Photo-Polymerization Damage Protection by Hydrogen Sulfide Donors for 3D-Cell Culture Systems Optimization
Abstract
:1. Introduction
Hydrogen Sulfide-Releasing Agents to Prevent Oxidative Damage
2. Results and Discussion
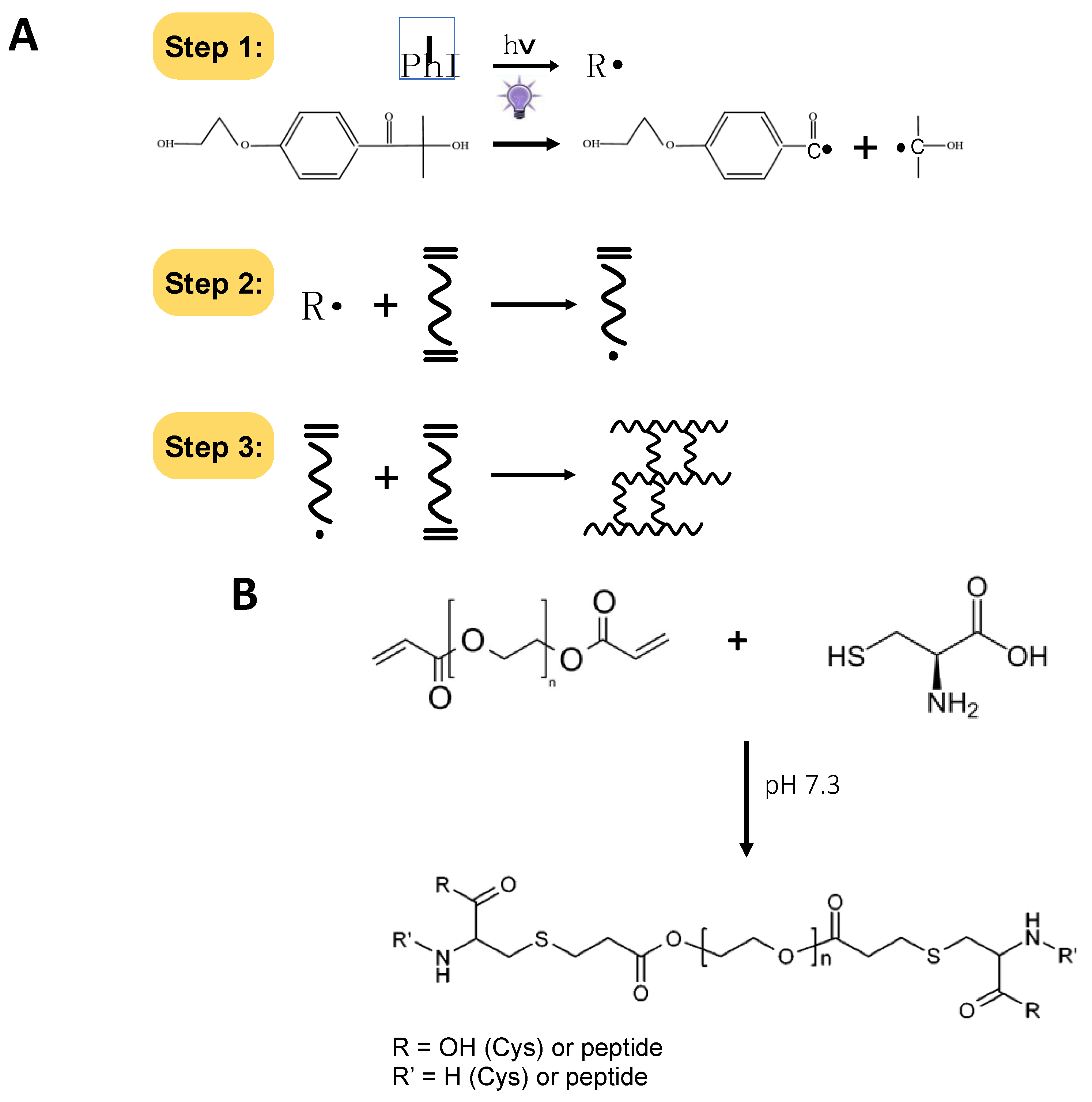
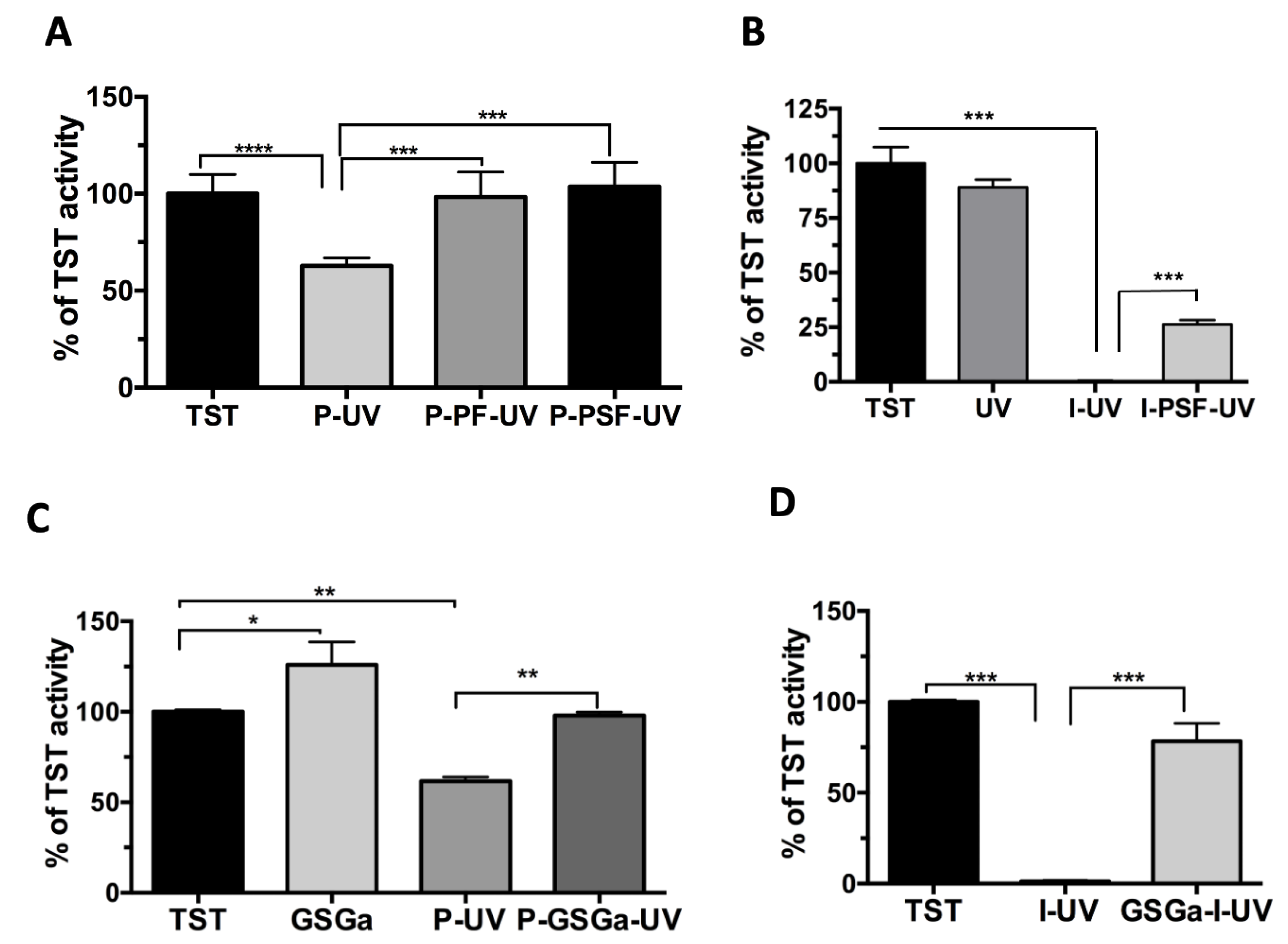
2.1. A Model Enzyme for Studying PhP-Damage
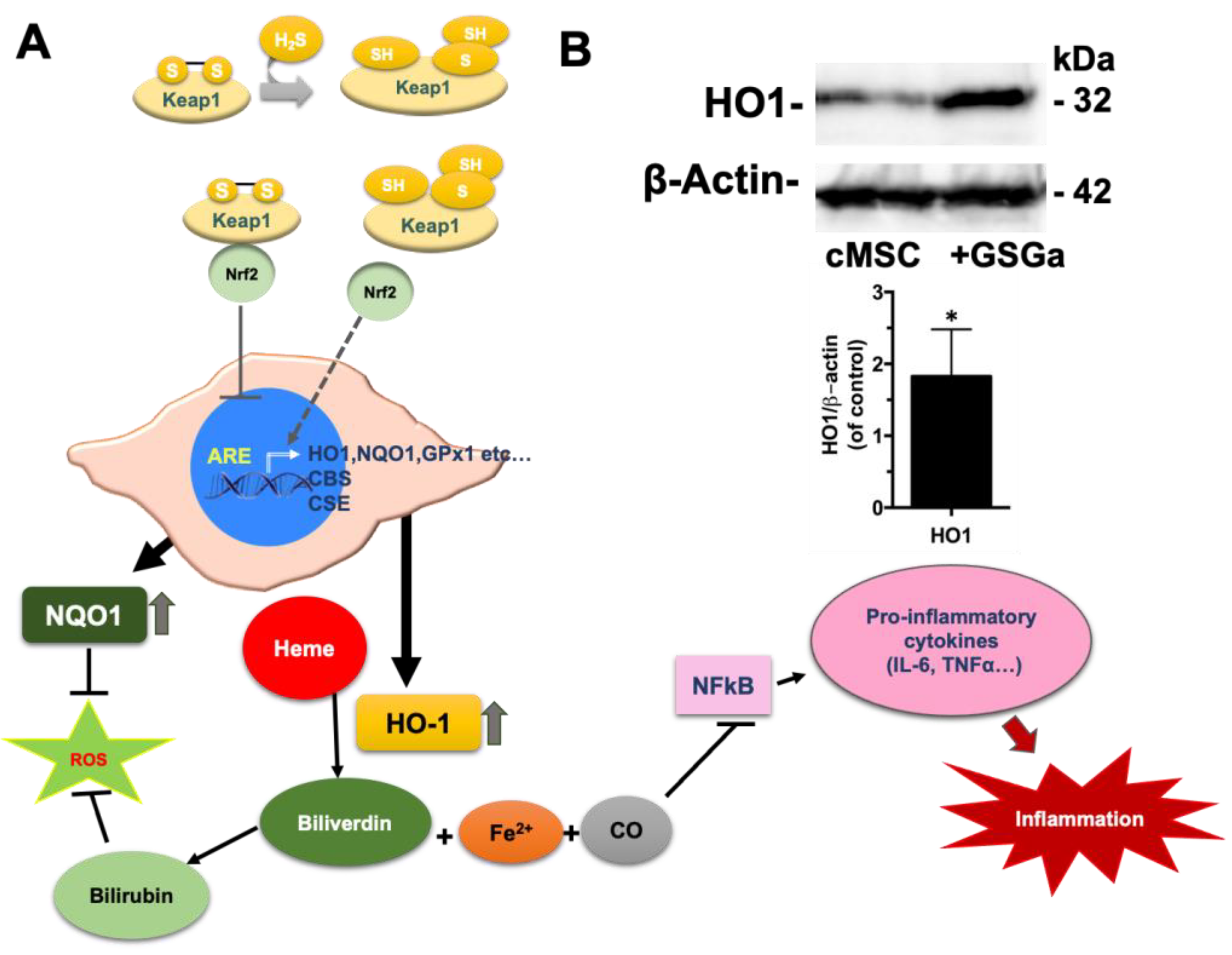
2.2. Preconditioning of cMSC with a Slow H2S-Donor: Effects on Antioxidant Enzyme Expression
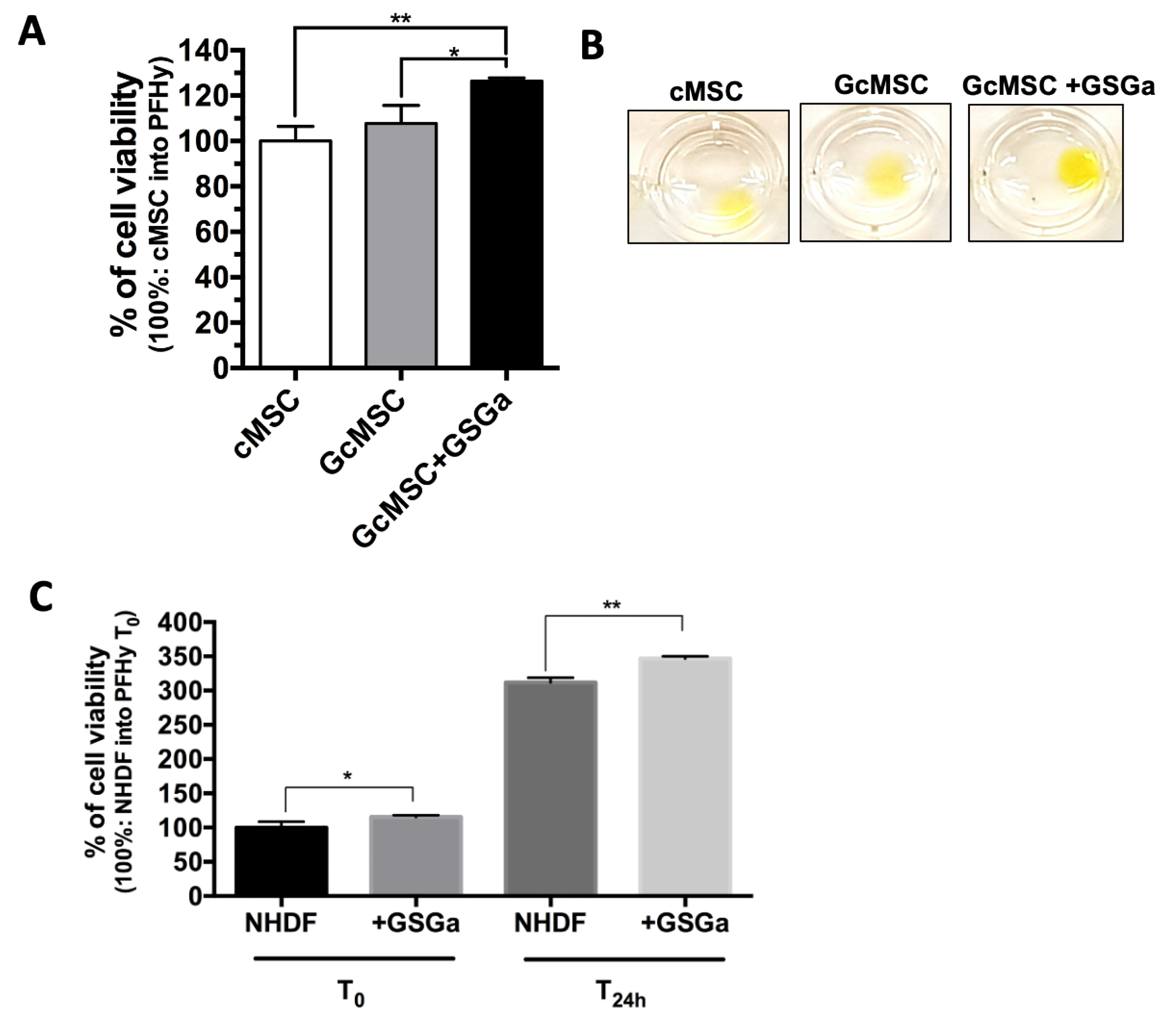
2.3. GSGa as a Preconditioning Agent for Reducing the PhP-Damage on cMSC
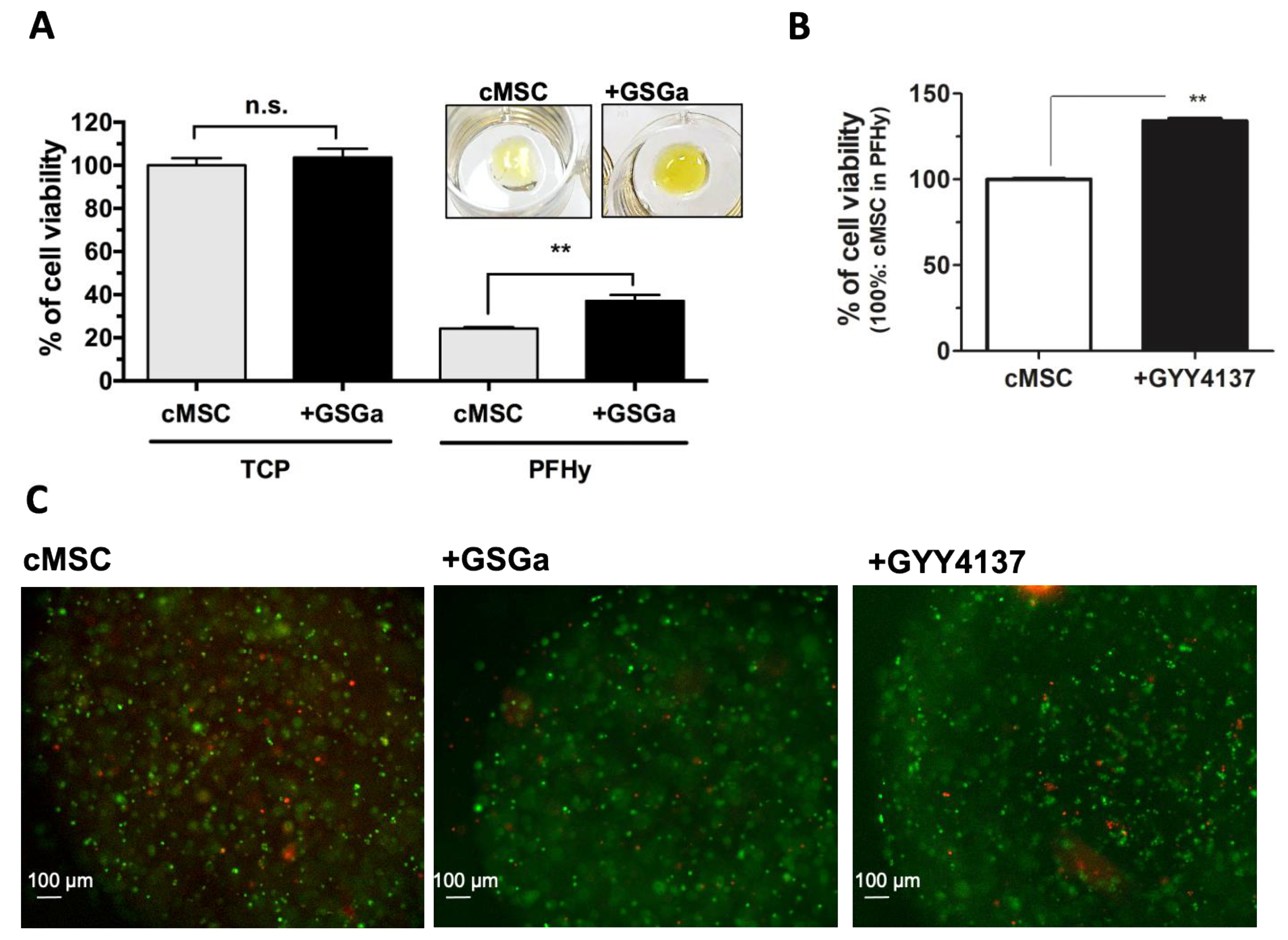
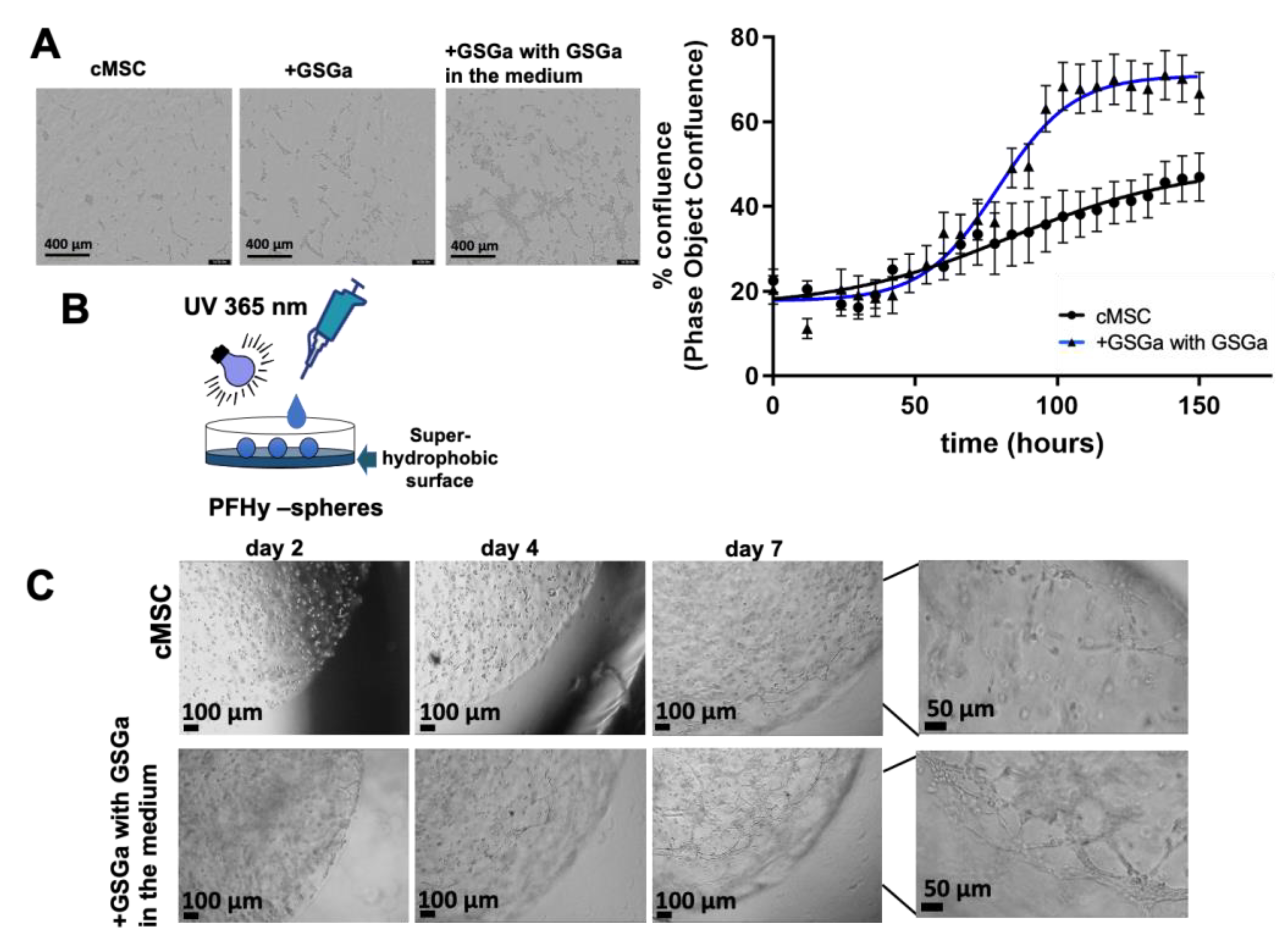
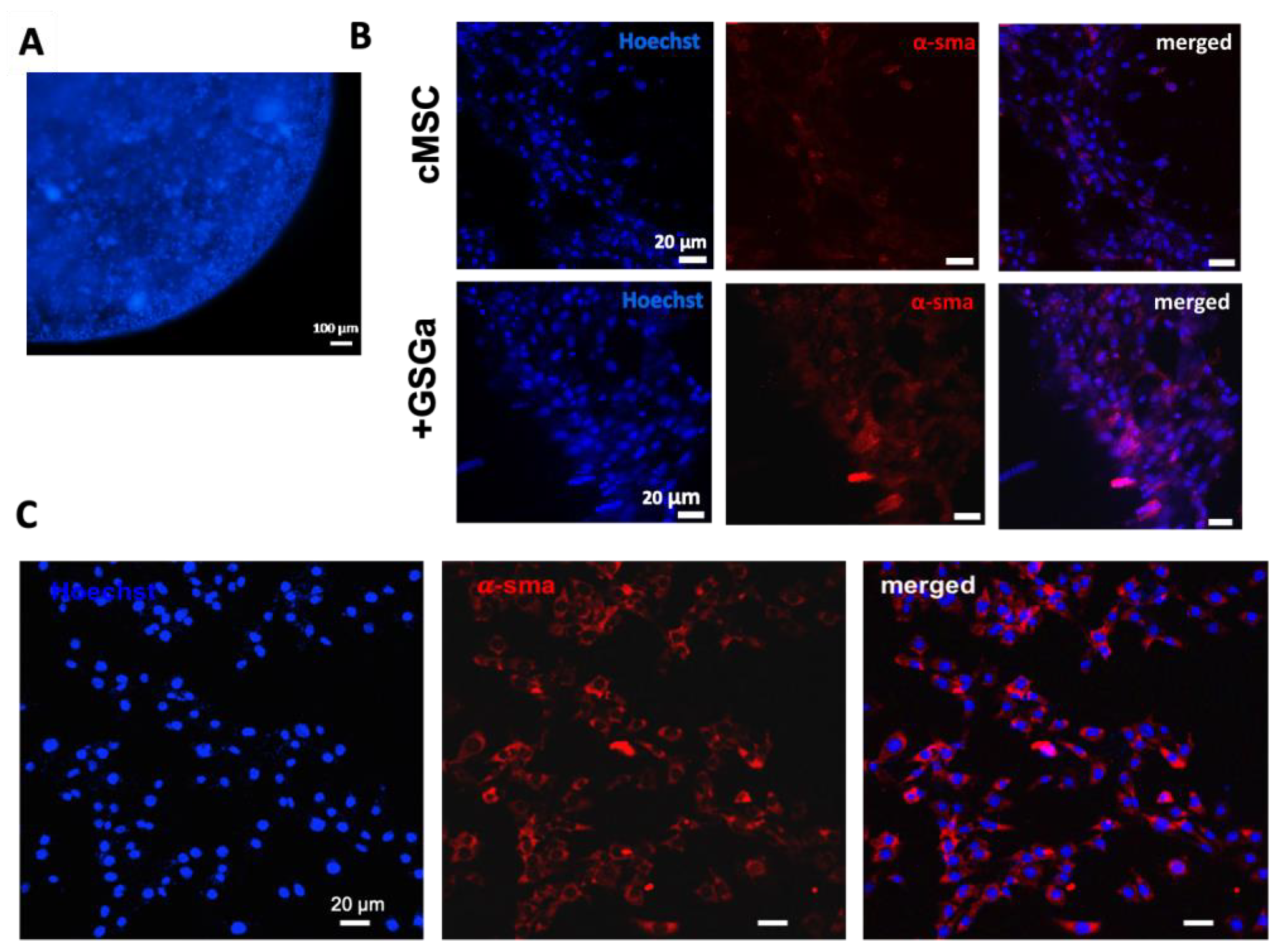
2.4. GSGa Treatment Improves cMSC Proliferation in 3D-Hydrogel Microspheres
3. Materials and Methods
3.1. Recombinant TST Production
3.2. TST Activity
3.3. Garlic Water-Soluble Extract Production from Allium sativum L.
3.4. MB-Assay for H2S Release
3.5. Western Blotting Analysis
3.6. Hydrogel Scaffold Preparation
3.7. 3D Cell Cultures and Cell Viability Assay
3.8. Immunofluorescence Microscopy
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | α-smooth muscle actin |
| ARE | Antioxidant response element |
| BCA | bicinchoninic acid |
| BM-MSC | bone marrow mesenchymal stem cells |
| cMSC | Sca-1+ Lin− human cardiac mesenchymal stem cells |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | Fetal Bovine Serum |
| H2S | hydrogen sulfide |
| HO-1 | heme oxygenase 1 |
| I | photo-initiator |
| Keep1 | Kelch ECH associating protein 1 |
| NHDF | normal human dermal fibroblasts |
| NQO1 | NAD(P)H quinoneoxidoreductase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OSCs | Organo-sulfur compounds |
| SCs | stem cells |
| PhP damage | photo-polymerization damage/photo-polymerization induced damage |
| TST | thiosulfate: cyanide sulfurtransferase enzyme |
References
