shen, D.; Luo, J.; Suman, V.J.; Wallis, J.W.; Van Tine, B.A.; Hoog, J.; Goiffon, R.; Goldstein, T.C.; et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nat. Cell Biol. 2012, 486, 353–360. [Google Scholar] [CrossRef]
- Kahlert, S.; Nuedling, S.; van Eickels, M.; Vetter, H.; Meyer, R.; Grohé, C. Estrogen Receptor α Rapidly Activates the IGF-1 Receptor Pathway. J. Biol. Chem. 2000, 275, 18447–18453. [Google Scholar] [CrossRef] [Green Version]
- Bourdeau, V.; Deschênes, J.; Métivier, R.; Nagai, Y.; Nguyen, D.; Bretschneider, N.; Gannon, F.; White, J.H.; Mader, S. Genome-Wide Identification of High-Affinity Estrogen Response Elements in Human and Mouse. Mol. Endocrinol. 2004, 18, 1411–1427. [Google Scholar] [CrossRef] [Green Version]
- Tsujihashi, H.; Nakanishi, A.; Matsuda, H.; Uejima, S.; Kurita, T. Cell Proliferation of Human Bladder Tumors Determined by Brdurd and Ki-67 Immunostaining. J. Urol. 1991, 145, 846–849. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Brehmer, B.; Biesterfeld, S.; Jakse, G. Expression of matrix metalloproteinases (MMP-2 and -9) and their inhibitors (TIMP-1 and -2) in prostate cancer tissue. Prostate Cancer Prostatic Dis. 2003, 6, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Lai, X.; Li, Q.; Wu, F.; Lin, J.; Chen, J.; Zheng, H.; Guo, L. Epithelial-Mesenchymal Transition and Metabolic Switching in Cancer: Lessons From Somatic Cell Reprogramming. Front. Cell Dev. Biol. 2020, 8, 760. [Google Scholar] [CrossRef]
- Tao, R.; Niu, W.; Dou, P.; Ni, S.; Yu, Y.; Cai, L.; Wang, X.; Li, S.; Zhang, C.; Luo, Z. Nucleobindin-2 enhances the epithelial-mesenchymal transition in renal cell carcinoma. Oncol. Lett. 2020, 19, 3653–3664. [Google Scholar] [CrossRef] [Green Version]
- Huo, X.; Wang, H.; Huo, B.; Wang, L.; Yang, K.; Wang, J.; Wang, L.; Wang, H. FTX contributes to cell proliferation and migration in lung adenocarcinoma via targeting miR-335-5p/NUCB2 axis. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Folgueira, C.; Barja-Fernandez, S.; Prado, L.; Al-Massadi, O.; Castelao, C.; Pena-Leon, V.; González-Sáenz, P.; Baltar, J.; Baamonde, I.; Leis, R.; et al. Pharmacological inhibition of cannabinoid receptor 1 stimulates gastric release of nesfatin-1 via the mTOR pathway. World J. Gastroenterol. 2017, 23, 6403–6411. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Li, Y.; Zhao, J.; Mulholland, M.W.; Zhang, W. mTOR-dependent Modulation of Gastric Nesfatin-1/NUCB2. Cell. Physiol. Biochem. 2012, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, J.; Chao, Y.; Zhang, L.; Jin, L.; Li, N.; He, R.; Ma, B.; Zhao, W.; Han, C. Regulation of the adaptation to ER stress by KLF4 facilitates melanoma cell metastasis via upregulating NUCB2 expression. J. Exp. Clin. Cancer Res. 2018, 37, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukumo, Y.; Tomida, A.; Kitahara, O.; Nakamura, Y.; Asada, S.; Mori, K.; Tsuruo, T. Nucleobindin 1 Controls the Unfolded Protein Response by Inhibiting ATF6 Activation. J. Biol. Chem. 2007, 282, 29264–29272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicari, D.; Fantuz, M.; Bellazzo, A.; Valentino, E.; Apollonio, M.; Pontisso, I.; Di Cristino, F.; Ferro, M.D.; Bicciato, S.; Del Sal, G.; et al. Mutant p53 improves cancer cells’ resistance to endoplasmic reticulum stress by sustaining activation of the UPR regulator ATF6. Oncogene 2019, 38, 6184–6195. [Google Scholar] [CrossRef]
- Dadey, D.Y.; Kapoor, V.; Khudanyan, A.; Urano, F.; Kim, A.H.; Thotala, D.; Hallahan, D.E. The ATF6 pathway of the ER stress response contributes to enhanced viability in glioblastoma. Oncotarget 2015, 7, 2080–2092. [Google Scholar] [CrossRef] [Green Version]
- Tay, K.H.; Luan, Q.; Croft, A.; Jiang, C.C.; Jin, L.; Zhang, X.D.; Tseng, H.-Y. Sustained IRE1 and ATF6 signaling is important for survival of melanoma cells undergoing ER stress. Cell. Signal. 2014, 26, 287–294. [Google Scholar] [CrossRef]
