Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments
Abstract
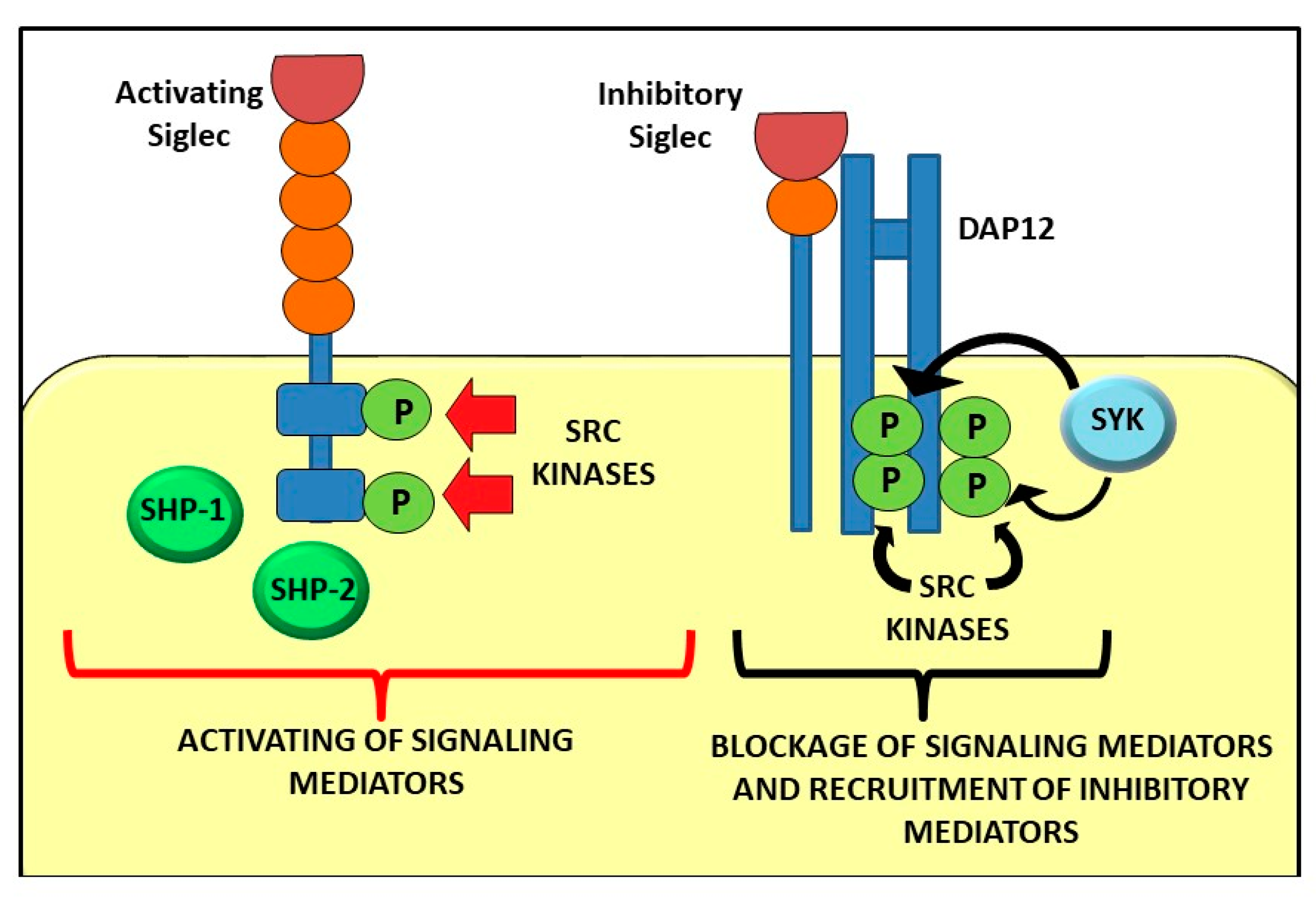
:1. Introduction: Biology of the Sialic Acid-Sialic Acid-Binding Immunoglobulin-Like Lectin (Siglec) Axis
2. Sialic Acid-Siglec Interactions in Human Diseases
2.1. Sialic Acid-Siglec Interactions in Immune-Mediated Diseases
2.2. Sialic Acid-Siglec in Cancer
3. Therapeutic Approaches Based on Siglecs
3.1. Therapeutic Targeting of Siglecs Using Antibody-Based Approaches
3.2. Therapeutic Targeting of Siglecs Using Glycan-Based Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varki, A. Glycan-Based Interactions Involving Vertebrate Sialic-Acid-Recognizing Proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef]
- Crocker, P.R.; Clark, E.A.; Filbin, M.; Gordon, S.; Jones, Y.; Kehrl, J.H.; Kelm, S.; Le Douarin, N.; Powell, L.; Roder, J.; et al. Siglecs: A Family of Sialic-Acid Binding Lectins. Glycobiology 1998, 8, v–vi. [Google Scholar] [CrossRef] [Green Version]
- Marth, J.D. A Unified Vision of the Building Blocks of Life. Nat. Cell Biol. 2008, 10, 1015–1016. [Google Scholar] [CrossRef]
- Schauer, R. Achievements and Challenges of Sialic Acid Research. Glycoconj. J. 2000, 17, 485–499. [Google Scholar] [CrossRef]
- Deng, L.; Chen, X.; Varki, A. Exploration of Sialic Acid Diversity and Biology Using Sialoglycan Microarrays. Biopolymers 2013, 99, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic Acids as Regulators of Molecular and Cellular Interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Varki, A. The Sialome—Far More than the Sum of Its Parts. OMICS 2010, 14, 455–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corfield, A.P.; Berry, M. Glycan Variation and Evolution in the Eukaryotes. Trends Biochem. Sci. 2015, 40, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and Immune Regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef] [Green Version]
- Varki, A. Colloquium Paper: Uniquely Human Evolution of Sialic Acid Genetics and Biology. Proc. Natl. Acad. Sci. USA 2010, 107, 8939–8946. [Google Scholar] [CrossRef] [Green Version]
- Samraj, A.N.; Pearce, O.M.; Laubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A Red Meat-Derived Glycan Promotes Inflammation and Cancer Progression. Proc. Natl. Acad. Sci. USA 2015, 112, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, K.; Dhar, C.; Do, R.; Varki, N.; Gordts, P.; Varki, A. Human Species-Specific Loss of CMP-N-Acetylneuraminic Acid Hydroxylase Enhances Atherosclerosis via Intrinsic and Extrinsic Mechanisms. Proc. Natl. Acad. Sci. USA 2019, 116, 16036–16045. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef] [Green Version]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and Their Roles in the Immune System. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- McMillan, S.J.; Sharma, R.S.; McKenzie, E.J.; Richards, H.E.; Zhang, J.; Prescott, A.; Crocker, P.R. Siglec-E is a Negative Regulator of Acute Pulmonary Neutrophil Inflammation and Suppresses CD11b β2-Integrin-Dependent Signaling. Blood 2013, 121, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-Mediated Regulation of Immune cell Function in Disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [Green Version]
- Adams, O.J.; Stanczak, M.A.; von Gunten, S.; Läubli, H. Targeting Sialic Acid-Siglec Interactions to Reverse Immune Suppression in Cancer. Glycobiology 2018, 28, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianchecchi, E.; Fierabracci, A. Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front. Immunol. 2018, 9, 2374. [Google Scholar] [CrossRef] [Green Version]
- Läubli, H.; Varki, A. Sialic Acid-Binding Immunoglobulin-Like Lectins (Siglecs) Detect Self-Associated Molecular Patterns to Regulate Immune Responses. Cell. Mol. Life Sci. 2020, 77, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Margulies, E.H.; Green, E.D.; Varki, A. Large-Scale Sequencing of the CD33-Related Siglec Gene Cluster in Five Mammalian Species Reveals Rapid Evolution by Multiple Mechanisms. Proc. Natl. Acad. Sci. USA 2004, 101, 13251–13256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlin, A.F.; Chang, Y.C.; Areschoug, T.; Lindahl, G.; Hurtado-Ziola, N.; King, C.C.; Varki, A.; Nizet, V. Group B Streptococcus Suppression of Phagocyte Functions by Protein-Mediated Engagement of Human Siglec-5. J. Exp. Med. 2009, 206, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Mikulak, J.; Di Vito, C.; Zaghi, E.; Mavilio, D. Host Immune Responses in HIV-1 Infection: The Emerging Pathogenic Role of Siglecs and Their Clinical Correlates. Front. Immunol. 