Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing
Abstract
:1. Ca2+ Handling in Skeletal Muscle Fibers
1.1. Excitation–Contraction (EC) Coupling
1.2. Excitation–Metabolism Coupling
1.3. Store-Operated Ca2+ Entry (SOCE)
2. Architecture of the Membrane Systems and Organelles Involved in Ca2+ Handling and Aerobic ATP Production
2.1. Calcium Release Units (CRUs), or Triads: The Sites of EC Coupling
- (a)
- (b)
- Inside the lumen of SR terminal cisternae, a dark matrix reveals the presence of calsequestrin (CASQ), the main SR Ca2+ buffer, which accumulates large amounts of Ca2+ in proximity of the sites of release [154,184,185,186,187,188]. The CASQ matrix appears to be anchored at the SR terminal cisterna via thin and long strands, which have been proposed to be constituted by triadin (Figure 2B,C) [189,190,191].
2.2. Mitochondria: The Powerhouse of the Cell
2.3. Calcium Entry Units (CEUs): The Dynamic Junctions That Mediate SOCE
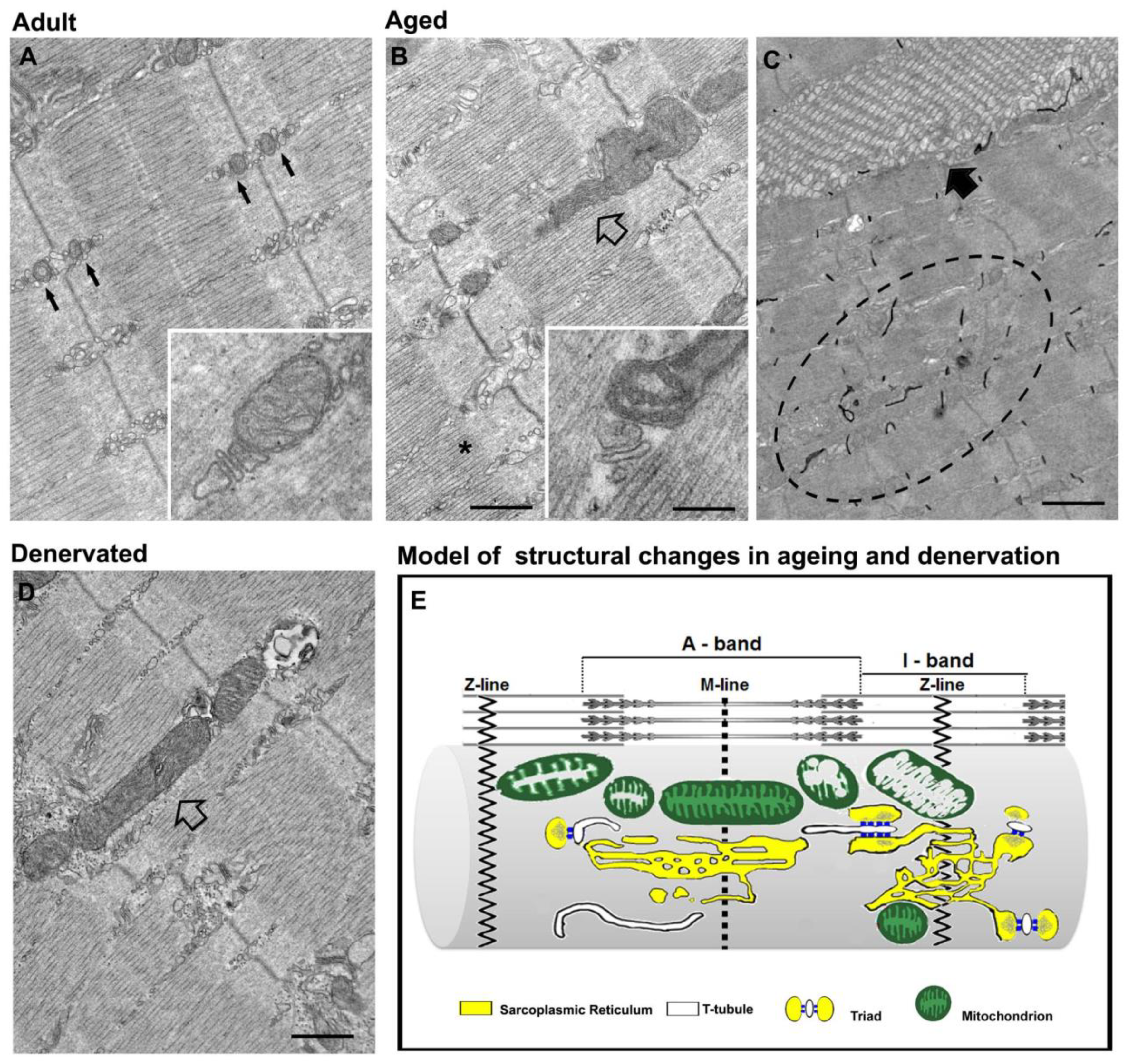
3. Disarray of Intracellular Organelles in Denervation and Ageing
3.1. Denervation Causes Disarray of EC Coupling and Mitochondrial Machineries
3.2. Misplacement of Intracellular Membranes and Organelles in Ageing
4. Are Alterations in Denervation and Ageing Mainly Caused by Inactivity?
4.1. Functional Electrical Stimulation (FES) Rescues the Ultrastructure of the EC Coupling Apparatus
- As a supportive measure to improve muscle function in healthy, but sedentary, seniors: in this study, FES was able to improve the muscle trophism, force, and cross-sectional area of fast muscle fibers thanks to the up-regulation of IGF-1 and the down-regulation of some atrogenes (atrogin and MuRF-1) [286];
- As an alternative therapy to improve muscle function and structure in a patient affected by a debilitating myopathy caused by a mutations in RYR1 (i.e., central core disease, CCD) [287].