- Satija, N.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, S.; Afrin, F.; Sharma, M.; Sharma, P.; Tripathi, R.P.; Gurudutta, G.U. Mesenchymal stem cell-based therapy: A new paradigm in regenerative medicine. J. Cell. Mol. Med. 2009, 13, 4385–4402. [Google Scholar] [CrossRef] [Green Version]
- Burdick, J.A.; Mauck, R.; Gerecht, S. To Serve and Protect: Hydrogels to Improve Stem Cell-Based Therapies. Cell Stem Cell 2016, 18, 13–15. [Google Scholar] [CrossRef] [Green Version]
- Sanina, C.; Hare, J.M. Mesenchymal Stem Cells as a Biological Drug for Heart Disease: Where Are We With Cardiac Cell-Based Therapy? Circ. Res. 2015, 117, 229–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copland, I.B. Mesenchymal stromal cells for cardiovascular disease. J. Cardiovasc. Dis. Res. 2011, 2, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Cha, M.-J.; Song, B.-W.; Kim, I.-K.; Chang, W.; Lim, S.; Choi, E.J.; Ham, O.; Lee, S.-Y.; Chung, N.; et al. Reactive Oxygen Species Inhibit Adhesion of Mesenchymal Stem Cells Implanted into Ischemic Myocardium via Interference of Focal Adhesion Complex. Stem Cells 2010, 28, 555–563. [Google Scholar] [CrossRef]
- Abdelwahid, E.; Kalvelyte, A.; Stulpinas, A.; De Carvalho, K.A.T.; Guarita-Souza, L.C.; Foldes, G. Stem cell death and survival in heart regeneration and repair. Apoptosis 2016, 21, 252–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amer, M.H.; Rose, F.R.A.J.; Shakesheff, K.M.; White, L.J. A biomaterials approach to influence stem cell fate in injectable cell-based therapies. Stem Cell Res. Ther. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Re-generative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Shim, W.; Gu, Y.; Shirhan, M.; Lim, K.P.; Tan, L.P.; Lim, C.H.; Sin, Y.K.; Wong, P. Hemodynamic Contribution of Stem Cell Scaffolding in Acute Injured Myocardium. Tissue Eng. Part A 2012, 18, 1652–1663. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Bryant, S.J.; Vernerey, F.J. Programmable Hydrogels for Cell Encapsulation and Neo-Tissue Growth to Enable Personalized Tissue Engineering. Adv. Health Mater. 2017, 7, 1700605. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Lui, K.O.; Tandon, N.; Chien, K.R. Bioengineering heart muscle: A paradigm for regenerative med-icine. Ann. Rev. Biomed. Eng 2011, 13, 245–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [Green Version]