- Morishima, N.; Nakanishi, K.; Nakano, A. Activating Transcription Factor-6 (ATF6) Mediates Apoptosis with Reduction of Myeloid Cell Leukemia Sequence 1 (Mcl-1) Protein via Induction of WW Domain Binding Protein 1*. J. Biol. Chem. 2011, 286, 35227–35235. [Google Scholar] [CrossRef] [Green Version]
- Valencia, C.A.; Cotten, S.W.; Duan, J.; Liu, R.; Cotten, S.W. Modulation of nucleobindin-1 and nucleobindin-2 by caspases. FEBS Lett. 2007, 582, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Yao, Y.; Hofmeister, R.; Tsien, R.Y.; Farquhar, M.G. Overexpression of CALNUC (Nucleobindin) Increases Agonist and Thapsigargin Releasable Ca2+ Storage in the Golgi. J. Cell Biol. 1999, 145, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Hirai, M.; Kanai, Y.; Kurosawa, Y. Organization of the Human Gene for Nucleobindin (NUC) and Its Chromosomal Assignment to 19q13.2–q13.4. Genomics 1996, 34, 181–186. [Google Scholar] [CrossRef]
- Nesselhut, J.; Jurgan, U.; Onken, E.; Gotz, H.; Barnikol, H.U.; Hirschfeld, G.; Barnikol-Watanabe, S.; Hilschmann, N. Gol-gi retention of human protein NEFA is mediated by its N-terminal Leu/Ile-rich region. FEBS Lett. 2001, 509, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- McClement, S. Cancer Anorexia-Cachexia Syndrome. J. Wound Ostomy Cont. Nurs. 2005, 32, 264–268. [Google Scholar] [CrossRef]
- Inui, A. Cancer anorexia-cachexia syndrome: Are neuropeptides the key? Cancer Res. 1999, 59, 4493–4501. [Google Scholar]
- Plata-Salamán, C.R. Central nervous system mechanisms contributing to the cachexia–anorexia syndrome. Nutrition 2000, 16, 1009–1012. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Massa, E.; Madeddu, C. Managing Cancer-Related Anorexia/Cachexia. Drugs 2001, 61, 499–514. [Google Scholar] [CrossRef]
- Burgos, J.R.; Iresjö, B.-M.; Smedh, U. MCG101-induced cancer anorexia-cachexia features altered expression of hypothalamic Nucb2 and Cartpt and increased plasma levels of cocaine- and amphetamine-regulated transcript peptides. Oncol. Rep. 2016, 35, 2425–2430. [Google Scholar] [CrossRef]
- Lonnroth, C.; Svaninger, G.; Gelin, J.; Cahlin, C.; Iresjö, B.-M.; Cvetkovska, E.; Edstrom, S.; Andersson, M.; Svanberg, E.; Lundholm, K. Effects related to indomethacin prolonged survival and decreased tumor growth in a mouse tumor model with cytokine dependent cancer cachexia. Int. J. Oncol. 1995, 7, 1405–1413. [Google Scholar] [CrossRef]
- Gelin, J.; Andersson, C.; Lundholm, K. Effects of indomethacin, cytokines, and cyclosporin A on tumor growth and the subsequent development of cancer cachexia. Cancer Res. 1991, 51, 880–885. [Google Scholar]
- Ezeoke, C.C.; Morley, J.E. Pathophysiology of anorexia in the cancer cachexia syndrome. J. Cachex Sarcopenia Muscle 2015, 6, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, T.; Elbelt, U.; Ahnis, A.; Rose, M.; Klapp, B.F.; Stengel, A. Sex-specific regulation of NUCB2/nesfatin-1: Differential implication in anxiety in obese men and women. Psychoneuroendocrinology 2015, 60, 130–137. [Google Scholar] [CrossRef]
- Ge, J.-F.; Xu, Y.-Y.; Qin, G.; Pan, X.-Y.; Cheng, J.-Q.; Chen, F.-H. Nesfatin-1, a potent anorexic agent, decreases exploration and induces anxiety-like behavior in rats without altering learning or memory. Brain Res. 2015, 1629, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.-M.; Li, J.-B.; Jiang, L.-L.; Shao, H.; Wang, B.-L. Plasma nesfatin-1 level is associated with severity of depression in Chinese depressive patients. BMC Psychiatry 2018, 18, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.-R.; Liang, J.; Cao, Y.; Shan, F.; Liu, Y.; Xu, Y.-Y. Increased plasma nesfatin-1 levels may be associated with corticosterone, IL-6, and CRP levels in patients with major depressive disorder. Clin. Chim. Acta 2018, 480, 107–111. [Google Scholar] [CrossRef]