4.2. Exercise Prevents/Rescues the Ultrastructural Modifications Caused by Inactive Ageing and Denervation
5. Final Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ca2+-induced Ca2+ release | CICR |
| calcium release unit | CRU |
| calcium entry unit | CEU |
| dihydropyridine receptor | DHPR |
| electron microscopy | EM |
| excitation–contraction | EC |
| functional electrical stimulation | FES |
| ryanodine receptor | RYR |
| sarcoplasmic-reticulum | SR |
| store-operated Ca2+ entry | SOCE |
| tubular aggregate | TA |
| transverse tubule | TT |
References
- Rasmussen, H.; Jensen, P.; Lake, W.; Goodman, D.B.P. Calcium ion as second messenger. Clin. Endocrinol. 1976, 5, 11S–27S. [Google Scholar] [CrossRef]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Endo, M. Calcium Ion as a Second Messenger with Special Reference to Excitation-Contraction Coupling. J. Pharmacol. Sci. 2006, 100, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmetsch, R. Excitation-transcription coupling: Signaling by ion channels to the nucleus. Sci. STKE 2003, 2003, PE4. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.F.; Chandler, W.K. Voltage dependent charge movement of skeletal muscle: A possible step in excitation-contraction coupling. Nature 1973, 242, 244–246. [Google Scholar] [CrossRef]
- Sandow, A.; Taylor, S.R.; Preiser, H. Role of the action potential in excitation-contraction coupling. Fed. Proc. 1965, 24, 1116–1123. [Google Scholar]
- Sandow, A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 1952, 25, 176–201. [Google Scholar] [PubMed]
- Rios, E.; Pizarro, G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol. Rev. 1991, 71, 849–908. [Google Scholar] [CrossRef] [PubMed]
- Calderon, J.C.; Bolanos, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef] [Green Version]
- Weber, A. Energized Calcium Transport and Relaxing Factors. Curr. Top. Bioenerg. 1966, 1, 203–254. [Google Scholar]
- Toyoshima, C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 2009, 1793, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Hasselbach, W.; Oetliker, H. Energetics and electrogenicity of the sarcoplasmic reticulum calcium pump. Annu. Rev. Physiol. 1983, 45, 325–339. [Google Scholar] [CrossRef]
- Hasselbach, W. Relaxation and the Sarcotubular Calcium Pump. Fed. Proc. 1964, 23, 909–912. [Google Scholar] [PubMed]
- Dulhunty, A.F.; Banyard, M.R.; Medveczky, C.J. Distribution of calcium ATPase in the sarcoplasmic reticulum of fast- and slow-twitch muscles determined with monoclonal antibodies. J. Membr. Biol. 1987, 99, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, P.; Guillen, A.; Rojas, H.; Boncompagni, S.; Caputo, C. The use of CalciumOrange-5N as a specific marker of mitochondrial Ca2+ in mouse skeletal muscle fibers. Pflug. Arch. 2008, 455, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ainbinder, A.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle. Cell Calcium 2015, 57, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Shkryl, V.M.; Shirokova, N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J. Biol. Chem. 2006, 281, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- Rudolf, R.; Mongillo, M.; Magalhaes, P.J.; Pozzan, T. In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction. J. Cell Biol. 2004, 166, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.E.; Boncompagni, S.; Dirksen, R.T. Sarcoplasmic reticulum-mitochondrial symbiosis: Bidirectional signaling in skeletal muscle. Exerc. Sport Sci. Rev. 2009, 37, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Parekh, A.B.; Penner, R. Store depletion and calcium influx. Physiol. Rev. 1997, 77, 901–930. [Google Scholar] [CrossRef]
- Wei-Lapierre, L.; Carrell, E.M.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 2013, 4, 2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyfenko, A.D.; Dirksen, R.T. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J. Physiol. 2008, 586, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Launikonis, B.S.; Rios, E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 2007, 583 Pt 1, 81–97. [Google Scholar] [CrossRef]
- Kurebayashi, N.; Ogawa, Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 2001, 533 Pt 1, 185–199. [Google Scholar] [CrossRef]
- Dirksen, R.T. Checking your SOCCs and feet: The molecular mechanisms of Ca2+ entry in skeletal muscle. J. Physiol. 2009, 587 Pt 13, 3139–3147. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, A.J.; Sandow, A. The potentiation of muscular contraction by the nitrate-ion. Science 1950, 112, 647–649. [Google Scholar] [CrossRef]
- Wray, S.; Ravens, U.; Verkhratsky, A.; Eisner, D. Two centuries of excitation-contraction coupling. Cell Calcium 2004, 35, 485–489. [Google Scholar] [CrossRef]
- Schneider, M.F. Control of calcium release in functioning skeletal muscle fibers. Annu. Rev. Physiol. 1994, 56, 463–484. [Google Scholar] [CrossRef]
- Pozzan, T.; Rizzuto, R.; Volpe, P.; Meldolesi, J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 1994, 74, 595–636. [Google Scholar] [CrossRef]
- Endo, M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977, 57, 71–108. [Google Scholar] [CrossRef]
- Weber, A. On the role of calcium in the activity of adenosine 5’-triphosphate hydrolysis by actomyosin. J. Biol. Chem. 1959, 234, 2764–2769. [Google Scholar] [CrossRef]
- Niedergerke, R. Local muscular shortening by intracellularly applied calcium. J. Physiol. 1955, 128, 12–13. [Google Scholar]
- Heilbrunn, L.V.; Wiercinski, F.J. The action of various cations on muscle protoplasm. J. Cell Comp. Physiol. 1947, 29, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Caputo, C.; Gimenez, M. Effects of external calcium deprivation on single muscle fibers. J. Gen. Physiol. 1967, 50, 2177–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, C.M.; Bezanilla, F.M.; Horowicz, P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim. Biophys. Acta 1972, 267, 605–608. [Google Scholar] [CrossRef]
- Winegrad, S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J. Gen. Physiol. 1968, 51, 65–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasselbach, W.; Makinose, M. The calcium pump of the “relaxing granules” of muscle and its dependence on ATP-splitting. Biochem. Z 1961, 333, 518–528. [Google Scholar]
- Stern, M.D.; Cheng, H. Putting out the fire: What terminates calcium-induced calcium release in cardiac muscle? Cell Calcium 2004, 35, 591–601. [Google Scholar] [CrossRef]
- Orkand, R.K.; Niedergerke, R. Heart Action Potential: Dependence on External Calcium and Sodium Ions. Science 1964, 146, 1176–1177. [Google Scholar] [CrossRef]
- Nabauer, M.; Callewaert, G.; Cleemann, L.; Morad, M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science 1989, 244, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Fabiato, A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985, 85, 291–320. [Google Scholar] [CrossRef] [Green Version]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef]
- Collier, M.L.; Thomas, A.P.; Berlin, J.R. Relationship between L-type Ca2+ current and unitary sarcoplasmic reticulum Ca2+ release events in rat ventricular myocytes. J. Physiol 1999, 516 Pt 1, 117–128. [Google Scholar] [CrossRef]
- Collier, M.L.; Ji, G.; Wang, Y.; Kotlikoff, M.I. Calcium-induced calcium release in smooth muscle: Loose coupling between the action potential and calcium release. J. Gen. Physiol. 2000, 115, 653–662. [Google Scholar] [CrossRef]
