Proteins Marking the Sequence of Genotoxic Signaling from Irradiated Mesenchymal Stromal Cells to CD34+ Cells
Abstract
1. Introduction
2. Results
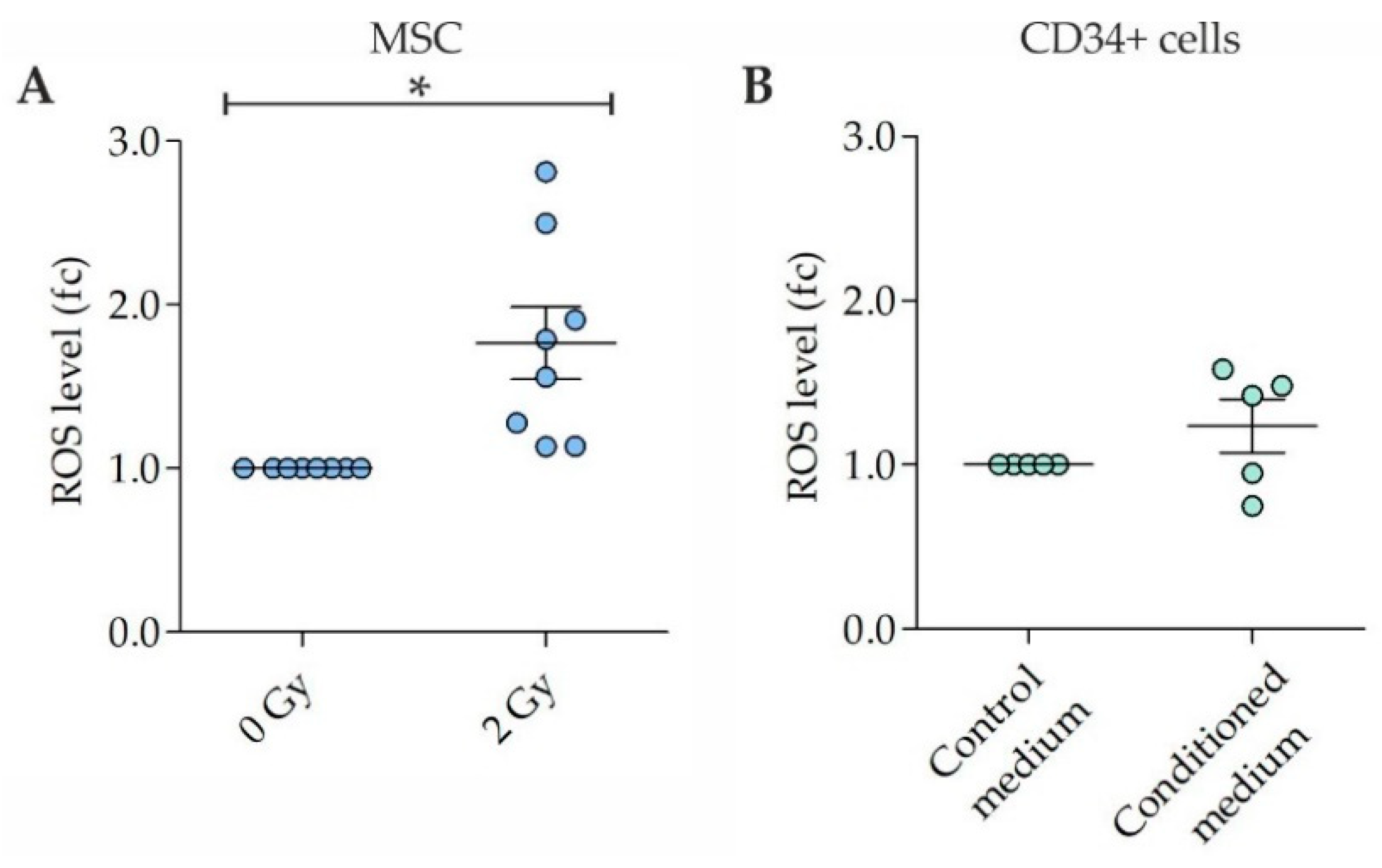
2.1. ROS in MSC and CD34+ Cells
2.2. DNA Damage in CD34+ Cells
2.3. Chromosomal Instability in CD34+ Cells
2.4. Viability of CD34+ Cells
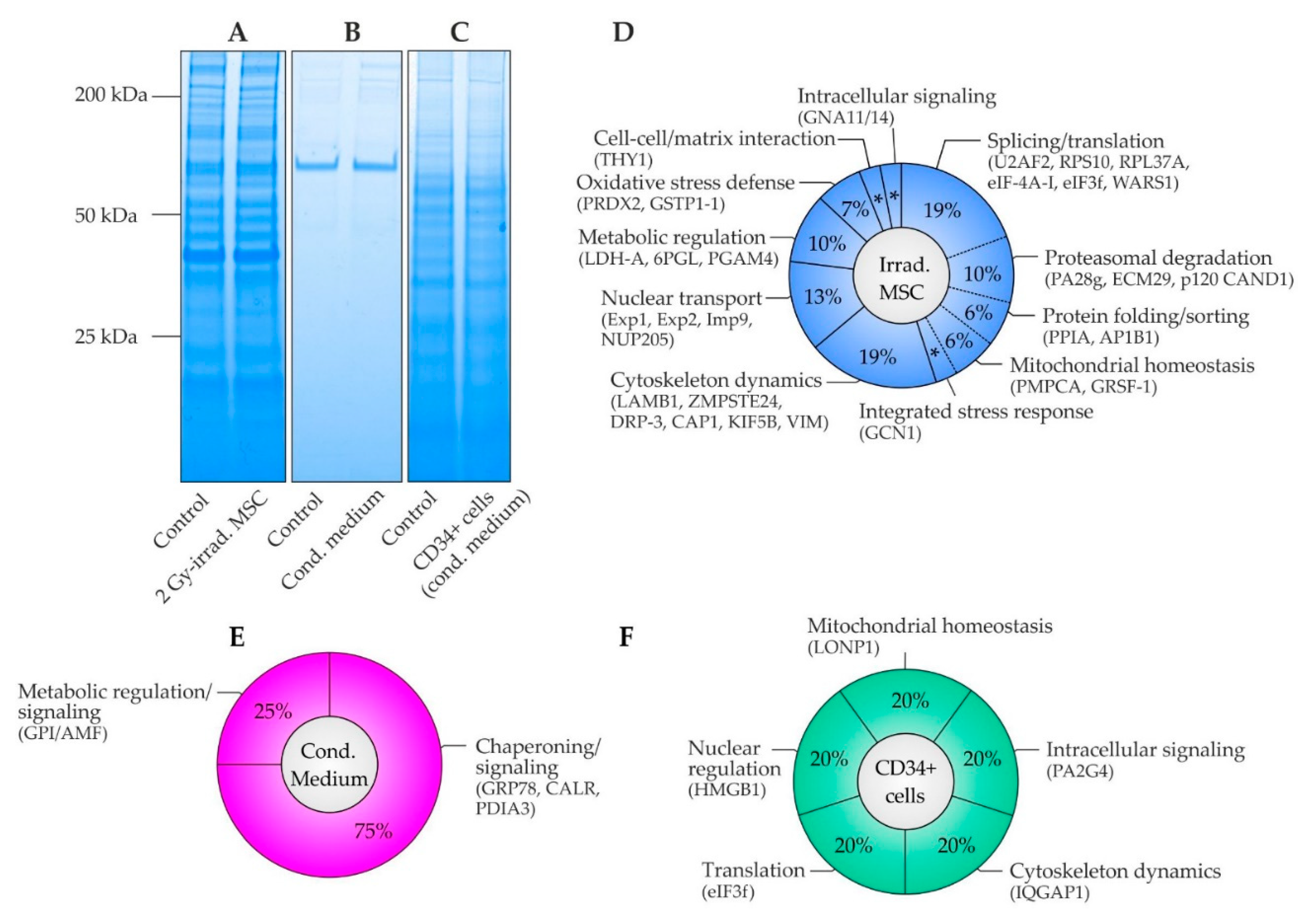
2.5. Proteome Analysis in MSC, MSC Conditioned Medium and CD34+ Cells
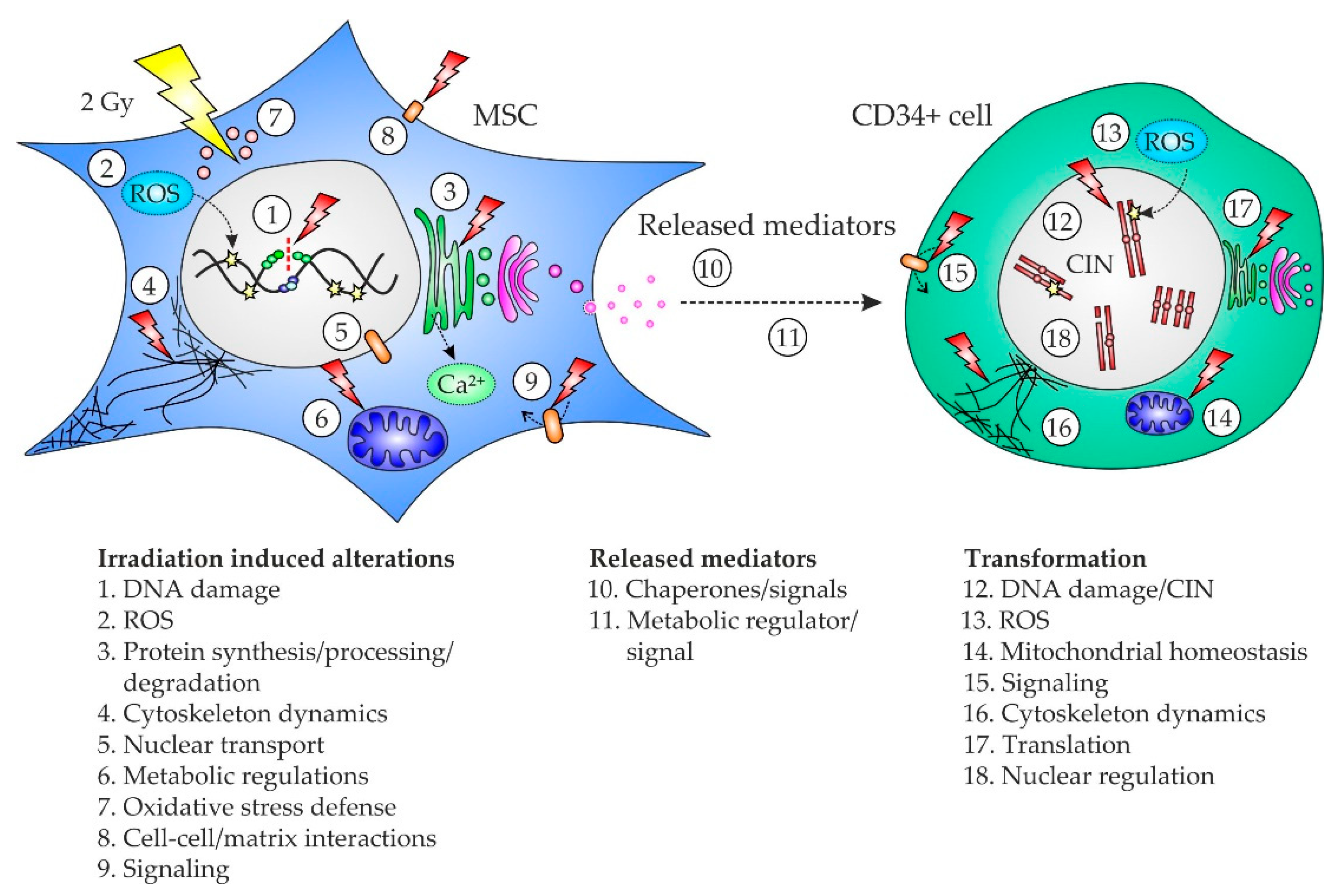
3. Discussion
4. Materials and Methods
4.1. Femoral Head Preparation
4.2. Preparation of MSC Conditioned Medium
4.3. NTE Analyses
4.4. Protein Quantitation Using Mass Spectrometry
4.5. Sample Preparation for Proteome Analysis
4.6. Sample Fractionation by SDS-PAGE and In-Gel Digestion
4.7. Mass Spectrometry
4.8. Comparative Proteome Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef]
- Nikitaki, Z.; Mavragani, I.V.; Laskaratou, D.A.; Gika, V.; Moskvin, V.P.; Theofilatos, K.; Vougas, K.; Stewart, R.D.; Georgakilas, A.G. Systemic mechanisms and effects of ionizing radiation: A new ‘old’ paradigm of how the bystanders and distant can become the players. Semin. Cancer Biol. 2016, 37, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Rusin, A.; Seymour, C. Relevance of Non-Targeted Effects for Radiotherapy and Diagnostic Radiology; A Historical and Conceptual Analysis of Key Players. Cancers 2019, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Prise, K.M.; O’Sullivan, J.M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 2009, 9, 351–360. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C.B. Radiation-induced bystander effects--implications for cancer. Nat. Rev. Cancer 2004, 4, 158–164. [Google Scholar]
- Lorimore, S.A.; McIlrath, J.M.; Coates, P.J.; Wright, E.G. Chromosomal instability in unirradiated hemopoietic cells resulting from a delayed in vivo bystander effect of gamma radiation. Cancer Res. 2005, 65, 5668–5673. [Google Scholar] [CrossRef] [PubMed]
- Lorimore, S.A.; Chrystal, J.A.; Robinson, J.I.; Coates, P.J.; Wright, E.G. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 2008, 68, 8122–8126. [Google Scholar] [CrossRef]
- Watson, G.E.; Lorimore, S.A.; Macdonald, D.A.; Wright, E.G. Chromosomal instability in unirradiated cells induced in vivo by a bystander effect of ionizing radiation. Cancer Res. 2000, 60, 5608–5611. [Google Scholar] [PubMed]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar] [PubMed]
- Zhou, H.; Randers-Pehrson, G.; Waldren, C.A.; Vannais, D.; Hall, E.J.; Hei, T.K. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc. Natl. Acad. Sci. USA 2000, 97, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; de Toledo, S.M.; Raaphorst, G.P.; Mitchel, R.E. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat. Res. 1996, 146, 369–373. [Google Scholar] [CrossRef]
