Well-Known and Novel Serum Biomarkers for Risk Stratification of Patients with Non-ischemic Dilated Cardiomyopathy
Abstract
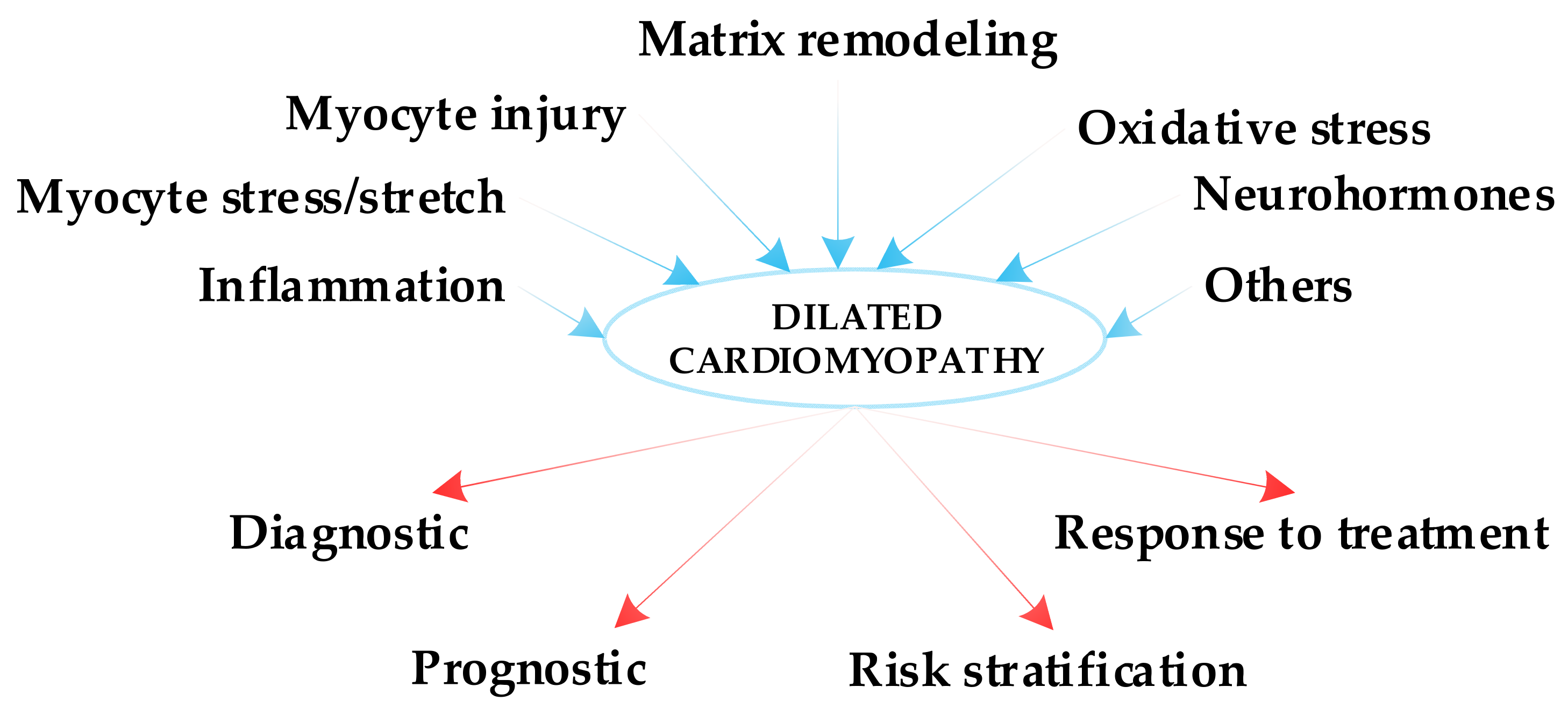
1. Introduction
- -
- class I: ordinary physical activity does not cause undue fatigue, palpitation or dyspnea.
- -
- class II: ordinary physical activity results in fatigue, palpitation or dyspnea.
- -
- class III: less than ordinary physical activity causes fatigue, palpitation or dyspnea.
- -
- class IV: unable to carry out any physical activity without discomfort and patients have symptoms of heart failure at rest [6].
- -
- The measurement of the biomarker can be repeated with cost-efficient methods.
- -
- It must bring more information compared to other tests already performed.
- -
- It should be clinically useful for decision making.
