Abstract
Proper skeletal muscle function is controlled by intracellular Ca2+ concentration and by efficient production of energy (ATP), which, in turn, depend on: (a) the release and re-uptake of Ca2+ from sarcoplasmic-reticulum (SR) during excitation–contraction (EC) coupling, which controls the contraction and relaxation of sarcomeres; (b) the uptake of Ca2+ into the mitochondrial matrix, which stimulates aerobic ATP production; and finally (c) the entry of Ca2+ from the extracellular space via store-operated Ca2+ entry (SOCE), a mechanism that is important to limit/delay muscle fatigue. Abnormalities in Ca2+ handling underlie many physio-pathological conditions, including dysfunction in ageing. The specific focus of this review is to discuss the importance of the proper architecture of organelles and membrane systems involved in the mechanisms introduced above for the correct skeletal muscle function. We reviewed the existing literature about EC coupling, mitochondrial Ca2+ uptake, SOCE and about the structural membranes and organelles deputed to those functions and finally, we summarized the data collected in different, but complementary, projects studying changes caused by denervation and ageing to the structure and positioning of those organelles: a. denervation of muscle fibers—an event that contributes, to some degree, to muscle loss in ageing (known as sarcopenia)—causes misplacement and damage: (i) of membrane structures involved in EC coupling (calcium release units, CRUs) and (ii) of the mitochondrial network; b. sedentary ageing causes partial disarray/damage of CRUs and of calcium entry units (CEUs, structures involved in SOCE) and loss/misplacement of mitochondria; c. functional electrical stimulation (FES) and regular exercise promote the rescue/maintenance of the proper architecture of CRUs, CEUs, and of mitochondria in both denervation and ageing. All these structural changes were accompanied by related functional changes, i.e., loss/decay in function caused by denervation and ageing, and improved function following FES or exercise. These data suggest that the integrity and proper disposition of intracellular organelles deputed to Ca2+ handling and aerobic generation of ATP is challenged by inactivity (or reduced activity); modifications in the architecture of these intracellular membrane systems may contribute to muscle dysfunction in ageing and sarcopenia.
1. Ca2+ Handling in Skeletal Muscle Fibers
Calcium ion (Ca2+) is an extremely versatile intracellular messenger that plays an important role in all cell types and in a variety of physiological functions. Transient elevations in intracellular Ca2+ concentration ([Ca2+]i) indeed serve as rapid signals to regulate cell communication, gene transcription, differentiation, metabolic regulation, neurotransmitter release, etc. [1,2,3,4]. In striated muscles, myofibril contraction and relaxation are controlled by Ca2+ release and re-uptake from the sarcoplasmic-reticulum (SR). Ca2+ release follows the depolarization of exterior membranes (sarcolemma and transverse tubules or TTs): the mechanism that links sarcolemmal depolarization to Ca2+ release from the SR is known as excitation–contraction (EC) coupling (Figure 1) [5,6,7,8,9]. The relaxation of muscle fibers after each cycle of contraction (either single twitch or tetanic) is achieved mainly by the action of specialized SR proteins known as SERCA-pumps (sarco-endoplasmic reticulum Ca2+ ATP-asis), which quickly reduces myoplasmic [Ca2+]i [10,11,12,13,14]. However, not all Ca2+ released during EC coupling is re-uptaken by the SR. A small amount of Ca2+ also enters the mitochondrial matrix to activate the mitochondrial respiratory chain and increase aerobic ATP production [15,16,17,18,19]: the effect of Ca2+ on the mitochondrial activity has been described as excitation–metabolism coupling (Figure 1). In addition, during repetitive and prolonged muscle stimulation, some Ca2+ is extruded in the extracellular matrix by plasma membrane (PM) Ca2+ ATPases (PMCA), causing a slight decrease in the SR Ca2+ content, a phenomenon known as SR depletion [20]. It has been demonstrated that SR depletion is the main trigger for activation of a mechanism known as store operated Ca2+ entry (SOCE) (Figure 1), which allows recovery of extracellular Ca2+ and replenishment of intracellular stores to counteract, or at least to limit, muscle fatigue [21,22,23,24,25,26].

Figure 1.
Ca2+ handling in skeletal muscle fibers. Intracellular Ca2+ concentrations in muscle fibers depend on: (A) the release and re-uptake of Ca2+ from intracellular SR stores during EC coupling, which controls the contraction and relaxation of sarcomeres; (B) the uptake of Ca2+ into the mitochondrial matrix during excitation–metabolism coupling, which stimulates aerobic ATP production; and finally (C) the entry of Ca2+ from the extracellular space via SOCE, a mechanism that is important to limit/delay muscle fatigue.
1.1. Excitation–Contraction (EC) Coupling
The term EC coupling was coined in 1952 by Alexander Sandow [6,7,27] to describe the physiological series of events that convert the electrical stimulus that propagates on external membranes of muscle cells (i.e., the action potential) into a mechanical response of muscle fibers due to the activation of contractile elements [9,28,29,30,31]. Before knowing anything about the molecular machinery involved in EC coupling, it was already shown that Ca2+ was the ion activating contraction of muscle cells [9,28,29,30,31,32,33,34], that Ca2+ activating contractile elements was released from the SR [35,36], and finally that relaxation of fibers occurred thanks to its re-uptake into the SR [13,37,38].
EC coupling in mammalian muscle cells has evolved in two main mechanisms: (a) Ca2+-induced Ca2+ release (CICR), which is used by cardiac and smooth muscle to link entry of Ca2+ from the extracellular space to Ca2+ release from internal SR stores [39,40,41,42,43,44,45,46,47]; and (b) mechanical coupling of skeletal muscle [5,8,29,48], a direct communication that does not require external Ca2+ [36]. Both mechanisms use the same proteins (though different isoforms) to allow communication between external (sarcolemma or TTs) and internal membranes (SR): (a) voltage-gated L-type Ca2+ channels of external membranes, also known as dihydropyridine receptors (DHPRs); and (b) SR Ca2+ release channels, i.e., the ryanodine receptors (RYRs) [49,50,51,52,53,54,55,56].
The main difference between the two systems of communication is that the alpha-1c subunit of the cardiac DHPR (also known as Ca.V 1.2) functions as a voltage-gated Ca2+ channel allowing entry of external Ca2+ when sarcolemma and TTs are depolarized [47,57,58], in turn activating the RYR type-2 channel. Indeed, in cardiac cells, DHPRs are not directly linked to RYR2 subunits [59,60,61,62]. On the other hand, the term mechanical coupling in skeletal muscle was coined because the alpha-1s subunit (also known as Ca.V 1.1) of voltage-gated L-type Ca2+ channels in TTs acts as a voltage sensor which directly activates the SR Ca2+ release channels from RYR type-1 [5,8,29,48,54,63,64,65,66,67]. This direct communication is allowed by the formation of tetrads, groups of four DHPRs associated with the four subunits of RYR type-1 [68,69,70,71,72,73,74,75]. Thanks to tetrads, the signaling between DHPR and RYR1 subunits during EC coupling has been proposed to be bi-directional: orthograde coupling (DHPR to RYR1) allows DHPR voltage sensors (activated by depolarization of the TT membrane) to open RYR1 and activate Ca2+ release, while retrograde coupling (RYR1 to DHPR) allows RYR1 to influence the Ca2+ conductance and gating properties of DHPRs [76,77,78].
As the RYRs open following depolarization, either activated by Ca2+ entry (during CICR in cardiac cells) or mechanically by conformational changes of the DHPR voltage sensors (in skeletal fibers), Ca2+ released from the SR rapidly diffuses into the cytoplasm to generate Ca2+ sparks, i.e., confined rises in Ca2+ concentration first described in cardiomyocytes [79,80]. Ca2+ sparks were later detected also in amphibian striated muscle fibers [81], and in mammalian fibers, but only under special conditions such as after permeabilization by saponine, osmotic stress, membrane damage, etc. [82,83,84]. Contrary to the initial idea of one spark representing the opening of a single RYR, Shtifman et al. [85] estimated the number of release channels contributing to the generation of one event to be between 2 and 4. In the end, though, the near synchronous activation of thousands of Ca2+ sparks during an action potential causes a cell-wide increase in [Ca2+]i, known as Ca2+ transient. The Ca2+ released into the cytosol during EC coupling will finally bind to troponin C, a regulatory protein localized in the thin filament of sarcomeres, to activate the crossbridge cycling of myosin by removing the inhibition of tropomyosin [86,87,88]. Contraction will end when myoplasmic [Ca2+]i will be lowered by removal, which is mainly operated by SERCA pumps [89,90].
Whereas DHPRs and RYRs are unquestionably the two main players in EC coupling, there are many other proteins that modulate and coordinate their interaction: calsequestrin, triadin, junctin, junctophilins, FK-506 binding protein-12 (FKBP-12), STAC3, histidine rich Ca2+ (HRC) binding protein, mitsugumins, etc. [91,92,93,94,95,96,97,98,99,100,101,102,103,104,105]. Most of those proteins are expressed in both cardiac and skeletal muscle cells (e.g., calsequestrin, triadin, junctin, junctophilins, junctate, FKBPs, HRC), while others are expressed only in one of the two (e.g., STAC3, JP45, and mitsugumins only in skeletal muscle). Together with RYR and DHPR, all those proteins constitute a macromolecular complex that coordinates the activation of SR Ca2+ release in skeletal fibers and cardiac cells.
1.2. Excitation–Metabolism Coupling
ATP is the source of energy for muscle fibers: (a) allows dissociation of the myosin heads from actin and its hydrolysis produces energy for force generation during the power stroke; (b) drives Ca2+ re-uptake by SERCA pumps during relaxation [106]; (c) is used by Na+/K+ ATPases to re-establish the proper balance of Na+ and K+ following the propagation of action potentials. As ATP is continuously used, several mechanisms are available to adequately match its availability: ATP may be produced anaerobically via hydrolysis of phosphocreatine, by anaerobic glycolysis [107,108], or by the aerobic metabolism within the mitochondrial matrix, which represents the major source of cellular ATP production in muscle [109]. A bi-directional interaction has been proposed between the SR and mitochondria in adult skeletal muscle fibers. Indeed this interaction involves an orthograde (SR to mitochondrion) and a retrograde (mitochondrion to SR) communication: (a) SR–mitochondrial signaling enhances aerobic ATP production through Ca2+-influx in the mitochondrial matrix [110,111,112]; whereas (b) mitochondrial-SR signaling would determine the ROS (reactive oxygen species)-dependent suppression of SR Ca2+ release [83,113].
Mitochondrial respiration and ATP production during muscle activity is controlled by a number of mechanisms [110,114]. Importantly, several mitochondrial dehydrogenases responsible for generating NADH (pyruvate, isocitrate, and 2-oxoglutarate dehydrogenases) and the ATP synthetic capacity of the F1F0-ATPase are stimulated by elevations in [Ca2+] in the mitochondrial matrix [110,111,114,115]. Thus, mitochondrial Ca2+ uptake during EC coupling (i.e., excitation–metabolism coupling) will stimulate aerobic ATP production to help keep pace with the increased ATP consumption associated with the augmented crossbridge cycling and the need for SERCA-mediated Ca2+ sequestration during muscle activity. An electrochemical gradient (>−180 mV) represents the driving force for mitochondrial Ca2+ entry. Several different mechanisms were postulated for entry of Ca2+ into the mitochondria: (i) a ruthenium red sensitive uniporter [116], (ii) a rapid mode Ca2+ transport mechanism [117], and (iii) a mitochondrial ryanodine receptor [118]. However, the recent identification of a mitochondrial calcium uniporter (MCU), a highly selective channel responsible for Ca2+ entry into mitochondria, may have unmasked the main pathway for mitochondrial Ca2+ uptake [119,120], even if the genetic ablation of MCU in the germline surprisingly displayed a mild phenotype [121].
The physiological relevance of mitochondrial Ca2+ uptake in skeletal fibers has long been debated, as the concentration of Ca2+ required for mitochondrial Ca2+ transport is considerably higher than the one achieved during global cytosolic Ca2+ transients elicited by EC coupling (~1–2 μM) [122,123,124]. A characteristic feature of the uniporter is its low affinity for Ca2+, with a Kd of around 10 μM in permeabilized cells [122,123,124]. MCU drives rapid and massive calcium entry, but only at cytosolic Ca2+ concentrations above the micromolar level (>10 μM) [123]. Nevertheless, elegant studies directly measuring mitochondrial Ca2+ clearly demonstrated a significant mitochondrial Ca2+ uptake in intact skeletal muscle [15,17,18,19]. Some studies proposed that this uptake occurs during both single twitches and tetanic stimulation [18], but these findings were not confirmed by others [125]. The apparent discrepancy between the low affinity mitochondrial Ca2+ transport and the magnitude of the Ca2+ transients could be reconciled by the concept of local Ca2+ microdomains (or nanodomains): strategic positioning of mitochondria in close proximity to sites of Ca2+ release would allow mitochondria to be exposed to a high pulse of Ca2+ before passive diffusion into myofibrils. An intimate structural interaction between mitochondria and endoplasmic/sarcoplasmic reticulum (ER/SR) have been demonstrated in non-muscle cells [126,127], in cardiomyocytes [128,129], and finally in skeletal muscle fibers [130].
