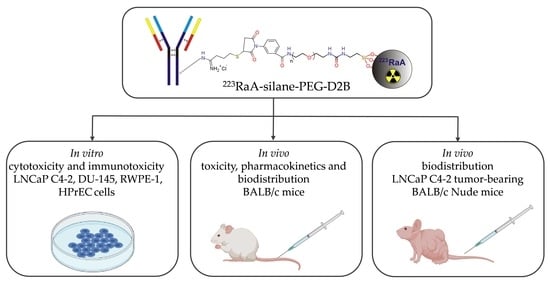
Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy—Part II. Toxicity, Pharmacokinetics and Biodistribution
Abstract
:1. Introduction
2. Results
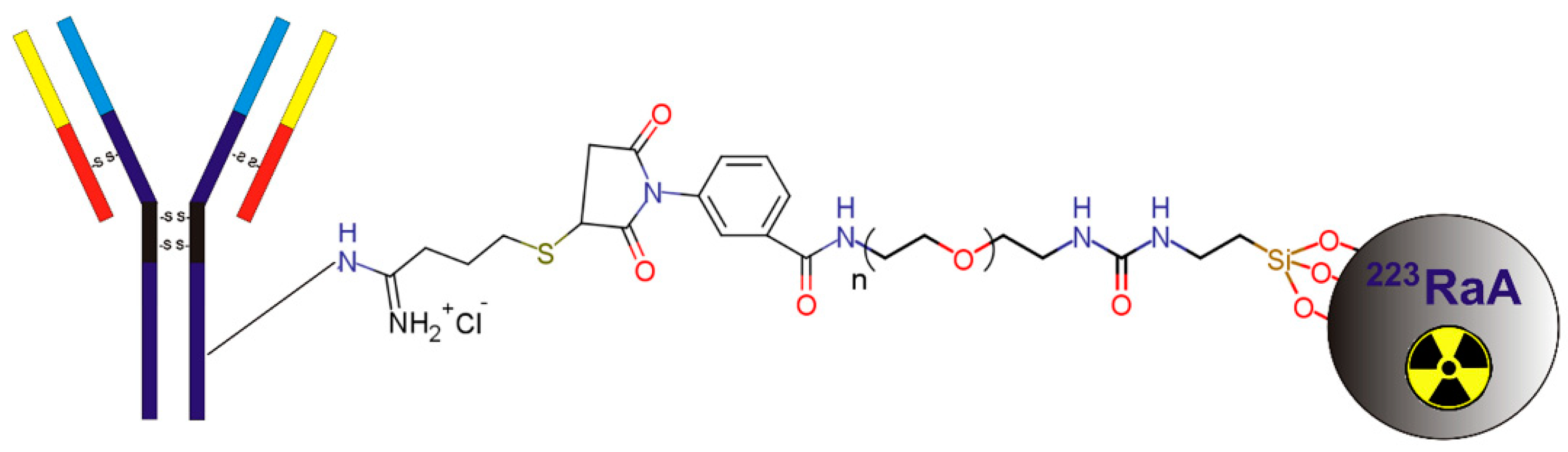
2.1. Synthesis and Physicochemical Characterization of 223RaA-Silane-PEG-D2B Radioconjugate
2.2. Analysis of Cell Death by the Annexin/Propidium Iodide Assay
2.3. Analysis of Apoptosis by Caspase 3/7 Assay
2.4. Gene Expression Profiling
2.5. Toxicity In Vivo
2.6. Biodistribution of 223RaA-Silane-PEG and 223RaA-Silane-PEG-D2B in Healthy BALB/c and Tumour Bearing BALB/c Nude Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis and Physicochemical Properties of 223RaA-Silane-PEG-D2B Radioimmunoconjugates
4.3. Cell Cultures
4.4. Analysis of Cell Death by the Annexin/IP Assay
4.5. Detection of Caspase 3/7 Activities
4.6. RNA Isolation, Reverse Transcription, and Real-Time PCR
4.7. Experimental Animals
4.8. Experimental Groups and Treatment
4.9. Pharmacokinetic Studies of 133BaA-Silane-PEG and 133BaA-Silane-PEG-D2B in BALB/c Mice
4.10. Toxicity Studies of 223RaA-Silane-PEG and 223RaA-Silane-PEG-D2B in BALB/c Mice
4.11. Measurements of Morphology Parameters
4.12. Measurements of Biochemical Parameters
4.13. Histopathology
4.14. Biodistribution of 223RaA-Silane-PEG and 223RaA-Silane-PEG-D2B in LNCaP C4-2 Tumor-Bearing BALB/c Nude Mice
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 0.5 h | 1 h | 4 h | 24 h | 48 h | 72 h | ||
| 133BaA-silane-PEG | Blood | 0.80 ± 0.31 | 1.16 ± 0.39 | 0.60 ± 0.28 | 0.16 ± 0.05 | 0.15 ± 0.04 | 0.14 ± 0.11 |
| Lungs | 37.55 ± 11.49 | 6.87 ± 2.11 | 5.54 ± 2.99 | 6.01 ± 2.24 | 0.31 ± 0.20 | 0.76 ± 0.55 | |
| Liver | 10.12 ± 3.67 | 19.47 ± 6.32 | 19.88 ± 6.84 | 14.41 ± 1.24 | 4.68 ± 1.82 | 2.72 ± 1.98 | |
| Spleen | 5.00 ± 2.69 | 5.17 ± 1.39 | 5.41 ± 3.10 | 4.44 ± 0.96 | 2.03 ± 0.99 | 1.26 ± 0.77 | |
| Kidneys | 6.71 ± 2.30 | 1.90 ± 0.86 | 0.51 ± 0.14 | 0.19 ± 0.09 | 0.08 ± 0.02 | 0.26 ± 0.17 | |
| small intestine | 4.42 ± 1.67 | 2.47 ± 0.71 | 0.64 ± 0.44 | 0.21 ± 0.08 | 0.05 ± 0.02 | 0.12 ± 0.05 | |
| large intestine | 0.98 ± 0.45 | 0.48 ± 0.18 | 1.74 ± 0.91 | 1.11 ± 0.22 | 0.17 ± 0.08 | 0.29 ± 0.14 | |
| Stomach | 3.06 ± 1.49 | 1.28 ± 0.60 | 0.90 ± 0.15 | 0.31 ± 0.15 | 0.03 ± 0.01 | 0.84 ± 0.58 | |
| Bone | 17.35 ± 3.76 | 9.52 ± 2.75 | 12.79 ± 4.41 | 17.45 ± 1.52 | 20.93 ± 4.20 | 13.96 ± 6.79 | |
| Muscle | 0.85± 0.24 | 0.38 ± 0.11 | 0.24 ± 0.08 | 0.14 ± 0.05 | 0.26 ± 0.13 | 0.63 ± 0.26 | |
| salivary gland | 4.22 ± 2.38 | 3.42 ± 1.11 | 1.09 ± 0.44 | 0.31 ± 0.11 | 0.04 ± 0.02 | 0.55 ± 0.26 | |
| urine + feces [%ID] | 4.61 ± 3.65 | 4.32 ± 2.47 | 7.59 ± 2.94 | 21.84 ± 2.68 | 25.99 ± 18.20 | 18.62 ± 10.57 | |
| 0.5 h | 1 h | 4 h | 24 h | 48 h | 72 h | ||
| 133BaA-silane-PEG-D2B | Blood | 1.60 ± 0.68 | 1.00 ± 0.25 | 0.67 ± 0.13 | 0.65 ± 0.29 | 0.26 ± 0.13 | 0.34 ± 0.05 |
| Lungs | 61.23 ± 29.34 | 59.71 ± 24.11 | 13.82 ± 5.81 | 5.03 ± 2.65 | 5.15 ± 2.04 | 9.52 ± 6.84 | |
| Liver | 22.97 ± 2.04 | 23.87 ± 3.65 | 17.61 ± 2.76 | 13.77 ± 1.93 | 10.35 ± 1.67 | 10.18 ± 1.18 | |
| Spleen | 10.26 ± 3.96 | 12.26 ± 3.38 | 10.35 ± 1.55 | 6.91 ± 0.90 | 5.60 ± 2.06 | 5.26 ± 0.09 | |
| Kidneys | 6.14 ± 1.00 | 3.69 ± 1.27 | 0.96 ± 0.17 | 0.50 ± 0.09 | 0.20 ± 0.09 | 0.39 ± 0.17 | |