2. Inflammatory Biomarkers
2.1. C-Reactive Protein (CRP) and High-Sensitivity-CRP (hs-CRP)
2.2. Neutrophil/Lymphocyte Ratio (NLR)
2.3. Galectin-3 (Gal-3)
2.4. Chemerin
2.5. Tumor Necrosis Factor-α (TNF-α)
3. Biomarkers of Myocyte Stress/Stretch
3.1. B-Type Natriuretic Peptide (BNP) and N-Terminal-pro Hormone BNP (NT-proBNP)
3.2. Soluble ST2
4. Biomarkers of Myocyte Injury
4.1. High-Sensitivity Cardiac Troponin T
4.2. Heart-Type Fatty Acid Binding Protein (H-FABP)
4.3. Myosin Binding Protein-C (MyBP-C)
5. Biomarkers of Extracellular Matrix Remodeling
Matrix Metalloproteinases (MMP)
6. Biomarkers of Oxidative Stress
Myeloperoxidase (MPO)
7. Neurohormones
8. Other Biomarkers
8.1. Endothelial Progenitor Cells (EPCs)
8.2. Bisphenol A (BPA)
8.3. MicroRNAs (miRNAs)
8.4. Syndecan-1
8.5. T-Cadherin
8.6. Growth Differentiation Factor-15 (GDF-15)
9. Emerging Approaches and Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marrow, B.A.; Cook, S.A.; Prasad, S.K.; McCann, G.P. Emerging techniques for risk stratification in nonischemic dilated cardiomyopathy: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 1196–1207. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Halliday, B.P.; Gulati, A.; Ali, A.; Newsome, S.; Lota, A.; Tayal, U.; Vassiliou, V.S.; Arzanauskaite, M.; Izgi, C.; Krishnathasan, K.; et al. Sex- and age- based differences in the natural history and outcome of dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 1392–1400. [Google Scholar] [CrossRef]
- Hammersley, D.J.; Halliday, B.P. Sudden cardiac death prediction in non-ischemic dilated cardiomyopathy: A multiparametric and dynamic approach. Curr. Cardiol. Rep. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Li, X.; Fan, X.; Li, S.; Sun, W.; Shivkumar, K.; Zhao, S.; Lu, M.; Yao, Y. A novel risk stratification score for sudden cardiac death prediction in middle-aged, nonischemic dilated cardiomyopathy patients: The ESTIMATED score. Can. J. Cardiol. 2020, 36, 1121–1129. [Google Scholar] [CrossRef]
- Morrow, D.A.; de Lemos, J.A. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation 2007, 115, 949–952. [Google Scholar] [CrossRef]
- Braunwald, E. Biomarkers in heart failure. N. Eng. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef]
- Dookhun, M.N.; Sun, Y.; Zou, H.; Cao, X.; Lu, X. Classification of new biomarkers of dilated cardiomyopathy based on pathogenesis—An update. Health 2018, 10, 300–312. [Google Scholar] [CrossRef][Green Version]
- Clapp, B.R.; Hirschfield, G.M.; Storry, C.; Gallimore, J.R.; Stidwill, R.P.; Singer, M.; Deanfield, J.E.; MacAllister, R.J.; Pepys, M.B.; Vallance, P.; et al. Inflammation and endothelial function: Direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation 2005, 111, 1530–1536. [Google Scholar] [CrossRef]
- Araújo, F.D.; Silva, R.M.F.; Oliveira, C.A.L.; Meira, Z.M.A. Neutrophil-to-lymphocyte ratio used as prognostic factor marker for dilated cardiomyopathy in childhood and adolescence. Ann. Pediatr. Card. 2019, 12, 18–24. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 1–6. [Google Scholar] [CrossRef]
- Alonso-Martinez, J.L.; Llorente-Diez, B.; Echegaray-Agara, M.; Olaz-Preciado, F.; Urbieta-Echezarreta, M.; Gonzalez-Arencibia, C. C-Reactive Protein as a predictor of improvement and readmission in heart failure. Eur. J. Heart Fail. 2002, 4, 331–336. [Google Scholar] [CrossRef]
- Yin, W.H.; Chen, J.W.; Jen, H.L.; Chiang, M.C.; Huang, W.P.; Feng, A.N.; Young, M.S.; Lin, S.J. Independent prognostic value of elevated high-sensitivity C-Reactive Protein in chronic heart failure. Am. Heart J. 2004, 147, 931–938. [Google Scholar] [CrossRef]
- Chitose, I.; Takayoshi, T.; Hiroshi, S.; Keijin, O.; Minoru, H. Plasma C-reactive protein is an independent prognostic predictor in patients with dilated cardiomyopathy. J. Card. Fail. 2004, 10, S161. [Google Scholar] [CrossRef]