In 1997, an elegant study reported how contractile relaxation was slower in more glycolytic fibers that contain fewer mitochondria than in mitochondria-rich slow twitch fibers [131]. It was argued that, although Ca2+ uptake by individual mitochondria is limited, a sufficiently large number of strategically positioned mitochondria may influence local and global Ca2+ transients elicited by EC coupling under physiological conditions. Other studies also demonstrated that the onset of local Ca2+ release events (Ca2+ sparks) in permeabilized skeletal muscle fibers was inversely proportional to the mitochondrial content, suggesting that mitochondria could inhibit spontaneous Ca2+ sparks [83,113]. Certainly, the evidence presented indicates that mitochondria are capable of physiological Ca2+ uptake, possibly during both single twitches and tetanic stimulation [16], though whether mitochondria accumulate significant amounts of Ca2+ and influence the size and temporal developments of Ca2+ transients remains a controversial issue.
1.3. Store-Operated Ca2+ Entry (SOCE)
During prolonged muscle activity a fraction of Ca2+ ions cycled by the SR during EC coupling is lost across the sarcolemma due to extrusion by Na+/Ca2+ exchangers of PM Ca2+ ATP-asis (PMCA) [132,133,134,135]. As loss of intracellular Ca2+, and a consequent reduction in the amount of Ca2+ stored in the SR (i.e., SR depletion), is one of the factors that may determine the onset of premature fatigue; muscle has developed a system to counteract this phenomenon and recover external Ca2+ during repetitive muscle activity [21,23,24,136,137,138]. This mechanism is known as SOCE, a pathway that allows extracellular Ca2+ to enter the cytosol and refill SR stores during muscle fatigue [138,139].
SOCE was first described in non-excitable cells, where its activation was triggered by the depletion of ER intracellular Ca2+ stores [20,140]. The molecular identity of the proteins responsible for SOCE, though, remained elusive for almost twenty years, until the two main proteins involved in this mechanism were discovered in patients affected by a severe immunodeficency: (a) stromal-interacting molecule-1 (STIM1), an ER protein which has an intraluminal portion that functions as a Ca2+ sensor [141,142,143]; and (b) ORAI1, a Ca2+ release activated channel (CRAC) of the PM [144,145,146,147]. The proposed mechanism for activation of SOCE involves depletion of intracellular stores that, in turn, induces Ca2+ dissociation from the luminal STIM1 N-terminal EF-hand domain and consequent conformational changes, dimerization, and relocation of STIM1 to ER sites of contact with external membranes [148,149,150]. Aggregated STIM1, in turn, recruits and traps ORAI1 channels into junctions, or puncta, formed by the association of STIM1-bearing ER with the external membrane. Finally, the STIM1-mediated opening of ORAI1 channels allows entry of Ca2+ into the cell from the extracellular space and replenishment of intracellular stores [151,152].
SOCE was reported in skeletal muscle in 2001 [24,137] when STIM1 and ORAI1 were not yet discovered. In 2008, though, Lyfenko and Dirksen [22] demonstrated that, also in skeletal muscle fibers, SOCE is mediated by interactions between STIM1, in the SR, and ORAI1 localized in the invaginations of the sarcolemma, the TTs, the specialized invaginations of the PM that carry the action potential [22,138,153]. In addition, calsequestrin-1 (CASQ1), a key SR protein that controls intracellular Ca2+ homeostasis [154] as a Ca2+ buffer and a modulator of RYR1, has been proposed to modulate SOCE [155,156,157]. SOCE in muscles developed some peculiarities with respect to other tissues, as a result of adaptation to the needs of skeletal fibers. For example, while, in non-muscle cells, the activation of CRAC channels needs over ~1 min [144], experiments in skinned skeletal muscle fibers have provided evidence that Ca2+ influx can be activated very quickly (<1 s) following Ca2+ store depletion [23,136,158]. One of the explanations, given a few years previously, for this rapid SOCE activation was that STIM1 and ORAI1 could be already clustered in pre-formed SR-TT junctions [25]. A STIM1 splice variant highly expressed in skeletal muscle, STIM1-long, was discovered and proposed to mediate rapid SOCE activation [159,160]. There is now general agreement that SOCE plays an important role in muscle physiology not only as a mechanism to counteract fatigue, but also in other aspects of muscle functions from differentiation to early development and late life. Carrell and colleagues reported that Orai-1 plays an important role in the maintenance of fatigue-resistant type I fibers [161]. In addition, impaired SOCE activity was proposed to contribute to muscle impairment in ageing [162,163,164], though this finding was not confirmed by others [165,166]. Finally, to explain the various roles of SOCE in skeletal muscle function, STIM1 has been proposed to act as a multipurpose stress transducer activated by different stimuli (depletion, oxidation, temperature, hypoxia and acidification) that, in turn, may regulate multiple downstream targets [167].
2. Architecture of the Membrane Systems and Organelles Involved in Ca2+ Handling and Aerobic ATP Production
In skeletal muscle fibers, the different functions that we described in Section 1 (i.e., EC coupling, cellular respiration, and SOCE) are executed by specific organelles: (a) EC coupling occurs at specialized junctions between SR and TT named Ca2+ release units (CRUs), or triads [9,168]; (b) aerobic ATP production takes place in mitochondria [169,170,171,172]; (c) SOCE is mediated by Ca2+ Entry Units (CEUs), again SR and TT junctions in continuity with membranes of CRUs, but with different morphologies and molecular components [173,174]. In adult fibers, each of these junctions/organelles retains a specific intracellular position, dictated somehow by the striation of contractile elements (see below). Cross striation is generated by the lateral alignment of contractile elements, long cylinders named myofibrils, which are constituted by repeating contractile units, the sarcomeres, in which thick and thin filaments generate alternating dark and pale bands—the A-band and the I-band, respectively.
2.1. Calcium Release Units (CRUs), or Triads: The Sites of EC Coupling
EC coupling in muscle fibers occurs in specialized structures, known as CRUs, formed by the close apposition of two membrane systems: TTs, carrying the sarcolemmal depolarization into the fiber interior [175], and the SR terminal cisternae, containing the Ca2+ needed for muscle activation [74]. CRUs in adult muscle are also named triads, as they are formed by three elements: a central TT—showing a narrow and flat profile—with two lateral terminal cisternae of the SR (Figure 2A,B) [73].
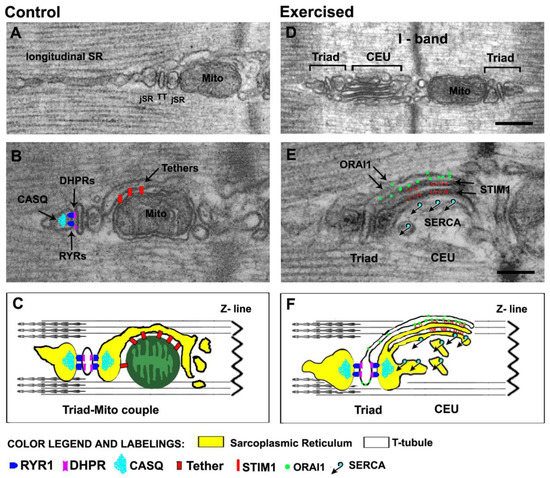
Figure 2.
Architecture of the membrane systems and organelles involved in Ca2+ handling and aerobic ATP production. (A–C) In adult mammalian fibers, triads (or CRUs) are placed approximately at the A–I band transition when sarcomeres are relaxed. The SR that does not participate in the formation of junctions with TT constitutes the longitudinal SR, placed either at the A or I band. Triads contain the molecular players of EC coupling (RYRs, DHPRs, and CASQ) shown in panel (B) and are tethered to mitochondria, which are preferentially placed at the I band. In panel C, the cartoon shows a mitochondrion-triad couple. (D–F) Following acute exercise, sarcotubular membranes at the I band (both SR and TTs), are capable of significant remodeling, which eventually leads to the assembly of CEUs. CEUs are formed by SR-stacks, coupled with a TT and contain the molecular players of SOCE (STIM1 and ORAI1) shown in panel (E). In panel (F), the cartoon shows a triad and a CEU, two intracellular junctions which are structurally and molecularly different. Scale bars: (D) 0.2 μm; (E) 0.1 μm.
The assembly of triads in adult skeletal muscle fibers represents the final result of a differentiation-maturation process in which CRUs have different morphologies [73,74,176]. (Step 1) During differentiation, i.e., in myotubes, the interaction between DHPRs and RYRs occurs in junctions between the SR and sarcolemma, in peripheral couplings (PCs). (Step 2) Later, when fibers present a more organized contractile apparatus, and once the sarcolemma forms the invaginations known as TTs, the junctional SR (jSR) associates with longitudinal TTs, i.e., parallel to the long axis of myofibrils. (Step 3) Finally, in adult fibers, most TTs become transversal and assume their final position, approximately, at the transition between the I and A bands of relaxed sarcomeres (2.2–2.4 μm long), forming two transversal networks, one at each side of the Z-lines [177,178]. This specific position seems to be the final location of triads in mammalian fibers as it is retained both in fast and slow twitch fibers, with some difference in number/area and size of the individual elements [74,176].
The depolarization coming from the neuromuscular junction spreads along the sarcolemma where it is detected by the DHPRs, voltage sensors specifically localized in the TTs [8,54,179]. DHPRs are in direct communication with the closely apposed Ca2+ release channels of the SR, the RYRs (Figure 2B,C) [67,180,181,182]. The physical/mechanical coupling between DHPRs and RYRs occurs in triads and results in the sudden opening of the RYR Ca2+ release channels with a consequent massive efflux of Ca2+ from the SR lumen into the myoplasm [5,29,65,75] (see Section 1 for additional details). The observation of triads at higher magnifications using electron microscopy (EM) makes it possible to appreciate other important details:
- (a)
- The gap between TT and SR is visually occupied by dark electron-densities, representing the cytoplasmic domain of RYRs, known as feet. RYR-feet in skeletal CRUs form ordered arrays in which RYRs touch each other corner-to-corner, forming ordered arrays [72,183].
- (b)
- Inside the lumen of SR terminal cisternae, a dark matrix reveals the presence of calsequestrin (CASQ), the main SR Ca2+ buffer, which accumulates large amounts of Ca2+ in proximity of the sites of release [154,184,185,186,187,188]. The CASQ matrix appears to be anchored at the SR terminal cisterna via thin and long strands, which have been proposed to be constituted by triadin (Figure 2B,C) [189,190,191].
Contrary to RYRs feet, DHPRs voltage sensors are not visible in standard EM as they are almost entirely embedded within the TT lipid bilayer. DHPRs have, however, been visualized as peculiar groups of four particles named tetrads, in a series of elegant papers by C. Franzini-Armstrong and colleagues, through the use of freeze fracture (FF) preparations, a technique that makes it possible to break the lipid bilayer of TTs [61,72,75]. The demonstration that DHPRs need association with RYR1 subunits to form tetrads came in experiments in which lack of RYR1 in dyspedic cells resulted in DHPRs being randomly disposed, as in cardiac cells [70,71]. Re-expression of Ryr1, but not of Ryr2 or Ryr3, two isoforms expressed mostly in cardiomyocytes and in the nervous system, restored tetrads’ arrangements [68,69].
2.2. Mitochondria: The Powerhouse of the Cell
In mammalian skeletal muscle fibers, myofibrils align laterally to generate a regular cross-striation, and sites of Ca2+ release occupy specific positions (see previous section). Interestingly, mitochondria, or at least a subpopulation of them, also seem to occupy a preferential disposition in skeletal muscle fibers. Ogata and Yamasaki, in EM studies, described a population of intermyofibrillar mitochondria surrounding myofibrils as incomplete rings in correspondence of the I band, on either side of the Z-line [192]. These I band mitochondria are present in fast, intermediate, and slow fibers of adult rat leg muscles. In addition to I-band mitochondria, slow-twitch fibers also contain large groups of mitochondria under the sarcolemma (mostly in proximity of capillaries) and often forming longitudinal columns between myofibrils, though columnar mitochondria are rare in fast-twitch fibers [192].
In 2009, we described the close proximity between mitochondria and sites of Ca2+ release [15,130], highlighting the presence of small electron-dense strands (generically named tethers) linking the outer mitochondrial membrane to the jSR (Figure 2A–C). Treatment with hypotonic solution, which caused swelling of jSR as well as mechanical stretching of tethers, was unable to disrupt the SR–mitochondria association [130]. However, despite these experiments suggesting that tethers may function as holders of the mitochondrial position next to CRUs, it is still unclear whether the EC coupling system is involved as a tether regulator. Even if a possible contribution of Mitofusin-2 in the formation was proposed [16], the molecular identity of tethers is still unknown.