- Sokolov, M.V.; Neumann, R.D. Radiation-induced bystander effects in cultured human stem cells. PLoS ONE 2010, 5, e14195. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Han, W.; Zhao, G.; Zhu, L.; Wang, J.; Bao, L.; Jiang, E.; Xu, A.; Hei, T.K.; et al. Mitochondria-dependent signalling pathway are involved in the early process of radiation-induced bystander effects. Br. J. Cancer 2008, 98, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Tartier, L.; Gilchrist, S.; Burdak-Rothkamm, S.; Folkard, M.; Prise, K.M. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007, 67, 5872–5879. [Google Scholar] [CrossRef]
- Shao, C.; Stewart, V.; Folkard, M.; Michael, B.D.; Prise, K.M. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003, 63, 8437–8442. [Google Scholar] [PubMed]
- Li, J.; He, M.; Shen, B.; Yuan, D.; Shao, C. Alpha particle-induced bystander effect is mediated by ROS via a p53-dependent SCO2 pathway in hepatoma cells. Int. J. Radiat. Biol. 2013, 89, 1028–1034. [Google Scholar] [CrossRef]
- Desai, S.; Kumar, A.; Laskar, S.; Pandey, B.N. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine 2013, 61, 54–62. [Google Scholar] [CrossRef]
- Shao, C.; Folkard, M.; Prise, K.M. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene 2008, 27, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Gow, M.D.; Seymour, C.B.; Ryan, L.A.; Mothersill, C.E. Induction of bystander response in human glioma cells using high-energy electrons: A role for TGF-beta1. Radiat. Res. 2010, 173, 769–778. [Google Scholar] [CrossRef]
- Shao, C.; Furusawa, Y.; Aoki, M.; Ando, K. Role of gap junctional intercellular communication in radiation-induced bystander effects in human fibroblasts. Radiat. Res. 2003, 160, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci. USA 2001, 98, 473–478. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, M.; Zheng, L.; Liang, Q.; Li, H.; Chen, J.T.; Guo, H.; Yoshina, S.; Chen, Y.Z.; Zhao, X.; et al. Cysteine protease cathepsin B mediates radiation-induced bystander effects. Nature 2017, 547, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Ding, N.; Hu, W.; Zhang, X.; Wang, B.; Hua, J.; Wei, W.; Zhu, Q. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015, 12, 1355–1363. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Miura, T.; Kasai, K.; Fujishima, Y.; Nakata, A.; Yoshida, M. Radiation-Induced Bystander Effect is Mediated by Mitochondrial DNA in Exosome-Like Vesicles. Sci. Rep. 2019, 9, 9103. [Google Scholar] [CrossRef]
- Kirolikar, S.; Prasannan, P.; Raghuram, G.V.; Pancholi, N.; Saha, T.; Tidke, P.; Chaudhari, P.; Shaikh, A.; Rane, B.; Pandey, R.; et al. Prevention of radiation-induced bystander effects by agents that inactivate cell-free chromatin released from irradiated dying cells. Cell Death Dis. 2018, 9, 1142. [Google Scholar] [CrossRef]
- Jella, K.K.; Moriarty, R.; McClean, B.; Byrne, H.J.; Lyng, F.M. Reactive oxygen species and nitric oxide signaling in bystander cells. PLoS ONE 2018, 13, e0195371. [Google Scholar] [CrossRef]