| small intestine | 3.20 ± 0.56 | 3.38 ± 0.66 | 1.35 ± 0.39 | 0.22 ± 0.09 | 0.10 ± 0.03 | 0.16 ± 0.12 | |
| large intestine | 0.74 ± 0.13 | 1.07 ± 0.35 | 2.92 ± 0.55 | 0.47 ± 0.29 | 0.22 ± 0.17 | 0.56 ± 0.43 | |
| Stomach | 2.38 ± 0.67 | 2.78 ± 1.16 | 0.69 ± 0.15 | 0.41 ± 0.22 | 0.06 ± 0.06 | 0.15 ± 0.12 | |
| Bone | 12.33 ± 3.35 | 17.99 ± 5.39 | 21.54 ± 4.58 | 22.87 ±5.03 | 15.02 ± 0.82 | 19.31 ± 1.98 | |
| Muscle | 1.09 ± 0.42 | 1.02 ± 0.54 | 0.88 ± 0.44 | 0.80 ± 0.27 | 0.25± 0.11 | 0.48 ± 0.24 | |
| salivary gland | 4.12 ± 1.09 | 2.76 ± 1.71 | 1.59 ± 0.33 | 0.58 ± 0.17 | 0.25 ± 0.11 | 0.31 ± 0.28 | |
| urine + feces [%ID] | 4.77 ± 2.86 | 3.78 ± 2.23 | 7.95 ± 3.71 | 32.02 ± 6.69 | 28.51 ± 4.39 | 37.80 ± 15.83 |


| Dunnett’s Multiple Comparisons Test | Significance | Adjusted p Value |
|---|---|---|
| femur | ||
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG (7 day) | Yes | 0.0024 |
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG-D2B (7 day) | Yes | <0.0001 |
| liver | ||
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG (7 day) | Yes | <0.0001 |
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG-D2B (7 day) | Yes | <0.0001 |
| spleen | ||
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG (7 day) | Yes | <0.0001 |
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG-D2B (7 day) | Yes | <0.0001 |
| kidneys | ||
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG (7 day) | No | 0.9900 |
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG-D2B (7 day) | No | 0.9742 |
| small intestine | ||
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG (7 day) | No | 0.9997 |
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG-D2B (7 day) | No | 0.9998 |
| large intestine | ||
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG (7 day) | No | 0.9876 |
| 223RaCl2 (7 day) Xofigo vs. 223Ra-silane-PEG-D2B (7 day) | No | 0.9991 |
| Group G3 | PBS | References Value [25] | ||
|---|---|---|---|---|
| 1st Day | 7th Day | p | ||
| RBC (×103/mcL) | 9.0 ± 2.57 | 11.1 ± 0.12 | 0.892 | 6.93–12.24 |
| HCT (%) | 40.3 ± 11.53 | 48.8 ± 0.35 | 0.892 | 42.1–68.3 |
| HGB (g/dL) | 13.7 ±3.69 | 16.2 ± 0.06 | 0..899 | 12.6–20.5 |
| MCV (fL) | 44.9 ± 0.15 | 43.9 ± 0.44 | 0.209 | 50.7–64.4 |
| MCH (pg) | 15.4 ± 0.36 | 14.5 ± 0.15 | 0.209 | 13.2–17.6 |
| MCHC (g/dL) | 34.2 ± 0.83 | 33.1 ± 0.17 | 0.611 | 23.3–32.7 |
| WBC (G/L) | 5.3 ± 0.48 | 4.1 ± 1.28 | 0.892 | 3.48–14.03 |
| NEUT (G/L) | 1.2 ± 0.24 | 1.0 ± 0.26 | 0.988 | - |
| NEUT (%) | 23.8 ± 6.94 | 26.0 ± 5.07 | 9.86–39.11 | |
| LYMPH (G/L) | 3.8 ± 0.73 | 2.9 ± 1.02 | 0.988 | - |
| LYMPH (%) | 71.0 ± 7.88 | 68.6 ± 4.87 | 48.81–83.19 | |
| MONO (G/L) | 0.1 ± 0.05 | 0.1 ± 0.03 | 0.981 | - |
| MONO (%) | 2.8 ± 1.04 | 3.2 ± 0.20 | 3.29–12.48 | |
| BASO (G/L) | 0.1 ± 0.02 | 0.1 ± 0.04 | 0.988 | - |
| BASO (%) | 2.2 ± 0.38 | 2.1 ± 0.40 | 0.00–1.84 | |
| EOS (G/L) | 0.01 ± 0.00 | 0.0 ± 0.00 | 0.709 | - |
| EOS (%) | 0.2 ± 0.06 | 0.1 ± 0.00 | 0.11–4.91 | |
| PLATELET (G/L) | 1095 ± 590.47 | 1216.3 ± 140.22 | 0.988 | 420–1698 |
| Group G4 | NaA-silane-PEG | References Value [25] | ||
|---|---|---|---|---|
| 1st Day | 7th Day | p | ||
| RBC (×103/mcL) | 10.8 ± 0.55 | 10.5 ± 0.36 | 0.761 | 6.93–12.24 |
| HCT (%) | 48.0 ± 2.61 | 47.3 ± 1.25 | 0.761 | 42.1–68.3 |