- Akhtar, M.; Elliott, P.M. Risk stratification for sudden cardiac death in non-ischaemic dilated cardiomyopathy. Curr. Cardiol. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Katz, S.D.; Hryniewicz, K.; Hriljac, I.; Balidemaj, K.; Dimayuga, C.; Hudaihed, A.; Yasskiy, A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 2005, 111, 310–314. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Gan, F.; Wang, Y.; Ding, L.; Hua, W. Plasma NT pro-BNP, hs-CRP and big-ET levels at admission as prognostic markers of survival in hospitalized patients with dilated cardiomyopathy: A single-center cohort study. BMC Cardiovasc. Disord. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Dookhun, M.N.; Zhang, J.N.; Lu, X. Circulating biomarkers of dilated cardiomyopathy—An analysis of new potential biomarkers. Int. J. Curr. Res. 2017, 9, 49420–49425. [Google Scholar]
- Sadahiro, T.; Kohsaka, S.; Okuda, S.; Inohara, T.; Shiraishi, Y.; Kohno, T.; Yoshikawa, T.; Fukuda, K. MRI and serum high-sensitivity C-reactive protein predict long-term mortality in non-ischaemic cardiomyopathy. Open Heart 2015, 2, e000298. [Google Scholar] [CrossRef]
- Avci, A.; Alizade, E.; Fidan, S.; Yesin, M.; Guler, Y.; Kargin, R.; Esen, A.M. Neutrophil/lymphocyte ratio is related to the severity of idiopathic dilated cardiomyopathy. Scand. Cardiovasc. J. 2014, 48, 202–208. [Google Scholar] [CrossRef]
- Fu, M. Inflammation in chronic heart failure: What is familiar, what is unfamiliar? Eur. J. Heart Fail. 2009, 11, 111–112. [Google Scholar] [CrossRef]
- Uthamalingam, S.; Patvardhan, E.A.; Subramanian, S.; Ahmed, W.; Martin, W.; Daley, M.; Capodilupo, R. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am. J. Cardiol. 2011, 107, 433–438. [Google Scholar] [CrossRef]
- Vergaro, G.; Del Franco, A.; Giannoni, A.; Prontera, C.; Ripoli, A.; Barison, A.; Masci, P.G.; Aquaro, G.D.; Solal, A.C.; Padeletti, L.; et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2015, 184, 96–100. [Google Scholar] [CrossRef]
- Imran, T.F.; Shin, H.J.; Mathenge, N.; Wang, F.; Kim, B.; Joseph, J.; Gaziano, J.M.; Djoussé, L. Meta-analysis of the usefulness of plasma galectin-3 to predict the risk of mortality in patients with heart failure and in the general population. Am. J. Cardiol. 2017, 119, 57–64. [Google Scholar] [CrossRef]
- Centurión, O.A.; Alderete, J.F.; Torales, J.M.; García, L.B.; Scavenius, K.E.; Miño, L.M. Myocardial fibrosis as a pathway of prediction of ventricular arrhythmias and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. Crit. Pathw. Cardiol. 2019, 18, 89–97. [Google Scholar] [CrossRef]
- Hu, D.J.; Xu, J.; Du, W.; Zhang, J.X.; Zhong, M.; Zhou, Y.N. Cardiac magnetic resonance and galectin-3 level as predictors of prognostic outcomes for non-ischemic cardiomyopathy patients. Int. J. Cardiovasc. Imaging 2016, 32, 1725–1733. [Google Scholar] [CrossRef]
- French, B.; Wang, L.; Ky, B.; Brandimarto, J.; Basuray, A.; Fang, J.C.; Sweitzer, N.K.; Cappola, T.P. Prognostic value of galectin-3 for adverse outcomes in chronic heart failure. J. Card. Fail. 2016, 22, 256–262. [Google Scholar] [CrossRef]
- Binas, D.; Daniel, H.; Richter, A.; Ruppert, V.; Schlüter, K.D.; Schieffer, B.; Pankuweit, S. The prognostic value of sST2 and galectin-3 considering different aetiologies in non-ischaemic heart failure. Open Heart 2018, 5, e000750. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Fu, J. Serum chemerin predicts the prognosis of patients with dilated cardiomyopathy. Heart Surg. Forum 2020, 23, E276–E280. [Google Scholar] [CrossRef]
- Gu, P.; Jiang, W.; Lu, B.; Shi, Z. Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin. Exp. Hypertens. 2014, 36, 326–332. [Google Scholar] [CrossRef]
- Zhang, O.; Ji, Q.; Lin, Y.; Wang, Z.; Huang, Y.; Lu, W.; Liu, X.; Zhang, J.; Liu, Y.; Zhou, Y.J. Circulating chemerin levels elevated in dilated cardiomyopathy patients with overt heart failure. Clin. Chim. Acta 2015, 448, 27–32. [Google Scholar] [CrossRef]