- Cannell, M.B.; Cheng, H.; Lederer, W.J. The control of calcium release in heart muscle. Science 1995, 268, 1045–1049. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Meissner, G.; Lu, X. Dihydropyridine receptor-ryanodine receptor interactions in skeletal muscle excitation-contraction coupling. Biosci. Rep. 1995, 15, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Takeshima, H.; Mikami, A.; Flockerzi, V.; Takahashi, H.; Kangawa, K.; Kojima, M.; Matsuo, H.; Hirose, T.; Numa, S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 1987, 328, 313–318. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Powell, J.A.; Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 1988, 336, 134–139. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Adams, B.A.; Niidome, T.; Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 1990, 346, 567–569. [Google Scholar] [CrossRef]
- Sorrentino, V.; Volpe, P. Ryanodine receptors: How many, where and why? Trends Pharm. Sci. 1993, 14, 98–103. [Google Scholar] [CrossRef]
- Sorrentino, V. The ryanodine receptor family of intracellular calcium release channels. Adv. Pharm. 1995, 33, 67–90. [Google Scholar]
- Rios, E.; Brum, G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 1987, 325, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Sekiguchi, N.; Rando, T.A.; Allen, P.D.; Beam, K.G. Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J. Biol. Chem. 1998, 273, 13403–13406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, G.; Sorrentino, V. Molecular structure and tissue distribution of ryanodine receptors calcium channels. Med. Res. Rev. 1995, 15, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Willard, H.F.; Khanna, V.K.; Zorzato, F.; Green, N.M.; MacLennan, D.H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 13472–13483. [Google Scholar] [CrossRef]
- Mikami, A.; Imoto, K.; Tanabe, T.; Niidome, T.; Mori, Y.; Takeshima, H.; Narumiya, S.; Numa, S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 1989, 340, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Protasi, F.; Takahashi, M.; Takeshima, H.; Ferguson, D.G.; Franzini-Armstrong, C. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J. Cell Biol. 1995, 129, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Sun, X.H.; Franzini-Armstrong, C. Formation and maturation of the calcium release apparatus in developing and adult avian myocardium. Dev. Biol. 1996, 173, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Protasi, F. Structural interaction between RYRs and DHPRs in calcium release units of cardiac and skeletal muscle cells. Front. Biosci. 2002, 7, d650–d658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flucher, B.E.; Franzini-Armstrong, C. Formation of junctions involved in excitation-contraction coupling in skeletal and cardiac muscle. Proc. Natl. Acad. Sci. USA 1996, 93, 8101–8106. [Google Scholar] [CrossRef] [Green Version]
- Zorzato, F.; Fujii, J.; Otsu, K.; Phillips, M.; Green, N.M.; Lai, F.A.; Meissner, G.; MacLennan, D.H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 2244–2256. [Google Scholar] [CrossRef]
- Takeshima, H.; Nishimura, S.; Matsumoto, T.; Ishida, H.; Kangawa, K.; Minamino, N.; Matsuo, H.; Ueda, M.; Hanaoka, M.; Hirose, T.; et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 1989, 339, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.; Ma, J.J.; Gonzalez, A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J. Muscle Res. Cell Motil. 1991, 12, 127–135. [Google Scholar] [CrossRef]
- Rios, E.; Karhanek, M.; Ma, J.; Gonzalez, A. An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 1993, 102, 449–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzini-Armstrong, C.; Protasi, F. Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol. Rev. 1997, 77, 699–729. [Google Scholar] [CrossRef]
- Protasi, F.; Takekura, H.; Wang, Y.; Chen, S.R.; Meissner, G.; Allen, P.D.; Franzini-Armstrong, C. RYR1 and RYR3 have different roles in the assembly of calcium release units of skeletal muscle. Biophys. J. 2000, 79, 2494–2508. [Google Scholar] [CrossRef] [Green Version]
- Protasi, F.; Paolini, C.; Nakai, J.; Beam, K.G.; Franzini-Armstrong, C.; Allen, P.D. Multiple regions of RyR1 mediate functional and structural interactions with alpha(1S)-dihydropyridine receptors in skeletal muscle. Biophys. J. 2002, 83, 3230–3244. [Google Scholar] [CrossRef] [Green Version]
- Protasi, F.; Franzini-Armstrong, C.; Flucher, B.E. Coordinated incorporation of skeletal muscle dihydropyridine receptors and ryanodine receptors in peripheral couplings of BC3H1 cells. J. Cell Biol. 1997, 137, 859–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protasi, F.; Franzini-Armstrong, C.; Allen, P.D. Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle. J. Cell Biol. 1998, 140, 831–842. [Google Scholar] [CrossRef]
- Paolini, C.; Protasi, F.; Franzini-Armstrong, C. The relative position of RyR feet and DHPR tetrads in skeletal muscle. J. Mol. Biol. 2004, 342, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Franzini-Armstrong, C.; Jorgensen, A.O. Structure and development of E-C coupling units in skeletal muscle. Annu. Rev. Physiol. 1994, 56, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Franzini-Armstrong, C. The sarcoplasmic reticulum and the control of muscle contraction. FASEB J. 1999, 13, S266–S270. [Google Scholar] [CrossRef]
- Block, B.A.; Imagawa, T.; Campbell, K.P.; Franzini-Armstrong, C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 1988, 107, 2587–2600. [Google Scholar] [CrossRef]
- Nakai, J.; Ogura, T.; Protasi, F.; Franzini-Armstrong, C.; Allen, P.D.; Beam, K.G. Functional nonequality of the cardiac and skeletal ryanodine receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 1019–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabner, M.; Dirksen, R.T.; Suda, N.; Beam, K.G. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the Bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 1999, 274, 21913–21919. [Google Scholar] [CrossRef] [Green Version]
- Dirksen, R.T. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front. Biosci. 2002, 7, d659–d670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Lederer, W.J.; Cannell, M.B. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 1993, 262, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lederer, W.J. Calcium sparks. Physiol Rev. 2008, 88, 1491–1545. [Google Scholar] [CrossRef] [Green Version]
- Tsugorka, A.; Rios, E.; Blatter, L.A. Imaging elementary events of calcium release in skeletal muscle cells. Science 1995, 269, 1723–1726. [Google Scholar] [CrossRef]
- Weisleder, N.; Zhou, J.; Ma, J. Detection of calcium sparks in intact and permeabilized skeletal muscle fibers. Methods Mol. Biol. 2012, 798, 395–410. [Google Scholar] [PubMed] [Green Version]
- Isaeva, E.V.; Shirokova, N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J. Physiol. 2003, 547 Pt 2, 453–462. [Google Scholar] [CrossRef]
- Apostol, S.; Ursu, D.; Lehmann-Horn, F.; Melzer, W. Local calcium signals induced by hyper-osmotic stress in mammalian skeletal muscle cells. J. Muscle Res. Cell Motil. 2009, 30, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Shtifman, A.; Ward, C.W.; Wang, J.; Valdivia, H.H.; Schneider, M.F. Effects of imperatoxin A on local sarcoplasmic reticulum Ca(2+) release in frog skeletal muscle. Biophys. J. 2000, 79, 814–827. [Google Scholar] [CrossRef] [Green Version]
- Ebashi, S.; Endo, M.; Otsuki, I. Control of muscle contraction. Q. Rev. Biophys. 1969, 2, 351–384. [Google Scholar] [CrossRef]
- Ebashi, S. Regulatory mechanism of muscle contraction with special reference to the Ca-troponin-tropomyosin system. Essays Biochem. 1974, 10, 1–36. [Google Scholar] [PubMed]
- Ebashi, F.; Ebashi, S. Removal of calcium and relaxation in actomyosin systems. Nature 1962, 194, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Kalyanasundaram, A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef]
