Cyclic Adenosine Monophosphate Eliminates Sex Differences in Estradiol-Induced Elastin Production from Engineered Dermal Substitutes
Abstract
1. Introduction
2. Results
2.1. Patient Demographics
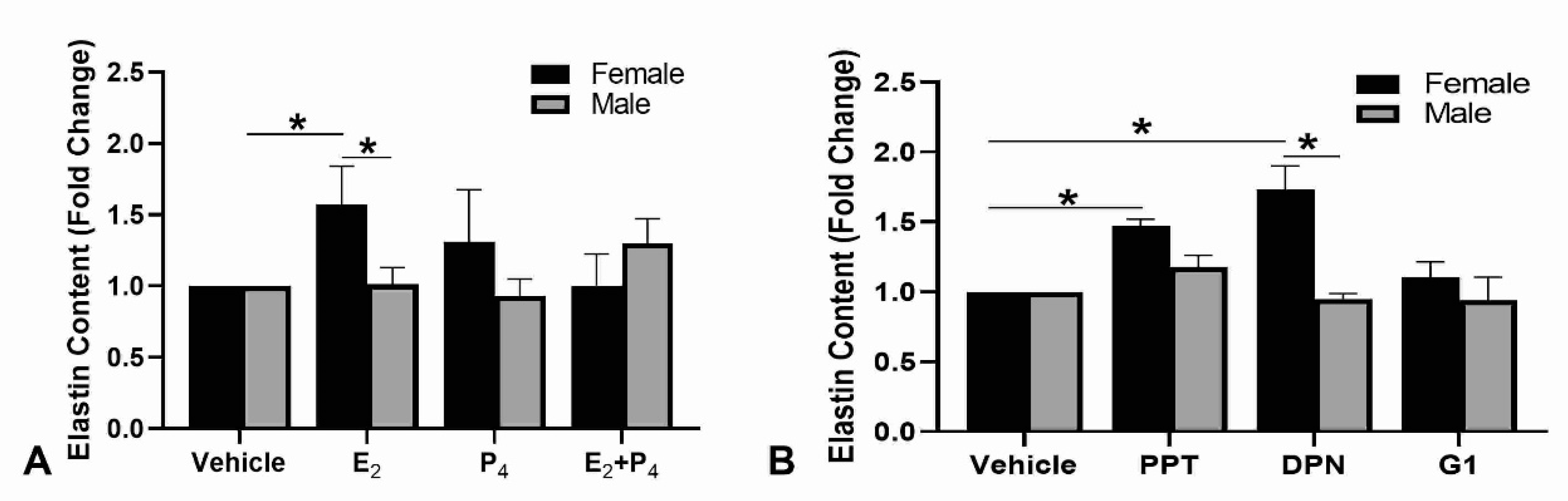
2.2. Elastin Contents in Engineered Dermal Substitutes due to Steroid Hormone Culture
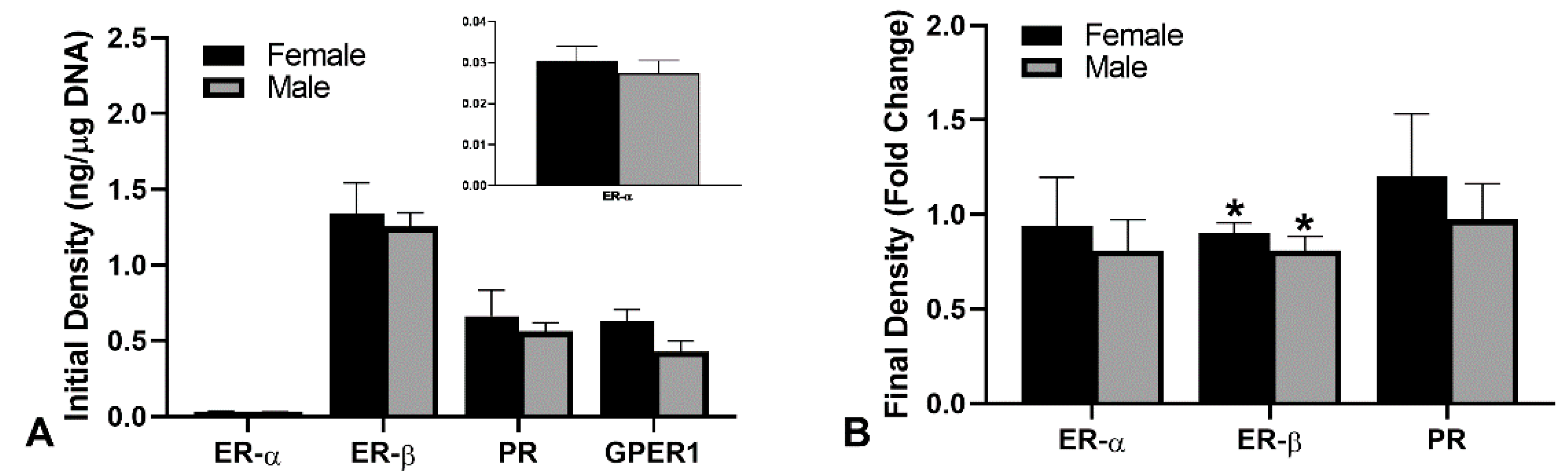
2.3. Receptor Densities in the Human Dermal Fibroblasts
2.4. Cellularity of the EDS
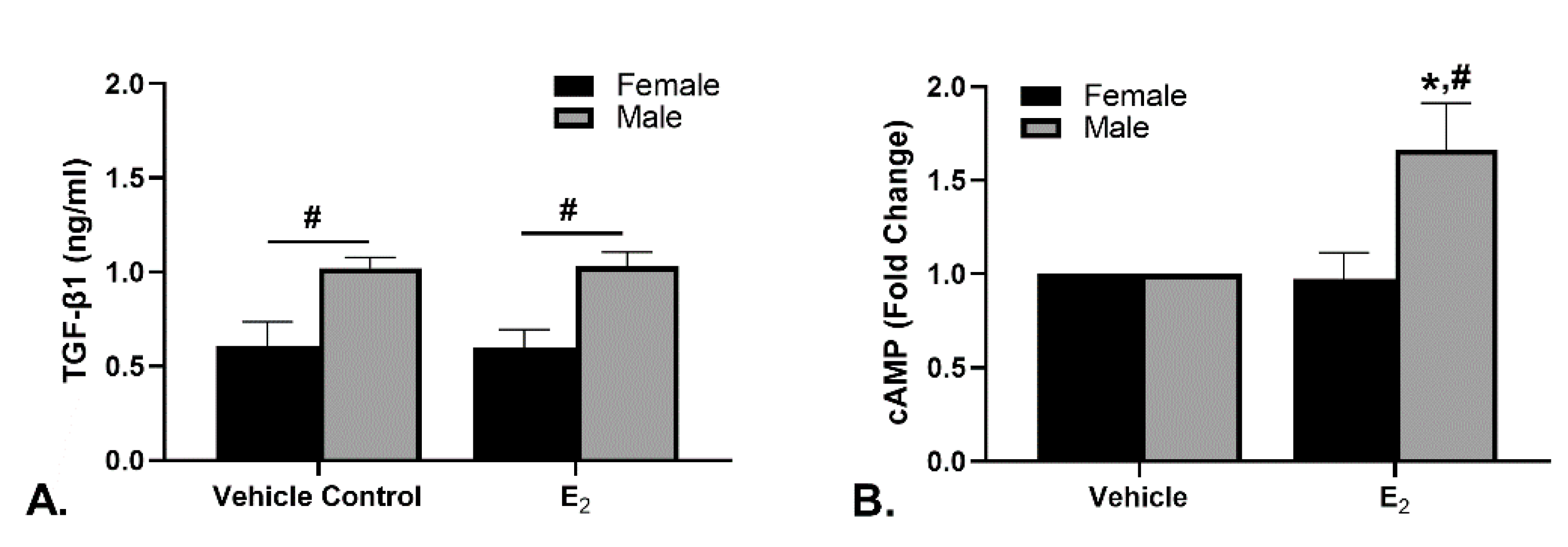
2.5. TGF-β1 and cAMP Concentrations in the Culture Medium after Culture with E2
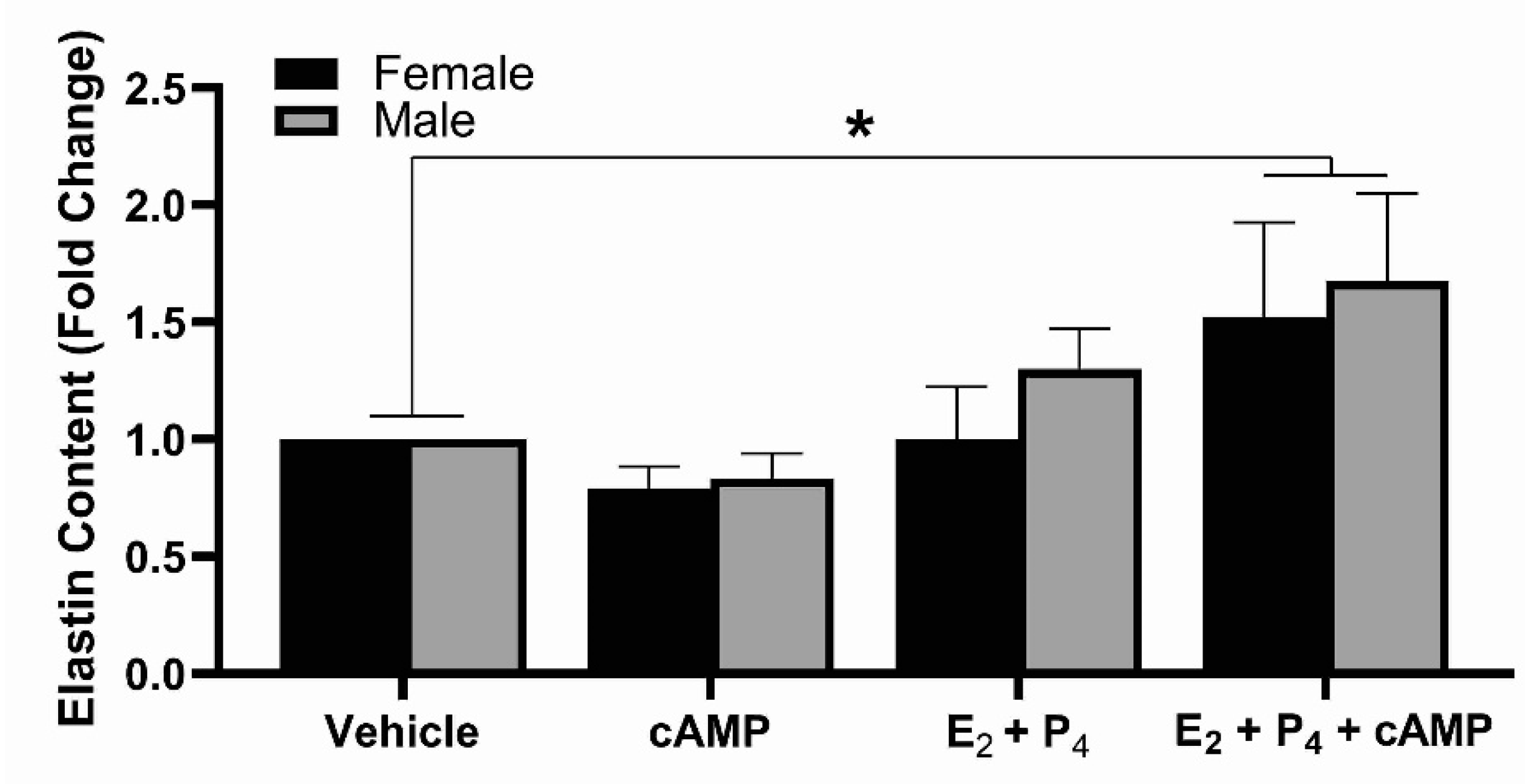
2.6. Elastin Content When Exposed to Steroid Hormones and cAMP
3. Discussion
4. Materials and Methods
4.1. Tissue Sources
4.2. Isolation and Culture of Primary Dermal Fibroblasts
4.3. Fabrication and Culture of Engineered Dermal Substitutes
4.4. Protein and DNA Quantification
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hult, A.M.; Goltz, R.W. The Measurement of Elastin in Human Skin and Its Quantity in Relation to Age. J. Invest. Dermatol. 1965, 44, 408–412. [Google Scholar] [CrossRef]
- Uitto, J.; Paul, J.L.; Brockley, K.; Pearce, R.H.; Clark, J.G. Elastic fibers in human skin: Quantitation of elastic fibers by computerized digital image analyses and determination of elastin by radioimmunoassay of desmosine. Lab. Invest. 1983, 49, 499–505. [Google Scholar]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Invest. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C.; Secrist, H.; Wu, L.C.; Mecham, R.P. Developmental regulation of tropoelastin isoforms. J. Biol. Chem. 1988, 263, 4416–4423. [Google Scholar] [CrossRef]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin signaling in wound repair. Birth Defects Res. C Embryo Today 2012, 96, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.C.M.; Schmelzer, C.E.