- Zhou, X.; Tao, Y.; Chen, Y.; Xu, W.; Qian, Z.; Lu, X. Serum chemerin as a novel prognostic indicator in chronic heart failure. J. Am. Heart Assoc. 2019, 8, e012091. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Y.F.; Yang, H.J.; Zhang, N.N.; Rui, Q.; Zhou, Y.F. Tumor necrosis factor-α gene polymorphism (G-308A) and dilated cardiomyopathy. Int. Heart J. 2019, 60, 656–664. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Xin, L.; Gao, N.; Liu, B. Association between rs1800629 polymorphism in tumor necrosis factor-α gene and dilated cardiomyopathy susceptibility: Evidence from case–control studies. Medicine 2018, 97, e13386. [Google Scholar] [CrossRef]
- Henriksen, P.A.; Newby, D.E. Therapeutic inhibition of tumour necrosis factor α in patients with heart failure: Cooling an inflamed heart. Heart 2003, 89, 14–18. [Google Scholar] [CrossRef]
- Lupón, J.; De Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Zamora, E.; Urrutia, A.; Bayes-Genis, A. Development of a novel heart failure risk tool: The Barcelona bio-heart failure risk calculator (BCN bio-HF calculator). PLoS ONE 2014, 9, e85466. [Google Scholar] [CrossRef]
- Chmielewski, P.; Michalak, E.; Kowalik, I.; Franaszczyk, M.; Sobieszczanska-Malek, M.; Truszkowska, G.; Stepien-Wojno, M.; Biernacka, E.K.; Foss-Nieradko, B.; Lewandowski, M.; et al. Can circulating cardiac biomarkers be helpful in the assessment of LMNA mutation carriers? J. Clin. Med. 2020, 9, 1443. [Google Scholar] [CrossRef]
- Tigen, K.; Karaahmet, T.; Cevik, C.; Gurel, E.; Pala, S.; Mutlu, B.; Basaran, Y. Prognostic utility of right ventricular systolic functions assessed by tissue doppler imaging in dilated cardiomyopathy and its correlation with plasma NT-pro-BNP levels. Congest. Heart Fail. 2009, 15, 234–239. [Google Scholar] [CrossRef]
- Shah, R.V.; Januzzi, J.L. ST2: A novel remodelling biomarker in acute and chronic heart failure. Curr. Heart Fail. Rep. 2010, 7, 9–14. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Felker, G.M. Surfing the biomarker tsunami at JACC: Heart failure. JACC Heart Fail. 2013, 1, 213–215. [Google Scholar] [CrossRef]
- Van Kimmenade, R.R.; Januzzi, J.L., Jr. Emerging biomarkers in heart failure. Clin. Chem. 2012, 58, 127–138. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Mebazaa, A.; Di Somma, S. ST2 and prognosis in acutely decompensated heart failure: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115, 26B–31B. [Google Scholar] [CrossRef]
- Shah, N.N.; Ayyadurai, P.; Saad, M.; Kosmas, C.E.; Dogar, M.U.; Patel, U.; Vittorio, T.J. Galactin-3 and soluble ST2 as complementary tools to cardiac MRI for sudden cardiac death risk stratification in heart failure: A review. JRSM Cardiovasc. Dis. 2020, 9, 2048004020957840. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Ordoñez-Llanos, J.; Tornel, P.L.; Vázquez, R.; Puig, T.; Valdés, M.; Cinca, J.; de Luna, A.B.; Bayes-Genis, A.; MUSIC Investigators. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 2009, 54, 2174–2179. [Google Scholar] [CrossRef]
- Broch, K.; Andreassen, A.K.; Ueland, T.; Michelsen, A.E.; Stueflotten, W.; Aukrust, P.; Aakhus, S.; Gullestad, L. Soluble ST2 reflects hemodynamic stress in non-ischemic heart failure. Int. J. Cardiol. 2015, 179, 378–384. [Google Scholar] [CrossRef]
- Wojciechowska, C.; Romuk, E.; Nowalany-Kozielska, E.; Jacheć, W. Serum Galectin-3 and ST2 as predictors of unfavorable outcome in stable dilated cardiomyopathy patients. Hell. J. Cardiol. 2017, 58, 350–359. [Google Scholar] [CrossRef]
- You, H.; Jiang, W.; Jiao, M.; Wang, X.; Jia, L.; You, S.; Li, Y.; Wen, H.; Jiang, H.; Yuan, H.; et al. Association of soluble ST2 serum levels with outcomes in paediatric dilated cardiomyopathy. Can. J. Cardiol. 2019, 35, 727–735. [Google Scholar] [CrossRef]