| HGB (g/dL) | 16.1 ± 0.96 | 15.3 ± 0.42 | 0.723 | 12.6–20.5 |
| MCV (fL) | 44.3 ± 0.65 | 45.1 ± 0.45 | 0.704 | 50.7–64.4 |
| MCH (pg) | 14.9 ± 0.21 | 14.6 ± 0.10 | 0.579 | 13.2–17.6 |
| MCHC (g/dL) | 33.6 ± 0.36 | 32.4 ± 0.29 | 0.113 | 23.3–32.7 |
| WBC (G/L) | 2.8 ± 0.79 | 8.6 ± 0.75 | 0.009 | 3.48–14.03 |
| NEUT (G/L) | 1.3 ± 0.38 | 2.2 ± 0.98 | 0.712 | - |
| NEUT (%) | 46.9 ± 2.48 | 25.4 ± 9.63 | 9.86–39.11 | |
| LYMPH (G/L) | 1.2 ± 0.29 | 5.7 ± 0.59 | 0.003 | - |
| LYMPH (%) | 44.1 ± 4.45 | 67.3 ± 9.39 | 48.81–83.19 | |
| MONO (G/L) | 0.2 ± 0.10 | 0.5 ± 0.10 | 0.1680 | - |
| MONO (%) | 6.3 ± 1.99 | 5.6 ± 0.83 | 6 | 3.29–12.48 |
| BASO (G/L) | 0.1 ± 0.04 | 0.1 ± 0.02 | 0.505 | - |
| BASO (%) | 2.6 ± 0.71 | 1.6 ± 0.17 | 0.00–1.84 | |
| EOS (G/L) | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.003 | - |
| EOS (%) | 0.1 ± 0.00 | 0.1 ± 0.00 | 0.11–4.91 | |
| PLATELET (G/L) | 841.7 ± 169.35 | 1048 ± 514.60 | 0.761 | 420–1698 |
| Group G6 | 223RaA-silane-PEG | References Value [25] | ||
|---|---|---|---|---|
| 1st Day | 7th Day | p | ||
| RBC (×103/mcL) | 11.1 ± 0.53 | 8.1 ± 0.71 | 0.030 | 6.93–12.24 |
| HCT (%) | 46.8 ± 2.26 | 34.0 ± 3.70 | 0.034 | 42.1–68.3 |
| HGB (g/dL) | 16.3 ± 0.40 | 11.7 ± 1.27 | 0.030 | 12.6–20.5 |
| MCV (fL) | 44.5 ± 0.55 | 42.9 ± 0.20 | 0.034 | 50.7–64.4 |
| MCH (pg) | 14.8 ± 0.21 | 14.7 ± 0.45 | 0.592 | 13.2–17.6 |
| MCHC (g/dL) | 33.4 ± 0.35 | 34.7 ± 0.50 | 0.067 | 23.3–32.7 |
| WBC (G/L) | 5.4 ± 0.42 | 0.5 ± 0.06 | 0.000 | 3.48–14.03 |
| NEUT (G/L) | 1.1 ± 0.32 | 0.1 ± 0.03 | - | |
| NEUT (%) | 28.0 ± 3.26 | 17.6 ± 2.49 | 0.030 | 9.86–39.11 |
| LYMPH (G/L) | 3.1 ± 0.67 | 0.3 ± 0.07 | - | |
| LYMPH (%) | 69.8 ± 4.86 | 76.0 ± 5.14 | 0.017 | 48.81–83.19 |
| MONO (G/L) | 0.2 ± 0.04 | 0.0 ± 0.00 | - | |
| MONO (%) | 2.5 ± 0.36 | 2.1 ± 0.25 | 0.011 | 3.29–12.48 |
| BASO (G/L) | 0.1 ± 0.01 | 0.0 ± 0.00 | - | |
| BASO (%) | 2.4 ± 0.25 | 3.3 ± 0.61 | 0.007 | 0.00–1.84 |
| EOS (G/L) | 0.0 ± 0.00 | 0.0 ± 0.00 | - | |
| EOS (%) | 0.1 ± 0.00 | 0.1 ± 0.00 | 0.067 | 0.11–4.91 |
| PLATELET (G/L) | 965.3 ± 104.00 | 355.3 ± 116.17 | 0.022 | 420–1698 |
| Group G5 | NaA-silane-PEG-D2B | References Value [25] | ||
|---|---|---|---|---|
| 1st Day | 7th Day | p | ||
| RBC (×103/mcL) | 11.4 ± 1.36 | 10.5 ± 0.31 | 0.969 | 6.93–12.24 |
| HCT (%) | 36.6 ± 20.18 | 46.4 ± 1.36 | 0.981 | 42.1–68.3 |
| HGB (g/dL) | 12.2 ± 6.55 | 15.4 ± 0.47 | 0.981 | 12.6–20.5 |
| MCV (fL) | 45.6 ± 0.44 | 44.4 ± 0.15 | 0.144 | 50.7–64.4 |
| MCH (pg) | 15.3 ± 0.36 | 14.7 ± 0.06 | 0.491 | 13.2–17.6 |
| MCHC (g/dL) | 33.6 ± 0.87 | 33.2 ± 0.23 | 0.981 | 23.3–32.7 |
| WBC (G/L) | 3.5 ± 1.47 | 4.4 ± 0.86 | 0.981 | 3.48–14.03 |
| NEUT (G/L) | 1.1 ± 0.30 | 1.2 ± 0.08 | 0.981 | - |
| NEUT (%) | 34.0 ± 11.36 | 27.7 ± 5.19 | 9.86–39.11 | |
| LYMPH (G/L) | 2.0 ± 1.07 | 3.0 ± 0.83 | 0.0961 | - |
| LYMPH (%) | 69.8 ± 4.86 | 76.0 ± 5.14 | 48.81–83.19 | |
| MONO (G/L) | 0.3 ± 0.22 | 0.1 ± 0.01 | 0.946 | - |
| MONO (%) | 7.7 ± 3.50 | 2.4 ± 0.50 | 3.29–12.48 | |
| BASO (G/L) | 0.1 ± 0.03 | 0.1 ± 0.05 | 0.950 | - |
| BASO (%) | 2.4 ± 0.29 | 2.8 ± 0.80 | 0.00–1.84 | |
| EOS (G/L) | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.981 | - |
| EOS (%) | 0.3 ± 0.20 | 0.2 ± 0.06 | 0.11–4.91 | |
| PLATELET (G/L) | 919.0 ± 466.12 | 1021.3 ± 458.82 | 0.980 | 420–1698 |