The intimate association between mitochondria and CRUs is achieved progressively during post-natal maturation of skeletal fibers in a process that also involves remodeling of the mitochondrial network. Specifically, before reaching their final position next to triads (triadic mitochondria), the majority of mitochondria for a few weeks after birth (in mice), are disposed longitudinally between myofibrils and/or under the sarcolemma. This longitudinal disposition is similar to that frequently found in adult slow-twitch fibers, but rarely in fast-twitch fibers. Interestingly, the shift of the mitochondrial network from longitudinal to transversal closely mimics the maturation of the EC coupling apparatus development, except for a temporal delay, as TTs are completely transversal when most mitochondria are still longitudinal.
2.3. Calcium Entry Units (CEUs): The Dynamic Junctions That Mediate SOCE
Activation of SOCE requires an interaction between ORAI1, a CRAC channel placed in external membranes (either PM or TT) and STIM1, a Ca2+ sensor of ER/SR membranes [141,142,144,145,193,194,195]. In non-muscle cells, several studies proposed ER-PM junctions that form following the depletion of intracellular stores as the sites of SOCE [144,196,197]. Perni and colleagues, using FF preparations, also visualized clusters of ORAI1 channels in the puncta of HEK-293 cells [196].
While activation of SOCE in non-muscle cells requires several seconds, the kinetics of SOCE activation in skeletal muscle has been proposed to be faster [23,158,198]. This rapid SOCE activation suggested that STIM1–ORAI1 complexes should already be assembled, or at least pre-localized, in existing junctions [25]. As in triad junctions, TTs (external membranes which contain ORAI1) and SR (which contains STIM1) are already associated with each other to mediate EC coupling this site would have been, in principle, the perfect location for SOCE [23,25]. For the above reasons, the triad has been suggested as the ideal site for SOCE. Although the conclusion is not illogical, it ignores the fact that there is no direct evidence for the presence of STIM1/ORAI1 in triads. In addition, some peculiar features of the triad junctions may have represented a limit for proper STIM1–ORAI1 interaction; thus, the possibility that SOCE may occur at sites different from the triads remained open. The jSR–TT gap at the triad is a fairly crowded space where many proteins, such as RYR and DHPR, junctophilins, FKBP12, triadin, junctin, mitsugumin, STAC3, etc., modulate EC coupling and participate in the assembly and maintenance of the triad junction [70,168,191,199,200,201]. The presence of this complex macromolecular machinery may result in only limited opportunity for migrations of STIM1 oligomers in the SR to recruit ORAI1 channels in the TT.
Recently published evidence suggests that the most likely site for SOCE may indeed not be the triad [173,174,202,203,]. First, colocalization of STIM1 and ORAI1 is minimal in control conditions, with most of STIM1 placed in the SR at the I band [21,174], while ORAI1 is mostly placed in TTs at the triad junction, colocalized with an EC coupling protein (i.e., RYR1). Second, a single bout of incremental exercise on treadmill designed to induce muscle fatigue triggered a significant re-modelling of both SR and TT at the I band, and the formation of new junctions that are structurally and molecularly different from triads, as they do not contain RYRs, but colocalized STIM1 and ORAI1. (Figure 2D–F). STIM1–ORAI1 colocalization was promoted by elongation/relocation of TT bearing ORAI1 to the I band region rich in STIM1 [174,]. These new junctions were proposed to function as site of Ca2+ entry during SOCE, as their presence was accompanied by increased resistance of isolated muscles to fatigue in the presence of external Ca2+, but blocked by SOCE inhibitors 3,5-bis(trifluoromethyl)pyrazole derivative (BTP-2) and 2-aminoethoxydiphenyl borate (2-APB) [204,205]. These SR-TT junctions at the I band, named Ca2+ Entry Units (CEUs) [173,174], are also present in control conditions, just significantly smaller and less frequent, which could be the reason why they were never characterized and reported earlier. Whether these are the sites that mediate rapid SOCE in control conditions or if some STIM1–ORAI1 interaction occurs also in triads, is still debated, even if the former hypothesis is, in our eyes, the most likely. Additional strong evidence that junctions assembling during exercise at the I band are indeed sites of SOCE came when we reported that assembly of SR-TT junctions at the I band during SOCE resulted in increased rate of Mn2+ quench, in a time course study where TT elongation during exercise and retraction during recovery controlled the functional entry of divalent cations in the myoplasm of isolated single fibers [203]. Two other manuscripts finally showed: (a) that CEUs are constitutively assembled in CASQ1-knockout fibers [202], which undergo deep depletion during repetitive stimulation [206]; (b) that SOCE is dysfunctional in fibers of aged mice, where CEUs are reduced in number, but is rescued by long-term exercise [207]. A commentary published in J. Gen. Physiol. proposed CEUs as the backdoor for Ca2+ ions in muscle cells, bringing new attention to the importance of external Ca2+ in the function skeletal fibers [208].
3. Disarray of Intracellular Organelles in Denervation and Ageing
Ageing is a phenomenon which causes structural and functional deterioration of body organs. Among them, a significant decline in neuromuscular function and performance greatly affects quality of life for elderly individuals [209,210,211,212,213,214,215,216], as well as their independence in everyday life, with dramatic increases in health care costs [217,218,219]. One of the main effects of ageing is a drastic reduction in muscle mass that occurs in various measures in most individuals [220,221,222,223]. This phenomenon is known as sarcopenia, and causes a loss of 40 to 50% of muscle mass in sedentary individuals between the ages of 30 and 70 [223,224,225,226,227]. This severe muscle wasting is the combined result of a variety of changes: loss of motor units due to progressive denervation, fast-to-slow fiber type switching, mitochondrial loss, oxidative stress, etc. [211,228,229,230,231,232].
In different, but complementary, projects we have collected several independent sets of data regarding the structural and functional decline of EC coupling and mitochondrial machineries caused either by long- and short-term denervation, or by sedentary ageing in human biopsies and animal models (rabbits, rats, and mice) (Figure 3). We have also collected some evidence of ultrastructural and functional changes occurring in the SOCE machinery of ageing mice.

Figure 3.
Disarray of intracellular organelles in denervation and ageing. (A–D) Representative EM images of EDL muscle fibers from adult (A), aged (B,C) and denervated mice (D). Ageing and denervation cause a similar misplacement and damage of mitochondria (B,D, empty arrows). In panel (C), a TA in ageing muscle (black arrow) and a disordered TT network (dashed oval), labelled in black with ferrocyanide, are shown. (E) A cartoon summarizing the main structural changes in ageing and denervation: loss of proper position of triads and mitochondria, and mitochondrial damage. Scale bar: (A,B,D) 0.5 μm; (C), 1 μm; insets, 0.1 μm.
3.1. Denervation Causes Disarray of EC Coupling and Mitochondrial Machineries
Among the numerous mechanisms that contribute to aged-related atrophy and degeneration of muscle tissue, denervation and loss of some motor units [233] seem to be important contributing factors. Several studies have shown that fast-twitch (type II) fibers are more affected by denervation than slow-twitch (type I) fibers [234,235] and that ageing leads to fast-to-slow transition in-fiber typing (thanks to cross innervation from other motor units), even if there is no general consensus on this finding.
Denervation of fast twitch motor units results in loss of muscle mass (atrophy), and functional and structural alterations of the fibers [236,237]. Between 2004 and 2019, we have studied the effect of short- and long-term denervation on muscle fibers in human biopsies from spinal cord injury (SCI) patients and in the muscle of rabbits and rats [238,239,240,241,242,243,244,245]. While a lot was known about the effect of denervation resulting in the reduction in the fiber diameter (atrophy) and in the disruption of contractile elements, its effect on the EC coupling system was completely overlooked. In two papers published in 2004 and 2007, we found that, following denervation, CRUs, which in adult mammalian fibers should be formed by three elements (i.e., triads; see Section 2 for additional details), lose proper orientation (becomes oblique or longitudinal), display altered morphology, and progressively disappear (their number is greatly reduced) [240,241]. This disarray of the EC coupling system was also confirmed in muscles from rabbits and rats, denervated for shorter periods (3–8 months) [243,244,245]. Interestingly, even after years of denervation, when fibers are only small tubes with a completely disrupted contractile apparatus, some deformed CRUs are still present [240,241]. The presence of some residual elements of the EC coupling machinery could explain why, even after 3–4 months of denervation, at least a subpopulation of muscle fibers maintains a partially functional EC coupling system [245]. It is worth noting that the changes in orientation, morphology, and number of CRUs detected in denervation resemble those of biopsies of elderly individuals (see next section below for additional details) [246].
Denervation in the muscles of rats and rabbits (3–8 months of denervation) also caused loss in proper disposition of mitochondria, which move from their preferential placement at the I band to become more longitudinal at the A band [243,244,245]. Interestingly, a similar pattern of de-modeling of the mitochondrial network was noted in muscle biopsies of elderly individuals and in extensor digitorum longus (EDL) muscle fibers of aged mice (see next section below for additional details) [247,248]. In Pietrangelo et al. 2019, we confirmed that denervation causes un-coupling of mitochondria from triads [238] and gathered some additional information about the timeframe causing these changes. Relocation of part of mitochondria in longitudinal columns between myofibrils is a relatively fast phenomenon, detected after 3–14 dd of denervation in mice, and after 15 dd of denervation in rats (Figure 3D). Moreover, it is worth noting that: (a) the loss of proper architecture of the metabolic machinery precedes the structural disarray of the EC coupling, suggesting that mitochondrial positioning is less stable than that of TT and SR (in Section 2, we described how, during post-natal maturation, the EC coupling system becomes transversal before the mitochondrial network; see also [130]); (b) the misplacement and un-coupling of mitochondria from CRUs is reversible and can be restored quite completely simply by muscle activity (an aspect that we will discuss more in depth in Section 4).
3.2. Misplacement of Intracellular Membranes and Organelles in Ageing
The diminished force output of ageing muscle is greater than the loss of mass, meaning that the specific force (SF) of ageing muscle (the force calculated by normalizing the maximal force produced by the muscle to its cross-sectional area) is smaller than the one measured in the adult. A possible explanation for the diminished SF could be a reduction in the supply of Ca2+ ions available for muscle contraction. An impairment in the events linking the action potential generated at the neuromuscular junction to the Ca2+ release from the SR has been proposed to explain age-related muscle weakness [229,249,250,251,252], and has been defined by Delbono and colleagues as EC un-coupling [250,253,254,255]. This un-coupling would be caused by a reduced expression/function of the α1SDHPR subunit, the voltage sensor of EC coupling in TTs (see Section 1.1). However, conflicting results can also be found in the literature, as a persistent expression of α1SDHPR in human ageing muscle was also reported [256].
To contribute to this controversy, we have studied the ultrastructure and geometry of the EC coupling (and of the mitochondrial) apparatus in human biopsies and murine muscles. Summaries of our results are given in the following paragraphs.
Boncompagni et al. 2006: contraction of muscle fibers is activated by a rapid increase in intracellular Ca2+ concentration known as Ca2+ transients. Transients, however, represent the final result of many individual Ca2+ release events, known as sparks [79,80,85], from different Ca2+ release sites [257,258,259], i.e. the triads, which in adult muscle are evenly distributed in the fiber interior, next to the I–A junction of each sarcomere (see Section 2.1. for additional details). To determine the possible reasons underlying the reduction in Ca2+ ions supply in ageing muscle [250,255], we analysed, using transmission EM, the ultrastructure and geometry of the EC coupling system in human muscle biopsies from vastus lateralis and glutaeus medius muscles of adult and aged individuals, and found that ageing causes significant disarray of membranes involved in EC coupling [246]. These alterations consisted primarily of: (a) progressive disarrangement of triads; and (b) reduction in the overall number of Ca2+ release sites available for triggering contraction. We reported a loss of 30–40% of release sites in aged specimens, a decrease that could explain the inefficient delivery of Ca2+ ions to the contractile apparatus and the impaired generation of force output. This finding provided a fairly sound structural explanation for the impaired transduction of the action potential into efficient increases in intracellular Ca2+ concentration reported in the literature [250,254].
Pietrangelo et al. 2015: in this study, we focused our attention on the association and functional crosstalk between CRUs and mitochondria using an integrated approach (not only EM, but also confocal microscopy, with functional and biochemical measurements of mitochondrial Ca2+ uptake, membrane potential and oxidative stress) [248]. This work reported: (a) that the n./volume of CRUs, mitochondria, and tethers decrease significantly with age, resulting in partial un-coupling between the two organelles; and (b) a fraction of mitochondria move from the I band position (Figure 3A) to being more longitudinally oriented at the A band (Figure 3B). Similar observations were also collected in human muscle biopsies from sedentary elderly individuals [247]. The age-related structural changes in number and disposition of CRUs and mitochondria with respect to myofibrils are shown in the cartoon in Figure 3E. The study also contains a correlation between structural modifications and functional changes, i.e., reduced Ca2+ transients and reduced mitochondrial Ca2+ uptake. The fact that these age-dependent changes are similar to those observed in human skeletal muscle biopsies [246,247] suggests that they may represent modifications that are specific to sedentary ageing. While mitochondrial modifications (structure, function, and number) have been widely reported both in age- and disease-related conditions [260,261,262,263,264], the data presented in this work draw attention to a previously unreported aspect: the fact that mitochondria tend to lose association to sites of Ca2+ release and position with respect to myofibrils.