- Lyng, F.M.; Howe, O.L.; McClean, B. Reactive oxygen species-induced release of signalling factors in irradiated cells triggers membrane signalling and calcium influx in bystander cells. Int. J. Radiat. Biol. 2011, 87, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ivanov, V.N.; Lien, Y.C.; Davidson, M.; Hei, T.K. Mitochondrial function and nuclear factor-kappaB-mediated signaling in radiation-induced bystander effects. Cancer Res. 2008, 68, 2233–2240. [Google Scholar] [CrossRef]
- Lyng, F.M.; Maguire, P.; McClean, B.; Seymour, C.; Mothersill, C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat. Res. 2006, 165, 400–409. [Google Scholar] [CrossRef]
- Sim, E.U.; Ang, C.H.; Ng, C.C.; Lee, C.W.; Narayanan, K. Differential expression of a subset of ribosomal protein genes in cell lines derived from human nasopharyngeal epithelium. J. Hum. Genet. 2010, 55, 118–120. [Google Scholar] [CrossRef][Green Version]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. gammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Arber, D.A.; Brunning, R.D.; Larson, R.A.; Matutes, E.; Baumann, I.; Kvasnicka, H.M. Therapy-Related Myeloid Neoplasms, 4th ed.; IARC: Lyon, France, 2017; pp. 153–155. [Google Scholar]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-related myeloid neoplasms: When genetics and environment collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Goto, H.; Nishimura, Y.; Kasahara, K.; Mizoguchi, A.; Inagaki, M. Tetraploidy in cancer and its possible link to aging. Cancer Sci. 2018, 109, 2632–2640. [Google Scholar] [CrossRef]
- Huang, L.; Wang, S.A.; DiNardo, C.; Li, S.; Hu, S.; Xu, J.; Zhou, W.; Goswami, M.; Medeiros, L.J.; Tang, G. Tetraploidy/near-tetraploidy acute myeloid leukemia. Leuk. Res. 2017, 53, 20–27. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012, 287, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kloss, F.R.; Brunauer, R.; Schimke, M.; Jamnig, A.; Greiderer-Kleinlercher, B.; Klima, G.; Rentenberger, J.; Auberger, T.; Hachl, O.; et al. Mesenchymal stem cells show radioresistance in vivo. J. Cell Mol. Med. 2012, 16, 877–887. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Meusser, B.; Hirsch, C.; Jarosch, E.; Sommer, T. ERAD: The long road to destruction. Nat. Cell Biol. 2005, 7, 766–772. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, Y.; Lee, A.S. Beyond the endoplasmic reticulum: Atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem. J. 2011, 434, 181–188. [Google Scholar] [CrossRef]
- Ge, R.; Kao, C. Cell Surface GRP78 as a Death Receptor and an Anticancer Drug Target. Cancers 2019, 11, 1787. [Google Scholar] [CrossRef]
- Vig, S.; Buitinga, M.; Rondas, D.; Crevecoeur, I.; van Zandvoort, M.; Waelkens, E.; Eizirik, D.L.; Gysemans, C.; Baatsen, P.; Mathieu, C.; et al. Cytokine-induced translocation of GRP78 to the plasma membrane triggers a pro-apoptotic feedback loop in pancreatic beta cells. Cell Death Dis. 2019, 10, 309. [Google Scholar] [CrossRef]
- Kern, J.; Untergasser, G.; Zenzmaier, C.; Sarg, B.; Gastl, G.; Gunsilius, E.; Steurer, M. GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood 2009, 114, 3960–3967. [Google Scholar] [CrossRef]