- Odermatt, A.; Becker, S.; Khanna, V.K.; Kurzydlowski, K.; Leisner, E.; Pette, D.; MacLennan, D.H. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 1998, 273, 12360–12369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzato, F.; Anderson, A.A.; Ohlendieck, K.; Froemming, G.; Guerrini, R.; Treves, S. Identification of a novel 45 kDa protein (JP-45) from rabbit sarcoplasmic-reticulum junctional-face membrane. Biochem. J. 2000, 351 Pt 2, 537–543. [Google Scholar] [CrossRef]
- Zhang, L.; Kelley, J.; Schmeisser, G.; Kobayashi, Y.M.; Jones, L.R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997, 272, 23389–23397. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, H.; Shimuta, M.; Komazaki, S.; Ohmi, K.; Nishi, M.; Iino, M.; Miyata, A.; Kangawa, K. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem. J. 1998, 331 Pt 1, 317–322. [Google Scholar] [CrossRef]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar]
- Rios, E.; Gyorke, S. Calsequestrin, triadin and more: The molecules that modulate calcium release in cardiac and skeletal muscle. J. Physiol. 2009, 587 Pt 13, 3069–3070. [Google Scholar] [CrossRef]
- Rebbeck, R.T.; Karunasekara, Y.; Board, P.G.; Beard, N.A.; Casarotto, M.G.; Dulhunty, A.F. Skeletal muscle excitation-contraction coupling: Who are the dancing partners? Int. J. Biochem. Cell Biol. 2014, 48, 28–38. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Wong, P.T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1971, 68, 1231–1235. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.C.; Caswell, A.H.; Talvenheimo, J.A.; Brandt, N.R. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry 1990, 29, 9281–9289. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.R.; Zhang, L.; Sanborn, K.; Jorgensen, A.O.; Kelley, J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J. Biol. Chem. 1995, 270, 30787–30796. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, S.L.; Goldstein, J.L.; Orth, K.; Moomaw, C.R.; Slaughter, C.A.; Brown, M.S. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J. Biol. Chem. 1989, 264, 18083–18090. [Google Scholar] [CrossRef]
- Guo, W.; Campbell, K.P. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J. Biol. Chem. 1995, 270, 9027–9030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froemming, G.R.; Pette, D.; Ohlendieck, K. The 90-kDa junctional sarcoplasmic reticulum protein forms an integral part of a supramolecular triad complex in skeletal muscle. Biochem. Biophys. Res. Commun. 1999, 261, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, A.F.; Wei-LaPierre, L.; Casarotto, M.G.; Beard, N.A. Core skeletal muscle ryanodine receptor calcium release complex. Clin. Exp. Pharm. Physiol. 2017, 44, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Caswell, A.H.; Brandt, N.R.; Brunschwig, J.P.; Purkerson, S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95,000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry 1991, 30, 7507–7513. [Google Scholar] [CrossRef]
- Anderson, A.A.; Treves, S.; Biral, D.; Betto, R.; Sandona, D.; Ronjat, M.; Zorzato, F. The novel skeletal muscle sarcoplasmic reticulum JP-45 protein. Molecular cloning, tissue distribution, developmental expression, and interaction with alpha 1.1 subunit of the voltage-gated calcium channel. J. Biol. Chem. 2003, 278, 39987–39992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, C.; Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 2002, 418, 605–611. [Google Scholar] [CrossRef]
- Sweeney, H.L. The importance of the creatine kinase reaction: The concept of metabolic capacitance. Med. Sci. Sports Exerc. 1994, 26, 30–36. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [Green Version]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Territo, P.R.; Mootha, V.K.; French, S.A.; Balaban, R.S. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: Role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000, 278, C423–C435. [Google Scholar] [CrossRef]
- McMillin, J.B.; Madden, M.C. The role of calcium in the control of respiration by muscle mitochondria. Med. Sci. Sports Exerc. 1989, 21, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G. Relation between cytosolic free Ca2+ concentration and the control of pyruvate dehydrogenase in isolated cardiac myocytes. Biochem. J. 1987, 241, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaeva, E.V.; Shkryl, V.M.; Shirokova, N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J. Physiol. 2005, 565 Pt 3, 855–872. [Google Scholar] [CrossRef]
- Territo, P.R.; French, S.A.; Balaban, R.S. Simulation of cardiac work transitions, in vitro: Effects of simultaneous Ca2+ and ATPase additions on isolated porcine heart mitochondria. Cell Calcium 2001, 30, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G. Role of calcium in respiratory control. Med. Sci. Sports Exerc. 1994, 26, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gunter, K.K.; Gunter, T.E. Transport of calcium by mitochondria. J. Bioenerg. Biomembr. 1994, 26, 471–485. [Google Scholar] [CrossRef]
- Buntinas, L.; Gunter, K.K.; Sparagna, G.C.; Gunter, T.E. The rapid mode of calcium uptake into heart mitochondria (RaM): Comparison to RaM in liver mitochondria. Biochim. Biophys. Acta 2001, 1504, 248–261. [Google Scholar] [CrossRef] [Green Version]
- Beutner, G.; Sharma, V.K.; Giovannucci, D.R.; Yule, D.I.; Sheu, S.S. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001, 276, 21482–21488. [Google Scholar] [CrossRef] [Green Version]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabo, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Liu, J.; Nguyen, T.; Liu, C.; Sun, J.; Teng, Y.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013, 15, 1464–1472. [Google Scholar] [CrossRef] [Green Version]
- Sembrowich, W.L.; Quintinskie, J.J.; Li, G. Calcium uptake in mitochondria from different skeletal muscle types. J. Appl. Physiol. 1985, 59, 137–141. [Google Scholar] [CrossRef]
- Scarpa, A.; Graziotti, P. Mechanisms for intracellular calcium regulation in heart. I. Stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J. Gen. Physiol. 1973, 62, 756–772. [Google Scholar] [CrossRef] [Green Version]
- Crompton, M.; Capano, M.; Carafoli, E. Respiration-dependent efflux of magnesium ions from heart mitochondria. Biochem. J. 1976, 154, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.E.; Boncompagni, S.; Wei, L.; Protasi, F.; Dirksen, R.T. Differential impact of mitochondrial positioning on mitochondrial Ca(2+) uptake and Ca(2+) spark suppression in skeletal muscle. Am. J. Physiol. Cell Physiol. 2011, 301, C1128–C1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannella, C.A.; Buttle, K.; Rath, B.K.; Marko, M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors 1998, 8, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Csordas, G.; Renken, C.; Varnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnoczky, G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006, 174, 915–921. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Ramesh, V.; Franzini-Armstrong, C.; Sheu, S.S. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 2000, 32, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta 2006, 1763, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Beznoussenko, G.V.; Polishchuk, R.S.; Dirksen, R.T.; Protasi, F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol. Biol. Cell 2009, 20, 1058–1067. [Google Scholar] [CrossRef]
- Gillis, J.M. Inhibition of mitochondrial calcium uptake slows down relaxation in mitochondria-rich skeletal muscles. J. Muscle Res. Cell Motil. 1997, 18, 473–483. [Google Scholar] [CrossRef]
- Hidalgo, C.; Gonzalez, M.E.; Garcia, A.M. Calcium transport in transverse tubules isolated from rabbit skeletal muscle. Biochim. Biophys. Acta 1986, 854, 279–286. [Google Scholar] [CrossRef]
