How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives
Abstract
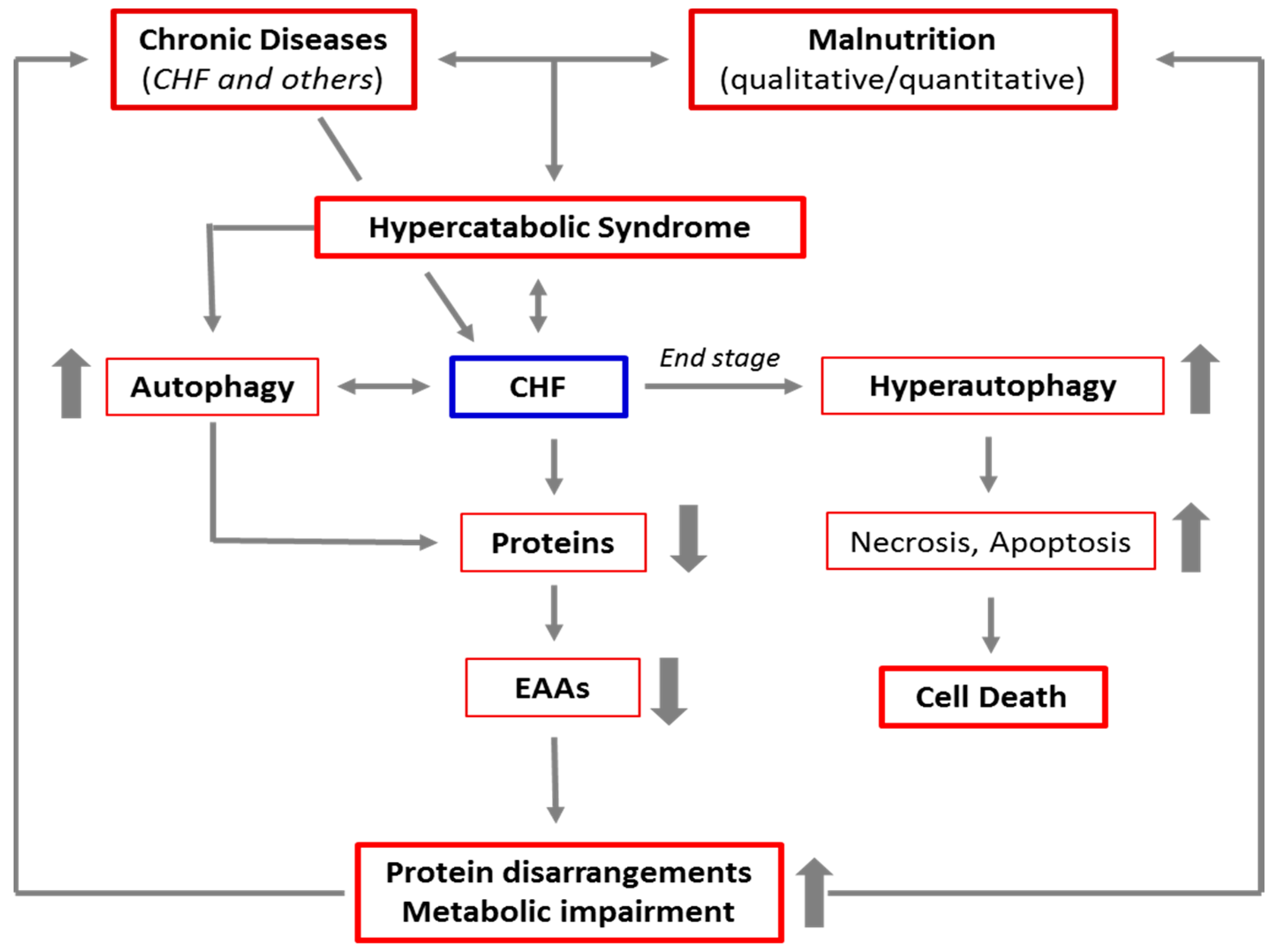
1. Chronic Diseases and Malnutrition: The Mortal Embrace
Nutritional Risk Assessment
2. Autophagy
3. Autophagy in the Heart
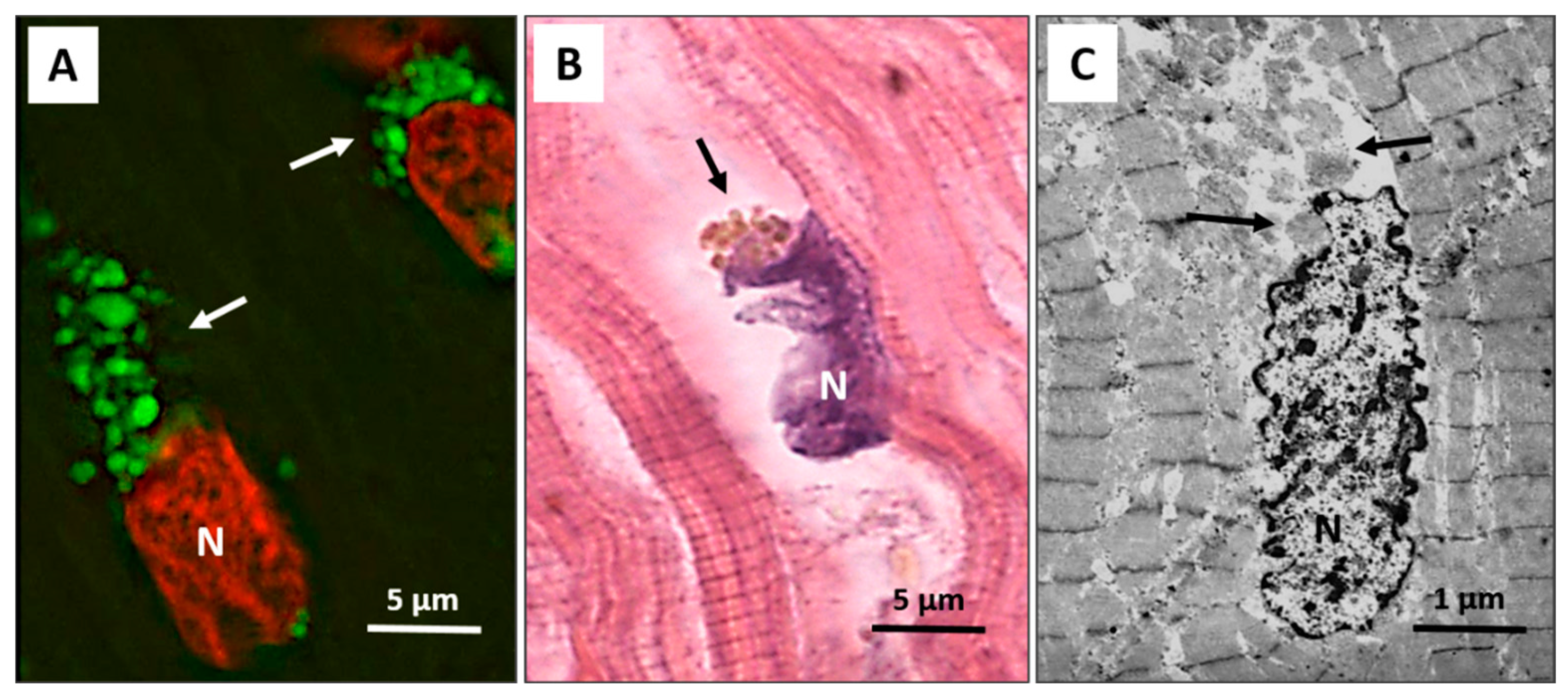
4. Autophagy in Heart Failure
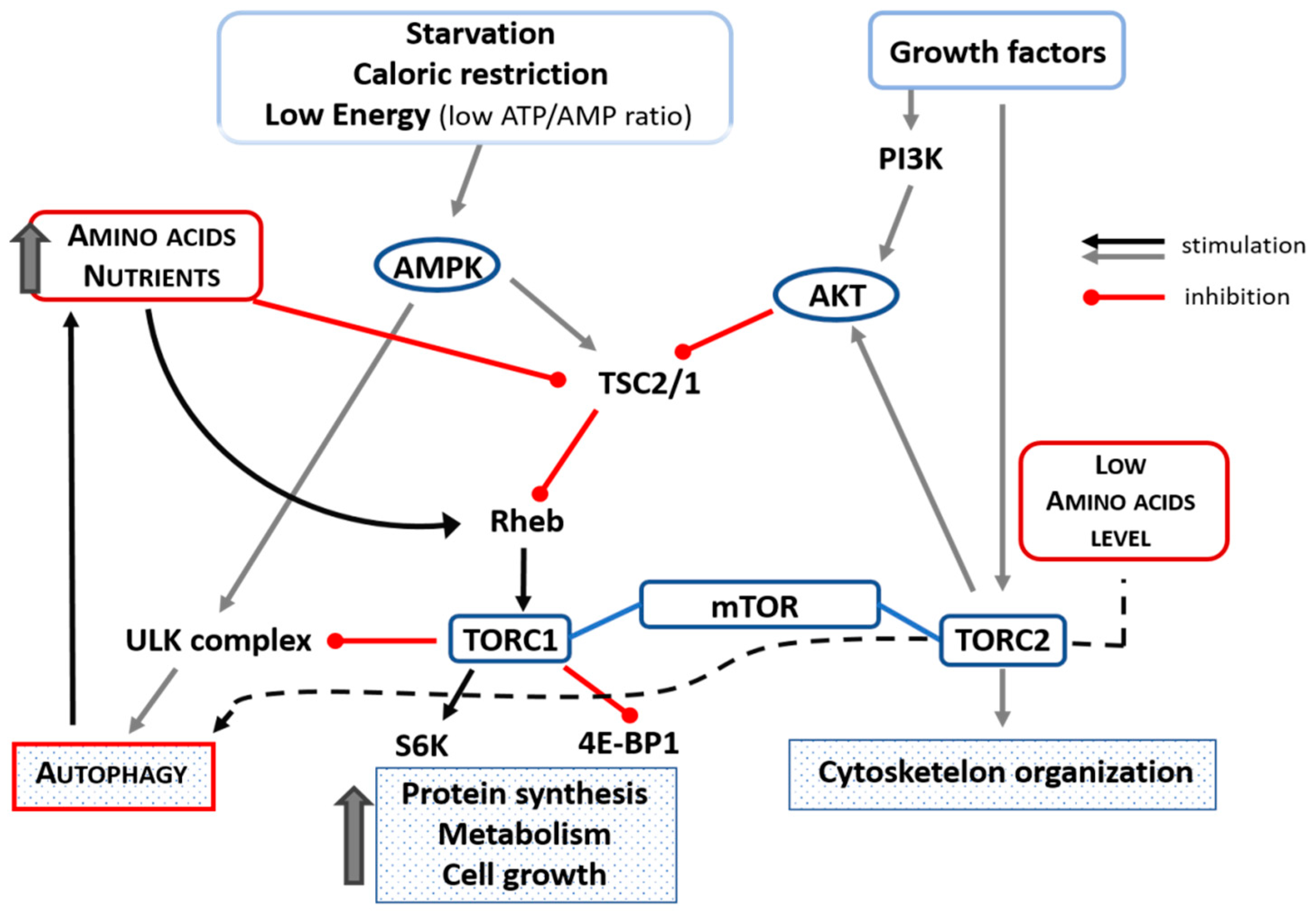
5. Role of Mammalian Target of Rapamycin (mTOR) in Autophagy
6. The Use of EAAs in Treatment and Prevention of Malnutrition under Increased Metabolic Demand
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacke, M.; Ebner, N.; Boschmann, M.; Jarius, A.; Valentova, M.; Fülster, S.; Sandek, A.; Schomburg, L.; Anker, S.D.; Doehner, W.; et al. Resting Energy Expenditure and the Effects of Muscle Wasting in Patients with Chronic Heart Failure: Results from the Studies Investigating Comorbidities Aggravating Heart Failure (SICA-HF). J. Am. Med. Dir. Assoc. 2013, 14, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Dioguardi, F.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatbolic syndrome: Molecular basis and effects of nutritional supplementation with aminoacids. Am. J. Cardiol. 2008, 101, S11–S15. [Google Scholar] [CrossRef]
- Anker, S.D.; Chua, T.P.; Ponikowski, P.; Harrington, D.; Swan, J.W.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J.S. Hormonal Changes and Catabolic/Anabolic Imbalance in Chronic Heart Failure and Their Importance for Cardiac Cachexia. Circulation 1997, 96, 526–534. [Google Scholar] [CrossRef]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of Dietary Proteins and Amino Acids in the Pathogenesis of Insulin Resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Dossena, M.; Iadarola, P.; Gualco, A.; Arcidiaco, P.; Viglio, S.; Boschi, F.; Verri, M.; Pasini, E. Increased skeletal muscle amino acid release with light exercise in deconditioned patients with heart failure. J. Am. Coll. Cardiol. 2005, 45, 158–160. [Google Scholar] [CrossRef]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.A.; Poole-Wilson, P.; Coats, A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation. Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef]