- Elisseeff, J.; Anseth, K.; Sims, D.; McIntosh, W.; Randolph, M.; Langer, R. Transdermal photopolymerization for minimally invasive implantation. Proc. Natl. Acad. Sci. USA 1999, 96, 3104–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, P.; Coelho, J.; Gil, M.H. Development of a new photocrosslinkable biodegradable bioadhesive. Int. J. Pharm. 2008, 352, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, J.L.; Hubbell, J.A. Polymeric Biomaterials with Degradation Sites for Proteases Involved in Cell Migration. Macromolecules 1999, 32, 241–244. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef]
- Ciocci, M.; Cacciotti, I.; Seliktar, D.; Melino, S. Injectable silk fibroin hydrogels functionalized with microspheres as adult stem cells-carrier systems. Int. J. Biol. Macromol. 2018, 108, 960–971. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J. The effect of photopolymeri-zation on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Click Chemistry-Based Injectable Hydrogels and Bioprinting Inks for Tissue Engineering Applications. Tissue Eng. Regen. Med. 2018, 15, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Carberry, B.J.; Worrell, B.T.; Dudaryeva, O.Y.; McBride, M.K.; Bowman, C.N.; Anseth, K.S. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 2018, 178, 496–503. [Google Scholar] [CrossRef]
- Nulty, J.; Freeman, F.E.; Browe, D.C.; Burdis, R.; Ahern, D.P.; Pitacco, P.; Bin Lee, Y.; Alsberg, E.; Kelly, D.J. 3D bioprinting of prevascularised implants for the repair of critically-sized bone defects. Acta Biomater. 2021, 126, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Fornetti, E.; Fuoco, C.; Riera, C.S.; Nie, M.; Bernardini, S.; Rainer, A.; Baldi, J.; Zoccali, C.; et al. Biofabricating murine and human myo-substitutes for rapid volumetric muscle loss restoration. EMBO Mol. Med. 2021, 13, e12778. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef]
