Antimicrobial Effect of Chitosan Films on Food Spoilage Bacteria
Abstract
:1. Introduction
2. Results and Discussion
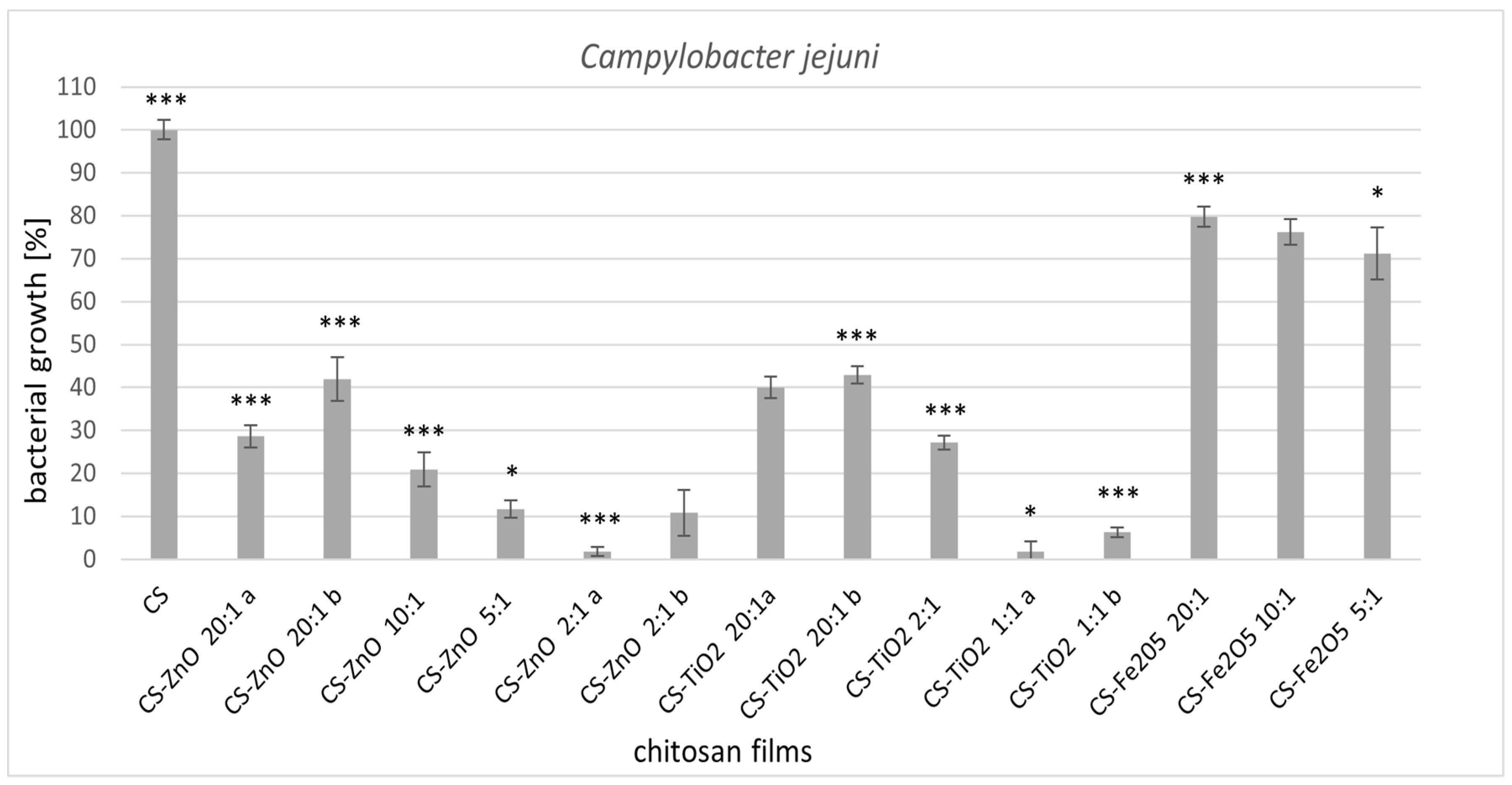
2.1. Antimicrobial Activity
2.1.1. Permeability of Bacterial Cell Membranes
2.1.2. Morphological Changes of L. monocytogenes and C. jejuni Cells Visualized by Scanning Electron Microscopy (SEM)
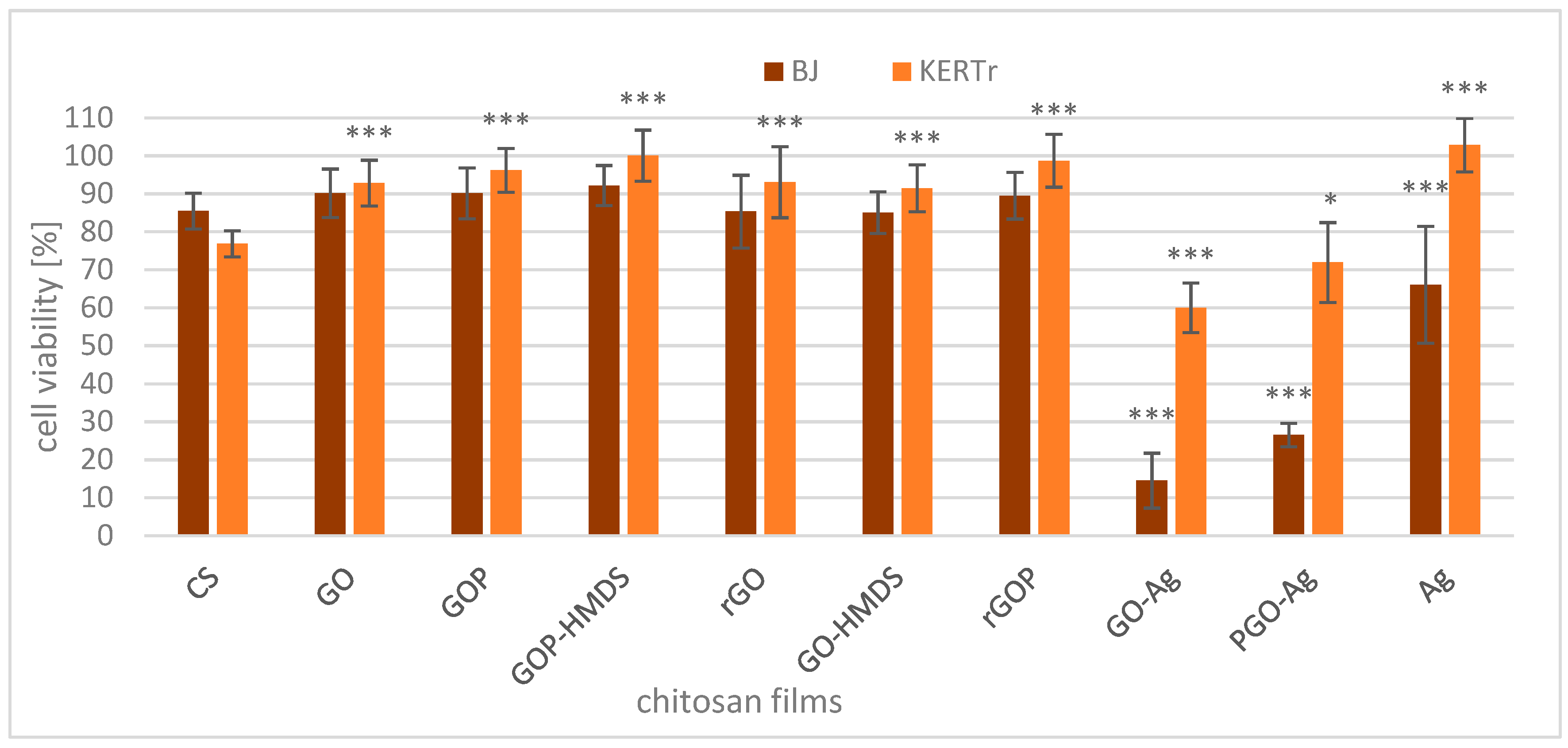
2.2. Cytotoxicity of Films
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan-Metal Oxide Films
3.3. Preparation of Functionalized Graphene Oxide Fillers
3.4. Preparation of Chitosan-Graphene Films
3.5. Determination of Antimicrobial Activity
3.6. Confocal Microscopy
3.7. Scanning Electron Microscopy (SEM)
3.8. Cell Culture
3.9. Cytotoxicity Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Saiz, P.; Soler, C.; Lagaron, J.M.; Ocio, M.J. Effects of chitosan films on the growth of Listeria monocytogenes, Staphylococcus aureus and Salmonella spp. in laboratory media and in fish soup. Int. J. Food Microbiol. 2010, 137, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Tan, T.K.; Wong, S.M.; Khor, E. The chitosan yield of Zygomycetes at their optimum harvesting time. Carbohydr. Polym. 1996, 30, 239–242. [Google Scholar] [CrossRef]
- Quin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 14, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Ali, M.A.; Gong, S.; Sun, Q.; Bi, X.; Liu, S.; Guo, L. Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control. 2018, 94, 289–294. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan composites in packaging industry-current trends and future challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial activity of chitosan nanoparticles: A review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Gossen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure-activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the microbial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farm. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984–109996. [Google Scholar] [CrossRef]
- Wrońska, N.; Anouar, A.; El Achaby, M.; Zawadzka, K.; Kędzierska, M.; Miłowska, K.; Katir, N.; Draoui, K.; Różalska, S.; Piwoński, I.; et al. Chitosan-functionalized graphene nanocomposites films: Interfacial interplay and biological activity. Materials 2020, 13, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammi, N.; Wrońska, N.; Katir, N.; Lisowska, K.; Marcotte, N.; Cacciaguerra, T.; Bryszewska, M.; El Kadib, A. Supramolecular chemistry-driven preparation of nanostructured transformable, and biologically active chitosan-clustered single, binary, and ternary metal oxide bioplastics. ACS Appl. Bio Mater. 2018, 2, 61–69. [Google Scholar] [CrossRef]