- Wey, S.; Luo, B.; Tseng, C.C.; Ni, M.; Zhou, H.; Fu, Y.; Bhojwani, D.; Carroll, W.L.; Lee, A.S. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood 2012, 119, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Miharada, K.; Karlsson, G.; Rehn, M.; Rorby, E.; Siva, K.; Cammenga, J.; Karlsson, S. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell 2011, 9, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and cancer. Cell Res. 2020, 31, 5–16. [Google Scholar] [CrossRef]
- Levine, R.L.; Pardanani, A.; Tefferi, A.; Gilliland, D.G. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat. Rev. Cancer 2007, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Bourdi, M.; Demady, D.; Martin, J.L.; Jabbour, S.K.; Martin, B.M.; George, J.W.; Pohl, L.R. cDNA cloning and baculovirus expression of the human liver endoplasmic reticulum P58: Characterization as a protein disulfide isomerase isoform, but not as a protease or a carnitine acyltransferase. Arch. Biochem. Biophys. 1995, 323, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; van der Wal, F.J.; Bulleid, N.J.; High, S. Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science 1997, 275, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Coe, H.; Jung, J.; Groenendyk, J.; Prins, D.; Michalak, M. ERp57 modulates STAT3 signaling from the lumen of the endoplasmic reticulum. J. Biol. Chem. 2010, 285, 6725–6738. [Google Scholar] [CrossRef]
- Lee, E.; Lee, D.H. Emerging roles of protein disulfide isomerase in cancer. BMB Rep. 2017, 50, 401–410. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Gupta, S.K.; Hogan, V.; Collard, J.G.; Raz, A. Activation of small GTPase Rho is required for autocrine motility factor signaling. Cancer Res. 2002, 62, 4484–4490. [Google Scholar] [PubMed]
- Araki, K.; Shimura, T.; Yajima, T.; Tsutsumi, S.; Suzuki, H.; Okada, K.; Kobayashi, T.; Raz, A.; Kuwano, H. Phosphoglucose isomerase/autocrine motility factor promotes melanoma cell migration through ERK activation dependent on autocrine production of interleukin-8. J. Biol. Chem. 2009, 284, 32305–32311. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Hogan, V.; Nabi, I.R.; Raz, A. Overexpression of the autocrine motility factor/phosphoglucose isomerase induces transformation and survival of NIH-3T3 fibroblasts. Cancer Res. 2003, 63, 242–249. [Google Scholar] [PubMed]
- Gibellini, L.; Losi, L.; De Biasi, S.; Nasi, M.; Lo Tartaro, D.; Pecorini, S.; Patergnani, S.; Pinton, P.; De Gaetano, A.; Carnevale, G.; et al. LonP1 Differently Modulates Mitochondrial Function and Bioenergetics of Primary Versus Metastatic Colon Cancer Cells. Front. Oncol. 2018, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.H.; Venkatesh, S.; Li, M.; Lee, J.; Lu, B.; Hilchey, S.P.; Morse, K.M.; Metcalfe, H.M.; Skalska, J.; Andreeff, M.; et al. The mitochondrial ATP-dependent Lon protease: A novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood 2012, 119, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Bianchi, F.; Ferguson, M.; Cesario, A.; Margaritora, S.; Granone, P.; Goldstraw, P.; Tetlow, M.; Ratcliffe, C.; Nicholson, A.G.; et al. Gene expression signature for angiogenic and nonangiogenic non-small-cell lung cancer. Oncogene 2005, 24, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Gala, K.; Chandarlapaty, S. Molecular pathways: HER3 targeted therapy. Clin. Cancer Res. 2014, 20, 1410–1416. [Google Scholar] [CrossRef]