- Sabnis, A.; Rahimi, M.; Chapman, C.; Nguyen, K.T. Cytocompatibility studies of an in situ photopolymerized ther-moresponsive hydrogel nanoparticle system using human aortic smooth muscle cells. J. Biomed. Mater. Res. A 2009, 91, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Zhang, L.; Yang, G.; Xu, C.; Wang, R. Butyrate-stimulated H2S Production in Colon Cancer Cells. Antioxid. Redox Signal. 2010, 12, 1101–1109. [Google Scholar] [CrossRef]
- Bhuiyan, A.I.; Papajani, V.T.; Paci, M.; Melino, S.M. Glutathione-Garlic Sulfur Conjugates: Slow Hydrogen Sulfide Releasing Agents for Therapeutic Applications. Molecules 2015, 20, 1731–1750. [Google Scholar] [CrossRef]
- Bolton, S.; Cerda, M.; Gilbert, A.K.; Pluth, M.D. Effects of sulfane sulfur content in benzyl polysulfides on thiol-triggered H2S release and cell proliferation. Free Radic Biol. Med. 2019, 131, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Moore, P.K.; Ming, S.H.; Nam, O.C.; Armstrong, J.S.; Whiteman, M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J. Gastroenterol. 2005, 11, 3990–3997. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, M.; Ju, L.; Wang, C.; Zhu, Y. Hydrogen sulfide induces human colon cancer cell proliferation: Role of Akt, ERK and p21. Cell Biol. Int. 2010, 34, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chuah, S.C.; Moore, P.K.; Zhu, Y.Z. S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am. J. Physiol. Circ. Physiol. 2007, 293, H2693–H2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deplancke, B.; Gaskins, H.R. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J. 2003, 17, 1310–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.-F.; Lu, M.; Wu, Z.-Y.; Wong, P.T.-H.; Bian, J.