Boncompagni et al. 2021: tubular aggregates (TAs) are abnormal accumulations of orderly disposed SR tubes which have been described in a variety of disorders [265,266,267,268,269,270], including TA myopathy (TAM), a disease recently linked to mutations in STIM1, ORAI1 [271,272,273,274,275,276], and CASQ1 [277]. Schiaffino et al. 1977 demonstrated that the formation of TAs is induced by anoxia in isolated rat muscle [278], and TAs have been also found in fast twitch fibers of male ageing mice (Figure 3C) [279,280,281]. In our knowledge, the presence of TAs has not been confirmed in aged human muscles.
In 2012, we showed how TAs, presumably representing improper remodeling of the SR, stain positive for CASQ1, but negative for RYRs, as triads are confined at their periphery [281]. In Boncompagni et al. 2021, TAs in EDL of aged mice were stained with antibodies against STIM1 and ORAI1, the two proteins that mediate SOCE: accumulation of STIM1 and ORAI1 in TAs correlates with an increased fatigability compared to adult mice [207]. Experiments in which extracellular Ca2+ was removed, unmasked the fact that muscles containing TAs seemed unable to use extracellular Ca2+ via SOCE during fatigue protocols [207]. These findings support previous works showing how a reduction in SOCE activity contributes to muscle weakness during ageing [162,163,164]. All of the above, together with the following additional two observations—(a) TAs accumulates, in addition to STIM1 and ORAI1, also CASQ1 [281], another protein that modulates SOCE [144,193,282], and (b) the presence of CEUs, the junctions that mediate SOCE, is reduced in aged fibers—led to the following conclusion: TAs must represent an SR demodeling that results in improper accumulation of proteins and dysfunctional SOCE.
4. Are Alterations in Denervation and Ageing Mainly Caused by Inactivity?
As described in detail in Section 2, both denervation and sedentary ageing result in severe disarray to EC coupling, mitochondrial, and SOCE machineries. However, in denervation, the injury causing muscle paralysis is not a direct insult to the muscle itself; hence, muscle should be, in principle, perfectly functional if appropriately stimulated. In ageing, on the other hand, one of the factors that needs to be taken into consideration is the progressive reduction in activity associated with ageing for both humans and animals. Hence, whether the alterations described in Section 3 are induced by denervation or by ageing per-se, or whether a lack of (or simply reduced) muscle activity would play a central role, remains to be determined.
4.1. Functional Electrical Stimulation (FES) Rescues the Ultrastructure of the EC Coupling Apparatus
We studied the effect of FES in different conditions: (a) in muscle of patients with SCI to restore muscle structure and mass; (b) as a supportive measure to improve muscle function in elderly individuals; and finally (c) as a therapy in a patient affected by a rare myopathy (central core disease, CCD) caused by a mutation in a protein involved in EC coupling (RYR1), and that causes mitochondrial damage and muscle weakness.
In a series of papers published between 2004 and 2010 [240,241,283,284], we used transmission EM to analyze muscle biopsies from patients who suffered complete lesion of the spinal cord. Their muscles were stimulated with electrical devices for prolonged periods (several years), but they started therapy at a time point in which alteration of muscle fibers due to denervation was already quite severe (a year or more). EM analysis of biopsies after therapy revealed a striking restoration of denervated muscles induced by home-based FES of those patients performing therapy 3–5 times a week [240,241,283,284]. The EM data published in Kern et al. 2004 and Boncompagni et al. 2007 [240,241] showed, beside FES-induced rescue of contractile elements, also rescue of the membrane elements that mediate EC coupling, with perfectly shaped triads placed in the correct sarcomeric position, i.e., at the transition between the I and the A bands. Retrospective analysis of EM micrographs also indicates that the mitochondrial position at the I band was rescued to some degree [130]. Experiments in denervated rabbits confirmed that FES is quite effective in rescuing the intracellular structure of skeletal fibers [244].
The mechanism underlying the recovery of skeletal fibers induced by FES in the absence of innervation continues to be debated. In Biral et al. 2008, we reported the presence of apparently atrophy-resistant fibers in permanently denervated human skeletal muscle [242]. In Squecco et al. 2009, experiments in denervated muscle from rats indicated that excitably of skeletal fibers persisted for prolonged periods after the denervation injury [245]. In Kern et al. 2004 and 2010, and in Boncompagni et al. 2007, we showed that some EC coupling units (i.e., triads), while deformed, are still present in muscle fibers even after years of denervation [240,241,284]. Finally, in Carraro et al. 2015, we reported how muscle fiber regeneration persists for years in long-term denervated muscle [285]. Altogether, these observations may provide the framework for the positive response of long-term denervated muscle to FES.
We also applied FES to different subjects or patients affected by muscle weakness:
- As a supportive measure to improve muscle function in healthy, but sedentary, seniors: in this study, FES was able to improve the muscle trophism, force, and cross-sectional area of fast muscle fibers thanks to the up-regulation of IGF-1 and the down-regulation of some atrogenes (atrogin and MuRF-1) [286];
- As an alternative therapy to improve muscle function and structure in a patient affected by a debilitating myopathy caused by a mutations in RYR1 (i.e., central core disease, CCD) [287].
4.2. Exercise Prevents/Rescues the Ultrastructural Modifications Caused by Inactive Ageing and Denervation
Recently, we studied the effect of long-term training on the function, structure and connectivity between EC coupling units and mitochondria in humans and mice, focusing on: (a) muscle biopsies from well-trained seniors (average of 70 years of age) and age-matched healthy controls; (b) EDL muscles from 2-year-old mice, either sedentary or trained for 1 year in wheel cages; and finally (c) short-term denervated and re-innervated EDL muscles from adult rats. In the first study, three groups of male participants were included: (i) a group of well-trained seniors (average of 70 years of age) who exercised regularly in the previous 30 years of their lives, (ii) age-matched healthy sedentary seniors, and finally (c) active young men (average of 27 years of age). The results collected in this study [247] indicate that lifelong physical exercise prevented the age-related structural decay of intracellular organization, which causes misalignment of myofibrils, and partial misplacement of EC coupling and mitochondria from their proper intracellular disposition. Furthermore, the mitochondrial volume, n./area of mitochondria, and number of properly coupled mitochondria to triads in exercised individuals (average of 70 years) are approximately double those of age-matched healthy sedentary seniors, and similar to those of active young men (average of 27 years) [247].
Similar results were collected in a murine model in which WT mice were allowed to age for up to 24 months [238]. Two groups of WT mice were studied: sedentary aged mice (housed for 24 months in regular cages) and aged-trained mice (housed for the first 12 months in regular cages, and from 12 to 24 months of age in wheel cages for voluntary running). Long-term voluntary exercise prevented the ultrastructural decay of the EC coupling and mitochondrial machineries caused by sedentary ageing. In addition, long-term exercise rescued muscle function, and reduced oxidative stress, which was proposed in Pietrangelo et al. 2015 to cause damage to membranes and organelles deputed to Ca2+ handling and aerobic ATP production [248]. Physical exercise was also shown to: (a) increase the expression levels of the MCU and affect mitochondria dynamics [288], and (b) promote muscle reinnervation with age in ageing human skeletal muscle [289].
In Pietrangelo et al. 2019, we also studied short-term denervated EDL muscles from adult rats before (15 dd) and after (15 + 30 dd) sufficient time was given to the nerve to reinnervate muscle fibers [238]. These experiments showed how the misplacement of mitochondria at the A band (and consequent un-coupling from triads) resulting from short-term denervation (15 dd) was perfectly rescued by reinnervation (15 + 30 dd), suggesting that the rapid loss of mitochondrial position due to complete muscle inactivity is reversible.
Some preliminary findings have been also collected regarding the effect of inactivity and exercise on the membrane systems handling SOCE. SOCE in muscle fibers is handled by CEUs, junctions which are formed at the I band during exercise through the association of TTs and SR-stacks (see Section 2.3 for additional details). In Boncompagni et al. 2021, we showed how sedentary ageing in mice results in loss of SOCE function and reduced presence of the structures needed for the assembly of functional CEUs (TTs at the I band) [207]. A significant amount of dysfunctional STIM1 and ORAI1 was accumulated in TAs, representing peculiar assembly of SR tubes; this was found in ageing and in several myopathies. Importantly, the study also demonstrated that long-term training of mice in wheel cages (a) reduced the formation of TAs, (b) prevented the loss of CEUs, and (c) rescued functional SOCE [207].
5. Final Remarks
Adult skeletal muscle fibers are marvelous multinucleated machines designed to efficiently produce force while burning energy. Beside constituting the fundamental units deputed to movement, in the end—if they work properly—they may represent the metabolic balance of the whole organism. However, to work properly, skeletal fibers need a specific arrangement of the intracellular organelles and membranes deputed to force production (myofibrils), Ca2+ handling (CRUs and CEUs), and aerobic ATP production (mitochondria). This arrangement (discussed in detail in Section 2) is achieved during a process of differentiation and post-natal maturation that may take months or years depending on the species we are considering (e.g., mice vs. humans).
In this review, we have focused our attention on an aspect that is not often discussed and that has attracted our attention. In skeletal muscle fibers, intracellular ordered disposition of organelles is lost quite quickly when muscle activity is absent or reduced (we have studied denervation and sedentary ageing; see Section 3), suggesting that the preservation of proper architecture requires muscle activity. This hypothesis is supported by the studies in which we used FES and exercise to prevent or reverse the remodeling caused by inactivity. Changes caused by muscle inactivity are transversal (from humans to animal models) and somehow similar in ageing and early-denervation, though more severe in the latter, especially when denervation is prolonged.
The studies reviewed in Section 3 and Section 4 support the idea that the intracellular architecture of fibers is quite important for the proper function, both contractile and metabolic, of muscle. Indeed, the disarray of membranes involved in EC coupling (CRUs), excitation–metabolism coupling (close association CRU-mitochondrion), and SOCE (CEUs) may contribute to impaired delivery of Ca2+ and the production of ATP (required for optimal force generation by myofibrils), and to muscle fatigue, thus causing reduced muscle performance and dysfunction.
