Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy
Abstract
:1. Biosynthesis and Expression of Cancer-Associated Gangliosides
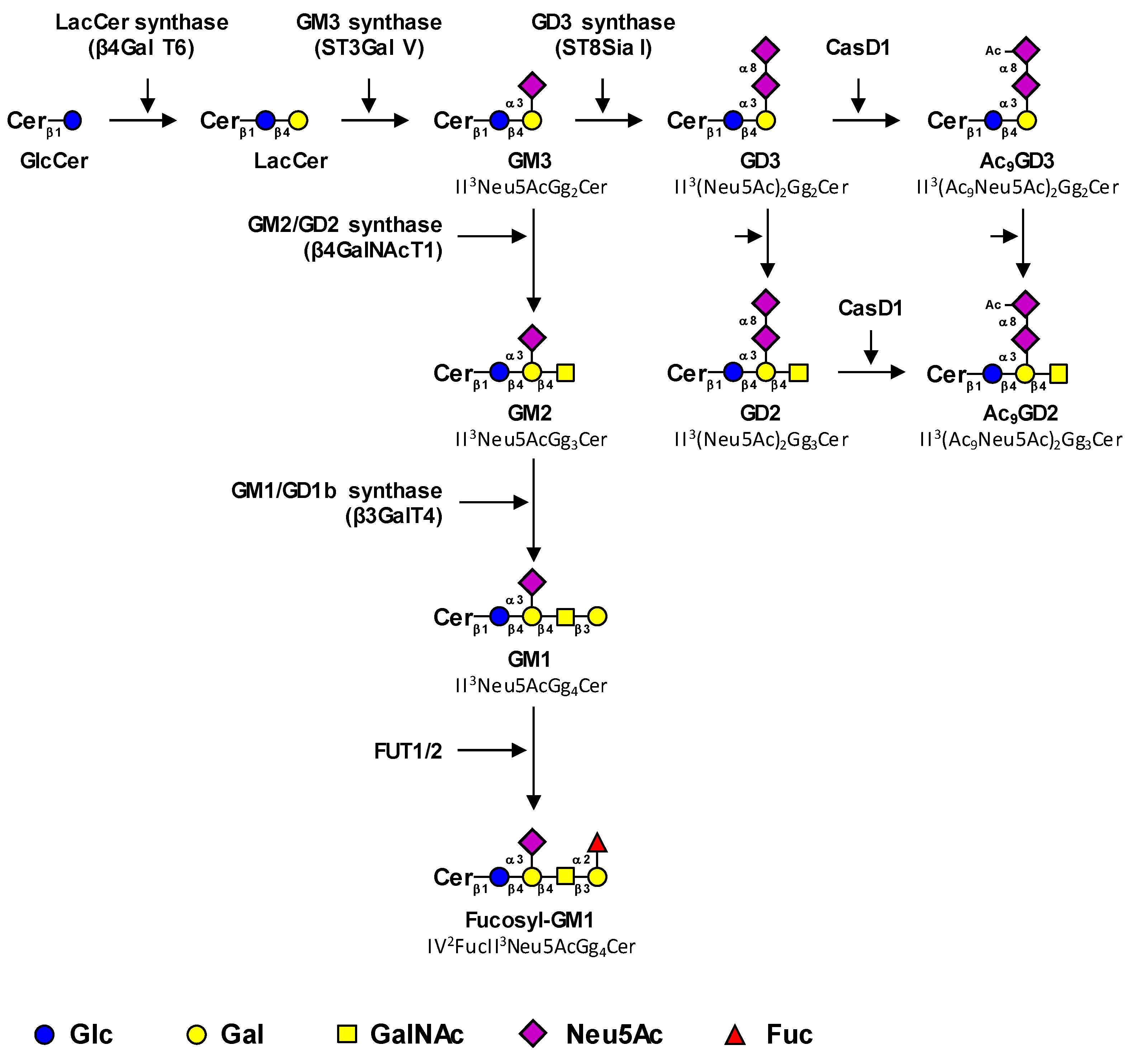
1.1. Biosynthesis Pathways of Cancer-Associated Gangliosides
1.2. Expression of Cancer-Associated Gangliosides in Healthy Cells/Tissues
2. Expression and Roles of Cancer-Associated Ganglioside in Malignant Properties
2.1. Expression of Cancer-Associated Gangliosides in Cancers
2.1.1. Expression of b-Series Gangliosides and Their O-Acetylated Derivatives in Cancer
2.1.2. Expression of Fuc-GM1 in Cancer
2.2. Roles of Gangliosides in Malignant Properties of Cancer Cells
2.2.1. Biological Roles of b-Series Gangliosides and Their O-Acetylated Derivatives in Cancer
2.2.2. Biological Roles of Fuc-GM1 in Cancer
3. Immunotherapy Targeting Cancer-Associated Gangliosides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishii, A.; Ikeda, T.; Hitoshi, S.; Fujimoto, I.; Torii, T.; Sakuma, K.; Nakakita, S.I.; Hase, S.; Ikenaka, K. Developmental changes in the expression of glycogenes and the content of N-glycans in the mouse cerebral cortex. Glycobiology 2007, 17, 261–276. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, A.; Hasegawa, Y.; Higai, K.; Matsumoto, K. Transcriptional regulation of human β-galactoside α2, 6-sialyltransferase (hST6Gal I) gene during differentiation of the HL-60 cell line. Glycobiology 2000, 10, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.I.; Toyota, M.; Kawashima, R.; Hagiwara, T.; Suzuki, H.; Imai, K.; Shinomura, Y.; Tokino, T.; Kannagi, R.; Dohi, T. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology 2008, 135, 142–151. [Google Scholar] [CrossRef]
- Ishii, A.; Ohta, M.; Watanabe, Y.; Matsuda, K.; Ishiyama, K.; Sakoe, K.; Nakamura, M.; Inokuchi, J.; Sanai, Y.; Saito, M. Expression cloning and functional characterization of human cDNA for ganglioside GM3 synthase. J. Biol. Chem. 1998, 273, 31652–31655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisada, S.; Yoshimura, Y.; Sakaguchi, K.; Uemura, S.; Go, S.; Ikeda, K.; Uchima, H.; Matsunaga, N.; Ogura, K.; Tai, T.; et al. Zebrafish and mouse α2,3-sialyltransferases responsible for synthesizing GM4 ganglioside. J. Biol. Chem. 2009, 284, 30534–30546. [Google Scholar] [CrossRef] [Green Version]
- Uemura, S.; Yoshida, S.; Shishido, F.; Inokuchi, J. The cytoplasmic tail of GM3 synthase defines its subcellular localization, stability, and in vivo activity. Mol. Biol. Cell 2009, 20, 3088–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathow, D.; Chessa, F.; Rabionet, M.; Kaden, S.; Jennemann, R.; Sandhoff, R.; Gröne, H.J.; Feuerborn, A. Zeb1 affects epithelial cell adhesion by diverting glycosphingolipid metabolism. EMBO Rep. 2015, 16, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Nagata, Y.; Yamashiro, S.; Yodoi, J.; Lloyd, K.O.; Shiku, H.; Furukawa, K. Expression cloning of β1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J. Biol. Chem. 1992, 267, 12082–12089. [Google Scholar] [CrossRef]
- Amado, M.; Almeida, R.; Carneiro, F.; Levery, S.B.; Holmes, E.H.; Nomoto, M.; Hollingsworth, M.A.; Hassan, H.; Schwientek, T.; Nielsen, P.A.; et al. A family of human β3-galactosyltransferases. Characterization of four members of a UDP-galactose, β-N-acetyl-glucosamine/β-N-acetyl-galactosamine β-1,3-galactosyltransferase family. J. Biol. Chem. 1998, 273, 12770–12778. [Google Scholar] [CrossRef] [Green Version]
- Iber, H.; Zacharias, C.; Sandhoff, K. The c-series gangliosides GT3, GT2 and GP1c are formed in rat liver Golgi by the same set of glycosyltransferases that catalyse the biosynthesis of asialo-, a- and b-series gangliosides. Glycobiology 1992, 2, 137–142. [Google Scholar] [CrossRef]