2017, 8, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 Selectively Repress Tissue Damage-Induced Immune Responses. Science 2009, 323, 1722–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Lu, Q.; Sanmanmed, M.F.; Wang, J. Siglec-15 as an Emerging Target for Next-generation Cancer Immunotherapy. Clin. Cancer Res. 2021, 27, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kitov, P.I.; Kitova, E.N.; Mozenah, F.; Rodrigues, E.; Chapla, D.G.; Moremen, K.W.; Macauley, M.S.; Klassen, J.S. CUPRA-ZYME: An Assay for Measuring Carbohydrate-Active Enzyme Activities, Pathways, and Substrate Specificities. Anal. Chem. 2020, 92, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Nizet, V. Siglecs at the Host-Pathogen Interface. Adv. Exp. Med. Biol. 2020, 1204, 197–214. [Google Scholar] [CrossRef]
- von Gunten, S.; Bochner, B.S. Basic and Clinical Immunology of Siglecs. Ann. N. Y. Acad. Sci. 2008, 1143, 61–82. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Yeh, Y.C.; Yang, K.D. Functions and Therapeutic Targets of Siglec-Mediated Infections, Inflammations and Cancers. J. Formos. Med. Assoc. 2021, 120, 5–24. [Google Scholar] [CrossRef]
- Sammar, M.; Siwetz, M.; Meiri, H.; Fleming, V.; Altevogt, P.; Huppertz, B. Expression of CD24 and Siglec-10 in First Trimester Placenta: Implications for Immune Tolerance at the Fetal-Maternal Interface. Histochem. Cell Biol. 2017, 147, 565–574. [Google Scholar] [CrossRef]
- Munday, J.; Floyd, H.; Crocker, P.R. Sialic Acid Binding Receptors (Siglecs) Expressed by Macrophages. J. Leukoc. Biol. 1999, 66, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Landig, C.S.; Hazel, A.; Kellman, B.P.; Fong, J.J.; Schwarz, F.; Agarwal, S.; Varki, N.; Massari, P.; Lewis, N.E.; Ram, S.; et al. Evolution of the Exclusively Human Pathogen Neisseria Gonorrhoeae: Human-Specific Engagement of Immunoregulatory Siglecs. Evol. Appl. 2019, 12, 337–349. [Google Scholar] [CrossRef]
- Murch, S.H. Common Determinants of Severe Covid-19 Infection Are Explicable by SARS-CoV-2 Secreted Glycoprotein Interaction with the CD33-Related Siglecs, Siglec-3 and Siglec-5/14. Med. Hypotheses 2020, 144, 110168. [Google Scholar] [CrossRef]
- Varki, A. Are humans prone to autoimmunity? Implications from evolutionary changes in hominin sialic acid biology. J. Autoimmun. 2017, 83, 134–142. [Google Scholar] [CrossRef]
- Clark, E.A.; Giltiay, N.V. CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity. Front. Immunol. 2018, 9, 2235. [Google Scholar] [CrossRef]
- Flores, R.; Zhang, P.; Wu, W.; Wang, X.; Ye, P.; Zheng, P.; Liu, Y. Siglec Genes Confer Resistance to Systemic Lupus Erythematosus in Humans and Mice. Cell. Mol. Immunol. 2019, 16, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Büll, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic Acids Sweeten a Tumor’s Life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel Insights into the Function of CD24: A Driving Force in Cancer. Int. J. Cancer. 2021, 148, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Koziol-White, C.J.; Jester, W.F., Jr.; Scott, A.; Smith, S.A.; Corwin, M.; Nycholat, C.M.; Macauley, M.S.; Panettieri, R.A., Jr.; Paulson, J.C. CD33 Recruitment Inhibits IgE-Mediated Anaphylaxis and Desensitizes Mast Cells to Allergen. J. Clin. Investig. 2019, 129, 1387–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Yu, K.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic Inactivation of CD33 in Hemato-Poietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell 2018, 173, 1439–1453.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, V.S.; Pillai, S. Sialic Acids and Autoimmune Disease. Immunol. Rev. 2016, 269, 145–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angata, T. Associations of Genetic Polymorphisms of Siglecs with Human Diseases. Glycobiology 2014, 24, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, A.A.D.; Gomaa, S.; Li, X.; Routledge, M.; Saigoh, K.; Numoto, N.; Angata, T.; Hitomi, Y.; Takematsu, H.; Tsuiji, M.; et al. A Guillain-Barré Syndrome-Associated SIGLEC10 Rare Variant Impairs Its Recognition of Gangliosides. J. Autoimmun. 2021, 116, 102571. [Google Scholar] [CrossRef]
- Hitomi, Y.; Tsuchiya, N.; Hasegawa, M.; Fujimoto, M.; Takehara, K.; Tokunaga, K.; Satoz, S. Association of CD22 Gene Polymorphism with Susceptibility to Limited Cutaneous Systemic Sclerosis. Tissue Antigens 2007, 69, 242–249. [Google Scholar] [CrossRef]