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128–129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Olsen, D.R.; Fazio, M.J.; Shamban, A.T.; Rosenbloom, J.; Uitto, J. Cutis laxa: Reduced elastin gene expression in skin fibroblast cultures as determined by hybridizations with a homologous cDNA and an exon 1-specific oligonucleotide. J. Biol. Chem. 1988, 263, 6465–6467. [Google Scholar] [CrossRef]
- Zhang, M.C.; He, L.; Giro, M.; Yong, S.L.; Tiller, G.E.; Davidson, J.M. Cutis laxa arising from frameshift mutations in exon 30 of the elastin gene (ELN). J. Biol. Chem. 1999, 274, 981–986. [Google Scholar] [CrossRef]
- Hu, Q.; Loeys, B.L.; Coucke, P.J.; De Paepe, A.; Mecham, R.P.; Choi, J.; Davis, E.C.; Urban, Z. Fibulin-5 mutations: Mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum. Mol. Genet. 2006, 15, 3379–3386. [Google Scholar] [CrossRef]
- Markova, D.; Zou, Y.; Ringpfeil, F.; Sasaki, T.; Kostka, G.; Timpl, R.; Uitto, J.; Chu, M.L. Genetic heterogeneity of cutis laxa: A heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am. J. Hum. Genet. 2003, 72, 998–1004. [Google Scholar] [CrossRef]
- Boss, G.R.; Seegmiller, J.E. Age-related physiological changes and their clinical significance. West. J. Med. 1981, 135, 434–440. [Google Scholar] [PubMed]
- Calleja-Agius, J.; Brincat, M. The effect of menopause on the skin and other connective tissues. Gynecol. Endocrinol. 2012, 28, 273–277. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. The role of estrogen in cutaneous ageing and repair. Maturitas 2017, 103, 60–64. [Google Scholar] [CrossRef]
- Rzepecki, A.K.; Murase, J.E.; Juran, R.; Fabi, S.G.; McLellan, B.N. Estrogen-deficient skin: The role of topical therapy. Int. J. Womens Dermatol. 2019, 5, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Son, E.D.; Lee, J.Y.; Lee, S.; Kim, M.S.; Lee, B.G.; Chang, I.S.; Chung, J.H. Topical application of 17beta-estradiol increases extracellular matrix protein synthesis by stimulating tgf-Beta signaling in aged human skin in vivo. J. Invest. Dermatol. 2005, 124, 1149–1161. [Google Scholar] [CrossRef]