- Yamashiro, S.; Haraguchi, M.; Furukawa, K.; Takamiya, K.; Yamamoto, A.; Nagata, Y.; Lloyd, K.O.; Shiku, H.; Furukawa, K. Substrate specificity of β1,4-N-acetylgalactosaminyltransferase in vitro and in cDNA-transfected cells. GM2/GD2 synthase efficiently generates asialo-GM2 in certain cells. J. Biol. Chem. 1995, 270, 6149–6155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraudo, C.G.; Daniotti, J.L.; Maccioni, H.J. Physical and functional association of glycolipid N-acetyl-galactosaminyl and galactosyl transferases in the Golgi apparatus. Proc. Natl. Acad. Sci. USA 2001, 98, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Rouquier, S.; Lowe, J.B.; Kelly, R.J.; Fertitta, A.L.; Lennon, G.; Giorgi, D. Molecular cloning of a human genomic region containing the H blood group α1,2-fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human Secretor blood group locus. J. Biol. Chem. 1995, 270, 4632–4639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Huante, K.; Salinas-Marín, R.; Mora-Montes, H.M.; Gonzalez, R.A.; Martínez-Duncker, I. Human adenovirus type 5 increases host cell fucosylation and modifies Ley antigen expression. Glycobiology 2019, 29, 469–478. [Google Scholar] [CrossRef]
- Tokuda, N.; Zhang, Q.; Yoshida, S.; Kusunoki, S.; Urano, T.; Furukawa, K.; Furukawa, K. Genetic mechanisms for the synthesis of fucosyl GM1 in small cell lung cancer cell lines. Glycobiology 2006, 16, 916–925. [Google Scholar] [CrossRef] [Green Version]
- Iwamori, M.; Domino, S.E. Tissue-specific loss of fucosylated glycolipids in mice with targeted deletion of α1,2-fucosyltransferase genes. Biochem. J. 2004, 380, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Ruan, S.; Raj, B.K.; Lloyd, K.O. Relationship of glycosyltransferases and mRNA levels to ganglioside expression in neuroblastoma and melanoma cells. J. Neurochem. 1999, 72, 514–521. [Google Scholar] [CrossRef]
- Ruan, S.; Lloyd, K.O. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells, relative glycosyltransferase levels determine ganglioside patterns. Cancer Res. 1992, 52, 5725–5731. [Google Scholar]
- Ruckhäberle, E.; Karn, T.; Rody, A.; Hanker, L.; Gätje, R.; Metzler, D.; Holtrich, U.; Kaufmann, M. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J. Cancer Res. Clin. Oncol. 2009, 135, 1005–1013. [Google Scholar] [CrossRef]
- Lo Piccolo, M.S.; Cheung, N.K.; Cheung, I.Y. GD2 synthase, a new molecular marker for detecting neuroblastoma. Cancer 2001, 92, 924–931. [Google Scholar] [CrossRef]
- Furukawa, K.; Horie, M.; Okutomi, K.; Sugano, S.; Furukawa, K. Isolation and functional analysis of the melanoma specific promoter region of human GD3 synthase gene. Biochim. Biophys. Acta 2003, 1627, 71–78. [Google Scholar] [CrossRef]
- Kang, N.Y.; Kim, C.H.; Kim, K.S.; Ko, J.H.; Lee, J.H.; Jeong, Y.K.; Lee, Y.C. Expression of the human CMP-NeuAc: GM3 α2,8-sialyltransferase (GD3 synthase) gene through the NF-κB activation in human melanoma SK-MEL-2 cells. Biochim. Biophys. Acta 2007, 1769, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Dae, H.M.; Kwon, H.Y.; Kang, N.Y.; Song, N.R.; Kim, K.S.; Kim, C.H.; Lee, J.H.; Lee, Y.C. Isolation and functional analysis of the human glioblastoma-specific promoter region of the human GD3 synthase (hST8Sia I) gene. Acta Biochim. Biophys. Sin. 2009, 41, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Bobowski, M.; Vincent, A.; Steenackers, A.; Colomb, F.; Van Seuningen, I.; Julien, S.; Delannoy, P. Estradiol represses the GD3 synthase gene ST8SIA1 expression in human breast cancer cells by preventing NF-κB binding to ST8SIA1 promoter. PLoS ONE 2013, 8, e62559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, A.M.T.; Bakkers, M.J.G.; Buettner, F.R.; Hartmann, M.; Grove, M.; Langereis, M.A.; de Groot, R.J.; Mühlenhoff, M. 9-O-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nat. Commun. 