- Thornhill, S.I.; Mak, A.; Lee, B.; Lee, H.Y.; Poidinger, M.; Connolly, J.E.; Fairhurst, A.M. Monocyte Siglec-14 Expression is Upregulated in Patients with Systemic Lupus Erythematosus and Correlates with Lupus Disease Activity. Rheumatology 2017, 56, 1025–1030. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Tsai, Y.J.; Grigoryev, D.N.; Barnes, K.C. Host Defense Genes in Asthma and Sepsis and the Role of the Environment. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Sajay-Asbaghi, M.; Sadeghi-Shabestrai, M.; Monfaredan, A.; Seyfizadeh, N.; Razavi, A.; Kazemi, T. Promoter Region Single Nucleotide Polymorphism of Siglec-8 Gene Associates with Susceptibility to Allergic Asthma. Personal. Med. 2020, 17, 195–201. [Google Scholar] [CrossRef]
- Ishii, T.; Angata, T.; Wan, E.S.; Cho, M.H.; Motegi, T.; Gao, C.; Ohtsubo, K.; Shinobu Kitazume, S.; Gemma, A.; Paré, P.D.; et al. Influence of SIGLEC9 Polymorphisms on COPD Phenotypes Including Exacerbation Frequency. Respirology 2017, 22, 684–690. [Google Scholar] [CrossRef]
- Angata, T.; Ishii, T.; Motegi, T.; Oka, R.; Taylor, R.E.; Campos Soto, P.; Chang, Y.; Secundino, I.; Gao, C.; Ohtsubo, K.; et al. Loss of Siglec-14 Reduces the Risk of Chronic Obstructive Pulmonary Disease Exacerbation. Cell Mol. Life Sci. 2013, 70, 3199–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.R.; Fong, J.J.; Carlin, A.F.; Busch, T.D.; Linden, R.; Angata, T.; Areschoug, T.; Parast, M.; Varki, N.; Murray, J.; et al. Siglec-5 and Siglec-14 Are Polymorphic Paired Receptors that Modulate Neutrophil and Amnion Signaling Responses to Group B Streptococcus. J. Exp. Med. 2014, 211, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Surolia, I.; Pirnie, S.P.; Chellappa, V.; Taylor, K.N.; Cariappa, A.; Moya, J.; Liu, H.; Bell, D.W.; Driscoll, D.R.; Diederichs, S.; et al. Functionally Defective Germline Variants of Sialic Acid Acetylesterase in Autoimmunity. Nature 2010, 466, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Iguchi, G.; Bando, H.; Fukuoka, H.; Suda, K.; Takahashi, M.; Nishizawa, H.; Matsumoto, R.; Tojo, K.; Mokubo, A.; et al. A Missense Single-Nucleotide Polymorphism in the Sialic Acid Acetylesterase (SIAE) Gene is Associated with Anti-PIT-1 Antibody Syndrome. Endocr. J. 2014, 61, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Nitschke, L.; Carsetti, R.; Ocker, B.; Köhler, G.; Lamers, M.C. CD22 is a Negative Regulator of B-Cell Re-Ceptor Signaling. Curr. Biol. 1997, 7, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.S.; Cheng, Y.; Lin, Q.S.; Wu, A.L.; Yu, J.; Li, C.; Sun, Y.; Zhong, R.-Q.; Wu, L.-J. Increased Expression of Siglec-1 on Peripheral Blood Monocytes and Its Role in Mononuclear Cell Reactivity to Autoantigen in Rheumatoid Arthritis. Rheumatology 2014, 53, 250–259. [Google Scholar] [CrossRef] [Green Version]
- York, M.R.; Nagai, T.; Mangini, A.J.; Lemaire, R.; van Seventer, J.M.; Lafyatis, R. A Macrophage Marker, Siglec-1, Is Increased on Circulating Monocytes in Patients with Systemic Sclerosis and Induced by Type I interferons and Toll-Like Receptor Agonists. Arthritis Rheum. 2007, 56, 1010–1020. [Google Scholar] [CrossRef]
- Wilhelm, T.R.; Taddeo, A.; Winter, O.; Schulz, A.R.; Malzer, J.N.; Domingo, C.; Biesen, R.; Alexander, T.; Thiel, A.; Radbruch, A.; et al. Siglec-1-Positive Plasmacytoid dendritic Cells (pDCs) in Human Peripheral Blood: A Semi-Mature and Myeloid-Like Subset Imbalanced during Protective and Autoimmune Responses. Clin. Immunol. 2016, 163, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Molineros, J.E.; Looger, L.L.; Zhou, X.-J.; Kim, K.; Okada, Y.; Ma, J.; Qi, Y.-Y.; Kim-Howard, X.; Motghare, P.; et al. High-Density Genotyping of Immune-Related Loci Identifies New SLE Risk Variants in Individuals with Asian Ancestry. Nat. Genet. 2016, 48, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Pfrengle, F.; Macauley, M.S.; Kawasaki, N.; Paulson, J.C. Copresentation of Antigen and Ligands of Siglec-G Induces B Cell Tolerance Independent of CD22. J. Immunol. 2013, 191, 1724–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, J.; Nitschke, L. The role of CD22 and Siglec-G in B-Cell Tolerance and Autoimmune Disease. Nat. Rev. Rheumatol. 2014, 10, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Madge, P.D.; Maggioni, A.; Pascolutti, M.; Amin, M.; Waespy, M.; Bellette, B.; Thomson, R.J.; Kelm, S.; von Itzstein, M.; Haselhorst, T. Structural Characterisation of High Affinity Siglec-2 (CD22) Ligands in Complex with Whole Burkitt’s Lymphoma (BL) Daudi Cells by NMR Spectroscopy. Sci. Rep. 2016, 6, 36012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jellusova, J.; Wellmann, U.; Amann, K.; Winkler, T.H.; Nitschke, L. CD22 x Siglec-G double-Deficient Mice Have Massively increased B1 Cell Numbers and Develop Systemic Autoimmunity. J. Immunol. 2010, 184, 3618–3627. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Completo, G.C.; Sigal, D.S.; Crocker, P.R.; Saven, A.; James, C.; Paulson, J.C. In Vivo Targeting of B-Cell Lymphoma with Glycan Ligands of CD22. Blood 2010, 115, 4778–4786. [Google Scholar] [CrossRef] [Green Version]