| Group G7 | 223RaA-silane-PEG-D2B | References Value [25] | ||
|---|---|---|---|---|
| 1st Day | 7th Day | p | ||
| RBC (×103/mcL) | 11.0 ± 0.12 | 7.9 ± 0.86 | 0.030 | 6.93–12.24 |
| HCT (%) | 49.4 ± 0.86 | 34.1 ± 3.67 | 0.024 | 42.1–68.3 |
| HGB (g/dL) | 16.5 ± 0.15 | 11.9 ± 1.36 | 0.034 | 12.6–20.5 |
| MCV (fL) | 44.8 ± 0.23 | 43.1 ± 0.15 | 0.005 | 50.7–64.4 |
| MCH (pg) | 14.9 ± 0.12 | 14.9 ± 0.23 | >0.990 | 13.2–17.6 |
| MCHC (g/dL) | 33.3 ± 0.38 | 34.7 ± 0.49 | 0.068 | 23.3–32.7 |
| WBC (G/L) | 4.6 ± 1.10 | 0.4 ± 0.10 | 0.026 | 3.48–14.03 |
| NEUT (G/L) | 1.1 ± 0.36 | 0.39 ± 0.10 | 0.039 | - |
| NEUT (%) | 24.8 ± 5.80 | 14.1 ± 8.18 | 9.86–39.11 | |
| LYMPH (G/L) | 3.3 ± 0.92 | 0.31 ± 0.10 | 0.035 | - |
| LYMPH (%) | 70.5 ± 6.68 | 78.3 ± 8.89 | 48.81–83.19 | |
| MONO (G/L) | 0.1 ± 0.05 | 0.01 ± 0.01 | 0.078 | - |
| MONO (%) | 2.2 ± 0.69 | 4.0 ± 2.98 | 3.29–12.48 | |
| BASO (G/L) | 0.1 ± 0.02 | 0.01 ± 0.01 | 0.020 | - |
| BASO (%) | 2.4 ± 0.49 | 3.5 ± 1.36 | 0.00–1.84 | |
| EOS (G/L) | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.088 | - |
| EOS (%) | 0.1 ± 0.06 | 0.1 ± 0.00 | 0.11–4.91 | |
| PLATELET (G/L) | 950.7 ± 183.07 | 457.7 ± 81.45 | 0.063 | 420–1698 |
References
- Dong, L.; Zieren, R.C.; Xue, W.; de Reijke, T.M.; Pienta, K.J. Metastatic prostate cancer remains incurable, why? Asian J. Urol. 2019, 6, 26–41. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bjartell, A.; Lumen, N.; Maroto, P.; Paiss, T.; Gomez-Veiga, F.; Birtle, A.; Kramer, G.; Kalinka, E.; Spaëth, D.; et al. Real-World Outcomes in First-Line Treatment of Metastatic Castration-Resistant Prostate Cancer: The Prostate Cancer Registry. Target. Oncol. 2020, 15, 301–315. [Google Scholar] [CrossRef]
- Czerwińska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A.; Fracasso, G.; Pruszyński, M.; Bilewicz, A.; Kruszewski, M.; Majkowska-Pilip, A.; et al. Targeted radionuclide therapy of prostate cancer-from basic research to clinical perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Solnes, L.B.; Gorin, M.A.; Wang, N.-Y.; Rowe, S.P. A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177–labeled Prostate-specific Membrane Antigen–targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, A.K.; Pienta, K.J.; Morris, M.J.; Bander, N.H.; Baum, R.P.; Fendler, W.P.; Goeckeler, W.; Gorin, M.A.; Hennekes, H.; Pomper, M.G.; et al. Meeting report from the Prostate Cancer Foundation PSMA-directed radionuclide scientific working group. Prostate 2018, 78, 775–789. [Google Scholar] [CrossRef]
- Satapathy, S.; Sood, A.; Das, C.K.; Mittal, B.R. Evolving role of 225Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer—A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Zalutsky, R.M.; Pruszynski, M. Astatine-211: Production and Availability. Curr. Radiopharm. 2012, 4, 177–185. [Google Scholar] [CrossRef]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and Actinium-225—Generator Performance and Evolving Therapeutic Applications of Two Generator-Derived Alpha-Emitting Radioisotopes. Curr. Radiopharm. 2012, 5, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Shishkin, D.N.; Krupitskii, S.V.; Kuznetsov, S.A. Extraction generator of 223Ra for nuclear medicine. Radiochemistry 2011, 53, 404–406. [Google Scholar] [CrossRef]