2015, 6, 7673. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, V.S.; Alsufyani, F.; Mattoo, H.; Rosenberg, I.; Pillai, S. Alterations in sialic-acid O-acetylation glycoforms during murine erythrocyte development. Glycobiology 2019, 29, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva-Cabello, T.M.; Mollicone, R.; Cruz-Muñoz, M.E.; López-Guerrero, D.V.; Martínez-Duncker, I. Activation of human naïve Th cells increases surface expression of GD3 and induces neoexpression of GD2 that colocalize with TCR clusters. Glycobiology 2015, 25, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Constantine-Paton, M.; Blum, A.S.; Mendez-Otero, R.; Barnstable, C.J. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature 1986, 324, 459–462. [Google Scholar] [CrossRef]
- Kotani, M.; Terashima, T.; Tai, T. Developmental changes of ganglioside expressions in postnatal rat cerebellar cortex. Brain Res. 1995, 700, 40–58. [Google Scholar] [CrossRef]
- Mendez-Otero, R.; Friedman, J.E. Role of acetylated gangliosides on neurite extension. Eur. J. Cell Biol. 1996, 71, 192–198. [Google Scholar]
- Erdmann, M.; Wipfler, D.; Merling, A.; Cao, Y.; Claus, C.; Kniep, B.; Sadick, H.; Bergler, W.; Vlasak, R.; Schwartz-Albiez, R. Differential surface expression and possible function of 9-O- and 7-O-acetylated GD3 (CD60 b and c) during activation and apoptosis of human tonsillar B and T lymphocytes. Glycoconj. J. 2006, 23, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, S.; Inoue, K.; Iwamori, M.; Nagai, Y.; Mannen, T. Fucosylated glycoconjugates in human dorsal root ganglion cells with unmyelinated axons. Neurosci. Lett. 1991, 126, 159–162. [Google Scholar] [CrossRef]
- Kusunoki, S.; Inoue, K.; Iwamori, M.; Nagai, Y.; Mannen, T.; Kanazawa, I. Developmental changes of fucosylated glycoconjugates in rabbit dorsal root ganglia. Neurosci. Res. 1992, 15, 74–80. [Google Scholar] [CrossRef]
- Hitoshi, S.; Koijima, N.; Kusunoki, S.; Inokuchi, J.; Kanazawa, I.; Tsuji, S. Expression of the β-galactoside α1,2-fucosyltransferase gene suppresses axonal outgrowth of neuro2a neuroblastoma cells. J. Neurochem. 1996, 66, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, Y.; Miyazaki, S.; Hamamura, K.; Kambe, M.; Miyata, M.; Tajima, O.; Ohmi, Y.; Yamauchi, Y.; Furukawa, K.; Furukawa, K. Ganglioside GD3 enhances adhesion signals and augments malignant properties of melanoma cells by recruiting integrins to glycolipid-enriched microdomains. J. Biol. Chem. 2010, 285, 27213–27223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasawa, T.; Zhang, P.; Ohkawa, Y.; Momota, H.; Wakabayashi, T.; Ohmi, Y.; Bhuiyan, R.H.; Furukawa, K.; Furukawa, K. Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2. Int. J. Oncol. 2018, 52, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Merritt, W.D.; Casper, J.T.; Lauer, S.J.; Reaman, G.H. Expression of GD3 ganglioside in childhood T-cell lymphoblastic malignancies. Cancer Res. 1987, 47, 1724–1730. [Google Scholar]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.K.V. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr. Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.R.; Cordon-Cardo, C.; Houghton, A.N.; Cheung, N.K.; Brennan, M.F. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer 1992, 70, 633–638. [Google Scholar] [CrossRef]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside GD2 in small cell lung cancer cell lines, enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar]
- Wu, Z.L.; Schwartz, E.; Seeger, R.; Ladisch, S. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res. 1986, 46, 440–443. [Google Scholar] [PubMed]