- Tsutamoto, T.; Kawahara, C.; Yamaji, M.; Nishiyama, K.; Fujii, M.; Yamamoto, T.; Horie, M. Relationship between renal function and serum cardiac troponin T in patients with chronic heart failure. Eur. J. Heart Fail. 2009, 11, 653–658. [Google Scholar] [CrossRef]
- Kawahara, C.; Tsutamoto, T.; Nishiyama, K.; Yamaji, M.; Sakai, H.; Fujii, M.; Yamamoto, T.; Horie, M. Prognostic role of high-sensitivity cardiac troponin T in patients with nonischemic dilated cardiomyopathy. Circ. J. 2011, 75, 656–661. [Google Scholar] [CrossRef]
- Baba, Y.; Kubo, T.; Yamanaka, S.; Hirota, T.; Tanioka, K.; Yamasaki, N.; Sugiura, T.; Kitaoka, H. Clinical significance of high-sensitivity cardiac troponin T in patients with dilated cardiomyopathy. Int. Heart J. 2015, 56, 309–313. [Google Scholar] [CrossRef][Green Version]
- Komamura, K.; Sasaki, T.; Hanatani, A.; Kim, J.; Hashimura, K.; Ishida, Y.; Ohkaru, Y.; Asayama, K.; Tanaka, T.; Ogai, A.; et al. Heart-type fatty acid binding protein is a novel prognostic marker in patients with non-ischaemic dilated cardiomyopathy. Heart 2006, 92, 615–618. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Takabatake, N.; Nozaki, N.; Hirono, O.; Watanabe, T.; Nitobe, J.; Harada, M.; Suzuki, S.; et al. Heart-type fatty acid-binding protein is more sensitive than troponin T to detect the ongoing myocardial damage in chronic heart failure patients. J. Card. Fail. 2007, 13, 120–127. [Google Scholar] [CrossRef]
- Arimoto, T.; Takeishi, Y.; Shiga, R.; Fukui, A.; Tachibana, H.; Nozaki, N.; Hirono, O.; Nitobe, J.; Miyamoto, T.; Hoit, B.D.; et al. Prognostic value of elevated circulating heart-type fatty acid binding protein in patients with congestive heart failure. J. Card. Fail. 2005, 11, 56–60. [Google Scholar] [CrossRef]
- Sun, Y.; Dookhun, M.N.; Zou, H.; Cao, X.; Zhang, Y.; Lu, X.Z. Research progress of cardiac myosin binding protein C in dilated cardiomyopathy and other cardiac conditions. World J. Cardiovas. Dis. 2018, 8, 452–461. [Google Scholar] [CrossRef]
- Doesch, A.O.; Mueller, S.; Nelles, M.; Konstandin, M.; Celik, S.; Frankenstein, L.; Goeser, S.; Kaya, Z.; Koch, A.; Zugck, C.; et al. Impact of troponin I-autoantibodies in chronic dilated and ischemic cardiomyopathy. Basic Res. Cardiol. 2011, 106, 25–35. [Google Scholar] [CrossRef]
- Kaya, Z.; Leib, C.; Katus, H.A. Autoantibodies in heart failure and cardiac dysfunction. Circ. Res. 2012, 110, 145–158. [Google Scholar] [CrossRef]
- Franz, M.; Berndt, A.; Neri, D.; Galler, K.; Grün, K.; Porrmann, C.; Reinbothe, F.; Mall, G.; Schlattmann, P.; Renner, A.; et al. Matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, B+ tenascin-C and ED-A+ fibronectin in dilated cardiomyopathy: Potential impact on disease progression and patients’ prognosis. Int. J. Cardiol. 2013, 168, 5344–5351. [Google Scholar] [CrossRef]
- Antonov, I.B.; Kozlov, K.L.; Pal’Tseva, E.M.; Polyakova, O.V.; Lin’Kova, N.S. Matrix metalloproteinases MMP-1 and MMP-9 and their inhibitor TIMP-1 as markers of dilated cardiomyopathy in patients of different age. Bull. Exp. Biol. Med. 2018, 164, 550–553. [Google Scholar] [CrossRef]
- Gedikli, O.; Kiris, A.; Hosoglu, Y.; Karahan, C.; Kaplan, S. Serum myeloperoxidase level is associated with heart-type fatty acid-binding protein but not troponin T in patients with chronic heart failure. Med. Princ. Pract. 2015, 24, 42–46. [Google Scholar] [CrossRef]
- La Rocca, G.; Di Stefano, A.; Eleuteri, E.; Anzalone, R.; Magno, F.; Corrao, S.; Loria, T.; Martorana, A.; Di Gangi, C.; Colombo, M.; et al. Oxidative stress induces myeloperoxidase expression in endocardial endothelial cells from patients with chronic heart failure. Basic Res. Cardiol. 2009, 104, 307–320. [Google Scholar] [CrossRef]
- Tang, W.W.; Brennan, M.L.; Philip, K.; Tong, W.; Mann, S.; Van Lente, F.; Hazen, S.L. Plasma myeloperoxidase levels in patients with chronic heart failure. Am. J. Cardiol. 2006, 98, 796–799. [Google Scholar] [CrossRef]