- Morris, M.J.; Corey, E.; Guise, T.A.; Gulley, J.L.; Kevin Kelly, W.; Quinn, D.I.; Scholz, A.; Sgouros, G. Radium-223 mechanism of action: Implications for use in treatment combinations. Nat. Rev. Urol. 2019, 16, 745–756. [Google Scholar] [CrossRef]
- Brito, A.E.; Etchebehere, E. Radium-223 as an Approved Modality for Treatment of Bone Metastases. Semin. Nucl. Med. 2020, 50, 177–192. [Google Scholar] [CrossRef]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, Ø.S.; Larsen, R.H. Targeting of osseous sites with α-emitting 223Ra: Comparison with the β-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259. [Google Scholar]
- Abou, D.S.; Thiele, N.A.; Gutsche, N.T.; Villmer, A.; Zhang, H.; Woods, J.J.; Baidoo, K.E.; Escorcia, F.E.; Wilson, J.J.; Thorek, D.L.J. Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand. Chem. Sci. 2021, 12, 3733–3742. [Google Scholar] [CrossRef]
- Henriksen, G.; Schoultz, B.W.; Michaelsen, T.E.; Bruland, S.; Larsen, R.H. Sterically stabilized liposomes as a carrier for α-emitting radium and actinium radionuclides. Nucl. Med. Biol. 2004, 31, 441–449. [Google Scholar] [CrossRef]
- Jonasdottir, T.J.; Fisher, D.R.; Borrebæk, J.; Bruland, Ø.S.; Larsen, R.H. First in vivo evaluation of liposome-encapsulated 223Ra as a potential alpha-particle-emitting cancer therapeutic agent. Anticancer Res. 2006, 26, 2841–2848. [Google Scholar]
- Mokhodoeva, O.; Vlk, M.; Málková, E.; Kukleva, E.; Mičolová, P.; Štamberg, K.; Šlouf, M.; Dzhenloda, R.; Kozempel, J. Study of 223Ra uptake mechanism by Fe3O4 nanoparticles: Towards new prospective theranostic SPIONs. J. Nanopart. Res. 2016, 18, 301. [Google Scholar] [CrossRef]
- Westrøm, S.; Malenge, M.; Jorstad, I.S.; Napoli, E.; Bruland, Ø.S.; Bønsdorff, T.B.; Larsen, R.H. Ra-224 labeling of calcium carbonate microparticles for internal α-therapy: Preparation, stability, and biodistribution in mice. J. Label. Compd. Radiopharm. 2018, 61, 472–486. [Google Scholar] [CrossRef] [Green Version]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Vlk, M.; Kozempel, J. Study of 223ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of ph. RSC Adv. 2020, 10, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Reissig, F.; Bauer, D.; Ullrich, M.; Kreller, M.; Pietzsch, J.; Mamat, C.; Kopka, K.; Pietzsch, H.J.; Walther, M. Recent insights in barium-131 as a diagnostic match for radium-223: Cyclotron production, separation, radiolabeling, and imaging. Pharmaceuticals 2020, 13, 272. [Google Scholar] [CrossRef]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with224,225Ra for targeted alpha therapy. J. Nanopart. Res. 2013, 15, 2082. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, A.; Męczyńska-Wielgosz, S.; Majkowska-Pilip, A.; Koźmiński, P.; Wójciuk, G.; Cędrowska, E.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with 223Ra for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.; Fracasso, G.; Pruszyński, M.; Bilewicz, A.; Kruszewski, M.; Majkowska-Pilip, A.; Lankoff, A. Design and evaluation of 223Ra-labeled and anti-PSMA targeted NaA nanozeolites for prostate cancer therapy-Part I. Materials 2020, 13, 3875. [Google Scholar] [CrossRef] [PubMed]
- BALB/C Mouse Hematology—Charles River Laboratories. Available online: https://www.criver.com (accessed on 26 May 2021).