- Balis, F.M.; Busch, C.M.; Desai, A.V.; Hibbitts, E.; Naranjo, A.; Bagatell, R.; Irwin, M.; Fox, E. The ganglioside GD2 as a circulating tumor biomarker for neuroblastoma. Pediatr. Blood Cancer 2020, 67, e28031. [Google Scholar] [CrossRef] [PubMed]
- Cheresh, D.A.; Reisfeld, R.A.; Varki, A.P. O-acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science 1984, 225, 844–846. [Google Scholar] [CrossRef] [Green Version]
- Natali, P.G.; Bigotti, A.; Nicotra, M.R.; Nardi, R.M.; Delovu, A.; Segatto, O.; Ferrone, S. Analysis of the antigenic profile of uveal melanoma lesions with anti-cutaneous melanoma-associated antigen and anti-HLA monoclonal antibodies. Cancer Res. 1989, 49, 1269–1274. [Google Scholar]
- Herlyn, M.; Thurin, J.; Balaban, G.; Bennicelli, J.L.; Herlyn, D.; Elder, D.E.; Bondi, E.; Guerry, D.; Nowell, P.; Clark, W.H.; et al. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985, 45, 5670–5676. [Google Scholar] [PubMed]
- Birks, S.M.; Danquah, J.O.; King, L.; Vlasak, R.; Gorecki, D.C.; Pilkington, G.J. Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro Oncol. 2011, 13, 950–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, K.; Chava, A.K.; Mandal, C.; Dey, S.N.; Kniep, B.; Chandra, S.; Mandal, C. O-acetylation of GD3 prevents its apoptotic effect and promotes survival of lymphoblasts in childhood acute lymphoblastic leukaemia. J. Cell Biochem. 2008, 105, 724–734. [Google Scholar] [CrossRef]
- Fuentes, R.; Allman, R.; Mason, M.D. Ganglioside expression in lung cancer cell lines. Lung Cancer 1997, 18, 21–33. [Google Scholar] [CrossRef]
- Thurin, J.; Herlyn, M.; Hindsgaul, O.; Strömberg, N.; Karlsson, K.A.; Elder, D.; Steplewski, Z.; Koprowski, H. Proton NMR and fast-atom bombardment mass spectrometry analysis of the melanoma-associated ganglioside 9-O-acetyl-GD3. J. Biol. Chem. 1985, 260, 14556–14563. [Google Scholar] [CrossRef]
- Cavdarli, S.; Yamakawa, N.; Clarisse, C.; Aoki, K.; Brysbaert, G.; Le Doussal, J.M.; Delannoy, P.; Guérardel, Y.; Groux-Degroote, S. Profiling of O-acetylated gangliosides expressed in neuroectoderm derived cells. Int. J. Mol. Sci. 2020, 21, 370. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Helling, F.; Lloyd, K.O.; Livingston, P.O. Increased tumor cell reactivity and complement-dependent cytotoxicity with mixtures of monoclonal antibodies against different gangliosides. Cancer Immunol. Immunother. 1995, 40, 88–94. [Google Scholar] [CrossRef]
- Vukelić, Z.; Kalanj-Bognar, S.; Froesch, M.; Bîndila, L.; Radić, B.; Allen, M.; Peter-Katalinić, J.; Zamfir, A.D. Human gliosarcoma-associated ganglioside composition is complex and distinctive as evidenced by high-performance mass spectrometric determination and structural characterization. Glycobiology 2007, 17, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Marquina, G.; Waki, H.; Fernandez, L.E.; Kon, K.; Carr, A.; Valiente, O.; Perez, R.; Ando, S. Gangliosides expressed in human breast cancer. Cancer Res. 1996, 56, 5165–5171. [Google Scholar]
- Gocht, A.; Rutter, G.; Kniep, B. Changed expression of 9-O-acetyl GD3 (CDw60) in benign and atypical proliferative lesions and carcinomas of the human breast. Histochem. Cell Biol. 1998, 110, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Fahr, C.; Schauer, R. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J. Investig. Dermatol. 2001, 116, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Ji, L.; Kurono, S.; Fujita, S.C.; Furuya, S.; Hirabayashi, Y. Developmentally regulated O-acetylated sialoglycans in the central nervous system revealed by a new monoclonal antibody 493D4 recognizing a wide range of O-acetylated glycoconjugates. Glycoconj. J. 1997, 14, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Okita, H.; Nakajima, H.; Iijima, K.; Ogasawara, N.; Miyagawa, Y.; Katagiri, Y.U.; Nakagawa, A.; Kiyokawa, N.; Sato, T.; et al. Neuroblastoma cells can be classified according to glycosphingolipid expression profiles identified by liquid chromatography-tandem mass spectrometry. Int. J. Oncol. 2010, 37, 1279–1288. [Google Scholar]