- Odedina, G.F.; Vongkamjan, K.; Voravuthikunchai, S.P. Potential bio-control agent from Rhodomyrtus tomentosa against Listeria monocytogenes. Nutrients 2015, 7, 7451–7468. [Google Scholar] [CrossRef] [Green Version]
- Linke, K.; Rückerl, I.; Brugger, K.; Karpiskova, R.; Walland, J.; Muri-Klinger, S.; Tichy, A.; Wagner, M.; Stress, B. Reservoirs of Listeria species in three environmental ecosystems. Appl. Environ. Microbiol. 2014, 80, 5583–5592. [Google Scholar] [CrossRef] [Green Version]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet. Res. 2020, 87, 1869–1889. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; Van Deun, K.; Martel, A.; Van Immerseel, F.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011, 42, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Waldenstrom, J.; Axelsson-Olsson, D.; Olsen, B.; Hasselquist, D.; Griekspoor, P.; Jansson, L.; Teneberg, S.; Svensson, L.; Ellstrom, P. Campylobacter jejuni colonization in wild birds: Results from an infection experiment. PLoS ONE 2010, 5, e9082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudra, L.L.; Sebranek, J.G.; Dickson, J.S.; Mendonca, A.; Zhang, Q.; Jackson, A.L.; Prusa, K.J. Control of Campylobacter jejuni in chicken breast meat by irradiation combined with modified atmosphere packaging including carbon monoxide. J. Food Prot. 2012, 75, 1728–1733. [Google Scholar] [CrossRef]
- Thames, H.T.; Sukumaran, A.T. A review of Salmonella and Campylobacter in broiler meat: Emerging challenges and food safety measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef]
- Gonzales-Fandos, E.; Martinez-Laorden, A.; Perez-Arnedo, I. Effect of decontamination treatments on Campylobacter jejuni in chicken. Foods 2020, 9, 1453. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.T. Anatase TiO2 nanocomposites for antimicrobial coatings. J. Phys. Chem. 2005, 109, 8889–8898. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O.; Komatsu, M.; Sawai, J.; Nakagawa, Z.E. Effect of lattice constant of zinc oxide on antibacterial characteristics. J. Mater. Sci. Mater. Med. 2004, 15, 847–851. [Google Scholar] [CrossRef]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-based sensors for human health monitoring. Front. Chem. 2019, 7, 399–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.; Tareen, A.K.; Aslam, M.; Wang, R.; Zhang, Y.; Mahmood, A.; Ouyang, Z.; Zhang, H.; Guo, Z. Recent developments in emerging two-dimensional materials and their applications. J. Mater. Chem. C 2020, 8, 387–440. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of titanium dioxide (TiO2) modification on its application to pollution treatment—A review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, h.; Yeh, J.; Zink, J.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 10, 2121–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 4, 866–870. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Zerefa, E.; Adimasu, Y. PVA assisted ZnO based mesoporous ternary metal oxides nanomaterials: Synthesis, optimization, and evaluation of antibacterial activity. Mater. Res. Express 2020, 7, 045011. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Madeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess. Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids—A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- El Kadib, A.; Molvinger, K.; Bousmina, M.; Brunel, D. Decoration of chitosan microspheres with inorganic oxide clusters: Rational design of hierarchically porous, stable and cooperative acid-base nanoreactors. J. Catal. 2010, 273, 147–155. [Google Scholar] [CrossRef]
- El Kadib, A.; Molvinger, K.; Bousmina, M.; Brunel, D. Improving catalytic activity by synergic effect between base and acid pairs in hierarchically porous chitosan and titania nanoreactors. Org. Lett. 2010, 12, 948–951. [Google Scholar] [CrossRef]
- Fontecha-Umana, F.; Rios-Castillo, A.G.; Ripolles-Avila, C.; Rodriguez-Jerez, J.J. Antimicrobial activity and prevention of bacterial biofilm formation of silver and zinc oxide nanoparticle-containing polyester surfaces at various concentrations for use. Foods 2020, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- De Kwaadstenied, M.; Botes, M.; Cloete, T.E. Application of nanotechnology in antimicrobial coatings in the water industry. Nano 2011, 5, 395–407. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.Z.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Lofti, M.; Tajik, H.; Moradi, M.; Forough, M.; Divsalar, E.; Kuswandi, B. Nanostructured chitosan/ monolaurin film: Preparation, characterization and antimicrobial activity against Listeria monocytogenes on ultrafiltered white cheese. LWT 2018, 92, 576–583. [Google Scholar] [CrossRef]
- Jovanović, G.D.; Klaus, A.S.; Nikšić, M.P. Antimicrobial activity of chitosan coatings and films against Listeria monocytogenes on black radish. Rev. Argent. Microbiol. 2016, 48, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 7, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Ganan, M.