-S. Hydrogen Sulfide Inhibits Rotenone-Induced Apoptosis via Preservation of Mitochondrial Function. Mol. Pharmacol. 2008, 75, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Pao, J.; Lin, S.; Sheen, L. Molecular mechanisms of garlic-derived allyl sulfides in the inhibition of skin cancer progression. Ann. N. Y. Acad. Sci. 2012, 1271, 44–52. [Google Scholar] [CrossRef]
- Li, M.; Min, J.-M.; Cui, J.-R.; Zhang, L.-H.; Wang, K.; Valette, A.; Davrinche, C.; Wright, M.; Leung-Tack, J. Z-Ajoene Induces Apoptosis of HL-60 Cells: Involvement of Bcl-2 Cleavage. Nutr. Cancer 2002, 42, 241–247. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, R.; Feng, C.; Zhang, J.; Liu, D.; Xu, K.; Wang, X.; Zhang, S.; Li, Z.; Liu, X.; et al. Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathways in human esophageal squamous cell carcinoma. Oncol Rep. 2014, 32, 1748–1756. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Herman-Antosiewicz, A.; Antosiewicz, J.; Xiao, H.; Brisson, M.; Lazo, J.S.; Singh, S.V. Diallyl trisulfide-induced G2–M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc25C. Oncogene 2005, 24, 6256–6268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; Zeng, Y.; Hahm, E.-R.; Kim, Y.-A.; Ramalingam, S.; Singh, S.V. Diallyl trisulfide selectively causes Bax- and Bak-mediated apoptosis in human lung cancer cells. Environ. Mol. Mutagen. 2009, 50, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murai, M.; Inoue, T.; Suzuki-Karasaki, M.; Ochiai, T.; Ra, C.; Nishida, S.; Suzuki-Karasaki, Y. Diallyl trisulfide sensitizes human melanoma cells to TRAIL-induced cell death by promoting endoplasmic reticulum-mediated apoptosis. Int. J. Oncol. 2012, 41, 2029–2037. [Google Scholar] [CrossRef]
- Chandra-Kuntal, K.; Lee, J.; Singh, S.V. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res. Treat. 2013, 138, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Dirsch, V.M.; Gerbes, A.L.; Vollmar, A.M. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kappaB. Mol. Pharmacol. 