- Heidenreich, P. Inflammation and heart failure: Therapeutic or diagnostic opportunity? J. Am. Coll. Cardiol. 2017, 69, 1286–1287. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Verri, M.; Boschi, F.; Febo, O.; Pasini, E.; Pastoris, O. Is nutritional intake adequate in chronic heart failure patients? J. Am. Coll. Cardiol. 2003, 42, 1218–1223. [Google Scholar] [CrossRef]
- Alberda, C.; Graf, A.; McCargar, L. Malnutrition: Etiology, consequences, and assessment of a patient at risk. Best Pr. Res. Clin. Gastroenterol. 2006, 20, 419–439. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Gheorghiade, M.; Dioguardi, F.S. Malnutrition, muscle wasting and cachexia in chronic heart failure: The nutritional approach. Ital. Heart J. 2003, 4, 232–235. [Google Scholar]
- Rondel, A.; Langius, J.; De Van Der Schueren, M.; Kruizenga, H. The new ESPEN diagnostic criteria for malnutrition predict overall survival in hospitalised patients. Clin. Nutr. 2018, 37, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Comini, L.; Dioguardi, F.S.; Grossetti, F.; Olivares, A.; Zanelli, E.; Aquilani, R.; Scalvini, S. Hypoalbuminemia as a marker of protein metabolism disarrangement in patients with stable chronic heart failure. Minerva Med. 2020, 111, 226–238. [Google Scholar] [CrossRef]
- Gariballa, E.S.; Parker, S.G.; Taub, N.; Castleden, C.M. Influence of nutritional status on clinical outcome after acute stroke. Am. J. Clin. Nutr. 1998, 68, 275–281. [Google Scholar] [CrossRef]
- Rady, M.Y.; Ryan, T.; Starr, N.J. Clinical Characteristics of Preoperative Hypoalbuminemia Predict Outcome of Cardiovascular Surgery. J. Parenter. Enter. Nutr. 1997, 21, 81–90. [Google Scholar] [CrossRef]
- Adejumo, A.C.; Adejumo, K.L.; Adegbala, O.M.; Chinedozi, I.; Ndansi, J.; Akanbi, O.; Onyeakusi, N.E.; Ogundipe, O.A.; Bob-Manuel, T.; Adeboye, A. Protein-Energy Malnutrition and Outcomes of Hospitalizations for Heart Failure in the USA. Am. J. Cardiol. 2019, 123, 929–935. [Google Scholar] [CrossRef]
- Liu, M.; Chan, C.-P.; Yan, B.P.; Zhang, Q.; Lam, Y.-Y.; Li, R.-J.; Sanderson, J.E.; Coats, A.J.; Sun, J.-P.; Yip, G.W.-K.; et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2012, 14, 39–44. [Google Scholar] [CrossRef]