It is important to underline that the loss and misplacement of CRUs, mitochondria, and CEUs appears to be a reversible process, as it can be prevented or rescued by muscle activity/exercise (discussed in Section 4), a fact that emphasizes the great plasticity of muscle fibers in response to an appropriate stimulation.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the following grants to FP: (1) GGP19231 from Italian Telethon ONLUS; (2) subcontract of AR059646-06 from the National Institutes of Health, USA; (3) PRIN #2015ZZR4W3 from the Italian Ministry of University and Research.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ca2+-induced Ca2+ release | CICR |
| calcium release unit | CRU |
| calcium entry unit | CEU |
| dihydropyridine receptor | DHPR |
| electron microscopy | EM |
| excitation–contraction | EC |
| functional electrical stimulation | FES |
| ryanodine receptor | RYR |
| sarcoplasmic-reticulum | SR |
| store-operated Ca2+ entry | SOCE |
| tubular aggregate | TA |
| transverse tubule | TT |
References
- Rasmussen, H.; Jensen, P.; Lake, W.; Goodman, D.B.P. Calcium ion as second messenger. Clin. Endocrinol. 1976, 5, 11S–27S. [Google Scholar] [CrossRef]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Endo, M. Calcium Ion as a Second Messenger with Special Reference to Excitation-Contraction Coupling. J. Pharmacol. Sci. 2006, 100, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Dolmetsch, R. Excitation-transcription coupling: Signaling by ion channels to the nucleus. Sci. STKE 2003, 2003, PE4. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.F.; Chandler, W.K. Voltage dependent charge movement of skeletal muscle: A possible step in excitation-contraction coupling. Nature 1973, 242, 244–246. [Google Scholar] [CrossRef]
- Sandow, A.; Taylor, S.R.; Preiser, H. Role of the action potential in excitation-contraction coupling. Fed. Proc. 1965, 24, 1116–1123. [Google Scholar]
- Sandow, A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 1952, 25, 176–201. [Google Scholar] [PubMed]
- Rios, E.; Pizarro, G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol. Rev. 1991, 71, 849–908. [Google Scholar] [CrossRef] [PubMed]
- Calderon, J.C.; Bolanos, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef]
- Weber, A. Energized Calcium Transport and Relaxing Factors. Curr. Top. Bioenerg. 1966, 1, 203–254. [Google Scholar]
- Toyoshima, C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 2009, 1793, 941–946. [Google Scholar] [CrossRef]
- Hasselbach, W.; Oetliker, H. Energetics and electrogenicity of the sarcoplasmic reticulum calcium pump. Annu. Rev. Physiol. 1983, 45, 325–339. [Google Scholar] [CrossRef]
- Hasselbach, W. Relaxation and the Sarcotubular Calcium Pump. Fed. Proc. 1964, 23, 909–912. [Google Scholar] [PubMed]
- Dulhunty, A.F.; Banyard, M.R.; Medveczky, C.J. Distribution of calcium ATPase in the sarcoplasmic reticulum of fast- and slow-twitch muscles determined with monoclonal antibodies. J. Membr. Biol. 1987, 99, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, P.; Guillen, A.; Rojas, H.; Boncompagni, S.; Caputo, C. The use of CalciumOrange-5N as a specific marker of mitochondrial Ca2+ in mouse skeletal muscle fibers. Pflug. Arch. 2008, 455, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ainbinder, A.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle. Cell Calcium 2015, 57, 14–24. [Google Scholar] [CrossRef]
- Shkryl, V.M.; Shirokova, N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J. Biol. Chem. 2006, 281, 1547–1554. [Google Scholar] [CrossRef]
- Rudolf, R.; Mongillo, M.; Magalhaes, P.J.; Pozzan, T. In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction. J. Cell Biol. 2004, 166, 527–536. [Google Scholar] [CrossRef]
- Rossi, A.E.; Boncompagni, S.; Dirksen, R.T. Sarcoplasmic reticulum-mitochondrial symbiosis: Bidirectional signaling in skeletal muscle. Exerc. Sport Sci. Rev. 2009, 37, 29–35. [Google Scholar] [CrossRef]
- Parekh, A.B.; Penner, R. Store depletion and calcium influx. Physiol. Rev. 1997, 77, 901–930. [Google Scholar] [CrossRef]
- Wei-Lapierre, L.; Carrell, E.M.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 2013, 4, 2805. [Google Scholar] [CrossRef] [PubMed]
- Lyfenko, A.D.; Dirksen, R.T. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J. Physiol. 2008, 586, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Launikonis, B.S.; Rios, E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 2007, 583 Pt 1, 81–97. [Google Scholar] [CrossRef]
- Kurebayashi, N.; Ogawa, Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 2001, 533 Pt 1, 185–199. [Google Scholar] [CrossRef]
- Dirksen, R.T. Checking your SOCCs and feet: The molecular mechanisms of Ca2+ entry in skeletal muscle. J. Physiol. 2009, 587 Pt 13, 3139–3147. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Kahn, A.J.; Sandow, A. The potentiation of muscular contraction by the nitrate-ion. Science 1950, 112, 647–649. [Google Scholar] [CrossRef]
- Wray, S.; Ravens, U.; Verkhratsky, A.; Eisner, D. Two centuries of excitation-contraction coupling. Cell Calcium 2004, 35, 485–489. [Google Scholar] [CrossRef]
- Schneider, M.F. Control of calcium release in functioning skeletal muscle fibers. Annu. Rev. Physiol. 1994, 56, 463–484. [Google Scholar] [CrossRef]
- Pozzan, T.; Rizzuto, R.; Volpe, P.; Meldolesi, J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 1994, 74, 595–636. [Google Scholar] [CrossRef]
- Endo, M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977, 57, 71–108. [Google Scholar] [CrossRef]
- Weber, A. On the role of calcium in the activity of adenosine 5’-triphosphate hydrolysis by actomyosin. J. Biol. Chem. 1959, 234, 2764–2769. [Google Scholar] [CrossRef]
- Niedergerke, R. Local muscular shortening by intracellularly applied calcium. J. Physiol. 1955, 128, 12–13. [Google Scholar]
- Heilbrunn, L.V.; Wiercinski, F.J. The action of various cations on muscle protoplasm. J. Cell Comp. Physiol. 1947, 29, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Caputo, C.; Gimenez, M. Effects of external calcium deprivation on single muscle fibers. J. Gen. Physiol. 1967, 50, 2177–2195. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Bezanilla, F.M.; Horowicz, P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim. Biophys. Acta 1972, 267, 605–608. [Google Scholar] [CrossRef]
- Winegrad, S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J. Gen. Physiol. 1968, 51, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Hasselbach, W.; Makinose, M. The calcium pump of the “relaxing granules” of muscle and its dependence on ATP-splitting. Biochem. Z 1961, 333, 518–528. [Google Scholar]
- Stern, M.D.; Cheng, H. Putting out the fire: What terminates calcium-induced calcium release in cardiac muscle? Cell Calcium 2004, 35, 591–601. [Google Scholar] [CrossRef]
- Orkand, R.K.; Niedergerke, R. Heart Action Potential: Dependence on External Calcium and Sodium Ions. Science 1964, 146, 1176–1177. [Google Scholar] [CrossRef]
- Nabauer, M.; Callewaert, G.; Cleemann, L.; Morad, M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science 1989, 244, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Fabiato, A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985, 85, 291–320. [Google Scholar] [CrossRef]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef]
- Collier, M.L.; Thomas, A.P.; Berlin, J.R. Relationship between L-type Ca2+ current and unitary sarcoplasmic reticulum Ca2+ release events in rat ventricular myocytes. J. Physiol 1999, 516 Pt 1, 117–128. [Google Scholar] [CrossRef]
- Collier, M.L.; Ji, G.; Wang, Y.; Kotlikoff, M.I. Calcium-induced calcium release in smooth muscle: Loose coupling between the action potential and calcium release. J. Gen. Physiol. 2000, 115, 653–662. [Google Scholar] [CrossRef]
- Cannell, M.B.; Cheng, H.; Lederer, W.J. The control of calcium release in heart muscle. Science 1995, 268, 1045–1049. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Meissner, G.; Lu, X. Dihydropyridine receptor-ryanodine receptor interactions in skeletal muscle excitation-contraction coupling. Biosci. Rep. 1995, 15, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Takeshima, H.; Mikami, A.; Flockerzi, V.; Takahashi, H.; Kangawa, K.; Kojima, M.; Matsuo, H.; Hirose, T.; Numa, S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 1987, 328, 313–318. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Powell, J.A.; Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 1988, 336, 134–139. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Adams, B.A.; Niidome, T.; Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 1990, 346, 567–569. [Google Scholar] [CrossRef]
- Sorrentino, V.; Volpe, P. Ryanodine receptors: How many, where and why? Trends Pharm. Sci. 1993, 14, 98–103. [Google Scholar] [CrossRef]
- Sorrentino, V. The ryanodine receptor family of intracellular calcium release channels. Adv. Pharm. 1995, 33, 67–90. [Google Scholar]
- Rios, E.; Brum, G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 1987, 325, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Sekiguchi, N.; Rando, T.A.; Allen, P.D.; Beam, K.G. Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J. Biol. Chem. 1998, 273, 13403–13406. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Sorrentino, V. Molecular structure and tissue distribution of ryanodine receptors calcium channels. Med. Res. Rev. 1995, 15, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Willard, H.F.; Khanna, V.K.; Zorzato, F.; Green, N.M.; MacLennan, D.H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 13472–13483. [Google Scholar] [CrossRef]
- Mikami, A.; Imoto, K.; Tanabe, T.; Niidome, T.; Mori, Y.; Takeshima, H.; Narumiya, S.; Numa, S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 1989, 340, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Protasi, F.; Takahashi, M.; Takeshima, H.; Ferguson, D.G.; Franzini-Armstrong, C. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J. Cell Biol. 1995, 129, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Sun, X.H.; Franzini-Armstrong, C. Formation and maturation of the calcium release apparatus in developing and adult avian myocardium. Dev. Biol. 1996, 173, 265–278. [Google Scholar] [CrossRef][Green Version]
- Protasi, F. Structural interaction between RYRs and DHPRs in calcium release units of cardiac and skeletal muscle cells. Front. Biosci. 2002, 7, d650–d658. [Google Scholar] [CrossRef] [PubMed]
- Flucher, B.E.; Franzini-Armstrong, C. Formation of junctions involved in excitation-contraction coupling in skeletal and cardiac muscle. Proc. Natl. Acad. Sci. USA 1996, 93, 8101–8106. [Google Scholar] [CrossRef]
- Zorzato, F.; Fujii, J.; Otsu, K.; Phillips, M.; Green, N.M.; Lai, F.A.; Meissner, G.; MacLennan, D.H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 2244–2256. [Google Scholar] [CrossRef]
- Takeshima, H.; Nishimura, S.; Matsumoto, T.; Ishida, H.; Kangawa, K.; Minamino, N.; Matsuo, H.; Ueda, M.; Hanaoka, M.; Hirose, T.; et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 1989, 339, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.; Ma, J.J.; Gonzalez, A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J. Muscle Res. Cell Motil. 1991, 12, 127–135. [Google Scholar] [CrossRef]
- Rios, E.; Karhanek, M.; Ma, J.; Gonzalez, A. An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 1993, 102, 449–481. [Google Scholar] [CrossRef] [PubMed]
- Franzini-Armstrong, C.; Protasi, F. Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol. Rev. 1997, 77, 699–729. [Google Scholar] [CrossRef]
- Protasi, F.; Takekura, H.; Wang, Y.; Chen, S.R.; Meissner, G.; Allen, P.D.; Franzini-Armstrong, C. RYR1 and RYR3 have different roles in the assembly of calcium release units of skeletal muscle. Biophys. J. 2000, 79, 2494–2508. [Google Scholar] [CrossRef]
- Protasi, F.; Paolini, C.; Nakai, J.; Beam, K.G.; Franzini-Armstrong, C.; Allen, P.D. Multiple regions of RyR1 mediate functional and structural interactions with alpha(1S)-dihydropyridine receptors in skeletal muscle. Biophys. J. 2002, 83, 3230–3244. [Google Scholar] [CrossRef]
- Protasi, F.; Franzini-Armstrong, C.; Flucher, B.E. Coordinated incorporation of skeletal muscle dihydropyridine receptors and ryanodine receptors in peripheral couplings of BC3H1 cells. J. Cell Biol. 1997, 137, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Franzini-Armstrong, C.; Allen, P.D. Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle. J. Cell Biol. 1998, 140, 831–842. [Google Scholar] [CrossRef]
- Paolini, C.; Protasi, F.; Franzini-Armstrong, C. The relative position of RyR feet and DHPR tetrads in skeletal muscle. J. Mol. Biol. 2004, 342, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Franzini-Armstrong, C.; Jorgensen, A.O. Structure and development of E-C coupling units in skeletal muscle. Annu. Rev. Physiol. 1994, 56, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Franzini-Armstrong, C. The sarcoplasmic reticulum and the control of muscle contraction. FASEB J. 1999, 13, S266–S270. [Google Scholar] [CrossRef]
- Block, B.A.; Imagawa, T.; Campbell, K.P.; Franzini-Armstrong, C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 1988, 107, 2587–2600. [Google Scholar] [CrossRef]