- Cavdarli, S.; Delannoy, P.; Groux-Degroote, S. O-acetylated gangliosides as targets for cancer immunotherapy. Cells 2020, 9, 741. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Cheung, N.K.V. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 2014, 588, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terme, M.; Dorvillius, M.; Cochonneau, D.; Chaumette, T.; Xiao, W.; Diccianni, M.B.; Barbet, J.; Yu, A.L.; Paris, F.; Sorkin, L.S.; et al. Chimeric antibody c.8B6 to O-acetyl-GD2 mediates the same efficient anti-neuroblastoma effects as therapeutic ch14.18 antibody to GD2 without antibody induced allodynia. PLoS ONE 2014, 9, e87210. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Rueda, N.; Desselle, A.; Cochonneau, D.; Chaumette, T.; Clemenceau, B.; Leprieur, S.; Bougras, G.; Supiot, S.; Mussini, J.M.; Barbet, J.; et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PLoS ONE 2011, 6, e25220. [Google Scholar] [CrossRef] [Green Version]
- Sjoberg, E.R.; Manzi, A.E.; Khoo, K.H.; Dell, A.; Varki, A. Structural and immunological characterization of O-acetylated GD2. Evidence that GD2 is an acceptor for ganglioside O-acetyltransferase in human melanoma cells. J. Biol. Chem. 1992, 267, 16200–16201. [Google Scholar] [CrossRef]
- Fleurence, J.; Cochonneau, D.; Fougeray, S.; Oliver, L.; Geraldo, F.; Terme, M.; Dorvillius, M.; Loussouarn, D.; Vallette, F.; Paris, F.; et al. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget 2016, 7, 41172–41185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazet, A.; Bobowski, M.; Rombouts, Y.; Lefebvre, J.; Steenackers, A.; Popa, I.; Guérardel, Y.; Le Bourhis, X.; Tulasne, D.; Delannoy, P. The ganglioside GD2 induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the GD3 synthase. Glycobiology 2012, 22, 806–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, E.H.; Hakomori, S.I. Enzymatic basis for changes in fucoganglioside during chemical carcinogenesis. Induction of a specific α-fucosyltransferase and status of an α-galactosyltransferase in precancerous rat liver and hepatoma. J. Biol. Chem. 1983, 258, 3706–3713. [Google Scholar] [CrossRef]
- Vangsted, A.J.; Clausen, H.; Kjeldsen, T.B.; White, T.; Sweeney, B.; Hakomori, S.; Drivsholm, L.; Zeuthen, J. Immunochemical detection of a small cell lung cancer-associated ganglioside (FucGM1) antigen in serum. Cancer Res. 1991, 51, 2879–2884. [Google Scholar] [PubMed]
- Brezicka, F.T.; Olling, S.; Nilsson, O.; Bergh, J.; Holmgren, J.; Sörenson, S.; Yngvason, F.; Lindholm, L. Immunohistological detection of fucosyl-GM1 ganglioside in human lung cancer and normal tissues with monoclonal antibodies. Cancer Res. 1989, 49, 1300–1305. [Google Scholar]
- Brezicka, T.; Bergman, B.; Olling, S.; Fredman, P. Reactivity of monoclonal antibodies with ganglioside antigens in human small cell lung cancer tissues. Lung Cancer 2000, 28, 29–36. [Google Scholar] [CrossRef]
- Wu, C.S.; Yen, C.J.; Chou, R.H.; Li, S.T.; Huang, W.C.; Ren, C.T.; Wu, C.Y.; Yu, Y.L. Cancer-associated carbohydrate antigens as potential biomarkers for hepatocellular carcinoma. PLoS ONE 2012, 7, e39466. [Google Scholar] [CrossRef]
- Lewartowska, A.; Pacuszka, T.; Adler, G.; Panasiewicz, M.; Wojciechowska, W. Ganglioside reactive antibodies of IgG and IgM class in sera of patients with differentiated thyroid cancer. Immunol. Lett. 2002, 80, 129–132. [Google Scholar] [CrossRef]