- Bonilla-Palomas, J.L.; Gámez-López, A.L.; Moreno-Conde, M.; López-Ibáñez, M.C.; Anguita-Sánchez, M.; De la Sacristana, Á.G.; García-Catalán, F.; Villar-Ráez, A. Hypoalbuminemia in Acute Heart Failure Patients: Causes and its Impact on Hospital and Long-Term Mortality. J. Card. Fail. 2014, 20, 350–358. [Google Scholar] [CrossRef]
- Ancion, A.; Allepaerts, S.; Oury, C.; Gori, A.-S.; Piérard, L.A.; Lancellotti, P. Serum albumin level and hospital mortality in acute non-ischemic heart failure. ESC Heart Fail. 2017, 4, 138–145. [Google Scholar] [CrossRef]
- Uthamalingam, S.; Kandala, J.; Daley, M.; Patvardhan, E.; Capodilupo, R.; Moore, S.A.; Januzzi, J.L. Serum albumin and mortality in acutely decompensated heart failure. Am. Heart J. 2010, 160, 1149–1155. [Google Scholar] [CrossRef]
- Kato, T.; Yaku, H.; Morimoto, T.; Inuzuka, Y.; Tamaki, Y.; Ozasa, N.; Yamamoto, E.; Yoshikawa, Y.; Kitai, T.; Taniguchi, R.; et al. Association of an increase in serum albumin levels with positive 1-year outcomes in acute decompensated heart failure: A cohort study. PLoS ONE 2020, 15, e0243818. [Google Scholar] [CrossRef]
- Aquilani, R.; La Rovere, M.T.; Corbellini, D.; Pasini, E.; Verri, M.; Barbieri, A.; Condino, A.M.; Boschi, F. Plasma Amino Acid Abnormalities in Chronic Heart Failure. Mechanisms, Potential Risks and Targets in Human Myocardium Metabolism. Nutrients 2017, 9, 1251. [Google Scholar] [CrossRef]
- Uchino, Y.; Watanabe, M.; Takata, M.; Amiya, E.; Tsushima, K.; Adachi, T.; Hiroi, Y.; Funazaki, T.; Komuro, I. Effect of Oral Branched-Chain Amino Acids on Serum Albumin Concentration in Heart Failure Patients with Hypoalbuminemia: Results of a Preliminary Study. Am. J. Cardiovasc. Drugs 2018, 18, 327–332. [Google Scholar] [CrossRef]
- Filippatos, G.S.; Desai, R.V.; Ahmed, M.I.; Fonarow, G.C.; Love, T.E.; Aban, I.B.; Iskandrian, A.E.; Konstam, M.A.; Ahmed, A. Hypoalbuminaemia and incident heart failure in older adults. Eur. J. Heart Fail. 2011, 13, 1078–1086. [Google Scholar] [CrossRef]
- Yin, J.; Lu, X.; Qian, Z.; Xu, W.; Zhou, X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics 2019, 9, 4019–4029. [Google Scholar] [CrossRef]
- Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan. Nutrients 2019, 11, 1367. [Google Scholar] [CrossRef]