- Cheng, A.J.; Place, N.; Westerblad, H. Molecular Basis for Exercise-Induced Fatigue: The Importance of Strictly Controlled Cellular Ca(2+) Handling. Cold Spring Harb. Perspect. Med. 2018, 8, a029710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brini, M.; Carafoli, E. The plasma membrane Ca(2)+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb. Perspect Biol. 2011, 3, a004168. [Google Scholar] [CrossRef]
- Balnave, C.D.; Allen, D.G. Evidence for Na+/Ca2+ exchange in intact single skeletal muscle fibers from the mouse. Am. J. Physiol. 1998, 274, C940–C946. [Google Scholar] [CrossRef] [PubMed]
- Launikonis, B.S.; Barnes, M.; Stephenson, D.G. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2941–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikemoto, N.; Ronjat, M.; Meszaros, L.G.; Koshita, M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry 1989, 28, 6764–6771. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, S.R.; Belyea, M.J.; Epstein, D.R. Fatigue-based subgroups of breast cancer survivors with insomnia. Cancer Nurs. 2009, 32, 404–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelucci, A.; Garcia-Castaneda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-mediated store-operated Ca(2+) entry in skeletal muscle physiology and disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Buchanan, J.; Luik, R.M.; Lewis, R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006, 174, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luik, R.M.; Wang, B.; Prakriya, M.; Wu, M.M.; Lewis, R.S. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 2008, 454, 538–542. [Google Scholar] [CrossRef]
- Liou, J.; Fivaz, M.; Inoue, T.; Meyer, T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl. Acad. Sci. USA 2007, 104, 9301–9306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.M.; Covington, E.D.; Lewis, R.S. Single-molecule analysis of diffusion and trapping of STIM1 and Orai1 at endoplasmic reticulum-plasma membrane junctions. Mol. Biol. Cell 2014, 25, 3672–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, P.G.; Rao, A. Store-operated calcium entry: Mechanisms and modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Barr, V.A.; Bernot, K.M.; Shaffer, M.H.; Burkhardt, J.K.; Samelson, L.E. Formation of STIM and Orai complexes: Puncta and distal caps. Immunol. Rev. 2009, 231, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.L.; Yeromin, A.V.; Zhang, X.H.; Yu, Y.; Safrina, O.; Penna, A.; Roos, J.; Stauderman, K.A.; Cahalan, M.D. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl. Acad. Sci. USA 2006, 103, 9357–9362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.S. The molecular choreography of a store-operated calcium channel. Nature 2007, 446, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Vaidya, A.; Rohloff, P.; Sun, Y.P.; Grad, Y. Interactive medical case. A patient with fevers and fatigue. N. Engl. J. Med. 2013, 368, e9. [Google Scholar] [CrossRef]
- MacLennan, D.; Campbell, K.; Reithmeier, R. Calsequestrin. Calcium Cell Funct. 1983, 4, 151–173. [Google Scholar]
- Zhang, L.; Wang, L.; Li, S.; Xue, J.; Luo, D. Calsequestrin-1 Regulates Store-Operated Ca2+ Entry by Inhibiting STIM1 Aggregation. Cell Physiol. Biochem. 2016, 38, 2183–2193. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, S.; Zheng, Y.; Yan, X.; Chen, M.; Wang, H.; Putney, J.W.; Luo, D. Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci. Rep. 2015, 5, 11349. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.W.; Pan, Z.; Kim, E.K.; Lee, J.M.; Bhat, M.B.; Parness, J.; Kim, D.H.; Ma, J. A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J. Biol. Chem. 2003, 278, 3286–3292. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.N.; Murphy, R.M.; Cully, T.R.; von Wegner, F.; Friedrich, O.; Launikonis, B.S. Ultra-rapid activation and deactivation of store-operated Ca(2+) entry in skeletal muscle. Cell Calcium 2010, 47, 458–467. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; Konig, S.; Jousset, H.; Bader, C.; Demaurex, N.; Bernheim, L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J. Biol. Chem. 2009, 284, 5370–5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbellay, B.; Arnaudeau, S.; Bader, C.R.; Konig, S.; Bernheim, L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol. 2011, 194, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrell, E.M.; Coppola, A.R.; McBride, H.J.; Dirksen, R.T. Orai1 enhances muscle endurance by promoting fatigue-resistant type I fiber content but not through acute store-operated Ca2+ entry. FASEB J. 2016, 30, 4109–4119. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Weisleder, N.; Thornton, A.; Oppong, Y.; Campbell, R.; Ma, J.; Brotto, M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 2008, 7, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, A.M.; Zhao, X.; Weisleder, N.; Brotto, L.S.; Bougoin, S.; Nosek, T.M.; Reid, M.; Hardin, B.; Pan, Z.; Ma, J.; et al. Store-operated Ca(2+) entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle. Aging (Albany NY) 2011, 3, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Brotto, M. Aging, sarcopenia and store-operated calcium entry: A common link? Cell Cycle 2011, 10, 4201–4202. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.M.; Jimenez-Moreno, R.; Wang, Z.M.; Messi, M.L.; Delbono, O. Role of Ca2+, membrane excitability, and Ca2+ stores in failing muscle contraction with aging. Exp. Gerontol. 2009, 44, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.N.; Blackmore, D.G.; Gilbert, D.F.; Murphy, R.M.; Launikonis, B.S. Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression. Aging Cell 2011, 10, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Soboloff, J.; Rothberg, B.S.; Madesh, M.; Gill, D.L. STIM proteins: Dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012, 13, 549–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Qusairi, L.; Laporte, J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet. Muscle 2011, 1, 26. [Google Scholar] [CrossRef]
- Picard, M.; Taivassalo, T.; Gouspillou, G.; Hepple, R.T. Mitochondria: Isolation, structure and function. J. Physiol. 2011, 589 Pt 18, 4413–4421. [Google Scholar] [CrossRef]
- Newmeyer, D.D.; Ferguson-Miller, S. Mitochondria: Releasing power for life and unleashing the machineries of death. Cell 2003, 112, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Liesa, M.; Palacin, M.; Zorzano, A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 2009, 89, 799–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernster, L.; Schatz, G. Mitochondria: A historical review. J. Cell Biol. 1981, 91, 227s–255s. [Google Scholar] [CrossRef]
- Protasi, F.; Pietrangelo, L.; Boncompagni, S. Calcium entry units (CEUs): Perspectives in skeletal muscle function and disease. J. Muscle Res. Cell Motil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, L.; Dirksen, R.T.; Protasi, F. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 2017, 7, 14286, Addendum in 2018, 8, 17463. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Porter, K.R. Sarcolemmal Invaginations Constituting the T System in Fish Muscle Fibers. J. Cell Biol. 1964, 22, 675–696. [Google Scholar] [CrossRef] [Green Version]
- Franzini-Armstrong, C.; Protasi, F.; Ramesh, V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann. N. Y. Acad. Sci. 1998, 853, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Takekura, H.; Sun, X.; Franzini-Armstrong, C. Development of the excitation-contraction coupling apparatus in skeletal muscle: Peripheral and internal calcium release units are formed sequentially. J. Muscle Res. Cell Motil. 1994, 15, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Flucher, B.E.; Takekura, H.; Franzini-Armstrong, C. Development of the excitation-contraction coupling apparatus in skeletal muscle: Association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev. Biol. 1993, 160, 135–147. [Google Scholar] [CrossRef]