- Nguyen le, X.T.; Zhu, L.; Lee, Y.; Ta, L.; Mitchell, B.S. Expression and Role of the ErbB3-Binding Protein 1 in Acute Myelogenous Leukemic Cells. Clin. Cancer Res. 2016, 22, 3320–3327. [Google Scholar] [CrossRef]
- Johnson, M.; Sharma, M.; Henderson, B.R. IQGAP1 regulation and roles in cancer. Cell Signal 2009, 21, 1471–1478. [Google Scholar] [CrossRef]
- Doldan, A.; Chandramouli, A.; Shanas, R.; Bhattacharyya, A.; Leong, S.P.; Nelson, M.A.; Shi, J. Loss of the eukaryotic initiation factor 3f in melanoma. Mol. Carcinog. 2008, 47, 806–813. [Google Scholar] [CrossRef]
- Doldan, A.; Chandramouli, A.; Shanas, R.; Bhattacharyya, A.; Cunningham, J.T.; Nelson, M.A.; Shi, J. Loss of the eukaryotic initiation factor 3f in pancreatic cancer. Mol. Carcinog. 2008, 47, 235–244. [Google Scholar] [CrossRef]
- Rapoport, B.L.; Steel, H.C.; Theron, A.J.; Heyman, L.; Smit, T.; Ramdas, Y.; Anderson, R. High Mobility Group Box 1 in Human Cancer. Cells 2020, 9, 1664. [Google Scholar] [CrossRef]
- Lange, S.S.; Mitchell, D.L.; Vasquez, K.M. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc. Natl. Acad. Sci. USA 2008, 105, 10320–10325. [Google Scholar] [CrossRef]
- Martin, O.A.; Yin, X.; Forrester, H.B.; Sprung, C.N.; Martin, R.F. Potential strategies to ameliorate risk of radiotherapy-induced second malignant neoplasms. Semin. Cancer Biol. 2016, 37, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Gao, W.; Zhou, Y.; Wey, S.; Mitra, S.K.; Krasnoperov, V.; Dong, D.; Liu, S.; Li, D.; et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin. Cancer Res. 2013, 19, 6802–6811. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Chandna, R.; Ghode, A.; Dsouza, C.; Chen, M.; Larsson, A.; Lim, S.H.; Wang, M.; Cao, Z.; Zhu, Y.; et al. Proapoptotic Cyclic Peptide BC71 Targets Cell-Surface GRP78 and Functions as an Anticancer Therapeutic in Mice. EBioMedicine 2018, 33, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- de Wynter, E.A.; Coutinho, L.H.; Pei, X.; Marsh, J.C.; Hows, J.; Luft, T.; Testa, N.G. Comparison of purity and enrichment of CD34+ cells from bone marrow, umbilical cord and peripheral blood (primed for apheresis) using five separation systems. Stem Cells 1995, 13, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Popp, H.D.; Naumann, N.; Brendel, S.; Henzler, T.; Weiss, C.; Hofmann, W.K.; Fabarius, A. Increase of DNA damage and alteration of the DNA damage response in myelodysplastic syndromes and acute myeloid leukemias. Leuk. Res. 2017, 57, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Popp, H.D.; Brendel, S.; Hofmann, W.K.; Fabarius, A. Immunofluorescence Microscopy of γH2AX and 53BP1 for Analyzing the Formation and Repair of DNA Double-strand Breaks. J. Vis. Exp. 2017, 129, 56617. [Google Scholar]