- Löhr, J.-M.; Panic, N.; Vujasinovic, M.; Verbeke, C.S. The ageing pancreas: A systematic review of the evidence and analysis of the consequences. J. Intern. Med. 2018, 283, 446–460. [Google Scholar] [CrossRef]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef]
- Corsetti, G.; Pasini, E.; Romano, C.; Calvani, R.; Picca, A.; Marzetti, E.; Flati, V.; Dioguardi, F.S. Body Weight Loss and Tissue Wasting in Late Middle-Aged Mice on Slightly Imbalanced Essential/Non-essential Amino Acids Diet. Front. Med. 2018, 5, 136. [Google Scholar] [CrossRef]
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sung, S.; Cheng, H.; Hsu, P.; Guo, C.; Yu, W.; Chen, C. Prognostic Nutritional Index and the Risk of Mortality in Patients with Acute Heart Failure. J. Am. Heart Assoc. 2017, 6, e004876. [Google Scholar] [CrossRef] [PubMed]
- Candeloro, M.; Di Nisio, M.; Balducci, M.; Genova, S.; Valeriani, E.; Pierdomenico, S.D.; Porreca, E. Prognostic nutritional index in elderly patients hospitalized for acute heart failure. ESC Heart Fail. 2020, 7, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Nishi, I.; Seo, Y.; Hamada-Harimura, Y.; Yamamoto, M.; Ishizu, T.; Sugano, A.; Sato, K.; Sai, S.; Obara, K.; Suzuki, S.; et al. Geriatric nutritional risk index predicts all-cause deaths in heart failure with preserved ejection fraction. ESC Heart Fail. 2019, 6, 396–405. [Google Scholar] [CrossRef]
- Iwakami, N.; Nagai, T.A.; Furukawa, T.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure. Int. J. Cardiol. 2017, 230, 529–536. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Joaquín, C.; Puig, R.; Gastelurrutia, P.; Lupón, J.; De Antonio, M.; Domingo, M.; Moliner, P.; Zamora, E.; Martin, M.; Alonso, N.; et al. Mini nutritional assessment is a better predictor of mortality than subjective global assessment in heart failure out-patients. Clin. Nutr. 2019, 38, 2740–2746. [Google Scholar] [CrossRef]