- Rios, E.; Pizarro, G. Voltage sensors and calcium channels of excitation-contraction coupling. News Physiol. Sci. 1988, 3, 223–227. [Google Scholar] [CrossRef]
- Wagenknecht, T.; Grassucci, R.; Frank, J.; Saito, A.; Inui, M.; Fleischer, S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature 1989, 338, 167–170. [Google Scholar] [CrossRef]
- Lai, F.A.; Erickson, H.P.; Rousseau, E.; Liu, Q.Y.; Meissner, G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature 1988, 331, 315–319. [Google Scholar]
- Fill, M.; Copello, J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002, 82, 893–922. [Google Scholar] [CrossRef] [Green Version]
- Franzini-Armstrong, C. Studies of the triad: I. Structure of the Junction in Frog Twitch Fibers. J. Cell Biol. 1970, 47, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Gamberucci, A.; Pierantozzi, E.; Amato, C.; Migliore, L.; Sorrentino, V. Calsequestrin, a key protein in striated muscle health and disease. J. Muscle Res. Cell Motil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Paolini, C.; Canato, M.; Reggiani, C.; Quarta, M. Lessons from calsequestrin-1 ablation in vivo: Much more than a Ca(2+) buffer after all. J. Muscle Res. Cell Motil. 2011, 32, 257–270. [Google Scholar] [CrossRef]
- Paolini, C.; Quarta, M.; Nori, A.; Boncompagni, S.; Canato, M.; Volpe, P.; Allen, P.D.; Reggiani, C.; Protasi, F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J. Physiol. 2007, 583 Pt 2, 767–784. [Google Scholar] [CrossRef]
- Meissner, G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim. Biophys. Acta 1975, 389, 51–68. [Google Scholar] [CrossRef]
- Campbell, K.P.; MacLennan, D.H.; Jorgensen, A.O.; Mintzer, M.C. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J. Biol. Chem. 1983, 258, 1197–1204. [Google Scholar] [CrossRef]
- Kobayashi, Y.M.; Alseikhan, B.A.; Jones, L.R. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J. Biol. Chem. 2000, 275, 17639–17646. [Google Scholar] [CrossRef] [Green Version]
- Franzini-Armstrong, C.; Kenney, L.J.; Varriano-Marston, E. The structure of calsequestrin in triads of vertebrate skeletal muscle: A deep-etch study. J. Cell Biol. 1987, 105, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Thomas, M.; Lopez, J.R.; Allen, P.D.; Yuan, Q.; Kranias, E.G.; Franzini-Armstrong, C.; Perez, C.F. Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis. PLoS ONE 2012, 7, e39962. [Google Scholar] [CrossRef] [Green Version]
- Ogata, T.; Yamasaki, Y. Scanning electron-microscopic studies on the three-dimensional structure of sarcoplasmic reticulum in the mammalian red, white and intermediate muscle fibers. Cell Tissue Res. 1985, 242, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Yeromin, A.V.; Zhang, S.L.; Jiang, W.; Yu, Y.; Safrina, O.; Cahalan, M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 2006, 443, 226–229. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J. Gen. Physiol. 2006, 128, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Dynes, J.L.; Yeromin, A.V.; Cahalan, M.D.; Franzini-Armstrong, C. Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry. Proc. Natl. Acad. Sci. USA 2015, 112, E5533–E5542. [Google Scholar] [CrossRef] [Green Version]
- Orci, L.; Ravazzola, M.; Le Coadic, M.; Shen, W.W.; Demaurex, N.; Cosson, P. From the Cover: STIM1-induced precortical and cortical subdomains of the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2009, 106, 19358–19362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Launikonis, B.S.; Stephenson, D.G.; Friedrich, O. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J. Physiol. 2009, 587 Pt 10, 2299–2312. [Google Scholar] [CrossRef]
- Tang, W.; Ingalls, C.P.; Durham, W.J.; Snider, J.; Reid, M.B.; Wu, G.; Matzuk, M.M.; Hamilton, S.L. Altered excitation-contraction coupling with skeletal muscle specific FKBP12 deficiency. FASEB J. 2004, 18, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.R.; Wu, F.; Liu, Y.; Anderson, D.M.; McAnally, J.; Lin, W.; Cannon, S.C.; Bassel-Duby, R.; Olson, E.N. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA 2013, 110, 11881–11886. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Komazaki, S.; Sasamoto, K.; Yoshida, M.; Nishi, M.; Kitamura, K.; Takeshima, H. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J. Cell Biol. 2001, 154, 1059–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Takano, T.; Protasi, F.; Dirksen, R.T. Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice. J. Gen. Physiol. 2020, 152, 10. [Google Scholar] [CrossRef]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Garcia-Castaneda, M.; Takano, T.; Malik, S.; Dirksen, R.T.; Protasi, F. Transverse tubule remodeling enhances Orai1-dependent Ca(2+) entry in skeletal muscle. eLife 2019, 8, e47576. [Google Scholar] [CrossRef] [PubMed]
- Zitt, C.; Strauss, B.; Schwarz, E.C.; Spaeth, N.; Rast, G.; Hatzelmann, A.; Hoth, M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 2004, 279, 12427–12437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002, 16, 1145–1150. [Google Scholar] [CrossRef] [Green Version]
- Canato, M.; Scorzeto, M.; Giacomello, M.; Protasi, F.; Reggiani, C.; Stienen, G.J. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl. Acad. Sci. USA 2010, 107, 22326–22331. [Google Scholar] [CrossRef] [Green Version]
- Boncompagni, S.; Pecorai, C.; Michelucci, A.; Pietrangelo, L.; Protasi, F. Long-Term Exercise Reduces Formation of Tubular Aggregates and Promotes Maintenance of Ca(2+) Entry Units in Aged Muscle. Front. Physiol. 2021, 11, 601057. [Google Scholar] [CrossRef]
- Melzer, W. ECC meets CEU-New focus on the backdoor for calcium ions in skeletal muscle cells. J. Gen. Physiol. 2020, 152, 10. [Google Scholar] [CrossRef]
- Vandervoort, A.A. Aging of the human neuromuscular system. Muscle Nerve 2002, 25, 17–25. [Google Scholar] [CrossRef]
- Roos, M.R.; Rice, C.L.; Vandervoort, A.A. Age-related changes in motor unit function. Muscle Nerve 1997, 20, 679–690. [Google Scholar] [CrossRef]
- Luff, A.R. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann. N. Y. Acad. Sci. 1998, 854, 92–101. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.Y.; Larsson, Y.; Bruhn, E.; Morsing, B. Heavy-resistance training in older Scandinavian men and women: Short- and long-term effects on arm and leg muscles. Scand. J. Med. Sci. Sports 1995, 5, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Grimby, G.; Saltin, B. The ageing muscle. Clin. Physiol. 1983, 3, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J.; Vandervoort, A.A.; Taylor, A.W.; Brown, W.F. Effects of motor unit losses on strength in older men and women. J. Appl. Physiol. 1993, 74, 868–874. [Google Scholar] [CrossRef]
- Clark, B.C. Neuromuscular Changes with Aging and Sarcopenia. J. Frailty Aging 2019, 8, 7–9. [Google Scholar]
- Billot, M.; Calvani, R.; Urtamo, A.; Sanchez-Sanchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. Clin. Interv. Aging 2020, 15, 1675–1690. [Google Scholar] [CrossRef]
- Schneider, E.L.; Guralnik, J.M. The aging of America. Impact on health care costs. JAMA 1990, 263, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Hughes, V.A. Sarcopenia: Current concepts. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M716–M724. [Google Scholar] [CrossRef] [Green Version]