- Alge, C.S.; Hauck, S.M.; Priglinger, S.G.; Kampik, A.; Ueffing, M. Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival. J. Proteome Res. 2006, 5, 862–878. [Google Scholar] [CrossRef] [PubMed]
- Męczyńska-Wielgosz, S.; Piotrowska, A.; Majkowska-Pilip, A.; Bilewicz, A.; Kruszewski, M. Effect of Surface Functionalization on the Cellular Uptake and Toxicity of Nanozeolite A. Nanoscale Res. Lett. 2016, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Thomassen, L.C.J.; Napierska, D.; Dinsdale, D.; Lievens, N.; Jammaer, J.; Lison, D.; Kirschhock, C.E.A.; Hoet, P.H.; Martens, J.A. Investigation of the cytotoxicity of nanozeolites A and y. Nanotoxicology 2012, 6, 472–485. [Google Scholar] [CrossRef]
- Kihara, T.; Zhang, Y.; Hu, Y.; Mao, Q.; Tang, Y.; Miyake, J. Effect of composition, morphology and size of nanozeolite on its in vitro cytotoxicity. J. Biosci. Bioeng. 2011, 111, 725–730. [Google Scholar] [CrossRef]
- Engin, A.B.; Hayes, A.W. The impact of immunotoxicity in evaluation of the nanomaterials safety. Toxicol. Res. Appl. 2018, 2, 239784731875557. [Google Scholar] [CrossRef] [Green Version]
- Mohammadpour, R.; Yazdimamaghani, M.; Cheney, D.L.; Jedrzkiewicz, J.; Ghandehari, H. Subchronic toxicity of silica nanoparticles as a function of size and porosity. J. Control. Release 2019, 304, 216–232. [Google Scholar] [CrossRef]
- Staal, J.; Beyaert, R. Inflammation and NF-κB Signaling in Prostate Cancer: Mechanisms and Clinical Implications. Cells 2018, 7, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baken, K.A.; Vandebriel, R.J.; Pennings, J.L.A.; Kleinjans, J.C.; Loveren, H. van Toxicogenomics in the assessment of immunotoxicity. Methods 2007, 41, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.; Sukumar, S. HOX genes and the NF-κB pathway: A convergence of developmental biology, inflammation and cancer biology. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188450. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M. BCL2A1: The underdog in the BCL2 family. Cell Death Differ. 2012, 19, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yang, C.S.; Ma, Z.X.; Chen, J.C.; Zheng, J.N.; Sun, X.Q.; Wang, J.Q. Inhibition of prostate cancer DU145 cell growth with small interfering RNA targeting the SATB1 gene. Exp. Ther. Med. 2018, 15, 3028–3033. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhang, Y.; Xu, M.; Zheng, X.; Lin, M.; Pan, J.; Ye, C.; Deng, Y.; Jiang, C.; Lin, Y.; et al. Demethylation and overexpression of CSF2 are involved in immune response, chemotherapy resistance, and poor prognosis in colorectal cancer. OncoTargets Ther. 2019, 12, 11255–11269. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.C.; Der Lee, K.; Tsai, Y.C. Roles of interleukin-1 receptor antagonist in prostate cancer progression. Biomedicines 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Made Joni, I.; Vanitha, M.; Panatarani, C.; Faizal, F.; Yang, G.; Zhang, X.; Liu, S.; Yeung, K.L.; Wang, J.; Biemmi, E.; et al. Study of 223Ra uptake mechanism by Fe3O4 nanoparticles: Towards new prospective theranostic SPIONs. J. Nanopart. Res. 2020, 10, 3659–3666. [Google Scholar]
- Jain, P.; Pawar, R.S.; Pandey, R.S.; Madan, J.; Pawar, S.; Lakshmi, P.K.; Sudheesh, M.S. In-vitro in-vivo correlation (IVIVC) in nanomedicine: Is protein corona the missing link? Biotechnol. Adv. 2017, 35, 889–904. [Google Scholar] [CrossRef]
- Woreta, T.A.; Alqahtani, S.A. Evaluation of abnormal liver tests. Med. Clin. N. Am. 2014, 98, 1–16. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, J.; Liang, S.; Du, Z.; Liu, J.; Sun, Z.; Duan, J. Metabolomic characteristics of hepatotoxicity in rats induced by silica nanoparticles. Ecotoxicol. Environ. Saf. 2021, 208, 111496. [Google Scholar] [CrossRef] [PubMed]
- Trip, A.K.; Sikorska, K.; Van Sandick, J.W.; Heeg, M.; Cats, A.; Boot, H.; Jansen, E.P.M.; Verheij, M. Radiation-induced dose-dependent changes of the spleen following postoperative chemoradiotherapy for gastric cancer. Radiother. Oncol. 2015, 116, 239–244. [Google Scholar] [CrossRef]
- Baldi, G.; Ravagli, C.; Mazzantini, F.; Loudos, G.; Adan, J.; Masa, M.; Psimadas, D.; Fragogeorgi, E.A.; Locatelli, E.; Innocenti, C.; et al. In vivo anticancer evaluation of the hyperthermic efficacy of anti-human epidermal growth factor receptor-targeted PEG-based nanocarrier containing magnetic nanoparticles. Int. J. Nanomed. 2014, 9, 3037–3047. [Google Scholar]
- Cędrowska, E.; Pruszyński, M.; Gawęda, W.; Zuk, M.; Krysiński, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.A.; Bouziotis, P.; Bilewicz, A. Trastuzumab conjugated superparamagnetic iron oxide nanoparticles labeled with 225AC as a perspective tool for combined α-radioimmunotherapy and magnetic hyperthermia of HER2-positive breast cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ko, C.H.; Mirkin, C.A. The Impact of Protein Corona Formation on the Macrophage Cellular Uptake and Biodistribution of Spherical Nucleic Acids. Small 2017, 13, 1603847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, P.G.; O’Sullivan, J.M. 223Ra and other bone-targeting radiopharmaceuticals? The translation of radiation biology into clinical practice. Br. J. Radiol. 2015, 88, 20140752. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, U.; Ojea Jimenez, I.; Calzolai, L. A random walk approach to estimate the confinement of α-particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm. Chem. 2018, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Center for Drug Evaluation and Pharmacology Review. 203971Orig1s000. Study 52158: Alpharadin (Radium-223) Single Dose Toxicity Study in Mice. IND 67,521. 2008; p. 87. Available online: https://www.accessdata.fda.gov/drugsatfda.cocs/nda/2013/203971Orig1a000PharmR.pdf (accessed on 16 May 2021).