- Zong, W.; Jiang, Y.; Zhao, J.; Zhang, J.; Gao, J.G. Estradiol plays a role in regulating the expression of lysyl oxidase family genes in mouse urogenital tissues and human Ishikawa cells. J. Zhejiang Univ. Sci. B 2015, 16, 857–864. [Google Scholar] [CrossRef]
- Punnonen, R.; Vaajalahti, P.; Teisala, K. Local oestriol treatment improves the structure of elastic fibers in the skin of postmenopausal women. Ann. Chir. Gynaecol. Suppl. 1987, 202, 39–41. [Google Scholar]
- Natoli, A.K.; Medley, T.L.; Ahimastos, A.A.; Drew, B.G.; Thearle, D.J.; Dilley, R.J.; Kingwell, B.A. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 2005, 46, 1129–1134. [Google Scholar] [CrossRef]
- Mangir, N.; Hillary, C.J.; Chapple, C.R.; MacNeil, S. Oestradiol-releasing Biodegradable Mesh Stimulates Collagen Production and Angiogenesis: An Approach to Improving Biomaterial Integration in Pelvic Floor Repair. Eur. Urol. Focus 2019, 5, 280–289. [Google Scholar] [CrossRef]
- Baron, Y.M.; Galea, R.; Brincat, M. Carotid artery wall changes in estrogen-treated and -untreated postmenopausal women. Obstet. Gynecol. 1998, 91, 982–986. [Google Scholar] [PubMed]
- Tyagi, T.; Alarab, M.; Leong, Y.; Lye, S.; Shynlova, O. Local oestrogen therapy modulates extracellular matrix and immune response in the vaginal tissue of post-menopausal women with severe pelvic organ prolapse. J. Cell Mol. Med. 2019, 23, 2907–2919. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Shin, S.C.; Park, G.C.; Lee, J.C.; Jeon, Y.K.; Ahn, S.J.; Thibeault, S.; Lee, B.J. Effect of sex hormones on extracellular matrix of lamina propria in rat vocal fold. Laryngoscope 2020, 130, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Yigit, O.; Sunter, A.V.; Edizer, D.T.; Dursun, N.; Okcu, O. Effects of Intracordal Estradiol and Dexamethasone Injection on Wound Healing in Vocal Fold Injuries. J. Voice 2019, 33, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, S.; Yoshida, K.; Akins, M.; Myers, K.; Iozzo, R.; Mahendroo, M. Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagen and Elastic Fibers. Endocrinology 2017, 158, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Rich, C.B.; Fontanilla, M.R.; Nugent, M.; Foster, J.A. Basic fibroblast growth factor decreases elastin gene transcription through an AP1/cAMP-response element hybrid site in the distal promoter. J. Biol. Chem. 1999, 274, 33433–33439. [Google Scholar] [CrossRef]
- Sakamoto, A.; Weinstein, L.S.; Plagge, A.; Eckhaus, M.; Kelsey, G. GNAS haploinsufficiency leads to subcutaneous tumor formation with collagen and elastin deposition and calcification. Endocr. Res. 2009, 34, 1–9. [Google Scholar] [CrossRef]
- Mecham, R.P.; Levy, B.D.; Morris, S.L.; Madaras, J.G.; Wrenn, D.S. Increased cyclic GMP levels lead to a stimulation of elastin production in ligament fibroblasts that is reversed by cyclic AMP. J. Biol. Chem. 1985, 260, 3255–3258. [Google Scholar] [CrossRef]
- Stevenson, S.; Nelson, L.D.; Sharpe, D.T.; Thornton, M.J. 17beta-estradiol regulates the secretion of TGF-beta by cultured human dermal fibroblasts. J. Biomater. Sci. Polym. Ed. 2008, 19, 1097–1109. [Google Scholar] [CrossRef]
- Bullough, W.S. The mitogenic actions of androgenic and oestrogenic hormones. Acta Physiol. Pharmacol. Neerl. 1950, 1, 357–358. [Google Scholar]