- Toubai, T.; Hou, G.; Mathewson, N.; Liu, C.; Wang, Y.; Oravecz-Wilson, K.; Cummings, E.; Rossi, C.; Evers, R.; Sun, Y.; et al. Siglec-G-CD24 Axis Controls the Severity of Graft-Versus-Host Disease in Mice. Blood 2014, 123, 3512–3523. [Google Scholar] [CrossRef] [Green Version]
- Muller, J.; Lunz, B.; Schwab, I.; Acs, A.; Nimmerjahn, F.; Daniel, C.; Nitschke, L. Siglec-G Deficiency Leads to Autoimmunity in Aging C57BL/6 Mice. J. Immunol. 2015, 195, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litschke, L. CD22 and Siglec-G Regulate Inhibition of B-cell Signaling by Sialic Acid Ligand Binding and Control B-Cell Tolerance. Glycobiology 2014, 24, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Matsushita, T.; Nguyen, V.; Tennichi, M.; Fujimoto, M.; Takehara, K.; Hamaguchi, Y. CD22 and CD72 Contribute to the Development of Scleroderma in a Murine Model. J. Dermatol. Sci. 2020, 97, 66–76. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Hamzeh-Cognasse, H.; Palle, S.; Anselme-Bertrand, I.; Arthaud, C.A.; Chavarin, P.; Pozzetto, B.; Garraud, O.; Cognasse, F. Role of Siglec-7 in Apoptosis in Human Platelets. PLoS ONE 2014, 9, e106239. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, K.; Asano, K.; Moriyama, S.; Ushiki, M.; Monya, M.; Iida, M.; Kuboki, E.; Hideo Yagita, H.; Uchida, K.; Nitta, K.; et al. Vascular-Resident CD169-Positive Monocytes and Macrophages Control Neutrophil Accumulation in the Kidney with Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2015, 26, 896–906. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Brown, N.K.; Wu, W.; Khedri, Z.; Yu, H.; Chen, X.; van de Vlekkert, D.; D’Azzo, A.; Zheng, P.; Liu, Y. Broad and Direct Interaction between TLR and Siglec Families of Pattern Recognition Receptors and its Regulation by Neu1. eLife 2014, 3, e04066. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.; Mezouar, S.; Pegon, J.; Muczynski, V.; Adam, F.; Bianchini, E.P.; Bazaa, A.; Proulle, V.; Alain Rupin, A.; Paysant, J.; et al. Soluble Siglec-5 Associates to PSGL-1 and Displays Anti-Inflammatory Activity. Sci. Rep. 2016, 6, 37953. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.J.; Sreedhara, K.; Deng, L.; Varki, N.M.; Angata, T.; Liu, Q.; Nizet, V.; Varki, A. Immunomodulatory Activity of Extracellular Hsp70 Mediated via Paired Receptors Siglec-5 and Siglec-14. EMBO J. 2015, 34, 2775–2788. [Google Scholar] [CrossRef]
- McMillan, S.J.; Sharma, R.S.; Richards, H.E.; Hegde, V.; Crocker, P.R. Siglec-E Promotes Beta2-Integrin-Dependent Nadph Oxidase Activation to Suppress Neutrophil Recruitment to the Lung. J. Biol. Chem. 2014, 289, 20370–20376. [Google Scholar] [CrossRef] [Green Version]
- von Gunten, S.; Yousefi, S.; Seitz, M.; Jakob, S.M.; Schaffner, T.; Seger, R.; Takala, J.; Villiger, P.M.; Simon, H.-U. Siglec-9 Transduces Apoptotic and Nonapoptotic Death Signals into Neutrophils Depending on the Proinflammatory Cytokine Environment. Blood 2005, 106, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, H.; Fernandes, S.M.; Wei, Y.; Gonzalez-Gil, A.; Motari, M.G.; Katarina Vajn, K.; Stevens, W.W.; Peters, A.T.; Bochner, B.S.; et al. Expression of Ligands for Siglec-8 and Siglec-9 in Human Airways and Airway Cells. J. Allergy Clin. Immunol. 2015, 135, 799–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banda, K.; Gregg, C.J.; Chow, R.; Varki, N.M.; Varki, A. Metabolism of Vertebrate Amino Sugars with N-Glycolyl Groups: Mechanisms Underlying Gastrointestinal Incorporation of the Non-Human Sialic Acid Xeno-Autoantigen N-Glycolylneuraminic Acid. J. Biol. Chem. 2012, 287, 28852–28864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.E.; Gregg, C.J.; Padler-Karavani, V.; Ghaderi, D.; Yu, H.; Huang, S.; Sorensen, R.U.; Xi Chen, X.; Inostroza, J.; Nizet, V.; et al. Novel Mechanism for the Generation of Human Xeno-Autoantibodies Against the Nonhuman Sialic Acid N-Glycolylneuraminic Acid. J. Exp. Med. 2010, 207, 1637–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boligan, K.F.; Mesa, C.; Fernandez, L.E.; von Gunten, S. Cancer Intelligence Acquired (CIA): Tumor Glycosylation and Sialylation Codes Dismantling Antitumor Defense. Cell. Mol. Life Sci. 2015, 72, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Tumor-Associated Carbohydrate Antigens. Annu. Rev. Immunol. 1984, 2, 103–126. [Google Scholar] [CrossRef]
- Amon, R.; Reuven, E.M.; Leviatan Ben-Arye, S.; Padler-Karavani, V. Glycans in Immune Recognition and Response. Carbohydr. Res. 2014, 389, 115–122. [Google Scholar] [CrossRef]