- Nakai, J.; Ogura, T.; Protasi, F.; Franzini-Armstrong, C.; Allen, P.D.; Beam, K.G. Functional nonequality of the cardiac and skeletal ryanodine receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Grabner, M.; Dirksen, R.T.; Suda, N.; Beam, K.G. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the Bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 1999, 274, 21913–21919. [Google Scholar] [CrossRef]
- Dirksen, R.T. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front. Biosci. 2002, 7, d659–d670. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lederer, W.J.; Cannell, M.B. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 1993, 262, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lederer, W.J. Calcium sparks. Physiol Rev. 2008, 88, 1491–1545. [Google Scholar] [CrossRef]
- Tsugorka, A.; Rios, E.; Blatter, L.A. Imaging elementary events of calcium release in skeletal muscle cells. Science 1995, 269, 1723–1726. [Google Scholar] [CrossRef]
- Weisleder, N.; Zhou, J.; Ma, J. Detection of calcium sparks in intact and permeabilized skeletal muscle fibers. Methods Mol. Biol. 2012, 798, 395–410. [Google Scholar] [PubMed]
- Isaeva, E.V.; Shirokova, N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J. Physiol. 2003, 547 Pt 2, 453–462. [Google Scholar] [CrossRef]
- Apostol, S.; Ursu, D.; Lehmann-Horn, F.; Melzer, W. Local calcium signals induced by hyper-osmotic stress in mammalian skeletal muscle cells. J. Muscle Res. Cell Motil. 2009, 30, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Shtifman, A.; Ward, C.W.; Wang, J.; Valdivia, H.H.; Schneider, M.F. Effects of imperatoxin A on local sarcoplasmic reticulum Ca(2+) release in frog skeletal muscle. Biophys. J. 2000, 79, 814–827. [Google Scholar] [CrossRef]
- Ebashi, S.; Endo, M.; Otsuki, I. Control of muscle contraction. Q. Rev. Biophys. 1969, 2, 351–384. [Google Scholar] [CrossRef]
- Ebashi, S. Regulatory mechanism of muscle contraction with special reference to the Ca-troponin-tropomyosin system. Essays Biochem. 1974, 10, 1–36. [Google Scholar] [PubMed]
- Ebashi, F.; Ebashi, S. Removal of calcium and relaxation in actomyosin systems. Nature 1962, 194, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Kalyanasundaram, A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef]
- Odermatt, A.; Becker, S.; Khanna, V.K.; Kurzydlowski, K.; Leisner, E.; Pette, D.; MacLennan, D.H. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 1998, 273, 12360–12369. [Google Scholar] [CrossRef] [PubMed]
- Zorzato, F.; Anderson, A.A.; Ohlendieck, K.; Froemming, G.; Guerrini, R.; Treves, S. Identification of a novel 45 kDa protein (JP-45) from rabbit sarcoplasmic-reticulum junctional-face membrane. Biochem. J. 2000, 351 Pt 2, 537–543. [Google Scholar] [CrossRef]
- Zhang, L.; Kelley, J.; Schmeisser, G.; Kobayashi, Y.M.; Jones, L.R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997, 272, 23389–23397. [Google Scholar] [CrossRef]
- Takeshima, H.; Shimuta, M.; Komazaki, S.; Ohmi, K.; Nishi, M.; Iino, M.; Miyata, A.; Kangawa, K. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem. J. 1998, 331 Pt 1, 317–322. [Google Scholar] [CrossRef]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar]
- Rios, E.; Gyorke, S. Calsequestrin, triadin and more: The molecules that modulate calcium release in cardiac and skeletal muscle. J. Physiol. 2009, 587 Pt 13, 3069–3070. [Google Scholar] [CrossRef]
- Rebbeck, R.T.; Karunasekara, Y.; Board, P.G.; Beard, N.A.; Casarotto, M.G.; Dulhunty, A.F. Skeletal muscle excitation-contraction coupling: Who are the dancing partners? Int. J. Biochem. Cell Biol. 2014, 48, 28–38. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Wong, P.T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1971, 68, 1231–1235. [Google Scholar] [CrossRef]
- Kim, K.C.; Caswell, A.H.; Talvenheimo, J.A.; Brandt, N.R. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry 1990, 29, 9281–9289. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.R.; Zhang, L.; Sanborn, K.; Jorgensen, A.O.; Kelley, J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J. Biol. Chem. 1995, 270, 30787–30796. [Google Scholar] [CrossRef]
- Hofmann, S.L.; Goldstein, J.L.; Orth, K.; Moomaw, C.R.; Slaughter, C.A.; Brown, M.S. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J. Biol. Chem. 1989, 264, 18083–18090. [Google Scholar] [CrossRef]
- Guo, W.; Campbell, K.P. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J. Biol. Chem. 1995, 270, 9027–9030. [Google Scholar] [CrossRef] [PubMed]
- Froemming, G.R.; Pette, D.; Ohlendieck, K. The 90-kDa junctional sarcoplasmic reticulum protein forms an integral part of a supramolecular triad complex in skeletal muscle. Biochem. Biophys. Res. Commun. 1999, 261, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, A.F.; Wei-LaPierre, L.; Casarotto, M.G.; Beard, N.A. Core skeletal muscle ryanodine receptor calcium release complex. Clin. Exp. Pharm. Physiol. 2017, 44, 3–12. [Google Scholar] [CrossRef]
- Caswell, A.H.; Brandt, N.R.; Brunschwig, J.P.; Purkerson, S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95,000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry 1991, 30, 7507–7513. [Google Scholar] [CrossRef]
- Anderson, A.A.; Treves, S.; Biral, D.; Betto, R.; Sandona, D.; Ronjat, M.; Zorzato, F. The novel skeletal muscle sarcoplasmic reticulum JP-45 protein. Molecular cloning, tissue distribution, developmental expression, and interaction with alpha 1.1 subunit of the voltage-gated calcium channel. J. Biol. Chem. 2003, 278, 39987–39992. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, C.; Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 2002, 418, 605–611. [Google Scholar] [CrossRef]
- Sweeney, H.L. The importance of the creatine kinase reaction: The concept of metabolic capacitance. Med. Sci. Sports Exerc. 1994, 26, 30–36. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Territo, P.R.; Mootha, V.K.; French, S.A.; Balaban, R.S. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: Role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000, 278, C423–C435. [Google Scholar] [CrossRef]
- McMillin, J.B.; Madden, M.C. The role of calcium in the control of respiration by muscle mitochondria. Med. Sci. Sports Exerc. 1989, 21, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G. Relation between cytosolic free Ca2+ concentration and the control of pyruvate dehydrogenase in isolated cardiac myocytes. Biochem. J. 1987, 241, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, E.V.; Shkryl, V.M.; Shirokova, N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J. Physiol. 2005, 565 Pt 3, 855–872. [Google Scholar] [CrossRef]
- Territo, P.R.; French, S.A.; Balaban, R.S. Simulation of cardiac work transitions, in vitro: Effects of simultaneous Ca2+ and ATPase additions on isolated porcine heart mitochondria. Cell Calcium 2001, 30, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G. Role of calcium in respiratory control. Med. Sci. Sports Exerc. 1994, 26, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gunter, K.K.; Gunter, T.E. Transport of calcium by mitochondria. J. Bioenerg. Biomembr. 1994, 26, 471–485. [Google Scholar] [CrossRef]
- Buntinas, L.; Gunter, K.K.; Sparagna, G.C.; Gunter, T.E. The rapid mode of calcium uptake into heart mitochondria (RaM): Comparison to RaM in liver mitochondria. Biochim. Biophys. Acta 2001, 1504, 248–261. [Google Scholar] [CrossRef]
- Beutner, G.; Sharma, V.K.; Giovannucci, D.R.; Yule, D.I.; Sheu, S.S. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001, 276, 21482–21488. [Google Scholar] [CrossRef]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabo, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef]
- Pan, X.; Liu, J.; Nguyen, T.; Liu, C.; Sun, J.; Teng, Y.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013, 15, 1464–1472. [Google Scholar] [CrossRef]
- Sembrowich, W.L.; Quintinskie, J.J.; Li, G. Calcium uptake in mitochondria from different skeletal muscle types. J. Appl. Physiol. 1985, 59, 137–141. [Google Scholar] [CrossRef]
- Scarpa, A.; Graziotti, P. Mechanisms for intracellular calcium regulation in heart. I. Stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J. Gen. Physiol. 1973, 62, 756–772. [Google Scholar] [CrossRef]
- Crompton, M.; Capano, M.; Carafoli, E. Respiration-dependent efflux of magnesium ions from heart mitochondria. Biochem. J. 1976, 154, 735–742. [Google Scholar] [CrossRef]
- Rossi, A.E.; Boncompagni, S.; Wei, L.; Protasi, F.; Dirksen, R.T. Differential impact of mitochondrial positioning on mitochondrial Ca(2+) uptake and Ca(2+) spark suppression in skeletal muscle. Am. J. Physiol. Cell Physiol. 2011, 301, C1128–C1139. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A.; Buttle, K.; Rath, B.K.; Marko, M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors 1998, 8, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Csordas, G.; Renken, C.; Varnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnoczky, G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006, 174, 915–921. [Google Scholar] [CrossRef]
- Sharma, V.K.; Ramesh, V.; Franzini-Armstrong, C.; Sheu, S.S. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 2000, 32, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta 2006, 1763, 542–548. [Google Scholar] [CrossRef]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Beznoussenko, G.V.; Polishchuk, R.S.; Dirksen, R.T.; Protasi, F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol. Biol. Cell 2009, 20, 1058–1067. [Google Scholar] [CrossRef]
- Gillis, J.M. Inhibition of mitochondrial calcium uptake slows down relaxation in mitochondria-rich skeletal muscles. J. Muscle Res. Cell Motil. 1997, 18, 473–483. [Google Scholar] [CrossRef]
- Hidalgo, C.; Gonzalez, M.E.; Garcia, A.M. Calcium transport in transverse tubules isolated from rabbit skeletal muscle. Biochim. Biophys. Acta 1986, 854, 279–286. [Google Scholar] [CrossRef]
- Cheng, A.J.; Place, N.; Westerblad, H. Molecular Basis for Exercise-Induced Fatigue: The Importance of Strictly Controlled Cellular Ca(2+) Handling. Cold Spring Harb. Perspect. Med. 2018, 8, a029710. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E. The plasma membrane Ca(2)+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb. Perspect Biol. 2011, 3, a004168. [Google Scholar] [CrossRef]
- Balnave, C.D.; Allen, D.G. Evidence for Na+/Ca2+ exchange in intact single skeletal muscle fibers from the mouse. Am. J. Physiol. 1998, 274, C940–C946. [Google Scholar] [CrossRef] [PubMed]
- Launikonis, B.S.; Barnes, M.; Stephenson, D.G. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, N.; Ronjat, M.; Meszaros, L.G.; Koshita, M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry 1989, 28, 6764–6771. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, S.R.; Belyea, M.J.; Epstein, D.R. Fatigue-based subgroups of breast cancer survivors with insomnia. Cancer Nurs. 2009, 32, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Garcia-Castaneda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-mediated store-operated Ca(2+) entry in skeletal muscle physiology and disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Buchanan, J.; Luik, R.M.; Lewis, R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006, 174, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Luik, R.M.; Wang, B.; Prakriya, M.; Wu, M.M.; Lewis, R.S. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 2008, 454, 538–542. [Google Scholar] [CrossRef]
- Liou, J.; Fivaz, M.; Inoue, T.; Meyer, T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl. Acad. Sci. USA 2007, 104, 9301–9306. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Covington, E.D.; Lewis, R.S. Single-molecule analysis of diffusion and trapping of STIM1 and Orai1 at endoplasmic reticulum-plasma membrane junctions. Mol. Biol. Cell 2014, 25, 3672–3685. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Rao, A. Store-operated calcium entry: Mechanisms and modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef]
- Barr, V.A.; Bernot, K.M.; Shaffer, M.H.; Burkhardt, J.K.; Samelson, L.E. Formation of STIM and Orai complexes: Puncta and distal caps. Immunol. Rev. 2009, 231, 148–159. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yeromin, A.V.; Zhang, X.H.; Yu, Y.; Safrina, O.; Penna, A.; Roos, J.; Stauderman, K.A.; Cahalan, M.D. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl. Acad. Sci. USA 2006, 103, 9357–9362. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S. The molecular choreography of a store-operated calcium channel. Nature 2007, 446, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Vaidya, A.; Rohloff, P.; Sun, Y.P.; Grad, Y. Interactive medical case. A patient with fevers and fatigue. N. Engl. J. Med. 2013, 368, e9. [Google Scholar] [CrossRef]
- MacLennan, D.; Campbell, K.; Reithmeier, R. Calsequestrin. Calcium Cell Funct. 1983, 4, 151–173. [Google Scholar]
- Zhang, L.; Wang, L.; Li, S.; Xue, J.; Luo, D. Calsequestrin-1 Regulates Store-Operated Ca2+ Entry by Inhibiting STIM1 Aggregation. Cell Physiol. Biochem. 2016, 38, 2183–2193. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, S.; Zheng, Y.; Yan, X.; Chen, M.; Wang, H.; Putney, J.W.; Luo, D. Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci. Rep. 2015, 5, 11349. [Google Scholar] [CrossRef]