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef]
- Dorff, T.B.; Fanti, S.; Farolfi, A.; Reiter, R.E.; Sadun, T.Y.; Sartor, O. The Evolving Role of Prostate-Specific Membrane Antigen–Based Diagnostics and Therapeutics in Prostate Cancer. Am. Soc. Clin. Oncol. Educ. B. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Larsen, R.H.; Saxtorph, H.; Skydsgaard, M.; Borrebæk, J.; Jonasdottir, T.J.; Bruland, Ø.S.; Klastrup, S.; Harling, R.; Ramdahl, T. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: Histology, clinical chemistry and hematology. In Vivo 2006, 20, 325–332. [Google Scholar]
- Lütje, S.; van Rij, C.M.; Franssen, G.M.; Fracasso, G.; Helfrich, W.; Eek, A.; Oyen, W.J.; Colombatti, M.; Boerman, O.C. Targeting human prostate cancer with 111In-labeled D2B IgG, F(ab′)2 and Fab fragments in nude mice with PSMA-expressing xenografts. Contrast Media Mol. Imaging 2015, 10, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug Delivery Systems Modulate Tumor Vessels to Increase the Enhanced Permeability and Retention Effect. J. Pers. Med. 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 26. [Google Scholar] [CrossRef]
- Bolkestein, M.; De Blois, E.; Koelewijn, S.J.; Eggermont, A.M.M.; Grosveld, F.; De Jong, M.; Koning, G.A. Investigation of factors determining the enhanced permeability and retention effect in subcutaneous xenografts. J. Nucl. Med. 2016, 57, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, N.; Fonge, H.; Cai, Z.; Scollard, D.; Lechtman, E.; Done, S.J.; Pignol, J.P.; Reilly, R.M. Role of antibody-mediated tumor targeting and route of administration in nanoparticle tumor accumulation in vivo. Mol. Pharm. 2012, 9, 2168–2179. [Google Scholar] [CrossRef]
- Yang, Y.; Neef, T.; Mittelholzer, C.; Garcia Garayoa, E.; Bläuenstein, P.; Schibli, R.; Aebi, U.; Burkhard, P. The biodistribution of self-assembling protein nanoparticles shows they are promising vaccine platforms. J. Nanobiotechnol. 2013, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Frigerio, B.; Fracasso, G.; Luison, E.; Cingarlini, S.; Mortarino, M.; Coliva, A.; Seregni, E.; Bombardieri, E.; Zuccolotto, G.; Rosato, A.; et al. A single-chain fragment against prostate specific membrane antigen as a tool to build theranostic reagents for prostate cancer. Eur. J. Cancer 2013, 49, 2223–2232. [Google Scholar] [CrossRef]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 2020. [Google Scholar] [CrossRef]
- Bioinformatics & Evolutionary Genomics. Calculate and Draw Custom Venn Diagrams. Available online: http://bioinformatics.psb.ugent.be/webtools/Venn (accessed on 26 May 2021).







| 223RaA-silane-PEG | 223RaA-silane-PEG-D2B | Reference Values [25] | |||||
|---|---|---|---|---|---|---|---|
| 1st Day | 7th Day | p | 1st Day | 7th Day | p | ||
| RBC (×103/mcL) | 11.1 ± 0.53 | 8.1 ± 0.71 | 0.030 * | 11.0 ± 0.12 | 7.9 ± 0.86 | 0.030 * | 6.93–12.24 |
| HCT (%) | 46.8 ± 2.26 | 34.0 ± 3.70 | 0.034 * | 49.4 ± 0.86 | 34.1 ± 3.67 | 0.024 * | 42.1–68.3 |
| HGB (g/dL) | 16.3 ± 0.40 | 11.7 ± 1.27 | 0.030 * | 16.5 ± 0.15 | 11.9 ± 1.36 | 0.034 * | 12.6–20.5 |
| MCV (fL) | 44.5 ± 0.55 | 42.9 ± 0.20 | 0.034 * | 44.8 ± 0.23 | 43.1 ± 0.15 | 0.005 * | 50.7–64.4 |
| MCH (pg) | 14.8 ± 0.21 | 14.7 ± 0.45 | 0.592 | 14.9 ± 0.12 | 14.9 ± 0.23 | >0.999 | 13.2–17.6 |
| MCHC (g/dL) | 33.4 ± 0.35 | 34.7 ± 0.50 | 0.067 | 33.3 ± 0.38 | 34.7 ± 0.49 | 0.068 | 23.3–32.7 |
| WBC (G/L) | 5.4 ± 0.42 | 0.5 ± 0.06 | 0.000 * | 4.6 ± 1.10 | 0.4 ± 0.10 | 0.028 * | 3.48–14.03 |
| NEUT (G/L) | 1.1 ± 0.32 | 0.05 ± 0.03 | 0.030 * | 1.1 ± 0.36 | 0.05 ± 0.04 | 0.039 * | - |
| LYMPH (G/L) | 3.1 ± 0.67 | 0.3 ± 0.07 | 0.017 * | 3.3 ± 0.92 | 0.3 ± 0.10 | 0.035 * | - |
| MONO (G/L) | 0.2 ± 0.04 | 0.01 ± 0.00 | 0.011 * | 0.1 ± 0.05 | 0.01 ± 0.01 | 0.078 | - |
| BASO (G/L) | 0.1 ± 0.01 | 0.02 ± 0.01 | 0.007 * | 0.1 ± 0.02 | 0.01 ± 0.01 | 0.020 * | - |
| EOS (G/L) | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.067 | 0.01 ± 0.00 | 0.0 ± 0.00 | 0.088 | - |
| PLATELET (G/L) | 965.3 ± 104.00 | 355.3 ± 116.17 | 0.022 * | 950.7 ± 183.07 | 457.7 ± 81.45 | 0.064 | 420–1698 |
| 24 h | 7 d | ||
| 223RaA-silane-PEG | Blood | 2.68 ± 2.54 | 0.90 ± 0.44 |