- Michowitz, Y.; Kisil, S.; Guzner-Gur, H.; Rubinstein, A.; Wexler, D.; Sheps, D.; Keren, G.; George, J. Usefulness of serum myeloperoxidase in prediction of mortality in patients with severe heart failure. Hypertension 2008, 173, 60–67. [Google Scholar]
- Tang, W.W.; Tong, W.; Troughton, R.W.; Martin, M.G.; Shrestha, K.; Borowski, A.; Jasper, S.; Hazen, S.L.; Klein, A.L. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 2364–2370. [Google Scholar] [CrossRef]
- Latini, R.; Masson, S.; Anand, I.; Salio, M.; Hester, A.; Judd, D.; Barlera, S.; Maggioni, A.P.; Tognoni, G.; Cohn, J.N. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur. Heart J. 2004, 25, 292–299. [Google Scholar] [CrossRef]
- Koglin, J.; Pehlivanli, S.; Schwaiblmair, M.; Vogeser, M.; Cremer, P.; Vonscheidt, W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J. Am. Coll. Cardiol. 2001, 38, 1934–1941. [Google Scholar] [CrossRef]
- Telgmann, R.; Harb, B.A.; Ozcelik, C.; Perrot, A.; Schönfelder, J.; Nonnenmacher, A.; Brand, M.; Schmidt-Petersen, K.; Dietz, R.; Kreutz, R.; et al. The G-231A polymorphism in the endothelin-A receptor gene is associated with lower aortic pressure in patients with dilated cardiomyopathy. Am. J. Hypertens. 2007, 20, 32–37. [Google Scholar] [CrossRef]
- Pacher, R.; Stanek, B.; Hülsmann, M.; Koller-Strametz, J.; Berger, R.; Schuller, M.; Hartter, E.; Ogris, E.; Frey, B.; Heinz, G.; et al. Prognostic impact of big endothelin-1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J. Am. Coll. Cardiol. 1996, 27, 633–641. [Google Scholar] [CrossRef]
- Herrmann, S.M.; Schmidt-Petersen, K.; Pfeifer, J.; Perrot, A.; Bit-Avragim, N.; Eichhorn, C.; Dietz, R.; Kreutz, R.; Paul, M.; Osterziel, K.J. A polymorphism in the endothelin-A receptor gene predicts survival in patients with idiopathic dilated cardiomyopathy. Eur. Heart J. 2001, 22, 1948–1953. [Google Scholar] [CrossRef]
- Roura, S.; Gálvez-Montón, C.; Fernández, M.A.; Lupón, J.; Bayes-Genis, A. Circulating endothelial progenitor cells: Potential biomarkers for idiopathic dilated cardiomyopathy. J. Cardiovasc. Transl. Res. 2016, 9, 80–84. [Google Scholar] [CrossRef]
- Poglajen, G.; Zemljič, G.; Frljak, S.; Cerar, A.; Andročec, V.; Sever, M.; Černelč, P. Stem cell therapy in patients with chronic nonischemic heart failure. Stem Cells Int. 2018, 2018, 6487812. [Google Scholar] [CrossRef]
- Gopal, D.M.; Sam, F. New and emerging biomarkers in left ventricular systolic dysfunction—insight into dilated cardiomyopathy. J. Cardiovasc. Transl. Res. 2013, 6, 516–527. [Google Scholar] [CrossRef][Green Version]
- Theiss, H.D.; David, R.; Engelmann, M.G.; Barth, A.; Schotten, K.; Naebauer, M.; Reichart, B.; Steinbeck, G.; Franz, W.M. Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM). Eur. Heart J. 2007, 28, 1258–1264. [Google Scholar] [CrossRef]
- Roura, S.; Planas, F.; Prat-Vidal, C.; Leta, R.; Soler-Botija, C.; Carreras, F.; Llach, A.; Hove-Madsen, L.; Lladó, G.P.; Farré, J.; et al. Idiopathic dilated cardiomyopathy exhibits defective vascularization and vessel formation. Eur. Heart J. Fail. 2007, 9, 995–1002. [Google Scholar] [CrossRef]
- Vrtovec, B.; Poglajen, G.; Lezaic, L.; Sever, M.; Socan, A.; Domanovic, D.; Cernelc, P.; Torre-Amione, G.; Haddad, F.; Wu, J.C. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation 2013, 128, S42–S49. [Google Scholar] [CrossRef]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Shan, C.; Wang, Y.; Qian, L.L.; Jia, D.D.; Zhang, Y.F.; Hao, X.D.; Xu, H.M. Cardiovascular toxicity and mechanism of bisphenol A and emerging risk of bisphenol S. Sci. Total Environ. 2020, 723, 137952. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, Y.A.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; et al. Bisphenol A in infant urine and baby-food samples among 9-to 15-month-olds. Sci. Total Environ. 2019, 697, 133861. [Google Scholar] [CrossRef]