- Varila, E.; Rantala, I.; Oikarinen, A.; Risteli, J.; Reunala, T.; Oksanen, H.; Punnonen, R. The effect of topical oestradiol on skin collagen of postmenopausal women. Br. J. Obstet. Gynaecol. 1995, 102, 985–989. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Dodsworth, J.; van Boxtel, E.; Tarnuzzer, R.W.; Horan, M.A.; Schultz, G.S.; Ferguson, M.W. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997, 3, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Ahola, T.M.; Purmonen, S.; Pennanen, P.; Zhuang, Y.H.; Tuohimaa, P.; Ylikomi, T. Progestin upregulates G-protein-coupled receptor 30 in breast cancer cells. Eur. J. Biochem. 2002, 269, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, M.; Bachi, T.; Meuli, M.; Altermatt, S.; Gobet, R.; Bruckner-Tuderman, L.; Steinmann, B. Fibrillin and elastin expression in skin regenerating from cultured keratinocyte autografts: Morphogenesis of microfibrils begins at the dermo-epidermal junction and precedes elastic fiber formation. J. Invest. Dermatol. 1996, 106, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Amadeu, T.P.; Braune, A.S.; Porto, L.C.; Desmouliere, A.; Costa, A.M. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen 2004, 12, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Kielty, C.M.; Horan, M.A.; Ferguson, M.W. Age-related changes in the temporal and spatial distributions of fibrillin and elastin mRNAs and proteins in acute cutaneous wounds of healthy humans. J. Pathol. 1997, 183, 80–89. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Weiss, A.S. Design of an elastin-layered dermal regeneration template. Acta Biomater. 2017, 52, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fantasia, J.; Lin, C.B.; Wiwi, C.; Kaur, S.; Hu, Y.P.; Zhang, J.; Southall, M.D. Differential levels of elastin fibers and TGF-beta signaling in the skin of Caucasians and African Americans. J. Dermatol. Sci. 2013, 70, 159–165. [Google Scholar] [CrossRef]