- Kannagi, R.; Sakuma, K.; Miyazaki, K.; Lim, K.T.; Yusa, A.; Yin, J.; Izawa, M. Altered Expression of Glycan Genes in Cancers Induced by Epigenetic Silencing and Tumor Hypoxia: Clues in the Ongoing Search for New Tumor Markers. Cancer Sci. 2010, 101, 586–593. [Google Scholar] [CrossRef]
- Schultz, M.J.; Swindall, A.F.; Bellis, S.L. Regulation of the Metastatic Cell Phenotype by Sialylated Glycans. Cancer Metastasis Rev. 2012, 31, 501–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoll, G.; Avril, T.; Lock, K.; Furukawa, K.; Bovin, N.; Crocker, P.R. Ganglioside GD3 Expression on Target Cells Can Modulate NK Cell Cytotoxicity via Siglec-7-Dependent and -Independent Mechanisms. Eur. J. Immunol. 2003, 33, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Krengel, U.; Bousquet, P.A. Molecular Recognition of Gangliosides and Their Potential for Cancer Immunotherapies. Front. Immunol. 2014, 5, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedarko, N.S.; Fohr, B.; Robey, P.G.; Young, M.F.; Fisher, L.W. Factor H Binding to Bone Sialoprotein and Osteopontin Enables Tumor Cell Evasion of Complement-Mediated Attack. J. Biol. Chem. 2000, 275, 16666–16672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx Engineering Reveals a Siglec-Based Mechanism for NK Cell Immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Demoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 Receptors and Ligands Influence NK Cell Dependent Tumor Immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef] [Green Version]
- Läubli, H.; Pearce, O.M.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of Myelomonocytic Siglecs by Tumor-Associated Ligands Modulates the Innate Immune Response to Cancer. Proc. Natl Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef] [Green Version]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.D.; Klausing, S.; Hillier, M.; Maher, J.; Noll, T.; Crocker, P.R.; et al. The Mucin MUC1 Modulates the Tumor Immunological Microenvironment through Engagement of the Lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Malykh, Y.N.; Schauer, R.; Shaw, L. N-Glycolylneuraminic Acid in Human Tumours. Biochimie 2001, 83, 623–634. [Google Scholar] [CrossRef]
- Hedlund, M.; Padler-Karavani, V.; Varki, N.M.; Varki, A. Evidence for a Human-Specific Mechanism for Diet and Antibody-Mediated Inflammation in Carcinoma Progression. Proc. Natl. Acad. Sci. USA 2008, 105, 18936–18941. [Google Scholar] [CrossRef] [Green Version]
- Bergfeld, A.K.; Pearce, O.M.; Diaz, S.L.; Lawrence, R.; Vocadlo, D.J.; Choudhury, B.; Esko, J.D.; Varkiet, A. Metabolism of Vertebrate Amino Sugars with N-Glycolyl Groups: Incorporation of N-Glycolylhexosamines into Mammalian Glycans by Feeding N-Glycolylgalactosamine. J. Biol. Chem. 2012, 287, 28898–28916. [Google Scholar] [CrossRef] [Green Version]
- Samraj, A.N.; Bertrand, K.A.; Luben, R.; Khedri, Z.; Yu, H.; Nguyen, D.; Gregg, C.J.; Diaz, S.L.; Sawyer, S.; Chen, X.; et al. Polyclonal Human Antibodies against Glycans Bearing Red Meat-Derived Non-Human Sialic Acid N-Glycolylneuraminic Acid Are Stable, Reproducible, Complex and Vary between Individuals: Total Antibody Levels are Associated with Colorectal Cancer Risk. PLoS ONE 2018, 13, e0197464. [Google Scholar] [CrossRef] [Green Version]
- Alisson-Silva, F.; Kawanishi, K.; Varki, A. Human Risk of Diseases Associated with Red Meat Intake: Analysis of Current Theories and Proposed Role for Metabolic Incorporation of a Non-Human Sialic Acid. Mol. Asp. Med. 2016, 51, 16–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito-Matsui, Y.; Takada, S.; Kano, Y.; Iyoda, T.; Sugai, M.; Shimizu, A.; Inaba, K.; Nitschke, L.; Tsubata, T.; Oka, S.; et al. Functional Evaluation of Activation-Dependent Alterations in the Sialoglycan Composition of T Cells. J. Biol. Chem. 2014, 289, 1564–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, S.; Sato, C.; Furuhata, K.; Kitajima, K. Oral Ingestion of Mannose Alters the Expression Level of Deaminoneuraminic Acid (KDN) in Mouse Organs. Glycoconj. J. 2006, 23, 411–421. [Google Scholar] [CrossRef]
- Inoue, S.; Lin, S.L.; Chang, T.; Wu, S.H.; Yao, C.W.; Chu, T.Y.; Troy, F.A.; Inoue, Y. Identification of Free Deaminated Sialic acid (2-Keto-3-Deoxy-D-Glycero-D-Galacto-Nononic Acid) in Human Red Blood Cells and Its Elevated Expression in Fetal Cord Red Blood Cells and Ovarian Cancer Cells. J. Biol Chem. 1998, 273, 27199–27204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Xie, B.; Wang, B.; Troy, F.A. LC-MS/MS Glycomic Analyses of Free and Conjugated Forms of the Sialic Acids, neu5ac, neu5gc and kdn in Human Throat Cancers. Glycobiology 2015, 25, 1362–1374. [Google Scholar] [CrossRef] [Green Version]
