Expression Profiles of ASIC1/2 and TRPV1/4 in Common Skin Tumors
Abstract
:1. Introduction
2. Results
3. Discussion
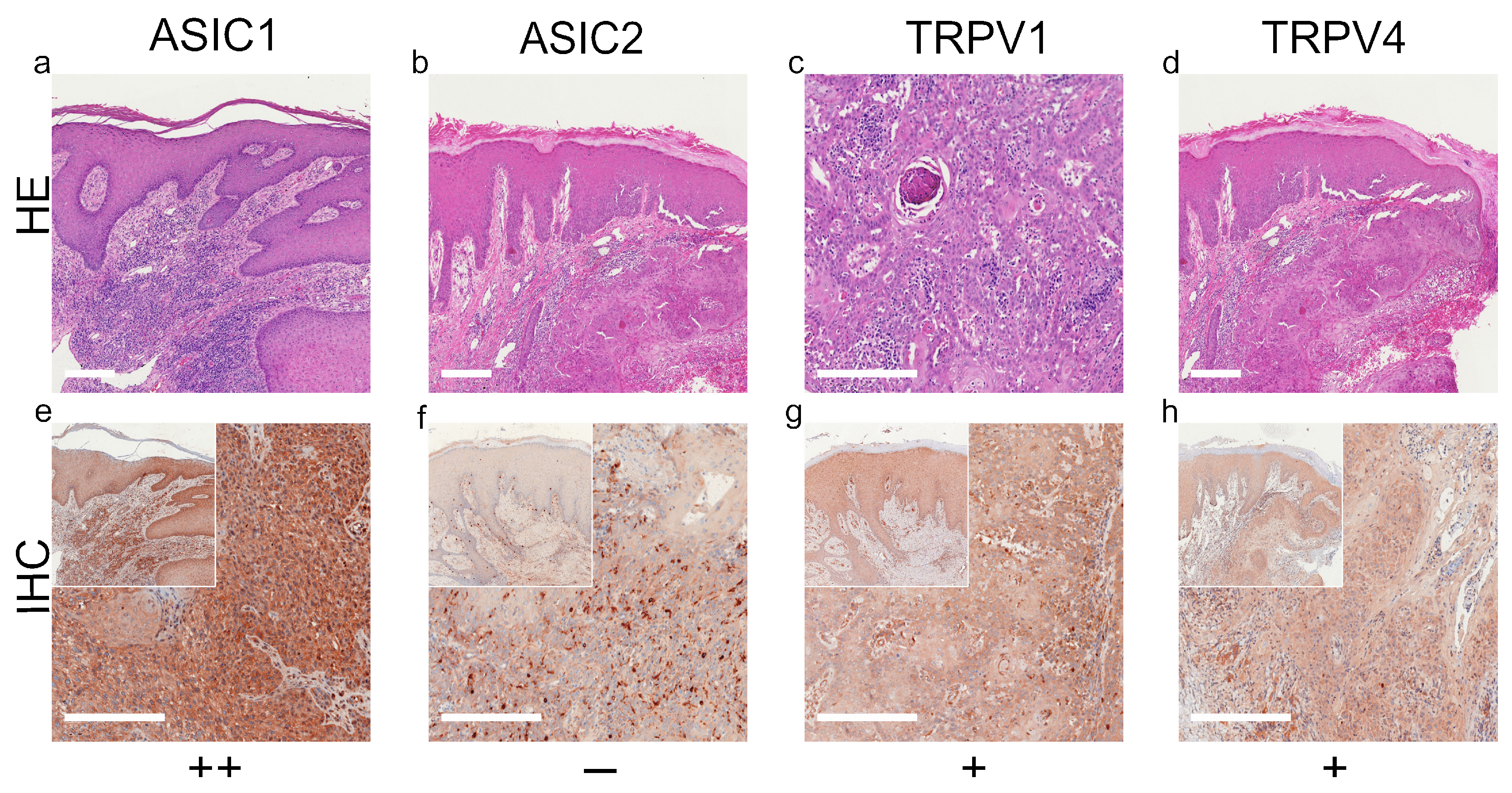
3.1. ASIC1
3.2. ASIC2
3.3. TRPV1
3.4. TRPV4
4. Materials and Methods
4.1. Immunohistochemistry
4.2. Tissue MiroArray (TMA)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, W.H.; Farma, J.M. (Eds.) Contributors. In Cutaneous Melanoma: Etiology and Therapy; Codon Publications: Singapore, 2017; pp. xi–xiv. [Google Scholar]
- Eisemann, N.; Waldmann, A.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Non-Melanoma Skin Cancer Incidence and Impact of Skin Cancer Screening on Incidence. J. Investig. Dermatol. 2014, 134, 43–50. [Google Scholar] [CrossRef] [Green Version]
- E Damsky, W.; Bosenberg, M. Melanocytic nevi and melanoma: Unraveling a complex relationship. Oncogene 2017, 36, 5771–5792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Gao, B.; Xiong, Q.-J.; Wang, Y.-C.; Huang, D.-K.; Wu, W.-N. Acid-sensing ion channels contribute to the effect of extracellular acidosis on proliferation and migration of A549 cells. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Nassios, A.; Wallner, S.; Haferkamp, S.; Klingelhöffer, C.; Brochhausen, C.; Schreml, S. Expression of proton-sensing G-protein-coupled receptors in selected skin tumors. Exp. Dermatol. 2018, 28, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohani, N.; Hao, L.; Alexis, M.S.; Joughin, B.A.; Krismer, K.; Moufarrej, M.N.; Soltis, A.R.; Lauffenburger, D.A.; Yaffe, M.B.; Burge, C.B.; et al. Acidification of Tumor at Stromal Boundaries Drives Transcriptome Alterations Associated with Aggressive Phenotypes. Cancer Res. 2019, 79, 1952–1966. [Google Scholar] [CrossRef] [Green Version]
- Edamaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef] [Green Version]
- Weiß, K.T.; Fante, M.; Köhl, G.; Schreml, J.; Haubner, F.; Kreutz, M.; Haverkampf, S.; Berneburg, M.; Schreml, S. Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp. Dermatol. 2017, 26, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Klatt, W.; Wallner, S.; Brochhausen, C.; Stolwijk, J.A.; Schreml, S. Expression profiles of proton-sensing G-protein coupled receptors in common skin tumors. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Tóth, B.I.; Oláh, A.; Szöllősi, A.G.; Bíró, T. TRP channels in the skin. Br. J. Pharmacol. 2014, 171, 2568–2581. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T. TRPV4 calcium entry channel: A paradigm for gating diversity. Am. J. Physiol. Physiol. 2004, 286, C195–C205. [Google Scholar] [CrossRef] [PubMed]