| Lungs | 18.13 ± 9.07 | 1 59 ± 1.48 | |
| Liver | 38.14 ± 2.22 | 20.06 ± 1.11 | |
| Spleen | 27.92 ± 4.77 | 40.71 ± 19.05 | |
| Kidneys | 2.46 ± 0.16 | 0.53 ± 0.09 | |
| small intestine | 0.25 ± 0.04 | 0.09 ± 0.01 | |
| large intestine | 1.13 ± 0.29 | 0.53 ± 0.16 | |
| Stomach | 0.59 ± 0.13 | 0.13 ± 0.08 | |
| Bone | 10.30 ± 1.13 | 19.52 ± 4.44 | |
| Muscle | 0.39 ± 0.14 | 0.16 ± 0.10 | |
| urine [%ID] | 14.75 ± 0.71 | ||
| mass of spleen [mg] | 70 ± 7 | 28 ± 10 | |
| 24 h | 7 d | ||
| Blood | 5.90 ± 3.81 | 1.42 ± 0.58 | |
| 223RaA-silane-PEG-D2B | Lungs | 33.78 ± 8.10 | 2.28 ± 0.94 |
| Liver | 57.34 ± 6.97 | 28.58 ± 0.91 | |
| Spleen | 69.34 ± 18.62 | 46.47 ± 18.16 | |
| Kidneys | 3.38 ± 0.02 | 0.80 ± 0.05 | |
| small intestine | 0.73 ± 0.85 | 0.08 ± 0.04 | |
| large intestine | 1.19 ± 0.50 | 0.17 ± 0.07 | |
| Stomach | 0.61 ± 0.08 | 0.10 ± 0.04 | |
| Bone | 17.74 ± 10.08 | 24.53 ± 3.87 | |
| Muscle | 0.33 ± 0.08 | 0.16 ± 0.07 | |
| urine [%ID] | 17.56 ± 1.71 | ||
| mass of spleen [mg] | 54 ± 15 | 35 ± 9 |
| 223RaA-silane-PEG | 4 h | 24 h | 72 h | 7 Days | |
| Blood | 3.13 ± 1.07 | 0.3 ± 0.19 | 2.62 ± 0.77 | 0.61 ± 0.30 | |
| Lungs | 61.50 ± 37.46 | 5.5 ± 5.27 | 14.75 ± 9.81 | 3.75 ± 2.11 | |
| Liver | 116.0 ± 28.45 | 28.0 ± 18.6 | 36.57 ± 11.48 | 31.46 ± 10.13 | |
| Spleen | 79.6 ± 22.26 | 15.2 ± 13.02 | 5.54 ± 4.28 | 49.69 ± 19.01 | |
| Kidneys | 5.8 ±2.16 | 1.3 ± 0.86 | 1.05 ± 0.41 | 0.69 ± 0.15 | |
| small intestine | 1.0 ± 0.20 | 0.2 ± 0.18 | 0.03 ± 0.01 | 0.10 ± 0.03 | |
| large intestine | 1.2 ± 0.26 | 0.7 ± 0.47 | 0.45 ± 0.12 | 0.13 ± 0.05 | |
| Stomach | 0.9 ± 0.45 | 0.2 ± 0.08 | 1.65 ± 1.97 | 0.10 ± 0.02 | |
| Bone | 4.2 ± 1.60 | 3.0 ± 1.87 | 10.62 ± 2.50 | 13.23 ± 4.85 | |
| Muscle | 0.5 ± 0.43 | 0.2 ± 0.11 | 0.12 ± 0.11 | 0.32 ± 0.12 | |
| Tumor | 0.71 ± 0.72 | 0.40 ± 0.18 | 0.05 ± 0.07 | 0.11 ± 0.06 | |
| 4 h | 24 h | 72 h | 7 Days | ||
| 223RaA-silane-PEG-D2B | Blood | 1.0 ± 0.30 | 0.9 ± 0.41 | 4.11 ± 1.15 | 0.63 ± 0.39 |
| Lungs | 20.2 ± 15.75 | 13.8 ± 13.51 | 33.80 ± 24.20 | 4.29 ± 1.58 | |
| Liver | 46.6 ± 7.11 | 62.5 ± 17.78 | 57.35 ± 8.68 | 43.55 ± 7.99 | |
| Spleen | 48.5 ± 5.24 | 36.6 ± 8.78 | 28.06 ± 24.74 | 128.57 ± 14.58 | |
| Kidneys | 2.3 ± 0.86 | 2.1 ± 0.37 | 1.81 ± 0.49 | 1.28 ± 0.29 | |
| small intestine | 0.6 ± 0.24 | 0.5 ± 0.09 | 0.09 ± 0.04 | 0.19 ± 0.10 | |
| large intestine | 0.7 ± 0.24 | 1.0 ± 0.66 | 0.61 ± 0.18 | 0.45 ± 0.22 | |
| Stomach | 0.3 ± 0.17 | 0.2 ± 0.10 | 1.29 ± 0.48 | 0.43 ± 0.35 | |
| Bone | 2.3 ± 1.42 | 5.7 ± 1.55 | 14.68 ± 6.40 | 15.02 ± 9.01 | |
| Muscle | 0.2 ± 0.07 | 0.3 ±0.19 | 0.27 ± 0.07 | 0.43 ± 0.16 | |
| Tumor | 0.23 ± 0.31 | 0.13 ± 0.19 | 0.24 ± 0.11 | 0.08 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lankoff, A.; Czerwińska, M.; Walczak, R.; Karczmarczyk, U.; Tomczyk, K.; Brzóska, K.; Fracasso, G.; Garnuszek, P.; Mikołajczak, R.; Kruszewski, M. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy—Part II. Toxicity, Pharmacokinetics and Biodistribution. Int. J. Mol. Sci. 2021, 22, 5702. https://doi.org/10.3390/ijms22115702
Lankoff A, Czerwińska M, Walczak R, Karczmarczyk U, Tomczyk K, Brzóska K, Fracasso G, Garnuszek P, Mikołajczak R, Kruszewski M. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy—Part II. Toxicity, Pharmacokinetics and Biodistribution. International Journal of Molecular Sciences. 2021; 22(11):5702. https://doi.org/10.3390/ijms22115702
Chicago/Turabian StyleLankoff, Anna, Malwina Czerwińska, Rafał Walczak, Urszula Karczmarczyk, Kamil Tomczyk, Kamil Brzóska, Giulio Fracasso, Piotr Garnuszek, Renata Mikołajczak, and Marcin Kruszewski. 2021. "Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy—Part II. Toxicity, Pharmacokinetics and Biodistribution" International Journal of Molecular Sciences 22, no. 11: 5702. https://doi.org/10.3390/ijms22115702
APA StyleLankoff, A., Czerwińska, M., Walczak, R., Karczmarczyk, U., Tomczyk, K., Brzóska, K., Fracasso, G., Garnuszek, P., Mikołajczak, R., & Kruszewski, M. (2021). Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy—Part II. Toxicity, Pharmacokinetics and Biodistribution. International Journal of Molecular Sciences, 22(11), 5702. https://doi.org/10.3390/ijms22115702