- Corfield, A.P.; Myerscough, N.; Warren, B.F.; Durdey, P.; Paraskeva, C.; Schauer, R. Reduction of Sialic Acid O-Acetylation in Human Colonic Mucins in the Adenoma-Carcinoma Sequence. Glycoconj. J. 1999, 16, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kohla, G.; Lrhorfi, A.L.; Sipos, B.; Kalthoff, H.; Gerwig, G.J.; Kamerling, J.P.; Schauer, R.; Tiralongo, J. O-Acetylation and De-O-Acetylation of Sialic Acids in Human Colorectal Carcinoma. Eur. J. Biochem. 2004, 271, 281–290. [Google Scholar] [CrossRef]
- Yu, Y.; Blokhuis, B.R.J.; Diks, M.A.P.; Keshavarzian, A.; Garssen, J.; Redegeld, F.A. Functional Inhibitory Siglec-6 Is Upregulated in Human Colorectal Cancer-Associated Mast Cells. Front. Immunol. 2018, 9, 2138. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Siddiqui, S.S.; Trefny, M.P.; Thommen, D.S.; Boligan, K.F.; von Gunten, S.; Tzankov, A.; Tietze, L.; Lardinois, D.; Heinzelmann-Schwarz, V.; et al. Self-Associated Molecular Patterns Mediate Cancer Immune Evasion by Engaging SIGLECS on T Cells. J. Clin. Investig. 2018, 128, 4912–4923. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an Immune Suppressor and Potential Target for Normalization Cancer Immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef]
- Santegoets, K.C.M.; Gielen, P.R.; Büll, C.; Schulte, B.M.; Kers-Rebel, E.D.; Küsters, B.; Bossman, S.A.J.F.H.; Ter Laan, M.; Wesseling, P.; Adema, G.J. Expression Profiling of Immune Inhibitory Siglecs and Their Ligands in Patients with Glioma. Cancer Immunol. Immunother. 2019, 68, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, E.; Boelaars, K.; Brown, K.; Li, R.J.E.; Kruijssen, L.; Bruijns, S.; van Ee, T.; Schetters, S.T.T.; Crommentuijn, M.H.W.; van der Horst, J.C.; et al. Sialic Acids in Pancreatic Cancer Cells Drive Tumour-Associated Macrophage Differentiation via the Siglec Receptors Sig-Lec-7 and Siglec-9. Nat. Commun. 2021, 12, 1270. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wang, S.; Yang, L.; Jiang, L.; Li, J.; Wang, X. Reduced Siglec-7 Expression on NK Cells Predicts NK Cell Dysfunction in Primary Hepatocellular Carcinoma. Clin. Exp. Immunol. 2020, 201, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Pei, H.; Li, X.; Li, J.; Yao, X.; Zhang, R. Serum Protein N-Glycosylation Signa-Tures of Neuroblastoma. Front. Oncol. 2021, 11, 603417. [Google Scholar] [CrossRef]
- Zahradnikova, M.; Ihnatova, I.; Lattova, E.; Uhrik, L.; Stuchlikova, E.; Nenutil, R.; Valik, D.; Nalezinska, M.; Chovanec, J.; Zdrahal, Z.; et al. N-Glycome Changes Reflecting Resistance to Platinum-Based Chemotherapy in Ovarian. J. Proteomic. 2021, 230, 103964. [Google Scholar] [CrossRef]
- Yamanaka, M.; Kato, Y.; Angata, T.; Narimatsu, H. Deletion Polymorphism of SIGLEC14 and Its Functional Implications. Glycobiology 2009, 19, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hazama, S.; Suzuki, N.; Xu, M.; Nakagami, Y.; Fujiwara, N.; Tsunedomi, R.; Yoshida, S.; Tomochika, S.; Matsukuma, S.; et al. Siglec-7 Is a Predictive Biomarker for the Efficacy of Cancer Vaccination against Metastatic Colorectal Cancer. Oncol. Lett. 2021, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Schanin, J.; Gebremeskel, S.; Korver, W.; Falahati, R.; Butuci, M.; Haw, T.J.; Nair, P.M.; Liu, G.; Hansbro, N.G.; Hansbro, P.M.; et al. A Monoclonal Antibody to Siglec-8 Suppresses Non-Allergic Airway Inflammation and Inhibits IgE-Independent Mast Cell Activation. Mucosal Immunol. 2021, 14, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Goldenberg, D.M.; Michel, R.; Rossi, D.L.; Wallace, D.J.; Chang, C.-H. Trogocytosis of Multiple B-Cell Surface Markers by cd22 Targeting with Epratuzumab. Blood 2013, 122, 3020–3029. [Google Scholar] [CrossRef] [Green Version]
- Sieger, N.; Fleischer, S.J.; Mei, H.E.; Reiter, K.; Shock, A.; Burmester, G.R.; Daridon, C.; Dörner, T. CD22 Ligation Inhibits Downstream B Cell Receptor Signaling and Ca2+ Flux Upon Activation. Arthritis Rheum. 2013, 65, 770–779. [Google Scholar] [CrossRef]
- A Study of Lirentelimab (AK002) in Patients With Active Eosinophilic Esophagitis (KRYPTOS). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04322708 (accessed on 27 May 2021).
- Alinari, L.; Lapalombella, R.; Andritsos, L.; Baiocchi, R.A.; Lin, T.S.; Byrd, J.C. Alemtuzumab (Campath-1H) in the Treatment of Chronic Lymphocytic Leukemia. Oncogene 2007, 26, 3644–3653. [Google Scholar] [CrossRef] [Green Version]
- A Study to Evaluate Safety and Effectiveness of mRNA-1273 Vaccine in Healthy Children Between 6 Months of Age and Less Than 12 Years of Age. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03665285 (accessed on 27 May 2021).