- McFarland, K.L.; Glaser, K.; Hahn, J.M.; Boyce, S.T.; Supp, D.M. Culture medium and cell density impact gene expression in normal skin and abnormal scar-derived fibroblasts. J. Burn Care Res. 2011, 32, 498–508. [Google Scholar] [CrossRef]
- Schutte, S.C.; Taylor, R.N. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil. Steril. 2012, 97, 997–1003. [Google Scholar] [CrossRef] [PubMed]
| Females | Males | p-Value | |
|---|---|---|---|
| Age (yrs) | 28.4 ± 17.4 | 22.1 ± 1.9 | 0.26 |
| Race | 0.58 | ||
| White | 2 | 3 | |
| Black | 3 | 2 | |
| Ethnicity | 0.93 | ||
| Hispanic | 1 | 0 | |
| Non-Hispanic/Not disclosed | 4 | 5 | |
| Biopsy Location | 0.17 | ||
| Abdomen | 1 | 3 | |
| Breast | 3 | 0 | |
| Foot | 0 | 1 | |
| Thigh | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moset Zupan, A.; Nietupski, C.; Schutte, S.C. Cyclic Adenosine Monophosphate Eliminates Sex Differences in Estradiol-Induced Elastin Production from Engineered Dermal Substitutes. Int. J. Mol. Sci. 2021, 22, 6358. https://doi.org/10.3390/ijms22126358
Moset Zupan A, Nietupski C, Schutte SC. Cyclic Adenosine Monophosphate Eliminates Sex Differences in Estradiol-Induced Elastin Production from Engineered Dermal Substitutes. International Journal of Molecular Sciences. 2021; 22(12):6358. https://doi.org/10.3390/ijms22126358
Chicago/Turabian StyleMoset Zupan, Andreja, Carolyn Nietupski, and Stacey C. Schutte. 2021. "Cyclic Adenosine Monophosphate Eliminates Sex Differences in Estradiol-Induced Elastin Production from Engineered Dermal Substitutes" International Journal of Molecular Sciences 22, no. 12: 6358. https://doi.org/10.3390/ijms22126358
APA StyleMoset Zupan, A., Nietupski, C., & Schutte, S. C. (2021). Cyclic Adenosine Monophosphate Eliminates Sex Differences in Estradiol-Induced Elastin Production from Engineered Dermal Substitutes. International Journal of Molecular Sciences, 22(12), 6358. https://doi.org/10.3390/ijms22126358