1998, 53, 402–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osipov, R.M.; Robich, M.P.; Chan, V.; Clements, R.T.; Deyo, R.J.; Feng, J.; Szabo, C.; Sellke, F.W. Effect of hydrogen sulfide on myocardial protection in the setting of cardioplegia and cardiopulmonary bypass? Interact. Cardiovasc. Thorac. Surg. 2010, 10, 506–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Liu, Y.; Shi, S. Hydrogen Sulfide Regulates Homeostasis of Mesenchymal Stem Cells and Regulatory T Cells. J. Dent. Res. 2016, 95, 1445–1451. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Sun, A.; Zhu, W.; Huang, Z.; Hu, X.; Jia, J.; Zou, Y.; Ge, J. Transplantation of Mesenchymal Stem Cells Preconditioned with Hydrogen Sulfide Enhances Repair of Myocardial Infarction in Rats. Tohoku J. Exp. Med. 2012, 226, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, S.; Li, T.; Yuan, L.; Liu, H.; Wang, X.; Wang, F.; Wang, S.; Hao, A.; Liu, D.; et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 2016, 7, 58089–58104. [Google Scholar] [CrossRef] [Green Version]
- Di Giovanni, E.; Buonvino, S.; Amelio, I.; Melino, S. Glutathione-Allylsulfur Conjugates as Mesenchymal Stem Cells Stim-ulating Agents for Potential Applications in Tissue Repair. Int. J. Mol. Sci. 2020, 21, 1638. [Google Scholar] [CrossRef] [Green Version]
- Seliktar, D.; Dikovsky, D.; Napadensky, E. Bioprinting and Tissue Engineering: Recent Advances and Future Perspectives. Isr. J. Chem. 2013, 53, 795–804. [Google Scholar] [CrossRef]
- Colnaghi, R.; Pagani, S.; Kennedy, C.; Drummond, M. Cloning, Sequence Analysis and Overexpression of the Rhodanese Gene of Azotobacter vinelandii. JBIC J. Biol. Inorg. Chem. 1996, 236, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sabelli, R.; Iorio, E.; De Martino, A.; Podo, F.; Ricci, A.; Viticchiè, G.; Rotilio, G.; Paci, M.; Melino, S.M. Rhodanese-thioredoxin system and allyl sulfur compounds. FEBS J. 2008, 275, 3884–3899. [Google Scholar] [CrossRef]