- Dualde, P.; Pardo, O.; Corpas-Burgos, F.; Kuligowski, J.; Gormaz, M.; Vento, M.; Pastor, A.; Yusà, V. Biomonitoring of bisphenols A, F, S in human milk and probabilistic risk assessment for breastfed infants. Sci. Total Environ. 2019, 668, 797–805. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.S. Impact of bisphenol A on the cardiovascular system—Epidemiological and experimental evidence and molecular mechanisms. Int. J. Environ. Res. Public Health 2014, 11, 8399–8413. [Google Scholar] [CrossRef]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef]
- Meeker, J.D.; Calafat, A.M.; Hauser, R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ. Sci. Technol. 2010, 44, 1458–1463. [Google Scholar] [CrossRef]
- Zhou, Q.; Miao, M.; Ran, M.; Ding, L.; Bai, L.; Wu, T.; Yuan, W.; Gao, E.; Wang, J.; Li, G.; et al. Serum bisphenol-A concentration and sex hormone levels in men. Fertil. Steril. 2013, 100, 478–482. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, X.; Shen, Y.; Yu, P.; Chen, S.; Hu, J.; Yu, J.; Li, J.; Wang, H.S.; Cheng, X.; et al. Elevated serum Bisphenol A level in patients with dilated cardiomyopathy. Int. J. Environ. Res. Public Health 2015, 12, 5329–5337. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Tornel, P.L.; Nicolás, F.; Sánchez-Más, J.; Martínez, M.D.; Gracia, M.R.; Garrido, I.P.; Ruipérez, J.A.; Valdés, M. Sex hormone-binding globulin: A new marker of disease severity and prognosis in men with chronic heart failure. Rev. Esp. Cardiol. 2019, 62, 1381–1387. [Google Scholar] [CrossRef]
- Tomasoni, D.; Adamo, M.; Anker, M.S.; von Haehling, S.; Coats, A.J.; Metra, M. Heart failure in the last year: Progress and perspective. ESC Heart Fail. 2020, 7, 3505–3530. [Google Scholar] [CrossRef]
- De Rosa, S.; Eposito, F.; Carella, C.; Strangio, A.; Ammirati, G.; Sabatino, J.; Abbate, F.G.; Iaconetti, C.; Liguori, V.; Pergola, V.; et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur. J. Heart Fail. 2018, 20, 1000–1010. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Mangas, A.; Belmonte, T.; Quezada-Feijoo, M.; Ramos, M.; Toro, R. Ischemic dilated cardiomyopathy pathophysiology through microRNA-16-5p. Rev. Esp. Cardiol. Engl. Ed. 2019, 10, S1885-5857(20)30398-4. [Google Scholar]
- Zaragoza, C.; Saura, M.; Hernández, I.; Ramirez-Carracedo, R.; García-García, F.; Zamorano, J.L.; Mangas, A.; Toro, R. Differential expression of circulating miRNAs as a novel tool to assess BAG3-associated familial dilated cardiomyopathy. BioSci. Rep. 2019, 39, BSR20180934. [Google Scholar] [CrossRef]
- Belmonte, T.; Mangas, A.; Calderon-Dominguez, M.; Quezada-Feijoo, M.; Ramos, M.; Campuzano, O.; Gomez, S.; Peña, M.L.; Cubillos-Arango, A.M.; Dominguez, F.; et al. Peripheral microRNA panels to guide the diagnosis of familial cardiomyopathy. Transl. Res. 2020, 218, 1–15. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Zheng, J.; Song, D.; Zheng, S.; Ren, L.; Wang, Y.; Yao, Y.; Wang, Y.; Liu, Y.; et al. Syndecan-1 as an independent risk factor for the incidence of adverse cardiovascular events in patients having stage C and D heart failure with non-ischemic dilated cardiomyopathy. Clin. Chim. Acta 2019, 490, 63–68. [Google Scholar] [CrossRef]
- Neves, F.M.; Meneses, G.C.; Sousa, N.E.A.; Parahyba, M.C.; Martins, A.M.C.; Libório, A.B. Syndecan-1 in acute decompensated heart failure–association with renal function and mortality. Circ. J. 2015, 79, 1511–1519. [Google Scholar] [CrossRef]
- Tromp, J.; van der Pol, A.; Klip, I.T.; de Boer, R.A.; Jaarsma, T.; van Gilst, W.H.; Voors, A.A.; van Veldhuisen, D.J.; van der Meer, P. Fibrosis marker syndecan-1 and outcome in patients with heart failure with reduced and preserved ejection fraction. Circ. Heart Fail. 2014, 7, 457–462. [Google Scholar] [CrossRef]