- Hoon, D.S.B.; Jung, T.; Naungayan, J.; Cochran, A.J.; Morton, D.L.; McBride, W.H. Modulation of human macrophage functions by gangliosides. Immunol. Lett. 1989, 20, 269–275. [Google Scholar] [CrossRef]
- Yoshida, H.; Koodie, L.; Jacobsen, K.; Hanzawa, K.; Miyamoto, Y.; Yamamoto, M. B4GALNT1 induces angiogenesis, anchorage independence growth and motility, and promotes tumorigenesis in melanoma by induction of ganglioside GM2/GD2. Sci. Rep. 2020, 10, 1199. [Google Scholar] [CrossRef] [Green Version]
- Lang, Z.; Guerrera, M.; Li, R.; Ladisch, S. Ganglioside GD1a enhances VEGF-induced endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 2001, 282, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Hamamura, K.; Ohkawa, Y.; Ohmi, Y.; Furukawa, K. Disialyl gangliosides enhance tumor phenotypes with differential modalities. Glycoconj. J. 2012, 29, 579–584. [Google Scholar] [CrossRef]
- Young, M.; Kester, M.; Wang, H.G. Sphingolipids, regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Dippold, W.G.; Lloyd, K.O.; Li, L.T.; Ikeda, H.; Oettgen, H.F.; Old, L.J. Cell surface antigens of human malignant melanoma, definition of six antigenic systems with mouse monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1980, 77, 6114–6118. [Google Scholar] [CrossRef] [Green Version]
- Birklé, S.; Gao, L.; Zeng, G.; Yu, R.K. Down-regulation of GD3 ganglioside and its O-acetylated derivative by stable transfection with antisense vector against GD3-synthase gene expression in hamster melanoma cells, effects on cellular growth, melanogenesis, and dendricity. J. Neurochem. 2000, 74, 547–554. [Google Scholar] [CrossRef]
- Zeng, G.; Gao, L.; Birklé, S.; Yu, R.K. Suppression of ganglioside GD3 expression in a rat F-11 tumor cell line reduces tumor growth, angiogenesis, and vascular endothelial growth factor production. Cancer Res. 2000, 60, 6670–6676. [Google Scholar]
- Sottocornola, E.; Colombo, I.; Vergani, V.; Taraboletti, G.; Berra, B. Increased tumorigenicity and invasiveness of C6 rat glioma cells transfected with the human α2,8 sialyltransferase cDNA. Invasion Metastasis 1998–1999, 18, 142–154. [Google Scholar] [CrossRef]
- Hedberg, K.M.; Dellheden, B.; Wikstrand, C.J.; Fredman, P. Monoclonal anti-GD3 antibodies selectively inhibit the proliferation of human malignant glioma cells in vitro. Glycoconj. J. 2000, 17, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamamura, K.; Tsuji, M.; Ohkawa, Y.; Nakashima, H.; Miyazaki, S.; Urano, T.; Yamamoto, N.; Ueda, M.; Furukawa, K.; Furukawa, K. Focal adhesion kinase as well as p130Cas and paxillin is crucially involved in the enhanced malignant properties under expression of ganglioside GD3 in melanoma cells. Biochim. Biophys. Acta 2008, 1780, 513–519. [Google Scholar] [CrossRef]
- Cazet, A.; Groux-Degroote, S.; Teylaert, B.; Kwon, K.M.; Lehoux, S.; Slomianny, C.; Kim, C.H.; Le Bourhis, X.; Delannoy, P. GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biol. Chem. 2009, 390, 601–609. [Google Scholar] [CrossRef]
- Cazet, A.; Lefebvre, J.; Adriaenssens, E.; Julien, S.; Bobowski, M.; Grigoriadis, A.; Tutt, A.; Tulasne, D.; Le Bourhis, X.; Delannoy, P. GD3 synthase expression enhances proliferation and tumor growth of MDA-MB-231 breast cancer cells through c-Met activation. Mol. Cancer Res. 2010, 8, 1526–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.J.; Ding, Y.; Levery, S.B.; Lobaton, M.; Handa, K.; Hakomori, S.I. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4968–4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, T.R.; Battula, V.L.; Werden, S.J.; Vijay, G.V.; Ramirez-Peña, E.Q.; Taube, J.H.; Chang, J.T.; Miura, N.; Porter, W.; Sphyris, N.; et al. GD3 synthase regulates epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene 2015, 34, 2958–2967. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.; Furukawa, K.; Takahashi, T.; Urano, T.; Sanai, Y.; Nagino, M.; Nimura, Y.; Furukawa, K. Fundamental study of small interfering RNAs for ganglioside GD3 synthase gene as a therapeutic target of lung cancers. Oncogene 2006, 25, 6924–6935. [Google Scholar] [CrossRef] [Green Version]