- Sörbo, B. A colorimetric method for the determination of thiosulfate. Biochim. Biophys. Acta Bioenerg. 1957, 23, 412–416. [Google Scholar] [CrossRef]
- Ciocci, M.; Iorio, E.; Carotenuto, F.; Khashoggi, H.A.; Nanni, F.; Melino, S. H2S-releasing nanoemulsions: A new formulation to inhibit tumor cells proliferation and improve tissue repair. Oncotarget 2016, 7, 84338–84358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.; Sylvester, S.L.; Choi, A.M. Hemoglobin provides protection against lethal endotoxemia in rats: The role of heme oxygenase-1. Am. J. Respir. Cell Mol. Biol. 1995, 13, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Kolls, J.K.; Mantell, L.L.; Cook, J.L.; Alam, J.; Choi, A.M. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J. Clin. Investig. 1999, 103, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.P.; Lu, H.T.; Wysk, M.A.; Davis, R.J.; Flavell, R.A.; Choi, A.M.K. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Lee, T.-S.; Chau, L.-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef]
- Altaany, Z.; Yang, G.; Wang, R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J. Cell. Mol. Med. 2013, 17, 879–888. [Google Scholar] [CrossRef]
- Sart, S.; Ma, T.; Li, Y. Preconditioning Stem Cells for In Vivo Delivery. BioRes. Open Access 2014, 3, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.; Rinaldi, B.; Sodano, L.; Berrino, L.; Rossi, F.; Finicelli, M.; Grossi, M.; Cobellis, G.; Botti, C.; De Feo, M.; et al. Stem Cell Therapy for Arterial Restenosis: Potential Parameters Contributing to the Success of Bone Marrow-Derived Mesenchymal Stromal Cells. Cardiovasc. Drugs Ther. 2011, 26, 9–21. [Google Scholar] [CrossRef]
- Koyanagi, M.; Kawakabe, S.; Arimura, Y. A comparative study of colorimetric cell proliferation assays in immune cells. Cytotechnology 2015, 68, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.-M.; Wang, Y.-P.; Wang, K.; Pu, L.-Y.; Zhang, F.; Li, X.-C.; Kong, L.-B.; Sun, B.-C.; Li, G.-Q.; Wang, X.