- Joaquín, C.; Alonso, N.; Lupón, J.; De Antonio, M.; Domingo, M.; Moliner, P.; Zamora, E.; Codina, P.; Ramos, A.; González, B.; et al. Mini Nutritional Assessment Short Form is a morbi-mortality predictor in outpatients with heart failure and mid-range left ventricular ejection fraction. Clin. Nutr. 2020, 39, 3395–3401. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Münz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Macian, F. Autophagy, nutrition and immunology. Mol. Asp. Med. 2012, 33, 2–13. [Google Scholar] [CrossRef]
- Lin, X.; Xiao, W.; Xiao, L.; Liu, M. Molecular mechanisms of autophagy in cardiac ischemia/reperfusion injury (Review). Mol. Med. Rep. 2018, 18, 675–683. [Google Scholar] [CrossRef]
- Li, W.-W.; Li, J.; Bao, J.-K. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Another way to die: Autophagic programmed cell death. Cell Death Differ. 2005, 12, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef]
- Kostin, S. Pathways of myocyte death: Implications for development of clinical laboratory biomarkers. Adv. Clin. Chem. 2005, 40, 37–98. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.E.; Ibacache, M.; Criollo, A.; Nemchenko, A.A.; Hill, J.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, G.R.Y.; De Keulenaer, G.W.; Martinet, W. Role of autophagy in heart failure associated with aging. Heart Fail. Rev. 2010, 15, 423–430. [Google Scholar] [CrossRef]
- Wohlgemuth, S.E.; Julian, D.; Akin, D.E.; Fried, J.; Toscano, K.; Leeuwenburgh, C.; Dunn, J.W.A. Autophagy in the Heart and Liver During Normal Aging and Calorie Restriction. Rejuvenation Res. 2007, 10, 281–292. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, J. Nutritional Status and Cardiac Autophagy. Diabetes Metab. J. 2013, 37, 30–35. [Google Scholar] [CrossRef][Green Version]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nat. Cell Biol. 2004, 432, 1032–1036. [Google Scholar] [CrossRef]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef]
- Loos, B.; Lochner, A.; Engelbrecht, A.M. Autophagy in heart disease: A strong hypothesis for an untouched metabolic reserve. Med. Hypotheses 2011, 77, 52–57. [Google Scholar] [CrossRef]
- Hein, S.; Arnon, E.; Kostin, S.; Schönburg, M.; Elsässer, A.; Polyakova, V.; Schaper, J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation 2003, 107, 984–991. [Google Scholar] [CrossRef]
- Ghosh, R.; Pattison, J.S. Macroautophagy and Chaperone-Mediated Autophagy in Heart Failure: The Known and the Unknown. Oxid. Med. Cell. Longev. 2018, 2018, 8602041. [Google Scholar] [CrossRef]