- Aixinjueluo, W.; Furukawa, K.; Zhang, Q.; Hamamura, K.; Tokuda, N.; Yoshida, S.; Ueda, R.; Furukawa, K. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies, roles of anoikis. J. Biol. Chem. 2005, 280, 29828–29836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, C.; Sarkar, S.; Chatterjee, U.; Schwartz-Albiez, R.; Mandal, C. Disialoganglioside GD3-synthase over expression inhibits survival and angiogenesis of pancreatic cancer cells through cell cycle arrest at S-phase and disruption of integrin-β1-mediated anchorage. Int. J. Biochem. Cell Biol. 2014, 53, 162–173. [Google Scholar] [CrossRef]
- Kniep, B.; Kniep, E.; Ozkucur, N.; Barz, S.; Bachmann, M.; Malisan, F.; Testi, R.; Rieber, E.P. 9-O-acetyl GD3 protects tumor cells from apoptosis. Int. J. Cancer 2006, 119, 67–73. [Google Scholar] [CrossRef]
- Cochonneau, D.; Terme, M.; Michaud, A.; Dorvillius, M.; Gautier, N.; Frikeche, J.; Alvarez-Rueda, N.; Bougras, G.; Aubry, J.; Paris, F.; et al. Cell cycle arrest and apoptosis induced by O-acetyl-GD2-specific monoclonal antibody 8B6 inhibits tumor growth in vitro and in vivo. Cancer Lett. 2013, 333, 194–204. [Google Scholar] [CrossRef]
- Yu, R.K.; Ariga, T.; Yoshino, H.; Katoh-Semba, R.; Ren, S. Differential effects of glycosphingolipids on Protein Kinase C activity in PC12D pheochromocytoma cells. J. Biomed. Sci. 1994, 1, 229–236. [Google Scholar] [CrossRef]
- Gan, C.Z.; Li, G.; Luo, Q.S.; Li, H.M. miR-339-5p downregulation contributes to Taxol resistance in small-cell lung cancer by targeting α1,2-fucosyltransferase 1. IUBMB Life 2017, 69, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Houghton, A.N.; Mintzer, D.; Cordon-Cardo, C.; Welt, S.; Fliegel, B.; Vadhan, S.; Carswell, E.; Melamed, M.R.; Oettgen, H.F.; Old, L.J. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside, a phase I trial in patients with malignant melanoma. Proc. Natl. Acad. Sci. USA 1985, 82, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Nasi, M.L.; Meyers, M.; Livingston, P.O.; Houghton, A.N.; Chapman, P.B. Anti-melanoma effects of R24, a monoclonal antibody against GD3 ganglioside. Melanoma Res. 1997, 7, S155–S162. [Google Scholar] [CrossRef]
- Krug, L.M. Vaccine therapy for small cell lung cancer. Semin. Oncol. 2004, 31, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Erratum to: Dinutuximab, first global approval. Drugs 2015, 75, 1831. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.; et al. Children’s Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, E.; Mueller, B.M.; Handgretinger, R.; Herter, M.; Yu, A.L.; Reisfeld, R.A. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991, 51, 144–149. [Google Scholar] [PubMed]
- Durbas, M.; Horwacik, I.; Boratyn, E.; Kamycka, E.; Rokita, H. GD2 ganglioside specific antibody treatment downregulates PI3K/Akt/mTOR signaling network in human neuroblastoma cell lines. Int. J. Oncol. 2015, 47, 1143–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Wu, Y.; Zhou, Y.; Peng, D. Endothelin A receptor antagonism enhances inhibitory effects of anti-ganglioside GD2 monoclonal antibody on invasiveness and viability of human osteosarcoma cells. PLoS ONE 2014, 9, e93576. [Google Scholar] [CrossRef]