- Roubenoff, R. Origins and clinical relevance of sarcopenia. Can. J. Appl. Physiol. 2001, 26, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Summary comments: Epidemiological and methodological problems in determining nutritional status of older persons. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Nikolic, M.; Malnar-Dragojevic, D.; Bobinac, D.; Bajek, S.; Jerkovic, R.; Soic-Vranic, T. Age-Related skeletal muscle atrophy in humans: An immunohistochemical and morphometric study. Coll. Antropol. 2001, 25, 545–553. [Google Scholar]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; A quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.J. What is sarcopenia? J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Alchin, D.R. Sarcopenia: Describing rather than defining a condition. J. Cachexia Sarcopenia Muscle 2014, 5, 265–268. [Google Scholar] [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Premkumar, P.; Ranjit, R.; Natarajan, K.S.; Ahn, B.; Riddle, K.; Claflin, D.R.; Richardson, A.; Brooks, S.V.; et al. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J. Cachexia Sarcopenia Muscle 2018, 9, 1003–1017. [Google Scholar] [CrossRef]
- Margreth, A.; Damiani, E.; Bortoloso, E. Sarcoplasmic reticulum in aged skeletal muscle. Acta Physiol. Scand 1999, 167, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L. The age-related motor disability: Underlying mechanisms in skeletal muscle at the motor unit, cellular and molecular level. Acta Physiol. Scand. 1998, 163, S27–S29. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fano, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Balagopal, P.; Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 1997, 273, E790–E800. [Google Scholar] [CrossRef]
- Aagaard, P.; Suetta, C.; Caserotti, P.; Magnusson, S.P.; Kjaer, M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scand. J. Med. Sci. Sports 2010, 20, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Evidence for nervous system degeneration with advancing age. J. Nutr. 1997, 127, 1011S–1013S. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.J.; Vukovic, J.; Dunlop, S.; Grounds, M.D.; Shavlakadze, T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS ONE 2011, 6, e28090. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, C.; Franzini, C. An Electron Microscope Study of Denervation Atrophy in Red and White Skeletal Muscle Fibers. J. Cell Biol. 1963, 17, 327–349. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.G.; Banker, B.Q.T. Myology, 3rd ed.; Engel, A.G., Franzini-Armstrong, C., Eds.; McGraw-Hill: New York, NY, USA, 2004; Volume 1, Chapter 31. [Google Scholar]
- Pietrangelo, L.; Michelucci, A.; Ambrogini, P.; Sartini, S.; Guarnier, F.A.; Fusella, A.; Zamparo, I.; Mammucari, C.; Protasi, F.; Boncompagni, S. Muscle activity prevents the uncoupling of mitochondria from Ca(2+) Release Units induced by ageing and disuse. Arch. Biochem. Biophys. 2019, 663, 22–33. [Google Scholar] [CrossRef]
- Kern, H.; Hofer, C.; Modlin, M.; Mayr, W.; Vindigni, V.; Zampieri, S.; Boncompagni, S.; Protasi, F.; Carraro, U. Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3- to 20-year SCI. Spinal Cord 2008, 46, 293–304. [Google Scholar] [CrossRef]
- Kern, H.; Boncompagni, S.; Rossini, K.; Mayr, W.; Fano, G.; Zanin, M.E.; Podhorska-Okolow, M.; Protasi, F.; Carraro, U. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): A role for myofiber regeneration? J. Neuropathol. Exp. Neurol. 2004, 63, 919–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boncompagni, S.; Kern, H.; Rossini, K.; Hofer, C.; Mayr, W.; Carraro, U.; Protasi, F. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 19339–19344. [Google Scholar] [CrossRef] [Green Version]
- Biral, D.; Kern, H.; Adami, N.; Boncompagni, S.; Protasi, F.; Carraro, U. Atrophy-resistant fibers in permanent peripheral denervation of human skeletal muscle. Neurol. Res. 2008, 30, 137–144. [Google Scholar] [CrossRef]
- Ashley, Z.; Sutherland, H.; Lanmuller, H.; Russold, M.F.; Unger, E.; Bijak, M.; Mayr, W.; Boncompagni, S.; Protasi, F.; Salmons, S.; et al. Atrophy, but not necrosis, in rabbit skeletal muscle denervated for periods up to one year. Am. J. Physiol. Cell Physiol. 2007, 292, C440–C451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, Z.; Salmons, S.; Boncompagni, S.; Protasi, F.; Russold, M.; Lanmuller, H.; Mayr, W.; Sutherland, H.; Jarvis, J.C. Effects of chronic electrical stimulation on long-term denervated muscles of the rabbit hind limb. J. Muscle Res. Cell Motil. 2007, 28, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Carraro, U.; Kern, H.; Pond, A.; Adami, N.; Biral, D.; Vindigni, V.; Boncompagni, S.; Pietrangelo, T.; Bosco, G.; et al. A subpopulation of rat muscle fibers maintains an assessable excitation-contraction coupling mechanism after long-standing denervation despite lost contractility. J. Neuropathol. Exp. Neurol. 2009, 68, 1256–1268. [Google Scholar] [CrossRef] [Green Version]
- Boncompagni, S.; d’Amelio, L.; Fulle, S.; Fano, G.; Protasi, F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: A possible role in the decline of muscle performance. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 995–1008. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Pond, A.; Grim-Stieger, M.; Cvecka, J.; Sedliak, M.; et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrangelo, L.; D’Incecco, A.; Ainbinder, A.; Michelucci, A.; Kern, H.; Dirksen, R.T.; Boncompagni, S.; Protasi, F. Age-dependent uncoupling of mitochondria from Ca2(+) release units in skeletal muscle. Oncotarget 2015, 6, 35358–35371. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, N.; Jones, D.L.; Xu, A.; Yu, J.C. Effects of aging on sarcoplasmic reticulum function and contraction duration in skeletal muscles of the rat. Am. J. Physiol. 1996, 271, C1032–C1040. [Google Scholar] [CrossRef]
- Delbono, O.; O’Rourke, K.S.; Ettinger, W.H. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J. Membr. Biol. 1995, 148, 211–222. [Google Scholar] [CrossRef]
- Danieli-Betto, D.; Betto, R.; Megighian, A.; Midrio, M.; Salviati, G.; Larsson, L. Effects of age on sarcoplasmic reticulum properties and histochemical composition of fast- and slow-twitch rat muscles. Acta Physiol. Scand. 1995, 154, 59–64. [Google Scholar] [CrossRef]
- Damiani, E.; Larsson, L.; Margreth, A. Age-related abnormalities in regulation of the ryanodine receptor in rat fast-twitch muscle. Cell Calcium 1996, 19, 15–27. [Google Scholar] [CrossRef]
- Ryan, M.; Ohlendieck, K. Excitation-Contraction Uncoupling and Sarcopenia. Basic Appl. Myol. 2004, 14, 141–154. [Google Scholar]
- Renganathan, M.; Messi, M.L.; Delbono, O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J. Membr. Biol. 1997, 157, 247–253. [Google Scholar] [CrossRef]
- Delbono, O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr. Aging Sci. 2011, 4, 248–259. [Google Scholar] [CrossRef]
- Ryan, M.; Butler-Browne, G.; Erzen, I.; Mouly, V.; Thornell, L.E.; Wernig, A.; Ohlendieck, K. Persistent expression of the alpha1S-dihydropyridine receptor in aged human skeletal muscle: Implications for the excitation-contraction uncoupling hypothesis of sarcopenia. Int. J. Mol. Med. 2003, 11, 425–434. [Google Scholar]
- Rios, E.; Brum, G. Ca2+ release flux underlying Ca2+ transients and Ca2+ sparks in skeletal muscle. Front. Biosci. 2002, 7, d1195–d1211. [Google Scholar]
- Hollingworth, S.; Zhao, M.; Baylor, S.M. The amplitude and time course of the myoplasmic free [Ca2+] transient in fast-twitch fibers of mouse muscle. J. Gen. Physiol. 1996, 108, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Csernoch, L.; Zhou, J.; Stern, M.D.; Brum, G.; Rios, E. The elementary events of Ca2+ release elicited by membrane depolarization in mammalian muscle. J. Physiol. 2004, 557 Pt 1, 43–58. [Google Scholar]
- Trounce, I.; Byrne, E.; Marzuki, S. Decline in skeletal muscle mitochondrial respiratory chain function: Possible factor in ageing. Lancet 1989, 1, 637–639. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef] [Green Version]