- Decker, R.S.; Wildenthal, K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am. J. Pathol. 1980, 98, 425–444. [Google Scholar]
- Liu, X.; Van Vleet, T.; Schnellmann, R.G. The role of calpain in oncotic cell death. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 349–370. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Chen, S.; Li, Y.; Cui, Z.; Ma, J. Knockdown of microRNA-122 protects H9c2 cardiomyocytes from hypoxia-induced apoptosis and promotes autophagy. Med. Sci. Monit. 2017, 23, 4284–4290. [Google Scholar] [CrossRef]
- Chen-Scarabelli, C.; Faggian, G.; Shah, M.; Saravolatz, L., II; Saravolatz, S.; Scarabelli, G.; Scarabelli, T.M. Warm blood cardioplegia induces myocyte autophagy, whose magnitude and severity are proportional to the duration of cardioplegic arrest. Circulation 2010, 122, A142. [Google Scholar]
- Corsetti, G.; Chen-Scarabelli, C.; Romano, C.; Pasini, E.; Dioguardi, F.S.; Onorati, F.; Knight, R.; Patel, H.; Saravolatz, L.; Faggian, G.; et al. Autophagy and Oncosis/Necroptosis Are Enhanced in Cardiomyocytes from Heart Failure Patients. Med. Sci. Monit. Basic Res. 2019, 25, 33–44. [Google Scholar] [CrossRef]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M.; Wei, Y.; Ginet, V.; Zhang, L.A.; Posner, B.; Tran, K.A.; Green, D.R.; Xavier, R.J.; et al. Autosis is a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.-P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef]
- Howell, J.J.; Manning, B.D. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 2011, 22, 94–102. [Google Scholar] [CrossRef]
- Flati, V.; Corsetti, G.; Pasini, E.; Rufo, A.; Romano, C.; Dioguardi, F.S. Nutrition, Nitrogen Requirements, Exercise and Chemotherapy-Induced Toxicity in Cancer Patients. A Puzzle of Contrasting Truths? Anti Cancer Agents Med. Chem. 2015, 16, 89–100. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Callier, V. A new mathematical approach for qualitative modeling of the insulin-TOR-MAPK network. Front. Physiol. 2013, 4, 245. [Google Scholar] [CrossRef]