- Gisselsson, D. Cancer Cytogenetics, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 9–16. [Google Scholar]
- McGowan-Jordan, J.; Simons, A.; Schmid, M. ISCN 2016 An International System for Human Cytogenetic Nomenclature (2016); Karger: Basel, Switzerland, 2016. [Google Scholar]

| Pt | Age/Sex | ROS Level (fc) Irrad. MSC | ROS Level (fc) CD34+ Cells Cond. Medium | γH2AX Foci (fc) per CD34+ Cell Cond. Medium | Cytogenetics (ISCN) CD34+ Cells Control Cond. Medium | Viability (fc) CD34+ Cells Cond. Medium | |
|---|---|---|---|---|---|---|---|
| #1 | 90/♂ | NA | NA | 13.0 | 46,XY | 46,XY[20] 46,XY,chtb(5q)[1] 46,XY,chtb(10q)[1] 92,XXYY[2] 184,XXXXYYYY[1] | 1.0 |
| #2 | 56/♂ | NA | NA | 1.9 | 46,XY | 46,XY[22] 92,XXYY[2] 184,XXXXYYYY[1] | 1.0 |
| #3 | 92/♀ | NA | 1.4 | 10.5 | 46,XX | 46,XX[18] 46,XX,chtb(13q)[1] 92,XXXX[2] 184,XXXXXXXX[3] 184,XXXXXXXX, chtb(11q)[1] | 0.8 |
| #4 | 58/♀ | NA | NA | 6.9 | 46,XX | 46,XX[19] 92,XXXX[3] 92,XXXX,chtb(6p)[1] 184,XXXXXXXX[2] | 0.9 |
| #5 | 85/♀ | 1.1 | 1.5 | 10.2 | 46,XX | 46,XX[24] 92,XXXX,chtb(7q)[1] | 1.1 |
| #6 | 67/♀ | 1.6 | NA | 2.1 | 46,XX | 46,XX[25] | 1.0 |
| #7 | 77/♂ | 1.9 | NA | 7.1 | 46,XY | 46,XY[21] 47,XYY[2] 92,XXYY[1] 90,XXYY,der(1) t(1;7)x2[1] | NA |
| #8 | 54/♀ | 2.5 | 1.6 | 3.9 | 46,XX | 46,XX[22] 92,XXXX[3] | 1.6 |
| #9 | 65/♂ | 1.3 | NA | NA | 46,XY | 46,XY[19] 45,X,-Y[2] 45,X,-Y,chtb(5q)[1] 92,XXYY[2] 184,XXXXYYYY[1] | 1.1 |
| #10 | 58/♀ | 1.8 | NA | 6.3 | 46,XX | 46,XX[20] 92,XXXX[2] 184,XXXXXXXX[3] | NA |
| #11 | 70/♂ | 1.1 | 1.0 | NA | 46,XY | 46,XY[19] 92,XXYY[5] 184,XXYY[1] | 1.3 |
| #12 | 59/♀ | 2.8 | 0.8 | NA | 46,XX | 46,XX[22] 92,XXXX[3] | 1.1 |
| Gp | Category | Accession No. | Protein | Function | Abundance Ratio | Abundance p Value | Coverage | No. of Unique Peptides | PSMs |
|---|---|---|---|---|---|---|---|---|---|
| Irradiated MSC | Protein synthesis/ processing/ degradation | P46783 | 40S ribosomal protein S10 (RPS10) | 40S ribosomal subunit | 4.3 | <0.0001 | 20 | 2 | 9 |
| O00303 | Eukaryotic translation initiation factor 3 subunit F (eIF3f) | Component of eIF-3 complex | 4.2 | <0.0001 | 13 | 3 | 11 | ||
| Q10713 | Mitochondrial-processing peptidase subunit alpha (PMPCA) | Subunit of essential mitochondrial processing protease | 3.8 | <0.0001 | 6 | 2 | 8 | ||
| Q92616 | eIF-2-alpha kinase activator GCN1 (GCN1) | Complex with EIF2AK4/GCN2 on translating ribosomes | 3.2 | <0.0001 | 6 | 9 | 45 | ||
| P62937 | Peptidyl-prolyl cis-trans isomerase A (PPIA) | Protein folding | 3.1 | 0.0037 | 58 | 9 | 67 | ||
| P61289 | Proteasome activator complex subunit 3 (PA28g) | Proteasome regulator | 2.8 | 0.0101 | 16 | 3 | 3 | ||
| P26368 | Splicing factor U2AF 65 kDa subunit (U2AF2) | pre-mRNA splicing and 3′-end processing | 2.7 | 0.0004 | 20 | 4 | 17 | ||