- Fusi, C.; Materazzi, S.; Minocci, D.; Maio, V.; Oranges, T.; Massi, D.; Nassini, R. Transient Receptor Potential Vanilloid 4 (TRPV4) Is Downregulated in Keratinocytes in Human Non-Melanoma Skin Cancer. J. Investig. Dermatol. 2014, 134, 2408–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity—Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Choong, L.Y.; Mon, N.N.; Lu, S.; Lin, Q.; Pang, B.; Yan, B.; Krishna, V.S.R.; Singh, H.; Tan, T.Z.; et al. TRPV4 Regulates Breast Cancer Cell Extravasation, Stiffness and Actin Cortex. Sci. Rep. 2016, 6, 27903. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Jiang, N.; Li, J.; Ji, Y.-H.; Xiong, Z.-G.; Zha, X.-M. Two aspects of ASIC function: Synaptic plasticity and neuronal injury. Neuropharmacology 2015, 94, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Boscardin, E.; Alijevic, O.; Hummler, E.; Frateschi, S.; Kellenberger, S. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na+channel (ENaC): IUPHAR Review 19. Br. J. Pharmacol. 2016, 173, 2671–2701. [Google Scholar] [CrossRef]
- Sherwood, T.W.; Frey, E.N.; Askwith, C.C. Structure and activity of the acid-sensing ion channels. Am. J. Physiol. Physiol. 2012, 303, C699–C710. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Lee, L.-Y. Acid-Sensing Ion Channels and Pain. Pharmaceuticals 2010, 3, 1411–1425. [Google Scholar] [CrossRef] [Green Version]
- Santoni, G.; Caprodossi, S.; Farfariello, V.; Liberati, S.; Gismondi, A.; Amantini, C. Antioncogenic Effects of Transient Receptor Potential Vanilloid 1 in the Progression of Transitional Urothelial Cancer of Human Bladder. ISRN Urol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdiev, B.K.; Xia, J.; McLean, L.A.; Markert, J.M.; Gillespie, G.Y.; Mapstone, T.B.; Naren, A.P.; Jovov, B.; Bubien, J.K.; Ji, H.-L.; et al. Acid-sensing Ion Channels in Malignant Gliomas. J. Biol. Chem. 2003, 278, 15023–15034. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Singh, R.; Asters, M.; Liu, J.; Zhang, X.; Pabbidi, M.R.; Watabe, K.; Mo, Y.-Y. Regulation of breast tumorigenesis through acid sensors. Oncogene 2016, 35, 4102–4111. [Google Scholar] [CrossRef] [PubMed]
- Vila-Carriles, W.H.; Kovacs, G.G.; Jovov, B.; Zhou, Z.-H.; Pahwa, A.K.; Colby, G.; Esimai, O.; Gillespie, G.Y.; Mapstone, T.B.; Markert, J.M.; et al. Surface Expression of ASIC2 Inhibits the Amiloride-sensitive Current and Migration of Glioma Cells. J. Biol. Chem. 2006, 281, 19220–19232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.-H.; Song, J.-W.; Li, W.; Liu, X.; Cao, L.; Wan, L.-M.; Tan, Y.-X.; Ji, S.-P.; Liang, Y.-M.; Gong, F. The acid-sensing ion channel, ASIC2, promotes invasion and metastasis of colorectal cancer under acidosis by activating the calcineurin/NFAT1 axis. J. Exp. Clin. Cancer Res. 2017, 36, 1–12. [Google Scholar] [CrossRef]