- Evans, D.S.; Kapahi, P.; Hsueh, W.-C.; Kockel, L. TOR signaling never gets old: Aging, longevity and TORC1 activity. Ageing Res. Rev. 2011, 10, 225–237. [Google Scholar] [CrossRef]
- Navé, B.T.; Ouwens, M.; Withers, D.J.; Alessi, D.R.; Shepherd, P.R. Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 1999, 344, 427–431. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Yu, L.; McPhee, C.K.; Zheng, L.; Mardones, G.A.; Rong, Y.; Peng, J.; Mi, N.; Zhao, Y.; Liu, Z.; Wan, F.; et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010, 465, 942–946. [Google Scholar] [CrossRef]
- Deldicque, L.; Theisen, D.; Francaux, M. Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 94, 1–10. [Google Scholar] [CrossRef]
- Proud, C.G. mTOR-mediated regulation of translation factors by amino acids. Biochem. Biophys. Res. Commun. 2004, 313, 429–436. [Google Scholar] [CrossRef]
- Flati, V.; Pasini, E.; D’Antona, G.; Speca, S.; Toniato, E.; Martinotti, S. Intracellular Mechanisms of Metabolism Regulation: The Role of Signaling via the Mammalian Target of Rapamycin Pathway and Other Routes. Am. J. Cardiol. 2008, 101, S16–S21. [Google Scholar] [CrossRef]
- Hara, K.; Yonezawa, K.; Weng, Q.-P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino Acid Sufficiency and mTOR Regulate p70 S6 Kinase and eIF-4E BP1 through a Common Effector Mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef]
- Wang, X.; Campbell, L.E.; Miller, C.M.; Proud, C.G. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem. J. 1998, 334, 261–267. [Google Scholar] [CrossRef]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the rec-ommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional Recommendations for the Management of Sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Short, K.R.; Campbell, W.W.; Volpi, E.; Wolfe, R.R. Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 2008, 87, S1562–S1566. [Google Scholar] [CrossRef]
- Sud, M.; Wang, X.; Austin, P.C.; Lipscombe, L.L.; Newton, G.E.; Tu, J.V.; Vasan, R.S.; Lee, U.S. Presentation blood glucose and death, hospitalization, and future diabetes risk in patients with acute heart failure syndromes. Eur. Heart J. 2015, 36, 924–931. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A.; et al. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. BioMed Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Viglio, S.; Iadarola, P.; Opasich, C.; Testa, A.; Dioguardi, F.S.; Pasini, E. Oral Amino Acid Supplements Improve Exercise Capacities in Elderly Patients with Chronic Heart Failure. Am. J. Cardiol. 2008, 101, S104–S110. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Gualco, A.; Verri, M.; Testa, A.; Pasini, E.; Viglio, S.; Iadarola, P.; Pastoris, O.; Dossena, M.; et al. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur. J. Heart Fail. 2008, 10, 1127–1135. [Google Scholar] [CrossRef]
- Scognamiglio, R.; Testa, A.; Aquilani, R.; Dioguardi, F.S.; Pasini, E. Impairment in walking capacity and myocardial function in the elderly: Is there a role for non-pharmacologic therapy with nutritional amino acid supplementation? Am. J. Cardiol. 2008, 101, S78–S81. [Google Scholar] [CrossRef]
- Vlahakis, A.; Powers, T. A role for TOR complex 2 signaling in promoting autophagy. Autophagy 2014, 10, 2085–2086. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsetti, G.; Pasini, E.; Romano, C.; Chen-Scarabelli, C.; Scarabelli, T.M.; Flati, V.; Saravolatz, L.; Dioguardi, F.S. How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives. Int. J. Mol. Sci. 2021, 22, 3332. https://doi.org/10.3390/ijms22073332
Corsetti G, Pasini E, Romano C, Chen-Scarabelli C, Scarabelli TM, Flati V, Saravolatz L, Dioguardi FS. How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives. International Journal of Molecular Sciences. 2021; 22(7):3332. https://doi.org/10.3390/ijms22073332
Chicago/Turabian StyleCorsetti, Giovanni, Evasio Pasini, Claudia Romano, Carol Chen-Scarabelli, Tiziano M. Scarabelli, Vincenzo Flati, Louis Saravolatz, and Francesco S. Dioguardi. 2021. "How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives" International Journal of Molecular Sciences 22, no. 7: 3332. https://doi.org/10.3390/ijms22073332
APA StyleCorsetti, G., Pasini, E., Romano, C., Chen-Scarabelli, C., Scarabelli, T. M., Flati, V., Saravolatz, L., & Dioguardi, F. S. (2021). How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives. International Journal of Molecular Sciences, 22(7), 3332. https://doi.org/10.3390/ijms22073332