- Shin, D.W.; Pan, Z.; Kim, E.K.; Lee, J.M.; Bhat, M.B.; Parness, J.; Kim, D.H.; Ma, J. A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J. Biol. Chem. 2003, 278, 3286–3292. [Google Scholar] [CrossRef]
- Edwards, J.N.; Murphy, R.M.; Cully, T.R.; von Wegner, F.; Friedrich, O.; Launikonis, B.S. Ultra-rapid activation and deactivation of store-operated Ca(2+) entry in skeletal muscle. Cell Calcium 2010, 47, 458–467. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; Konig, S.; Jousset, H.; Bader, C.; Demaurex, N.; Bernheim, L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J. Biol. Chem. 2009, 284, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Darbellay, B.; Arnaudeau, S.; Bader, C.R.; Konig, S.; Bernheim, L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol. 2011, 194, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Carrell, E.M.; Coppola, A.R.; McBride, H.J.; Dirksen, R.T. Orai1 enhances muscle endurance by promoting fatigue-resistant type I fiber content but not through acute store-operated Ca2+ entry. FASEB J. 2016, 30, 4109–4119. [Google Scholar] [CrossRef]
- Zhao, X.; Weisleder, N.; Thornton, A.; Oppong, Y.; Campbell, R.; Ma, J.; Brotto, M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 2008, 7, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.M.; Zhao, X.; Weisleder, N.; Brotto, L.S.; Bougoin, S.; Nosek, T.M.; Reid, M.; Hardin, B.; Pan, Z.; Ma, J.; et al. Store-operated Ca(2+) entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle. Aging (Albany NY) 2011, 3, 621–634. [Google Scholar] [CrossRef]
- Brotto, M. Aging, sarcopenia and store-operated calcium entry: A common link? Cell Cycle 2011, 10, 4201–4202. [Google Scholar] [CrossRef]
- Payne, A.M.; Jimenez-Moreno, R.; Wang, Z.M.; Messi, M.L.; Delbono, O. Role of Ca2+, membrane excitability, and Ca2+ stores in failing muscle contraction with aging. Exp. Gerontol. 2009, 44, 261–273. [Google Scholar] [CrossRef][Green Version]
- Edwards, J.N.; Blackmore, D.G.; Gilbert, D.F.; Murphy, R.M.; Launikonis, B.S. Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression. Aging Cell 2011, 10, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Soboloff, J.; Rothberg, B.S.; Madesh, M.; Gill, D.L. STIM proteins: Dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012, 13, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Al-Qusairi, L.; Laporte, J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet. Muscle 2011, 1, 26. [Google Scholar] [CrossRef]
- Picard, M.; Taivassalo, T.; Gouspillou, G.; Hepple, R.T. Mitochondria: Isolation, structure and function. J. Physiol. 2011, 589 Pt 18, 4413–4421. [Google Scholar] [CrossRef]
- Newmeyer, D.D.; Ferguson-Miller, S. Mitochondria: Releasing power for life and unleashing the machineries of death. Cell 2003, 112, 481–490. [Google Scholar] [CrossRef]
- Liesa, M.; Palacin, M.; Zorzano, A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 2009, 89, 799–845. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Schatz, G. Mitochondria: A historical review. J. Cell Biol. 1981, 91, 227s–255s. [Google Scholar] [CrossRef]
- Protasi, F.; Pietrangelo, L.; Boncompagni, S. Calcium entry units (CEUs): Perspectives in skeletal muscle function and disease. J. Muscle Res. Cell Motil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, L.; Dirksen, R.T.; Protasi, F. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 2017, 7, 14286, Addendum in 2018, 8, 17463. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Porter, K.R. Sarcolemmal Invaginations Constituting the T System in Fish Muscle Fibers. J. Cell Biol. 1964, 22, 675–696. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Protasi, F.; Ramesh, V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann. N. Y. Acad. Sci. 1998, 853, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Takekura, H.; Sun, X.; Franzini-Armstrong, C. Development of the excitation-contraction coupling apparatus in skeletal muscle: Peripheral and internal calcium release units are formed sequentially. J. Muscle Res. Cell Motil. 1994, 15, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Flucher, B.E.; Takekura, H.; Franzini-Armstrong, C. Development of the excitation-contraction coupling apparatus in skeletal muscle: Association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev. Biol. 1993, 160, 135–147. [Google Scholar] [CrossRef]
- Rios, E.; Pizarro, G. Voltage sensors and calcium channels of excitation-contraction coupling. News Physiol. Sci. 1988, 3, 223–227. [Google Scholar] [CrossRef]
- Wagenknecht, T.; Grassucci, R.; Frank, J.; Saito, A.; Inui, M.; Fleischer, S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature 1989, 338, 167–170. [Google Scholar] [CrossRef]
- Lai, F.A.; Erickson, H.P.; Rousseau, E.; Liu, Q.Y.; Meissner, G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature 1988, 331, 315–319. [Google Scholar]
- Fill, M.; Copello, J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002, 82, 893–922. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C. Studies of the triad: I. Structure of the Junction in Frog Twitch Fibers. J. Cell Biol. 1970, 47, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Gamberucci, A.; Pierantozzi, E.; Amato, C.; Migliore, L.; Sorrentino, V. Calsequestrin, a key protein in striated muscle health and disease. J. Muscle Res. Cell Motil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Paolini, C.; Canato, M.; Reggiani, C.; Quarta, M. Lessons from calsequestrin-1 ablation in vivo: Much more than a Ca(2+) buffer after all. J. Muscle Res. Cell Motil. 2011, 32, 257–270. [Google Scholar] [CrossRef]
- Paolini, C.; Quarta, M.; Nori, A.; Boncompagni, S.; Canato, M.; Volpe, P.; Allen, P.D.; Reggiani, C.; Protasi, F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J. Physiol. 2007, 583 Pt 2, 767–784. [Google Scholar] [CrossRef]
- Meissner, G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim. Biophys. Acta 1975, 389, 51–68. [Google Scholar] [CrossRef]
- Campbell, K.P.; MacLennan, D.H.; Jorgensen, A.O.; Mintzer, M.C. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J. Biol. Chem. 1983, 258, 1197–1204. [Google Scholar] [CrossRef]
- Kobayashi, Y.M.; Alseikhan, B.A.; Jones, L.R. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J. Biol. Chem. 2000, 275, 17639–17646. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Kenney, L.J.; Varriano-Marston, E. The structure of calsequestrin in triads of vertebrate skeletal muscle: A deep-etch study. J. Cell Biol. 1987, 105, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Thomas, M.; Lopez, J.R.; Allen, P.D.; Yuan, Q.; Kranias, E.G.; Franzini-Armstrong, C.; Perez, C.F. Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis. PLoS ONE 2012, 7, e39962. [Google Scholar] [CrossRef]
- Ogata, T.; Yamasaki, Y. Scanning electron-microscopic studies on the three-dimensional structure of sarcoplasmic reticulum in the mammalian red, white and intermediate muscle fibers. Cell Tissue Res. 1985, 242, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Yeromin, A.V.; Zhang, S.L.; Jiang, W.; Yu, Y.; Safrina, O.; Cahalan, M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 2006, 443, 226–229. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J. Gen. Physiol. 2006, 128, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Dynes, J.L.; Yeromin, A.V.; Cahalan, M.D.; Franzini-Armstrong, C. Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry. Proc. Natl. Acad. Sci. USA 2015, 112, E5533–E5542. [Google Scholar] [CrossRef]
- Orci, L.; Ravazzola, M.; Le Coadic, M.; Shen, W.W.; Demaurex, N.; Cosson, P. From the Cover: STIM1-induced precortical and cortical subdomains of the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2009, 106, 19358–19362. [Google Scholar] [CrossRef] [PubMed]
- Launikonis, B.S.; Stephenson, D.G.; Friedrich, O. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J. Physiol. 2009, 587 Pt 10, 2299–2312. [Google Scholar] [CrossRef]
- Tang, W.; Ingalls, C.P.; Durham, W.J.; Snider, J.; Reid, M.B.; Wu, G.; Matzuk, M.M.; Hamilton, S.L. Altered excitation-contraction coupling with skeletal muscle specific FKBP12 deficiency. FASEB J. 2004, 18, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.R.; Wu, F.; Liu, Y.; Anderson, D.M.; McAnally, J.; Lin, W.; Cannon, S.C.; Bassel-Duby, R.; Olson, E.N. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA 2013, 110, 11881–11886. [Google Scholar] [CrossRef]
- Ito, K.; Komazaki, S.; Sasamoto, K.; Yoshida, M.; Nishi, M.; Kitamura, K.; Takeshima, H. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J. Cell Biol. 2001, 154, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Takano, T.; Protasi, F.; Dirksen, R.T. Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice. J. Gen. Physiol. 2020, 152, 10. [Google Scholar] [CrossRef]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Garcia-Castaneda, M.; Takano, T.; Malik, S.; Dirksen, R.T.; Protasi, F. Transverse tubule remodeling enhances Orai1-dependent Ca(2+) entry in skeletal muscle. eLife 2019, 8, e47576. [Google Scholar] [CrossRef] [PubMed]
- Zitt, C.; Strauss, B.; Schwarz, E.C.; Spaeth, N.; Rast, G.; Hatzelmann, A.; Hoth, M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 2004, 279, 12427–12437. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002, 16, 1145–1150. [Google Scholar] [CrossRef]
- Canato, M.; Scorzeto, M.; Giacomello, M.; Protasi, F.; Reggiani, C.; Stienen, G.J. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl. Acad. Sci. USA 2010, 107, 22326–22331. [Google Scholar] [CrossRef]
- Boncompagni, S.; Pecorai, C.; Michelucci, A.; Pietrangelo, L.; Protasi, F. Long-Term Exercise Reduces Formation of Tubular Aggregates and Promotes Maintenance of Ca(2+) Entry Units in Aged Muscle. Front. Physiol. 2021, 11, 601057. [Google Scholar] [CrossRef]
- Melzer, W. ECC meets CEU-New focus on the backdoor for calcium ions in skeletal muscle cells. J. Gen. Physiol. 2020, 152, 10. [Google Scholar] [CrossRef]
- Vandervoort, A.A. Aging of the human neuromuscular system. Muscle Nerve 2002, 25, 17–25. [Google Scholar] [CrossRef]
- Roos, M.R.; Rice, C.L.; Vandervoort, A.A. Age-related changes in motor unit function. Muscle Nerve 1997, 20, 679–690. [Google Scholar] [CrossRef]
- Luff, A.R. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann. N. Y. Acad. Sci. 1998, 854, 92–101. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.Y.; Larsson, Y.; Bruhn, E.; Morsing, B. Heavy-resistance training in older Scandinavian men and women: Short- and long-term effects on arm and leg muscles. Scand. J. Med. Sci. Sports 1995, 5, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Grimby, G.; Saltin, B. The ageing muscle. Clin. Physiol. 1983, 3, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J.; Vandervoort, A.A.; Taylor, A.W.; Brown, W.F. Effects of motor unit losses on strength in older men and women. J. Appl. Physiol. 1993, 74, 868–874. [Google Scholar] [CrossRef]
- Clark, B.C. Neuromuscular Changes with Aging and Sarcopenia. J. Frailty Aging 2019, 8, 7–9. [Google Scholar]
- Billot, M.; Calvani, R.; Urtamo, A.; Sanchez-Sanchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. Clin. Interv. Aging 2020, 15, 1675–1690. [Google Scholar] [CrossRef]
- Schneider, E.L.; Guralnik, J.M. The aging of America. Impact on health care costs. JAMA 1990, 263, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Hughes, V.A. Sarcopenia: Current concepts. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M716–M724. [Google Scholar] [CrossRef]
- Roubenoff, R. Origins and clinical relevance of sarcopenia. Can. J. Appl. Physiol. 2001, 26, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Summary comments: Epidemiological and methodological problems in determining nutritional status of older persons. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Nikolic, M.; Malnar-Dragojevic, D.; Bobinac, D.; Bajek, S.; Jerkovic, R.; Soic-Vranic, T. Age-Related skeletal muscle atrophy in humans: An immunohistochemical and morphometric study. Coll. Antropol. 2001, 25, 545–553. [Google Scholar]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; A quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Evans, W.J. What is sarcopenia? J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Alchin, D.R. Sarcopenia: Describing rather than defining a condition. J. Cachexia Sarcopenia Muscle 2014, 5, 265–268. [Google Scholar] [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Premkumar, P.; Ranjit, R.; Natarajan, K.S.; Ahn, B.; Riddle, K.; Claflin, D.R.; Richardson, A.; Brooks, S.V.; et al. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J. Cachexia Sarcopenia Muscle 2018, 9, 1003–1017. [Google Scholar] [CrossRef]
- Margreth, A.; Damiani, E.; Bortoloso, E. Sarcoplasmic reticulum in aged skeletal muscle. Acta Physiol. Scand 1999, 167, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L. The age-related motor disability: Underlying mechanisms in skeletal muscle at the motor unit, cellular and molecular level. Acta Physiol. Scand. 1998, 163, S27–S29. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fano, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Balagopal, P.; Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 1997, 273, E790–E800. [Google Scholar] [CrossRef]