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antimicrobial activity of chitosan against Campylobacter spp. and other microorganisms and its mechanism of action. J. Food Prot. 2009, 72, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Hamal, D.B.; Haggstrom, J.A.; Marchin, G.L.; Ikenberry, M.A.; Hohn, K.; Klabunde, K.J. A multifunctional biocide/sporocide and photocatalyst based on titanium dioxide (TiO2) codoped with silver, carbon, and sulfur. Langmuir 2010, 26, 2805–2810. [Google Scholar] [CrossRef]
- Cheng, C.L.; Sun, D.S.; Chu, W.C.; Tseng, Y.H.; Ho, H.C.; Wang, J.B.; Chung, P.H.; Chen, J.H.; Tsai, P.J.; Lin, N.T.; et al. The effects of the bacterial interaction with visible-light responsive titania photocatalyst on the bactericidal performance. J. Biomed. Sci. 2009, 16, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Medina-Ramirez, I.; Mernaugh, R.; Liu, J. Nanocharacterization and bactericidal performance of silver modified titania photocatalyst. Colloids Surf. B Biointerfaces 2010, 77, 82–89. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir 2007, 23, 4982–4987. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Stelle, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Davaran, S.; Younes, G. Impacts of iron oxide nanoparticles on the invasion power of Listeria monocytogenes. Curr. Nanosci. 2014, 10, 382–388. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813–14825. [Google Scholar] [CrossRef] [Green Version]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014, 79, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Akhavan, O.; Simchi, A. Flexible bactericidal graphene oxide-chitosan layers for stem cell proliferation. Appl. Surf. Sci. 2014, 301, 456–462. [Google Scholar] [CrossRef]
- Konwar, A.; Kalita, S.; Kotoky, J.; Chowdhury, D. Chitosan–iron oxide coated graphene oxide nanocomposite hydrogel: A robust and soft antimicrobial biofilm. ACS Appl. Mat. Int. 2016, 8, 20625–20634. [Google Scholar] [CrossRef]
- Gajjar, P.; Pettee, B.; Britt, D.W.; Huang, W.; Johnson, W.P.; Anderson, A.J. Antimicrobial activities of commercial nanoparticles against an environmental soil microbe, Pseudomonas putida KT2440. J. Biol. Eng. 2009, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A. A Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.M.; Dringen, R.; Robinson, S.R. Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radic. Biol. Med. 2007, 42, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.; Prucek, R.; Panacek, A.; Avci-Adali, M.; Nolte, A.; Straub, A.; Zboril, R.; Wendel, H.P.; Kvitek, L. Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified chandler-loop in vitro assay on human blood. Acta Biomater. 2013, 9, 7460–7468. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Arasu, M.V.; Vincent, S.; Prakash, N.U.; Choi, S.H.; Oh, Y.-K.; Choi, K.C.; Kim, K.H. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: An in vitro study. Int. J. Nanomed. 2014, 9, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Yang, W.X. Nanoparticles and spermatogenesis: How do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier. Nanomedicine 2012, 7, 579–596. [Google Scholar] [CrossRef]
- Guo, D.; Wu, C.; Jiang, H.; Li, Q.; Wang, X.; Chen, B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J. Photochem. Photobiol. B Biol. 2008, 93, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J. Chitosan-based zinc oxide nanoparticle for enhanced anticancer effect in cervical cancer: A physicochemical and biological perspective. Saudi. Pharm. J. 2018, 26, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.R.; Martins, F.; Costa, M.E.V.; Senos, A.M.R.; Silva, O.A.B.D.C.E.; Pereira, M.L.; Rebelo, S. In vitro cytotoxicity effects of zinc oxide nanoparticles on spermatogonia cells. Cells 2020, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paunovic, J.; Vucevic, D.; Radosavljevic, T.; Mandić-Rajčević, S.; Pantic, I. Iron-based nanoparticles and their potential toxicity: Focus on oxidative stress and apoptosis. Chem. Biol. Interact. 2020, 316, 108935–108939. [Google Scholar] [CrossRef]
- Browning, C.L.; The, T.; Mason, M.D.; Wise, J.P., Sr. Titanium dioxide nanoparticles are not cytotoxic or clastogenic in human skin cells. J. Environ. Anal. Toxicol. 2014, 4, 239–254. [Google Scholar] [CrossRef]
- Kiss, B.; Bíró, T.; Czifra, G.; Tóth, B.I.; Kertész, Z.; Szikszai, Z.; Kiss, A.Z.; Juhász, I.; Zouboulis, C.C.; Hunyadi, J. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cell. Exp. Derm. 2008, 17, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, P.; Wang, C.; Ma, L.; Su, M. Reducing X-ray induced oxidative damages in fibroblasts with graphene oxide. Nanomaterials 2014, 4, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.H.; Ye, M.K.; Kim, H.S.; Kang, H.S. The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int. Immunopharmacol. 2007, 7, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, Y.K.; Jung, M.; Kim, K.H.; Chung, N.A.; Eun, K.L.; Young, L.; Lee, K.H. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal. Toxicol. 2008, 19, 59–65. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Anouar, A.; Katir, N.; Mamede, A.S.; Aboulaich, A.; Draoui, K.; Royer, S.; El Kadib, A. Synthesis and multifaceted use of phosphorylated graphene oxide: Growth of titanium dioxide clusters, interplay with gold nanoparticles and exfoliated sheets in bioplastics. Mater. Chem. Front. 2018, 3, 242–250. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]