-H. Exogenous Biliverdin Ameliorates Ischemia-Reperfusion Injury in Small-for-Size Rat Liver Grafts. Transplant. Proc. 2007, 39, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, R.; Tanaka, Y.; Noda, K.; Kawamura, T.; Toyoda, Y.; Billiar, T.R.; McCurry, K.R.; Nakao, A. Preservation solution supplemented with biliverdin prevents lung cold ischaemia/reperfusion injury. Eur. J. Cardio-Thorac. Surg. 2012, 42, 1035–1041. [Google Scholar] [CrossRef]
- Yamashita, K.; McDaid, J.; Öllinger, R.; Tsui, T.; Berberat, P.O.; Usheva, A.; Csizmadia, E.; Smith, R.N.; Soares, M.P.; Bach, F.H. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004, 18, 765–767. [Google Scholar] [CrossRef]
- Pileggi, A.; Molano, R.D.; Berney, T.; Cattan, P.; Vizzardelli, C.; Oliver, R.; Fraker, C.; Ricordi, C.; Pastori, R.L.; Bach, F.H.; et al. Heme Oxygenase-1 Induction in Islet Cells Results in Protection From Apoptosis and Improved In Vivo Function After Transplantation. Diabetes 2001, 50, 1983–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lee, S.S.; Gao, W.; Czismadia, E.; McDaid, J.; Ollinger, R.; Soares, M.P.; Yamashita, K.; Bach, F.H. Donor Treatment With Carbon Monoxide Can Yield Islet Allograft Survival and Tolerance. Diabetes 2005, 54, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonvino, S.; Ciocci, M.; Seliktar, D.; Melino, S. Photo-Polymerization Damage Protection by Hydrogen Sulfide Donors for 3D-Cell Culture Systems Optimization. Int. J. Mol. Sci. 2021, 22, 6095. https://doi.org/10.3390/ijms22116095
Buonvino S, Ciocci M, Seliktar D, Melino S. Photo-Polymerization Damage Protection by Hydrogen Sulfide Donors for 3D-Cell Culture Systems Optimization. International Journal of Molecular Sciences. 2021; 22(11):6095. https://doi.org/10.3390/ijms22116095
Chicago/Turabian StyleBuonvino, Silvia, Matteo Ciocci, Dror Seliktar, and Sonia Melino. 2021. "Photo-Polymerization Damage Protection by Hydrogen Sulfide Donors for 3D-Cell Culture Systems Optimization" International Journal of Molecular Sciences 22, no. 11: 6095. https://doi.org/10.3390/ijms22116095