- Kantarjian, H.; Thomas, D.; Jorgensen, J.; Jabbour, E.; Kebriaei, P.; Rytting, M.; York, S.; Ravandi, F.; Kwari, M.; Faderl, S.; et al. Inotuzumab Ozogamicin, an Anti-CD22-Calecheamicin Conjugate, for Refractory and Relapsed Acute Lymphocytic Leukaemia: A Phase 2 Study. Lancet Oncol. 2012, 13, 403–411. [Google Scholar] [CrossRef]
- Yilmaz, M.; Richard, S.; Jabbour, E. The Clinical Potential of Inotuzumab Ozogamicin in Relapsed and Refractory Acute Lymphocytic Leukemia. Ther. Adv. Hematol. 2015, 6, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Cowan, A.J.; Laszlo, G.S.; Estey, E.H.; Walter, R.B. Antibody-Based Therapy of Acute Myeloid Leukemia with Gemtuzumab Ozogamicin. Front. Biosci. 2013, 18, 1311–1334. [Google Scholar] [CrossRef] [Green Version]
- Laszlo, G.S.; Estey, E.H.; Walter, R.B. The Past and Future of CD33 as Therapeutic Target in Acute Myeloid Leukemia. Blood Rev. 2014, 28, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Brischwein, K.; Parr, L.; Pflanz, S.; Volkland, J.; Lumsden, J.; Klinger, M.; Locher, M.; Hammond, S.A.; Kiener, P.; Kufer, P.; et al. Strictly Target Cell-Dependent Activation of T Cells by Bispecific Single-Chain Antibody Constructs of the BiTE Class. J. Immunother. 2007, 30, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Frankel, A.E.; Qing Cao, Q.; Lewis, D.; Grzywacz, B.; Verneris, M.R.; Ustun, C.; Lazaryan, A.; McClune, B.; Warlick, E.D.; et al. Phase I Study of a Bispecific Ligand-Directed Toxin Targeting cd22 and cd19 (dt2219) for Refractory B-Cell Malignancies. Clin. Cancer Res. 2015, 21, 1267–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, D.; Singh, N.; Porter, D.L.; Grupp, S.A.; June, C.H. Chimeric Antigen Receptor Therapy for Cancer. Annu. Rev. Med. 2014, 65, 333–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haso, W.; Lee, D.W.; Shah, N.N.; Stetler-Stevenson, M.; Yuan, C.M.; Pastan, I.H.; Dimitrov, D.S.; Morgan, R.A.; FitzGerald, D.J.; Barrett, D.M.; et al. Anti-CD22-Chimeric Antigen Receptors Targeting B-Cell Precursor Acute Lymphoblastic Leukemia. Blood 2013, 121, 1165–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzitola, I.; Anjos-Afonso, F.; Rouault-Pierre, K.; Lassailly, F.; Tettamanti, S.; Spinelli, O.; Biondi, A.; Biagi, E.; Bonnet, D. Chimeric Antigen Receptors Against CD33/CD123 Antigens Efficiently Target Primary Acute Myeloid Leukemia Cells In Vivo. Leukemia 2014, 28, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Hai-yan, L.; Qing-wang, H.; Fan, F.; Guo, B.; Wang, L.; Han, W. Treatment of CD33-Directed Chimeric Antigen Receptor-Modified T Cells in One Patient with Relapsed and Refractory Acute Myeloid Leukemia. Mol. Ther. 2015, 23, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Spence, S.; Greene, M.K.; Fay, F.; Hams, E.; Saunders, S.P.; Hamid, U.; Fitzgerald, M.; Beck, J.; Bains, B.K.; Smyth, P.; et al. Targeting Siglecs with a Sialic Acid Decorated Nanoparticle Abrogates Inflammation. Sci. Transl. Med. 2015, 7, 303ra140. [Google Scholar] [CrossRef] [Green Version]
- Büll, C.; Heise, T.; Adema, G.J.; Boltje, T.J. Sialic Acid Mimetics to Target the Sialic Acid-Siglec Axis. Trends Biochem. Sci. 2016, 41, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Mi-Croenvironment. Bioact. Mater. 2020, 6, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Nycholat, C.M.; Rademacher, C.; Kawasaki, N.; Paulson, J.C. In Silico-Aided Design of a Glycan Ligand of Sialoadhesin for in Vivo Targeting of Macrophages. J. Am. Chem. Soc. 2012, 134, 15696–15699. [Google Scholar] [CrossRef] [Green Version]
- Rillahan, C.D.; Macauley, M.S.; Schwartz, E.; He, Y.; McBride, R.; Arlian, B.M.; Rangarajan, J.; Fokin, V.V.; Paulson, J.C. Disubstituted Sialic Acid Ligands Targeting Siglecs CD33 and CD22 Associated with Myeloid Leukaemias and B Cell Lymphomas. Chem. Sci. 2014, 5, 2398–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ayala-Orozco, C.; Rauta, P.R.; Krishnan, S. The Application of Nanotechnology in Enhancing Immunotherapy for Cancer Treat-Ment: Current Effects and Perspective. Nanoscale 2019, 11, 17157–17178. [Google Scholar] [CrossRef]
- O’Reilly, M.K.; Paulson, J.C. Siglecs as Targets for Therapy in Immune-Cell-Mediated Disease. Trends Pharmacol. Sci. 2009, 30, 240–248. [Google Scholar] [CrossRef] [Green Version]
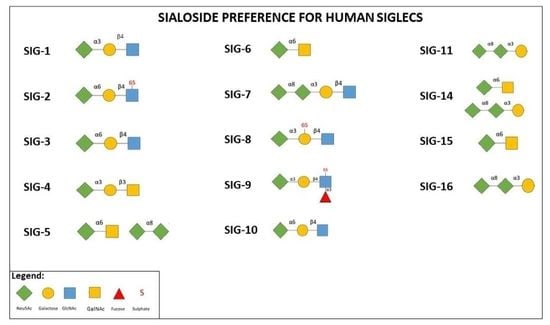
| Siglec | Expression | Suggested Biological Function | Pathological Condition |
|---|---|---|---|
| Sig-1 (CD169) | Macrophages, Monocytes | Myeloid cell differentiation, antigen presentation, host defense | RA, systemic sclerosis (SSc), systemic lupus erythematosus (SLE), Group B streptococci, (GBS) defense, HIV permissive infection |
| Sig-2 (CD22) | B cells | B cell differentiation and tolerance | B-cell lymphomas, SSc |