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of Apoptosis in disease. Aging 2012, 4, 330–349. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.M.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Mph, K.D.M.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Rodríguez-Penas, D.; García-Rúa, V.; Mosquera-Leal, A.; Abu-Assi, E.; Portoles, M.; Roselló-Lletí, E.; Rivera, M.; Diéguez, C.; González-Juanatey, J.R.; et al. 24 h nesfatin-1 treatment promotes apoptosis in cardiomyocytes. Endocrine 2015, 51, 551–555. [Google Scholar] [CrossRef]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef] [Green Version]
- Thampi, A.; Shah, E.; Elshimy, G.; Correa, R. Adrenocortical carcinoma: A literature review. Transl. Cancer Res. 2020, 9, 1253–1264. [Google Scholar] [CrossRef]
- Pereira, S.S.; Monteiro, M.P.; Antonini, S.R.; Pignatelli, D. Apoptosis regulation in adrenocortical carcinoma. Endocr. Connect. 2019, 8, R91–R104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanjaneya, M.; Tan, B.K.; Rucinski, M.; Kawan, M.; Hu, J.; Kaur, J.; Patel, V.H.; Malendowicz, L.K.; Komarowska, H.; Lehnert, H.; et al. Nesfatin-1 inhibits proliferation and enhances apoptosis of human adrenocortical H295R cells. J. Endocrinol. 2015, 226, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, D.N.; Reddy, E.P. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer 2017, 8, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Chapman, E.J.; Knowles, M.A. Necdin: A multi functional protein with potential tumor suppressor role? Mol. Carcinog. 2009, 48, 975–981. [Google Scholar] [CrossRef]
- Taniura, H.; Matsumoto, K.; Yoshikawa, K. Physical and Functional Interactions of Neuronal Growth Suppressor Necdin with p53. J. Biol. Chem. 1999, 274, 16242–16248. [Google Scholar] [CrossRef] [Green Version]
- Kuwako, K.-I.; Taniura, H.; Yoshikawa, K. Necdin-related MAGE Proteins Differentially Interact with the E2F1 Transcription Factor and the p75 Neurotrophin Receptor. J. Biol. Chem. 2004, 279, 1703–1712. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Kirton, H.M.; MacDougall, D.A.; Boyle, J.P.; Deuchars, J.; Frater, B.; Ponnambalam, S.; Hardy, M.E.; White, E.; Calaghan, S.C.; et al. The Golgi apparatus is a functionally distinct Ca2+store regulated by the PKA and Epac branches of the β1-adrenergic signaling pathway. Sci. Signal. 2015, 8, ra101. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF Receptor Superfamilies. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Hawari, F.; Alsaaty, S.; Lawrence, M.; Combs, C.A.; Geng, W.; Rouhani, F.N.; Miskinis, D.; Levine, S.J. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J. Clin. Investig. 2002, 110, 515–526. [Google Scholar] [CrossRef]
- Garcia-Marcos, M.; Kietrsunthorn, P.S.; Wang, H.; Ghosh, P.; Farquhar, M.G. G Protein Binding Sites on Calnuc (Nucleobindin 1) and NUCB2 (Nucleobindin 2) Define a New Class of Gαi-regulatory Motifs. J. Biol. Chem. 2011, 286, 28138–28149. [Google Scholar] [CrossRef] [Green Version]
- Maziarz, M.; Broselid, S.; DiGiacomo, V.; Park, J.-C.; Luebbers, A.; Garcia-Navarrete, L.; Blanco-Canosa, J.B.; Baillie, G.S.; Garcia-Marcos, M. A biochemical and genetic discovery pipeline identifies PLCδ4b as a nonreceptor activator of heterotrimeric G-proteins. J. Biol. Chem. 2018, 293, 16964–16983. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Garcia-Marcos, M.; Farquhar, M.G. GIV/Girdin is a rheostat that fine-tunes growth factor signals during tumor progression. Cell Adhes. Migr. 2011, 5, 237–248. [Google Scholar] [CrossRef] [Green Version]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Lappano, R.; Maggiolini, M. GPCRs and cancer. Acta Pharmacol. Sin. 2012, 33, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Marcos, M.; Ghosh, P.; Farquhar, M.G.; Zhao, H.; Chiaro, C.R.; Zhang, L.; Smith, P.B.; Chan, C.Y.; Pedley, A.M.; Pugh, R.J.; et al. GIV/Girdin Transmits Signals from Multiple Receptors by Triggering Trimeric G Protein Activation. J. Biol. Chem. 2015, 290, 6697–6704. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Jiang, P.; Cui, S.-P.; Ren, Y.-L.; Zhu, S.-N.; Yang, J.-P.; Du, J.; Zhang, Y.; Liu, J.-Y.; Zhang, B. Clinical Implications for Girdin Protein Expression in Breast Cancer. Cancer Investig. 2011, 29, 405–410. [Google Scholar] [CrossRef]