| Sample Code | Metal Precursors | Molar Ratio NH2:Metal Precursor |
|---|---|---|
| CS | ||
| CS-ZnO 1:1 | Zinc acetate | 1:1 |
| CS-ZnO 2:1 | Zinc acetate | 2:1 |
| CS-ZnO 5:1 | Zinc acetate | 5:1 |
| CS-ZnO 10:1 | Zinc acetate | 10:1 |
| CS-ZnO 20:1 a | Zinc acetate | 20:1 |
| CS-ZnO 20:1 b | Zinc chloride | 20:1 |
| CS-TiO2 1:1 a | Titanium diisopropoxide bis(acac) | 1:1 |
| CS-TiO2 1:1 b | Titanium isopropoxide | 1:1 |
| CS-TiO2 2:1 | Titanium diisopropoxide bis(acac) | 2:1 |
| CS-TiO2 20:1a | Titanium diisopropoxide bis(acac) | 20:1 |
| CS-TiO2 20:1 b | Titanium isopropoxide | 20:1 |
| CS-Fe2O3 5:1 | Iron(III) acetylacetonate | 5:1 |
| CS-Fe2O3 10:1 | Iron(III) acetylacetonate | 10:1 |
| CS-Fe2O3 20:1 | Iron(III) acetylacetonate | 20:1 |
| Sample Code | Functionalized Graphene Fillers | Metal Precursors |
|---|---|---|
| CS-GO | GO (3 wt%) | - |
| CS-GOP | GO (3 wt%) | - |
| CS-GOP-HMDS | GOP-HMDS (3 wt%) | - |
| CS-rGO | rGO (3 wt%) | - |
| CS-GO-HMDS | GO-HMDS (3 wt%) | - |
| CS-rGOP | rGOP (3 wt%) | - |
| CS-GO-Ag | GO(3 wt%) | Silver nitrate (3%) |
| CS-GOP-Ag | GOP (3%) | Silver nitrate (3%) |
| CS-Ag | - | Silver nitrate (3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrońska, N.; Katir, N.; Miłowska, K.; Hammi, N.; Nowak, M.; Kędzierska, M.; Anouar, A.; Zawadzka, K.; Bryszewska, M.; El Kadib, A.; et al. Antimicrobial Effect of Chitosan Films on Food Spoilage Bacteria. Int. J. Mol. Sci. 2021, 22, 5839. https://doi.org/10.3390/ijms22115839
Wrońska N, Katir N, Miłowska K, Hammi N, Nowak M, Kędzierska M, Anouar A, Zawadzka K, Bryszewska M, El Kadib A, et al. Antimicrobial Effect of Chitosan Films on Food Spoilage Bacteria. International Journal of Molecular Sciences. 2021; 22(11):5839. https://doi.org/10.3390/ijms22115839
Chicago/Turabian StyleWrońska, Natalia, Nadia Katir, Katarzyna Miłowska, Nisrine Hammi, Marta Nowak, Marta Kędzierska, Aicha Anouar, Katarzyna Zawadzka, Maria Bryszewska, Abdelkrim El Kadib, and et al. 2021. "Antimicrobial Effect of Chitosan Films on Food Spoilage Bacteria" International Journal of Molecular Sciences 22, no. 11: 5839. https://doi.org/10.3390/ijms22115839
APA StyleWrońska, N., Katir, N., Miłowska, K., Hammi, N., Nowak, M., Kędzierska, M., Anouar, A., Zawadzka, K., Bryszewska, M., El Kadib, A., & Lisowska, K. (2021). Antimicrobial Effect of Chitosan Films on Food Spoilage Bacteria. International Journal of Molecular Sciences, 22(11), 5839. https://doi.org/10.3390/ijms22115839