- Zeytin, H.E.; Tripathi, P.K.; Bhattacharya-Chatterjee, M.; Foon, K.A.; Chatterjee, S.K. Construction and characterization of DNA vaccines encoding the single-chain variable fragment of the anti-idiotype antibody 1A7 mimicking the tumor-associated antigen disialoganglioside GD2. Cancer Gene Ther. 2000, 7, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Horta, Z.P.; Goldberg, J.L.; Sondel, P.M. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy 2016, 8, 1097–1117. [Google Scholar] [CrossRef] [Green Version]
- Tivnan, A.; Heilinger, T.; Ramsey, J.M.; O’Connor, G.; Pokorny, J.L.; Sarkaria, J.N.; Stringer, B.W.; Day, B.W.; Boyd, A.W.; Kim, E.L.; et al. Anti-GD2-ch14.18/CHO coated nanoparticles mediate glioblastoma (GBM)-specific delivery of the aromatase inhibitor, Letrozole, reducing proliferation, migration and chemoresistance in patient-derived GBM tumor cells. Oncotarget 2017, 8, 16605–16620. [Google Scholar] [CrossRef]
- Gargett, T.; Yu, W.; Dotti, G.; Yvon, E.S.; Christo, S.N.; Hayball, J.D.; Lewis, I.D.; Brenner, M.K.; Brown, M.P. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol. Ther. 2016, 24, 1135–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charan, M.; Dravid, P.; Cam, M.; Audino, A.; Gross, A.C.; Arnold, M.A.; Roberts, R.D.; Cripe, T.P.; Pertsemlidis, A.; Houghton, P.J.; et al. GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevent primary tumor growth and metastasis in Ewing sarcoma. Int. J. Cancer 2020, 146, 3184–3195. [Google Scholar] [CrossRef]
- Golinelli, G.; Grisendi, G.; Prapa, M.; Bestagno, M.; Spano, C.; Rossignoli, F.; Bambi, F.; Sardi, I.; Cellini, M.; Horwitz, E.M.; et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2020, 27, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef]
- Ponath, P.; Menezes, D.; Pan, C.; Chen, B.; Oyasu, M.; Strachan, D.; LeBlanc, H.; Sun, H.; Wang, X.T.; Rangan, V.S.; et al. A novel, fully human anti-fucosyl-GM1 antibody demonstrates potent in vitro and in vivo antitumor activity in preclinical models of small cell lung cancer. Clin. Cancer Res. 2018, 24, 5178–5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickler, M.N.; Ragupathi, G.; Liu, N.X.; Musselli, C.; Martino, D.J.; Miller, V.A.; Kris, M.G.; Brezicka, F.T.; Livingston, P.O.; Grant, S.C. Immunogenicity of a fucosyl-GM1-keyhole limpet hemocyanin conjugate vaccine in patients with small cell lung cancer. Clin. Cancer Res. 1999, 5, 2773–2779. [Google Scholar] [PubMed]
- Nagorny, P.; Kim, W.H.; Wan, Q.; Lee, D.; Danishefsky, S.J. On the emerging role of chemistry in the fashioning of biologics, synthesis of a bidomanial fucosyl GM1-based vaccine for the treatment of small cell lung cancer. J. Org. Chem. 2009, 74, 5157–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, P.O.; Hood, C.; Krug, L.M.; Warren, N.; Kris, M.G.; Brezicka, T.; Ragupathi, G. Selection of GM2, fucosyl GM1, globo H and polysialic acid as targets on small cell lung cancers for antibody mediated immunotherapy. Cancer Immunol. Immunother. 2005, 54, 1018–1025. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groux-Degroote, S.; Delannoy, P. Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 6145. https://doi.org/10.3390/ijms22116145
Groux-Degroote S, Delannoy P. Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy. International Journal of Molecular Sciences. 2021; 22(11):6145. https://doi.org/10.3390/ijms22116145
Chicago/Turabian StyleGroux-Degroote, Sophie, and Philippe Delannoy. 2021. "Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy" International Journal of Molecular Sciences 22, no. 11: 6145. https://doi.org/10.3390/ijms22116145
APA StyleGroux-Degroote, S., & Delannoy, P. (2021). Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy. International Journal of Molecular Sciences, 22(11), 6145. https://doi.org/10.3390/ijms22116145