- Aagaard, P.; Suetta, C.; Caserotti, P.; Magnusson, S.P.; Kjaer, M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scand. J. Med. Sci. Sports 2010, 20, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Evidence for nervous system degeneration with advancing age. J. Nutr. 1997, 127, 1011S–1013S. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.J.; Vukovic, J.; Dunlop, S.; Grounds, M.D.; Shavlakadze, T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS ONE 2011, 6, e28090. [Google Scholar] [CrossRef]
- Pellegrino, C.; Franzini, C. An Electron Microscope Study of Denervation Atrophy in Red and White Skeletal Muscle Fibers. J. Cell Biol. 1963, 17, 327–349. [Google Scholar] [CrossRef]
- Engel, A.G.; Banker, B.Q.T. Myology, 3rd ed.; Engel, A.G., Franzini-Armstrong, C., Eds.; McGraw-Hill: New York, NY, USA, 2004; Volume 1, Chapter 31. [Google Scholar]
- Pietrangelo, L.; Michelucci, A.; Ambrogini, P.; Sartini, S.; Guarnier, F.A.; Fusella, A.; Zamparo, I.; Mammucari, C.; Protasi, F.; Boncompagni, S. Muscle activity prevents the uncoupling of mitochondria from Ca(2+) Release Units induced by ageing and disuse. Arch. Biochem. Biophys. 2019, 663, 22–33. [Google Scholar] [CrossRef]
- Kern, H.; Hofer, C.; Modlin, M.; Mayr, W.; Vindigni, V.; Zampieri, S.; Boncompagni, S.; Protasi, F.; Carraro, U. Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3- to 20-year SCI. Spinal Cord 2008, 46, 293–304. [Google Scholar] [CrossRef]
- Kern, H.; Boncompagni, S.; Rossini, K.; Mayr, W.; Fano, G.; Zanin, M.E.; Podhorska-Okolow, M.; Protasi, F.; Carraro, U. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): A role for myofiber regeneration? J. Neuropathol. Exp. Neurol. 2004, 63, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Kern, H.; Rossini, K.; Hofer, C.; Mayr, W.; Carraro, U.; Protasi, F. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 19339–19344. [Google Scholar] [CrossRef]
- Biral, D.; Kern, H.; Adami, N.; Boncompagni, S.; Protasi, F.; Carraro, U. Atrophy-resistant fibers in permanent peripheral denervation of human skeletal muscle. Neurol. Res. 2008, 30, 137–144. [Google Scholar] [CrossRef]
- Ashley, Z.; Sutherland, H.; Lanmuller, H.; Russold, M.F.; Unger, E.; Bijak, M.; Mayr, W.; Boncompagni, S.; Protasi, F.; Salmons, S.; et al. Atrophy, but not necrosis, in rabbit skeletal muscle denervated for periods up to one year. Am. J. Physiol. Cell Physiol. 2007, 292, C440–C451. [Google Scholar] [CrossRef] [PubMed]
- Ashley, Z.; Salmons, S.; Boncompagni, S.; Protasi, F.; Russold, M.; Lanmuller, H.; Mayr, W.; Sutherland, H.; Jarvis, J.C. Effects of chronic electrical stimulation on long-term denervated muscles of the rabbit hind limb. J. Muscle Res. Cell Motil. 2007, 28, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Carraro, U.; Kern, H.; Pond, A.; Adami, N.; Biral, D.; Vindigni, V.; Boncompagni, S.; Pietrangelo, T.; Bosco, G.; et al. A subpopulation of rat muscle fibers maintains an assessable excitation-contraction coupling mechanism after long-standing denervation despite lost contractility. J. Neuropathol. Exp. Neurol. 2009, 68, 1256–1268. [Google Scholar] [CrossRef]
- Boncompagni, S.; d’Amelio, L.; Fulle, S.; Fano, G.; Protasi, F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: A possible role in the decline of muscle performance. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 995–1008. [Google Scholar] [CrossRef]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Pond, A.; Grim-Stieger, M.; Cvecka, J.; Sedliak, M.; et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, L.; D’Incecco, A.; Ainbinder, A.; Michelucci, A.; Kern, H.; Dirksen, R.T.; Boncompagni, S.; Protasi, F. Age-dependent uncoupling of mitochondria from Ca2(+) release units in skeletal muscle. Oncotarget 2015, 6, 35358–35371. [Google Scholar] [CrossRef]
- Narayanan, N.; Jones, D.L.; Xu, A.; Yu, J.C. Effects of aging on sarcoplasmic reticulum function and contraction duration in skeletal muscles of the rat. Am. J. Physiol. 1996, 271, C1032–C1040. [Google Scholar] [CrossRef]
- Delbono, O.; O’Rourke, K.S.; Ettinger, W.H. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J. Membr. Biol. 1995, 148, 211–222. [Google Scholar] [CrossRef]
- Danieli-Betto, D.; Betto, R.; Megighian, A.; Midrio, M.; Salviati, G.; Larsson, L. Effects of age on sarcoplasmic reticulum properties and histochemical composition of fast- and slow-twitch rat muscles. Acta Physiol. Scand. 1995, 154, 59–64. [Google Scholar] [CrossRef]
- Damiani, E.; Larsson, L.; Margreth, A. Age-related abnormalities in regulation of the ryanodine receptor in rat fast-twitch muscle. Cell Calcium 1996, 19, 15–27. [Google Scholar] [CrossRef]
- Ryan, M.; Ohlendieck, K. Excitation-Contraction Uncoupling and Sarcopenia. Basic Appl. Myol. 2004, 14, 141–154. [Google Scholar]
- Renganathan, M.; Messi, M.L.; Delbono, O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J. Membr. Biol. 1997, 157, 247–253. [Google Scholar] [CrossRef]
- Delbono, O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr. Aging Sci. 2011, 4, 248–259. [Google Scholar] [CrossRef]
- Ryan, M.; Butler-Browne, G.; Erzen, I.; Mouly, V.; Thornell, L.E.; Wernig, A.; Ohlendieck, K. Persistent expression of the alpha1S-dihydropyridine receptor in aged human skeletal muscle: Implications for the excitation-contraction uncoupling hypothesis of sarcopenia. Int. J. Mol. Med. 2003, 11, 425–434. [Google Scholar]
- Rios, E.; Brum, G. Ca2+ release flux underlying Ca2+ transients and Ca2+ sparks in skeletal muscle. Front. Biosci. 2002, 7, d1195–d1211. [Google Scholar]
- Hollingworth, S.; Zhao, M.; Baylor, S.M. The amplitude and time course of the myoplasmic free [Ca2+] transient in fast-twitch fibers of mouse muscle. J. Gen. Physiol. 1996, 108, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Csernoch, L.; Zhou, J.; Stern, M.D.; Brum, G.; Rios, E. The elementary events of Ca2+ release elicited by membrane depolarization in mammalian muscle. J. Physiol. 2004, 557 Pt 1, 43–58. [Google Scholar]
- Trounce, I.; Byrne, E.; Marzuki, S. Decline in skeletal muscle mitochondrial respiratory chain function: Possible factor in ageing. Lancet 1989, 1, 637–639. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Paolini, C.; Quarta, M.; Wei-LaPierre, L.; Michelucci, A.; Nori, A.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Oxidative stress, mitochondrial damage, and cores in muscle from calsequestrin-1 knockout mice. Skelet. Muscle 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Mitochondrial Impairment in Sarcopenia. Biology 2021, 10, 31. [Google Scholar] [CrossRef]
- Vissing, J.; Schmalbruch, H.; Haller, R.G.; Clausen, T. Muscle phosphoglycerate mutase deficiency with tubular aggregates: Effect of dantrolene. Ann. Neurol. 1999, 46, 274–277. [Google Scholar] [CrossRef]
- Rosenberg, N.L.; Neville, H.E.; Ringel, S.P. Tubular aggregates. Their association with neuromuscular diseases, including the syndrome of myalgias/cramps. Arch. Neurol. 1985, 42, 973–976. [Google Scholar] [CrossRef]
- Pierobon-Bormioli, S.; Armani, M.; Ringel, S.P.; Angelini, C.; Vergani, L.; Betto, R.; Salviati, G. Familial neuromuscular disease with tubular aggregates. Muscle Nerve 1985, 8, 291–298. [Google Scholar] [CrossRef]
- Morgan-Hughes, J.A. Tubular aggregates in skeletal muscle: Their functional significance and mechanisms of pathogenesis. Curr. Opin. Neurol. 1998, 11, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Engel, W.K.; Bishop, D.W.; Cunningham, G.G. Tubular aggregates in type II muscle fibers: Ultrastructural and histochemical correlation. J. Ultrastruct. Res. 1970, 31, 507–525. [Google Scholar] [CrossRef]
- De Groot, J.G.; Arts, W.F. Familial myopathy with tubular aggregates. J. Neurol. 1982, 227, 35–41. [Google Scholar] [CrossRef]
- Walter, M.C.; Rossius, M.; Zitzelsberger, M.; Vorgerd, M.; Muller-Felber, W.; Ertl-Wagner, B.; Zhang, Y.; Brinkmeier, H.; Senderek, J.; Schoser, B. 50 years to diagnosis: Autosomal dominant tubular aggregate myopathy caused by a novel STIM1 mutation. Neuromuscul. Disord. 2015, 25, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Okuma, H.; Saito, F.; Mitsui, J.; Hara, Y.; Hatanaka, Y.; Ikeda, M.; Shimizu, T.; Matsumura, K.; Shimizu, J.; Tsuji, S.; et al. Tubular aggregate myopathy caused by a novel mutation in the cytoplasmic domain of STIM1. Neurol. Genet. 2016, 2, e50. [Google Scholar] [CrossRef]
- Nesin, V.; Wiley, G.; Kousi, M.; Ong, E.C.; Lehmann, T.; Nicholl, D.J.; Suri, M.; Shahrizaila, N.; Katsanis, N.; Gaffney, P.M.; et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc. Natl. Acad. Sci. USA 2014, 111, 4197–4202. [Google Scholar] [CrossRef]
- Endo, Y.; Noguchi, S.; Hara, Y.; Hayashi, Y.K.; Motomura, K.; Miyatake, S.; Murakami, N.; Tanaka, S.; Yamashita, S.; Kizu, R.; et al. Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca(2)(+) channels. Hum. Mol. Genet. 2015, 24, 637–648. [Google Scholar] [CrossRef]
- Bohm, J.; Chevessier, F.; Maues De Paula, A.; Koch, C.; Attarian, S.; Feger, C.; Hantai, D.; Laforet, P.; Ghorab, K.; Vallat, J.M.; et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am. J. Hum. Genet. 2013, 92, 271–278. [Google Scholar] [CrossRef]
- Bohm, J.; Bulla, M.; Urquhart, J.E.; Malfatti, E.; Williams, S.G.; O’Sullivan, J.; Szlauer, A.; Koch, C.; Baranello, G.; Mora, M.; et al. ORAI1 Mutations with Distinct Channel Gating Defects in Tubular Aggregate Myopathy. Hum. Mutat. 2017, 38, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Del Re, V.; Gamberucci, A.; Polverino, V.; Galli, L.; Rossi, D.; Costanzi, E.; Toniolo, L.; Berti, G.; Malandrini, A.; et al. Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum. Mutat. 2017, 38, 1761–1773. [Google Scholar] [CrossRef]
- Schiaffino, S.; Severin, E.; Cantini, M.; Sartore, S. Tubular aggregates induced by anoxia in isolated rat skeletal muscle. Lab. Investig. 1977, 37, 223–228. [Google Scholar] [PubMed]
- Salviati, G.; Pierobon-Bormioli, S.; Betto, R.; Damiani, E.; Angelini, C.; Ringel, S.P.; Salvatori, S.; Margreth, A. Tubular aggregates: Sarcoplasmic reticulum origin, calcium storage ability, and functional implications. Muscle Nerve 1985, 8, 299–306. [Google Scholar] [CrossRef]
- Chevessier, F.; Marty, I.; Paturneau-Jouas, M.; Hantai, D.; Verdiere-Sahuque, M. Tubular aggregates are from whole sarcoplasmic reticulum origin: Alterations in calcium binding protein expression in mouse skeletal muscle during aging. Neuromuscul. Disord. 2004, 14, 208–216. [Google Scholar] [CrossRef]
- Boncompagni, S.; Protasi, F.; Franzini-Armstrong, C. Sequential stages in the age-dependent gradual formation and accumulation of tubular aggregates in fast twitch muscle fibers: SERCA and calsequestrin involvement. Age 2012, 34, 27–41. [Google Scholar] [CrossRef]
- Luik, R.M.; Wu, M.M.; Buchanan, J.; Lewis, R.S. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 2006, 174, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Modlin, M.; Forstner, C.; Hofer, C.; Mayr, W.; Richter, W.; Carraro, U.; Protasi, F.; Kern, H. Electrical stimulation of denervated muscles: First results of a clinical study. Artif. Organs 2005, 29, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kern, H.; Carraro, U.; Adami, N.; Hofer, C.; Loefler, S.; Vogelauer, M.; Mayr, W.; Rupp, R.; Zampieri, S. One year of home-based daily FES in complete lower motor neuron paraplegia: Recovery of tetanic contractility drives the structural improvements of denervated muscle. Neurol. Res. 2010, 32, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Carraro, U.; Kern, H.; Gava, P.; Hofer, C.; Loefler, S.; Gargiulo, P.; Mosole, S.; Zampieri, S.; Gobbo, V.; Ravara, B.; et al. Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products. Eur. J. Transl. Myol. 2015, 25, 221–230. [Google Scholar] [CrossRef]
- Kern, H.; Barberi, L.; Lofler, S.; Sbardella, S.; Burggraf, S.; Fruhmann, H.; Carraro, U.; Mosole, S.; Sarabon, N.; Vogelauer, M.; et al. Electrical stimulation counteracts muscle decline in seniors. Front. Aging Neurosci. 2014, 6, 189. [Google Scholar] [CrossRef]
- Iodice, P.; Boncompagni, S.; Pietrangelo, L.; Galli, L.; Pierantozzi, E.; Rossi, D.; Fusella, A.; Caulo, M.; Kern, H.; Sorrentino, V. Functional Electrical Stimulation: A Possible Strategy to Improve Muscle Function in Central Core Disease? Front. Neurol. 2019, 10, 479. [Google Scholar] [CrossRef]
- Zampieri, S.; Mammucari, C.; Romanello, V.; Barberi, L.; Pietrangelo, L.; Fusella, A.; Mosole, S.; Gherardi, G.; Hofer, C.; Lofler, S.; et al. Physical exercise in aging human skeletal muscle increases mitochondrial calcium uniporter expression levels and affects mitochondria dynamics. Physiol. Rep. 2016, 4, e13005. [Google Scholar] [CrossRef]
- Mosole, S.; Carraro, U.; Kern, H.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Mayr, W.; Krenn, M.; Paternostro-Sluga, T.; et al. Long-term high-level exercise promotes muscle reinnervation with age. J. Neuropathol. Exp. Neurol. 2014, 73, 284–294. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).