- Paolini, C.; Quarta, M.; Wei-LaPierre, L.; Michelucci, A.; Nori, A.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Oxidative stress, mitochondrial damage, and cores in muscle from calsequestrin-1 knockout mice. Skelet. Muscle 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Mitochondrial Impairment in Sarcopenia. Biology 2021, 10, 31. [Google Scholar] [CrossRef]
- Vissing, J.; Schmalbruch, H.; Haller, R.G.; Clausen, T. Muscle phosphoglycerate mutase deficiency with tubular aggregates: Effect of dantrolene. Ann. Neurol. 1999, 46, 274–277. [Google Scholar] [CrossRef]
- Rosenberg, N.L.; Neville, H.E.; Ringel, S.P. Tubular aggregates. Their association with neuromuscular diseases, including the syndrome of myalgias/cramps. Arch. Neurol. 1985, 42, 973–976. [Google Scholar] [CrossRef]
- Pierobon-Bormioli, S.; Armani, M.; Ringel, S.P.; Angelini, C.; Vergani, L.; Betto, R.; Salviati, G. Familial neuromuscular disease with tubular aggregates. Muscle Nerve 1985, 8, 291–298. [Google Scholar] [CrossRef]
- Morgan-Hughes, J.A. Tubular aggregates in skeletal muscle: Their functional significance and mechanisms of pathogenesis. Curr. Opin. Neurol. 1998, 11, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Engel, W.K.; Bishop, D.W.; Cunningham, G.G. Tubular aggregates in type II muscle fibers: Ultrastructural and histochemical correlation. J. Ultrastruct. Res. 1970, 31, 507–525. [Google Scholar] [CrossRef]
- De Groot, J.G.; Arts, W.F. Familial myopathy with tubular aggregates. J. Neurol. 1982, 227, 35–41. [Google Scholar] [CrossRef]
- Walter, M.C.; Rossius, M.; Zitzelsberger, M.; Vorgerd, M.; Muller-Felber, W.; Ertl-Wagner, B.; Zhang, Y.; Brinkmeier, H.; Senderek, J.; Schoser, B. 50 years to diagnosis: Autosomal dominant tubular aggregate myopathy caused by a novel STIM1 mutation. Neuromuscul. Disord. 2015, 25, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Okuma, H.; Saito, F.; Mitsui, J.; Hara, Y.; Hatanaka, Y.; Ikeda, M.; Shimizu, T.; Matsumura, K.; Shimizu, J.; Tsuji, S.; et al. Tubular aggregate myopathy caused by a novel mutation in the cytoplasmic domain of STIM1. Neurol. Genet. 2016, 2, e50. [Google Scholar] [CrossRef] [Green Version]
- Nesin, V.; Wiley, G.; Kousi, M.; Ong, E.C.; Lehmann, T.; Nicholl, D.J.; Suri, M.; Shahrizaila, N.; Katsanis, N.; Gaffney, P.M.; et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc. Natl. Acad. Sci. USA 2014, 111, 4197–4202. [Google Scholar] [CrossRef] [Green Version]
- Endo, Y.; Noguchi, S.; Hara, Y.; Hayashi, Y.K.; Motomura, K.; Miyatake, S.; Murakami, N.; Tanaka, S.; Yamashita, S.; Kizu, R.; et al. Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca(2)(+) channels. Hum. Mol. Genet. 2015, 24, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Bohm, J.; Chevessier, F.; Maues De Paula, A.; Koch, C.; Attarian, S.; Feger, C.; Hantai, D.; Laforet, P.; Ghorab, K.; Vallat, J.M.; et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am. J. Hum. Genet. 2013, 92, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Bohm, J.; Bulla, M.; Urquhart, J.E.; Malfatti, E.; Williams, S.G.; O’Sullivan, J.; Szlauer, A.; Koch, C.; Baranello, G.; Mora, M.; et al. ORAI1 Mutations with Distinct Channel Gating Defects in Tubular Aggregate Myopathy. Hum. Mutat. 2017, 38, 426–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, V.; Del Re, V.; Gamberucci, A.; Polverino, V.; Galli, L.; Rossi, D.; Costanzi, E.; Toniolo, L.; Berti, G.; Malandrini, A.; et al. Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum. Mutat. 2017, 38, 1761–1773. [Google Scholar] [CrossRef]
- Schiaffino, S.; Severin, E.; Cantini, M.; Sartore, S. Tubular aggregates induced by anoxia in isolated rat skeletal muscle. Lab. Investig. 1977, 37, 223–228. [Google Scholar] [PubMed]
- Salviati, G.; Pierobon-Bormioli, S.; Betto, R.; Damiani, E.; Angelini, C.; Ringel, S.P.; Salvatori, S.; Margreth, A. Tubular aggregates: Sarcoplasmic reticulum origin, calcium storage ability, and functional implications. Muscle Nerve 1985, 8, 299–306. [Google Scholar] [CrossRef]
- Chevessier, F.; Marty, I.; Paturneau-Jouas, M.; Hantai, D.; Verdiere-Sahuque, M. Tubular aggregates are from whole sarcoplasmic reticulum origin: Alterations in calcium binding protein expression in mouse skeletal muscle during aging. Neuromuscul. Disord. 2004, 14, 208–216. [Google Scholar] [CrossRef]
- Boncompagni, S.; Protasi, F.; Franzini-Armstrong, C. Sequential stages in the age-dependent gradual formation and accumulation of tubular aggregates in fast twitch muscle fibers: SERCA and calsequestrin involvement. Age 2012, 34, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Luik, R.M.; Wu, M.M.; Buchanan, J.; Lewis, R.S. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 2006, 174, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Modlin, M.; Forstner, C.; Hofer, C.; Mayr, W.; Richter, W.; Carraro, U.; Protasi, F.; Kern, H. Electrical stimulation of denervated muscles: First results of a clinical study. Artif. Organs 2005, 29, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kern, H.; Carraro, U.; Adami, N.; Hofer, C.; Loefler, S.; Vogelauer, M.; Mayr, W.; Rupp, R.; Zampieri, S. One year of home-based daily FES in complete lower motor neuron paraplegia: Recovery of tetanic contractility drives the structural improvements of denervated muscle. Neurol. Res. 2010, 32, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Carraro, U.; Kern, H.; Gava, P.; Hofer, C.; Loefler, S.; Gargiulo, P.; Mosole, S.; Zampieri, S.; Gobbo, V.; Ravara, B.; et al. Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products. Eur. J. Transl. Myol. 2015, 25, 221–230. [Google Scholar] [CrossRef]
- Kern, H.; Barberi, L.; Lofler, S.; Sbardella, S.; Burggraf, S.; Fruhmann, H.; Carraro, U.; Mosole, S.; Sarabon, N.; Vogelauer, M.; et al. Electrical stimulation counteracts muscle decline in seniors. Front. Aging Neurosci. 2014, 6, 189. [Google Scholar] [CrossRef] [Green Version]
- Iodice, P.; Boncompagni, S.; Pietrangelo, L.; Galli, L.; Pierantozzi, E.; Rossi, D.; Fusella, A.; Caulo, M.; Kern, H.; Sorrentino, V. Functional Electrical Stimulation: A Possible Strategy to Improve Muscle Function in Central Core Disease? Front. Neurol. 2019, 10, 479. [Google Scholar] [CrossRef]
- Zampieri, S.; Mammucari, C.; Romanello, V.; Barberi, L.; Pietrangelo, L.; Fusella, A.; Mosole, S.; Gherardi, G.; Hofer, C.; Lofler, S.; et al. Physical exercise in aging human skeletal muscle increases mitochondrial calcium uniporter expression levels and affects mitochondria dynamics. Physiol. Rep. 2016, 4, e13005. [Google Scholar] [CrossRef]
- Mosole, S.; Carraro, U.; Kern, H.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Mayr, W.; Krenn, M.; Paternostro-Sluga, T.; et al. Long-term high-level exercise promotes muscle reinnervation with age. J. Neuropathol. Exp. Neurol. 2014, 73, 284–294. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protasi, F.; Pietrangelo, L.; Boncompagni, S. Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing. Int. J. Mol. Sci. 2021, 22, 6195. https://doi.org/10.3390/ijms22126195
Protasi F, Pietrangelo L, Boncompagni S. Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing. International Journal of Molecular Sciences. 2021; 22(12):6195. https://doi.org/10.3390/ijms22126195
Chicago/Turabian StyleProtasi, Feliciano, Laura Pietrangelo, and Simona Boncompagni. 2021. "Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing" International Journal of Molecular Sciences 22, no. 12: 6195. https://doi.org/10.3390/ijms22126195
APA StyleProtasi, F., Pietrangelo, L., & Boncompagni, S. (2021). Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing. International Journal of Molecular Sciences, 22(12), 6195. https://doi.org/10.3390/ijms22126195