| Q12849 | G-rich sequence factor 1 (GRSF-1) | Post-transcriptional mitochondrial gene expression | 2.3 | 0.0041 | 11 | 2 | 7 | ||
| Q86VP6 | Cullin-associated NEDD8-dissociated protein 1 (p120 CAND1) | Key assembly factor of SCF E3 ubiquitin ligase complexes | 2.3 | 0.0084 | 9 | 7 | 26 | ||
| P60842 | Eukaryotic initiation factor 4A-(eIF-4A-I) | RNA helicase subunit of eIF4F complex | 2.1 | 0.0114 | 39 | 8 | 63 | ||
| Q5VYK3 | Proteasome adapter and scaffold protein ECM29 (ECM29) | Binds to 26S proteasome | 2.1 | 0.0172 | 1 | 2 | 9 | ||
| P23381 | Tryptophan-tRNA ligase, cytoplasmic (WARS1) | Aminoacylation of tRNA | 2.0 | 0.0156 | 24 | 6 | 15 | ||
| Q10567 | AP-1 complex subunit beta-1 (AP1B1) | Protein sorting in trans-Golgi network and/or endosomes | 2.0 | 0.0278 | 14 | 2 | 57 | ||
| P61513 | 60S ribosomal protein L37a (RPL37A) | 60S ribosomal subunit | 0.39 | 0.0008 | 17 | 2 | 8 | ||
| Cytoskeleton dynamics | P07942 | Laminin subunit beta-1 (LAMB1) | Component of basal membrane | 2.6 | 0.0012 | 5 | 5 | 28 | |
| O75844 | CAAX prenyl protease 1 homolog (ZMPSTE24) | Cleavage of prelamin to lamin A | 2.5 | 0.0027 | 5 | 2 | 4 | ||
| Q14195 | Dihydropyrimidinase-related protein 3 (DRP-3) | Remodeling of cytoskeleton | 2.2 | 0.0054 | 14 | 4 | 17 | ||
| Q01518 | Adenylyl cyclase-associated protein 1 (CAP1) | Regulator of filament dynamics | 2.2 | 0.0070 | 40 | 11 | 66 | ||
| P33176 | Kinesin-1 heavy chain (KIF5B) | Microtubule-dependent motor | 2.1 | 0.0194 | 4 | 2 | 4 | ||
| P08670 | Vimentin (VIM) | Intermediate filaments | 0.29 | <0.0001 | 12 | 4 | 15 | ||
| Nuclear transport | O14980 | Exportin-1 (Exp1) | Nuclear export of proteins and RNA | 5.1 | <0.0001 | 5 | 3 | 11 | |
| Q96P70 | Importin-9 (Imp9) | Nuclear transport receptor | 4.5 | <0.0001 | 4 | 2 | 3 | ||
| Q92621 | Nuclear pore complex protein Nup205 (NUP205) | Component of nuclear pore complex (NPC) | 3.4 | <0.0001 | 4 | 3 | 7 | ||
| P55060 | Exportin-2 (Exp2) | Importin-alpha re-export from nucleus to cytoplasm | 2.5 | 0.0032 | 11 | 6 | 17 | ||
| Metabolic regulation | P00338 | L-lactate dehydrogenase A chain (LDH-A) | Synthesizes (S)-lactate from pyruvate | 3.2 | <0.0001 | 12 | 3 | 6 | |
| O95336 | 6-phosphogluconolactonase (6PGL) | Pentose phosphate pathway | 2.7 | 0.0125 | 11 | 2 | 9 | ||
| Q8N0Y7 | Probable phosphoglycerate mutase 4 (PGAM4) | Glycolysis | 2.6 | 0.0191 | 22 | 4 | 27 | ||
| Oxidative stress defense | P32119 | Peroxiredoxin-2 (PRDX2) | Thiol-specific peroxidase | qualitative | <0.0001 | 23 | 2 | 12 | |
| P09211 | Glutathione S-transferase P (GSTP1-1) | Conjugation of reduced glutathione | 2.4 | 0.0340 | 22 | 3 | 12 | ||
| Cell-cell/matrix interactions | P04216 | Thy-1 membrane glycoprotein (THY1) | Cell-cell and cell-matrix interactions, signaling (cis/trans) | 3.9 | 0.0003 | 12 | 3 | 19 | |
| Signaling | O95837/P29992 | Guanine nucleotide-binding protein subunit alpha-11/14 (GNA11/14) | Activation of PLC-β: IP3 → calcium/PKC | 3.8 | <0.0001 | 6 | 2 | 8 | |