- Marincsák, R.; I Tóth, B.; Czifra, G.; Márton, I.; Redl, P.; Tar, I.; Tóth, L.; Kovács, L.; Bíró, T. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral Dis. 2009, 15, 328–335. [Google Scholar] [CrossRef]
- Kiss, F.; Pohóczky, K.; Szállási, A.; Helyes, Z. Transient Receptor Potential (TRP) Channels in Head-and-Neck Squamous Cell Carcinomas: Diagnostic, Prognostic, and Therapeutic Potentials. Int. J. Mol. Sci. 2020, 21, 6374. [Google Scholar] [CrossRef]
- Park, M.; Naidoo, A.A.; Burns, A.; Choi, J.K.; Gatfield, K.M.; Vidgeon-Hart, M.; Bae, I.-H.; Lee, C.S.; Choi, G.; Powell, A.; et al. Do TRPV1 antagonists increase the risk for skin tumourigenesis? A collaborative in vitro and in vivo assessment. Cell Biol. Toxicol. 2018, 34, 143–162. [Google Scholar] [CrossRef]
- Huang, R.; Wang, F.; Yang, Y.; Ma, W.; Lin, Z.; Cheng, N.; Long, Y.; Deng, S.; Li, Z. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio 2018, 9, 206–225. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, B.; Wang, X.; Mao, J.; Li, W.; Sun, Y.; Yuan, Y.; Ben, Q.; Hua, L.; Qian, A. TRPV4 Overexpression Promotes Metastasis Through Epithelial–Mesenchymal Transition in Gastric Cancer and Correlates with Poor Prognosis. Onco Targets Ther. 2020, 13, 8383–8394. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ackermann, K.; Wallner, S.; Brochhausen, C.; Schreml, S. Expression Profiles of ASIC1/2 and TRPV1/4 in Common Skin Tumors. Int. J. Mol. Sci. 2021, 22, 6024. https://doi.org/10.3390/ijms22116024
Ackermann K, Wallner S, Brochhausen C, Schreml S. Expression Profiles of ASIC1/2 and TRPV1/4 in Common Skin Tumors. International Journal of Molecular Sciences. 2021; 22(11):6024. https://doi.org/10.3390/ijms22116024
Chicago/Turabian StyleAckermann, Kirsten, Susanne Wallner, Christoph Brochhausen, and Stephan Schreml. 2021. "Expression Profiles of ASIC1/2 and TRPV1/4 in Common Skin Tumors" International Journal of Molecular Sciences 22, no. 11: 6024. https://doi.org/10.3390/ijms22116024
APA StyleAckermann, K., Wallner, S., Brochhausen, C., & Schreml, S. (2021). Expression Profiles of ASIC1/2 and TRPV1/4 in Common Skin Tumors. International Journal of Molecular Sciences, 22(11), 6024. https://doi.org/10.3390/ijms22116024