- Liu, C.; Xue, H.; Lu, Y.; Chi, B. Stem cell gene Girdin: A potential early liver metastasis predictor of colorectal cancer. Mol. Biol. Rep. 2012, 39, 8717–8722. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, Y.; Cai, Z.; Ye, X.; Li, L.; Luo, X.; Yu, C. Girdin regulates the proliferation and apoptosis of pancreatic cancer cells via the PI3K/Akt signalling pathway. Oncol. Rep. 2018, 40, 599–608. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Gao, W.; Wang, S.; Wu, K.; Lu, C.; Luo, X.; Li, L.; Yu, C. Girdin interaction with vimentin induces EMT and promotes the growth and metastasis of pancreatic ductal adenocarcinoma. Oncol. Rep. 2020, 44, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Barbazan, J.; Dunkel, Y.; Li, H.; Nitsche, U.; Janssen, K.-P.; Messer, K.; Ghosh, P. Prognostic Impact of Modulators of G proteins in Circulating Tumor Cells from Patients with Metastatic Colorectal Cancer. Sci. Rep. 2016, 6, 22112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagunas-Rangel, F.A. KDM6B (JMJD3) and its dual role in cancer. Biochimie 2021, 184, 63–71. [Google Scholar] [CrossRef]
- Tang, B.; Qi, G.; Tang, F.; Yuan, S.; Wang, Z.; Liang, X.; Li, B.; Yu, S.; Liu, J.; Huang, Q.; et al. Aberrant JMJD3 Expression Upregulates Slug to Promote Migration, Invasion, and Stem Cell–Like Behaviors in Hepatocellular Carcinoma. Cancer Res. 2016, 76, 6520–6532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Shi, X.; Tian, F.; Fang, Y.; Wu, J.B.; Mrdenovic, S.; Nian, X.; Ji, J.; Xu, H.; Kong, C.; et al. KDM6B is an androgen regulated gene and plays oncogenic roles by demethylating H3K27me3 at cyclin D1 promoter in prostate cancer. Cell Death Dis. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Bhattarai, A.; Emerson, I.A. Dynamic conformational flexibility and molecular interactions of intrinsically disordered proteins. J. Biosci. 2020, 45, 1–17. [Google Scholar] [CrossRef]
| Cancer Type | Signaling Pathway | Role of Nucb2 | Types of Studies | References |
|---|---|---|---|---|
| Adrenocortical carcinoma | JNK-1/2/p38MAPK Ras/Raf/MEK/ERK | Apoptosis induction | in vitro | [72] |
| Bladder cancer cells | MMPs | Cancer migration and invasiveness promotion | in vitro | [26] |
| Colon cancer | LKB1/AMPK/mTORC1/ZEB1 G protein signaling | EMT promotion and cancer metastasis | in vitro and in vivo | [19,94] |
| Melanoma cells | Adaptation to ER stress | in vitro and in vivo | [42] | |
| Non-small cell lung carcinoma | FTX/ miR-335-5p/ Akt/mTOR | EMT induction and cancer metastasis | in vitro and in vivo | [39] |
| Ovarian epithelial cell carcinoma | mTOR/RhoA/ROCK | Apoptosis enhancement | in vitro | [15] |
| Renal cell carcinoma | AMPK/mTORC1/ZEB1 | EMT induction | in vitro and in vivo | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorupska, A.; Lenda, R.; Ożyhar, A.; Bystranowska, D. The Multifaceted Nature of Nucleobindin-2 in Carcinogenesis. Int. J. Mol. Sci. 2021, 22, 5687. https://doi.org/10.3390/ijms22115687
Skorupska A, Lenda R, Ożyhar A, Bystranowska D. The Multifaceted Nature of Nucleobindin-2 in Carcinogenesis. International Journal of Molecular Sciences. 2021; 22(11):5687. https://doi.org/10.3390/ijms22115687
Chicago/Turabian StyleSkorupska, Anna, Rafał Lenda, Andrzej Ożyhar, and Dominika Bystranowska. 2021. "The Multifaceted Nature of Nucleobindin-2 in Carcinogenesis" International Journal of Molecular Sciences 22, no. 11: 5687. https://doi.org/10.3390/ijms22115687