| MSC conditioned medium | Chaperoning/oncogenic signaling | P11021 | Endoplasmic reticulum chaperone BiP (GRP78) | Unfolded protein response (UPR), endoplasmic reticulum protein degradation (ERAD) pathway | 3.5 | 0.0227 | 29 | 13 | 40 |
| P27797 | Calreticulin (CALR) | Calreticulin/calnexin cycle, calcium-binding protein | 2.4 | 0.0036 | 13 | 4 | 19 | ||
| P30101 | Protein disulfide-isomerase A3 (PDIA3) | Rearrangement of -S-S- bonds in proteins | 2.0 | 0.0225 | 21 | 10 | 29 | ||
| Metabolic regulation/oncogenic signaling | P06744 | Glucose-6-phosphate isomerase (GPI)/autocrine motility factor (AMF) | Glycolysis-related enzyme, ligand of AMF receptor | 2.4 | 0.0006 | 9 | 4 | 7 | |
| CD34+ cells | Mitochondrial homeostasis | P36776 | Lon protease homolog, mitochondrial (LONP1) | Degradation of misfolded or damaged polypeptides | 4.1 | <0.0001 | 7 | 2 | 8 |
| Signaling | Q9UQ80 | Proliferation-associated protein 2G4 (PA2G4) | ERBB3 signaling, growth regulation, increased in AML | 2.2 | <0.0001 | 18 | 4 | 4 | |
| Cytoskeleton dynamcis | P46940 | Ras GTPase-activating-like protein IQGAP1 (IQGAP1) | Dynamics and assembly of actin cytoskeleton | 0.48 | <0.0001 | 3 | 2 | 5 | |
| Translation | O00303 | Eukaryotic translation initiation factor 3 subunit F (eIF3f) | Component of eIF-3 complex, decreased in cancers | 0.40 | <0.0001 | 8 | 2 | 12 | |
| Nuclear regulations | P09429 | High mobility group protein B1 (HMGB1) | DNA chaperone, replication, transcription, chromatin remodeling, p38-MAPK/NF-kappa B activation | 0.35 | <0.0001 | 18 | 3 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohl, V.; Drews, O.; Costina, V.; Bierbaum, M.; Jawhar, A.; Roehl, H.; Weiss, C.; Brendel, S.; Kleiner, H.; Flach, J.; et al. Proteins Marking the Sequence of Genotoxic Signaling from Irradiated Mesenchymal Stromal Cells to CD34+ Cells. Int. J. Mol. Sci. 2021, 22, 5844. https://doi.org/10.3390/ijms22115844
Kohl V, Drews O, Costina V, Bierbaum M, Jawhar A, Roehl H, Weiss C, Brendel S, Kleiner H, Flach J, et al. Proteins Marking the Sequence of Genotoxic Signaling from Irradiated Mesenchymal Stromal Cells to CD34+ Cells. International Journal of Molecular Sciences. 2021; 22(11):5844. https://doi.org/10.3390/ijms22115844
Chicago/Turabian StyleKohl, Vanessa, Oliver Drews, Victor Costina, Miriam Bierbaum, Ahmed Jawhar, Henning Roehl, Christel Weiss, Susanne Brendel, Helga Kleiner, Johanna Flach, and et al. 2021. "Proteins Marking the Sequence of Genotoxic Signaling from Irradiated Mesenchymal Stromal Cells to CD34+ Cells" International Journal of Molecular Sciences 22, no. 11: 5844. https://doi.org/10.3390/ijms22115844
APA StyleKohl, V., Drews, O., Costina, V., Bierbaum, M., Jawhar, A., Roehl, H., Weiss, C., Brendel, S., Kleiner, H., Flach, J., Spiess, B., Seifarth, W., Nowak, D., Hofmann, W.-K., Fabarius, A., & Popp, H. D. (2021). Proteins Marking the Sequence of Genotoxic Signaling from Irradiated Mesenchymal Stromal Cells to CD34+ Cells. International Journal of Molecular Sciences, 22(11), 5844. https://doi.org/10.3390/ijms22115844