- Nauta, J.F.; Hummel, Y.M.; Tromp, J.; Ouwerkerk, W.; van der Meer, P.; Jin, X.; Lam, C.S.P.; Bax, J.J.; Metra, M.; Samani, N.J.; et al. Concentric vs. Eccentric remodelling in heart failure with reduced ejection fraction: Clinical characteristics, pathophysiology and response to treatment. Eur. J. Heart Fail. 2020, 22, 1147–1155. [Google Scholar] [CrossRef]
- Baltrūnienė, V.; Rinkūnaitė, I.; Bogomolovas, J.; Bironaitė, D.; Kažukauskienė, I.; Šimoliūnas, E.; Ručinskas, K.; Puronaitė, R.; Bukelskienė, V.; Grabauskienė, V. The role of cardiac T-cadherin in the indicating heart failure severity of patients with non-ischemic dilated cardiomyopathy. Medicina 2020, 56, 27. [Google Scholar] [CrossRef]
- Lok, S.I.; Winkens, B.; Goldschmeding, R.; van Geffen, A.J.; Nous, F.M.; van Kuik, J.; van der Weide, P.; Klöpping, C.; Kirkels, J.H.; Lahpor, J.R.; et al. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur. J. Heart Fail. 2012, 14, 1249–1256. [Google Scholar] [CrossRef]
- Nair, N.; Gongora, E. Correlations of GDF-15 with sST2, MMPs, and worsening functional capacity in idiopathic dilated cardiomyopathy: Can we gain new insights into the pathophysiology? J. Circ. Biomark. 2018, 7, 1849454417751735. [Google Scholar] [CrossRef]
- Stojkovic, S.; Kaider, A.; Koller, L.; Brekalo, M.; Wojta, J.; Diedrich, A.; Demyanets, S.; Pezawas, T. GDF-15 is a better complimentary marker for risk stratification of arrhythmic death in non-ischaemic, dilated cardiomyopathy than soluble ST2. J. Cell Mol. Med. 2018, 22, 2422–2429. [Google Scholar] [CrossRef]
| Scheme | Population and Follow-Up | Characteristics | Clinical End Points | Results |
|---|---|---|---|---|
| High-sensitivity Cardiac Troponin-T (hs-cTnT) | ||||
| Kawahara et al. [51] |
|
|
|
|
| Baba et al. [52] |
|
|
|
|
| N-terminal-pro Hormone BNP (NT-proBNP) | ||||
| Tigen et al. [40] |
|
|
|
|
| Li et al. [19] |
|
|
|
|
| Soluble ST2 (sST2) | ||||
| Binas et al. [30] |
|
|
|
|
| Broch et al. [47] |
|
|
|
|
| Wojciechowska et al. [48] |
|
|
|
|
| Galectin-3 (Gal-3) | ||||
| Vergano et al. [25] |
|
|
|
|
| Hu et al. [28] |
|
|
|
|
| Scheme | Population and Follow-Up | Characteristics | Clinical End Points | Results | Limitations |
|---|---|---|---|---|---|
| High-sensitivity-CRP (hs-CRP) | |||||
| Li et al. [19] |
|
|
|
|
|
| Chitose et al. [16] |
|
|
|
|
|
| Neutrophil/Lymphocyte ratio (NLR) | |||||
| Avci et al. [22] |
|
|
|
|
|
| Araujo et al. [12] |
|
|
|
|
|
| Chemerin | |||||
| Chen et al. [31] |
|
|
|
|
|
| Tumor Necrosis Factor-α (TNF-α) | |||||
| Chen et al. [35] |
|
|
|
|
|
| Heart-type Fatty Acid Binding Protein (H-FABP) | |||||
| Komamura et al. [53] |
|
|
|
|
|
| Matrix Metalloproteinases (MMP) | |||||
| Franz et al. [59] |
|
|
|
|
|
| Endothelin-A (ETA) | |||||
| Herrmann et al. [70] |
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, L.; Sascău, R.; Zota, I.M.; Stătescu, C. Well-Known and Novel Serum Biomarkers for Risk Stratification of Patients with Non-ischemic Dilated Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 5688. https://doi.org/10.3390/ijms22115688
Anghel L, Sascău R, Zota IM, Stătescu C. Well-Known and Novel Serum Biomarkers for Risk Stratification of Patients with Non-ischemic Dilated Cardiomyopathy. International Journal of Molecular Sciences. 2021; 22(11):5688. https://doi.org/10.3390/ijms22115688
Chicago/Turabian StyleAnghel, Larisa, Radu Sascău, Ioana Mădălina Zota, and Cristian Stătescu. 2021. "Well-Known and Novel Serum Biomarkers for Risk Stratification of Patients with Non-ischemic Dilated Cardiomyopathy" International Journal of Molecular Sciences 22, no. 11: 5688. https://doi.org/10.3390/ijms22115688
APA StyleAnghel, L., Sascău, R., Zota, I. M., & Stătescu, C. (2021). Well-Known and Novel Serum Biomarkers for Risk Stratification of Patients with Non-ischemic Dilated Cardiomyopathy. International Journal of Molecular Sciences, 22(11), 5688. https://doi.org/10.3390/ijms22115688