| Sig-3 (CD33) | Myeloid progenitors, Macrophages, Monocytes, DCs, Microglia, Granulocytes | Myeloid differentiation progenitors, Regulation of inflammatory response upon Pathogen Associated Molecular Patterns (PAMP) or Damage- Associated Molecular Patterns (DAMP) antigen exposure | Leukemia, degeneration |
| Sig-4 | Myelin of nerves | Maintenance of myelinated axons, Suppression of axonal regeneration after injury | Latent infection, Neuron degeneration |
| Sig-5 | Neutrophils, Monocytes | Recognition and internalization of sialylated pathogens, Inhibition of immune cell activation (Co-paired with Siglec-14) | Prematurity, chronic obstructive pulmonary disease (COPD) |
| Sig-6 | Trophoblasts, Mast cells, Intestine | Regulation of trophoblast proliferation and invasiveness, Inflammation | Preeclampsia, Allergy |
| Sig-7 | NK cells, Neutrophils, Monocytes, Mast cells, Platelets | Regulation of pathways of apoptosis in human platelets, Immunosuppression IgE-mediated | Tumor evasion, Allergy, HIV infection |
| Sig-8 | Eosinophils, Mast cells, Basophils | Induction of apoptosis in eosinophils | Allergic asthma |
| Sig-9 | Neutrophils, Monocytes, DCs, NK and B cells | Inhibition of NK cell and neutrophil activation and function, Immune modulation of myeloid cells; Induction of neutrophil apoptosis, Infections, Checkpoint blocker, Modulation of the tumor immunological microenvironment | Sepsis, cancer progression, COPD, Allergy |
| Sig-10 | B cells, DCs, NKs | Immune tolerance | Tumor Immunity, Graft Versus Host Disease (GVHD), Safe pregnancy |
| Sig-11 | B cells, Macrophages, Microglia, Ovary stroma | Immunosuppression | Ovary cancer, Neuroprotection |
| Sig-12 | Macrophages | Unknown | Hypertension treatment outcome |
| Sig-14 | Neutrophils, Monocytes | Activation of proinflammatory pathway in monocytes, Recognition of sialylated pathogens | COPD, Prematurity |
| Sig-15 | Osteoclasts, Macrophages | Regulation of osteoclast differentiation and bone resorption, Immune modulation of macrophages | Osteoporosis, Cancer |
| Sig-16 | Microglia | E. coli defense, Neuroprotection | E. coli defense, Neuroprotection |
| Human Siglec Target | Application |
|---|---|
| Sig-2 (CD22) | Epratuzumab (anti-CD22) for Sjögren’s syndrome, B cell leukemia and SLE; Inotuzumab ozogamicin (anti-CD22 monoclonal antibody (MoAb) conjugated with a toxin (calicheamicin)); DT2219 and chimeric antigen receptors (CARs) for the treatment of B cell acute lymphoblastic leukemia (B-ALL); Moxetumomab pasudotox (LumoxitiTM) for hairy cell leukemia; CD22 binding peptide (PV3) for malignant B cells; CARs in B-cell acute lymphoblastic leukemia (BCP-ALL) |
| Sig-3 (CD33) | CARs for the treatment of AML; blinatumomab for B-cell acute lymphoblastic leukemia (ALL); Anti-CD33 (Siglec-3) BI 836,858 ( MoAb) for acute myeloid leukemia (AML), myelodysplasia syndrome (MDS); Anti-CD33 lintuzumab (HuM195) (MoAb) for AML; CD16/IL-15/CD33 Tri-Specific Killer Engagers (TriKEs) (Combined peptides) for AML, MDS, mast cell leukemia; gentuzumab ozogamicin (mylotarg) (MoAb) for newly diagnosed and relapsed AML patients; Anti-CD33/CD3 BiTE (AMG330, Amgen) for AML; JNJ-67571244 for not responding AML patients at high risk of myelodysplastic syndrome |
| Sig-7 | Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via a Siglec-7-dependent mechanisms |
| Sig-8 | Lirentelimab (AK002) (MoAb) for active eosinophilic esophagitis and Chronic urticarial; Monoclonal antibody towards Siglec-8 (anti-S8) halted non-allergic airway inflammation and inhibited IgE-independent mast cell activation in two in vivo models |
| Sig-9 | [68Ga]-DOTA-Siglec-9 (radioisotope-peptide imaging) for RA |
| Sig-10 | Alemtuzumab for CLL and MS |
| Sig-11 | PolySia avDP20 reduced vascular leakage of laser injury in humanized transgenic mice expressing Siglec-11 |
| Sig-15 | Anti-Siglec-15 NC318 (MoAb) in patient with advanced or metastatic solid tumors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianchecchi, E.; Arena, A.; Fierabracci, A. Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments. Int. J. Mol. Sci. 2021, 22, 5774. https://doi.org/10.3390/ijms22115774
Gianchecchi E, Arena A, Fierabracci A. Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments. International Journal of Molecular Sciences. 2021; 22(11):5774. https://doi.org/10.3390/ijms22115774
Chicago/Turabian StyleGianchecchi, Elena, Andrea Arena, and Alessandra Fierabracci. 2021. "Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments" International Journal of Molecular Sciences 22, no. 11: 5774. https://doi.org/10.3390/ijms22115774
APA StyleGianchecchi, E., Arena, A., & Fierabracci, A. (2021). Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments. International Journal of Molecular Sciences, 22(11), 5774. https://doi.org/10.3390/ijms22115774