Novel Techniques to Improve Precise Cell Injection
Abstract
1. Introduction
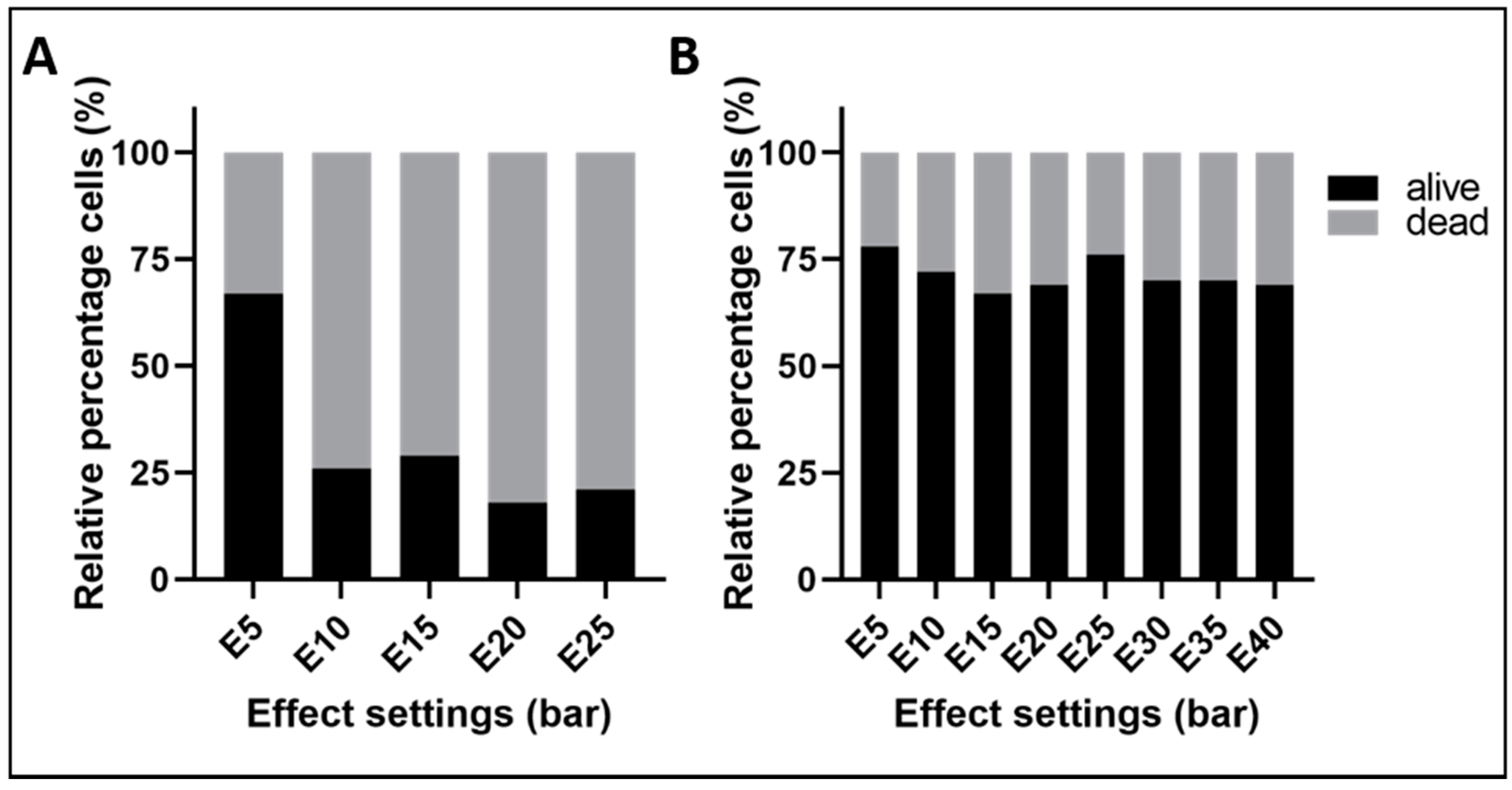
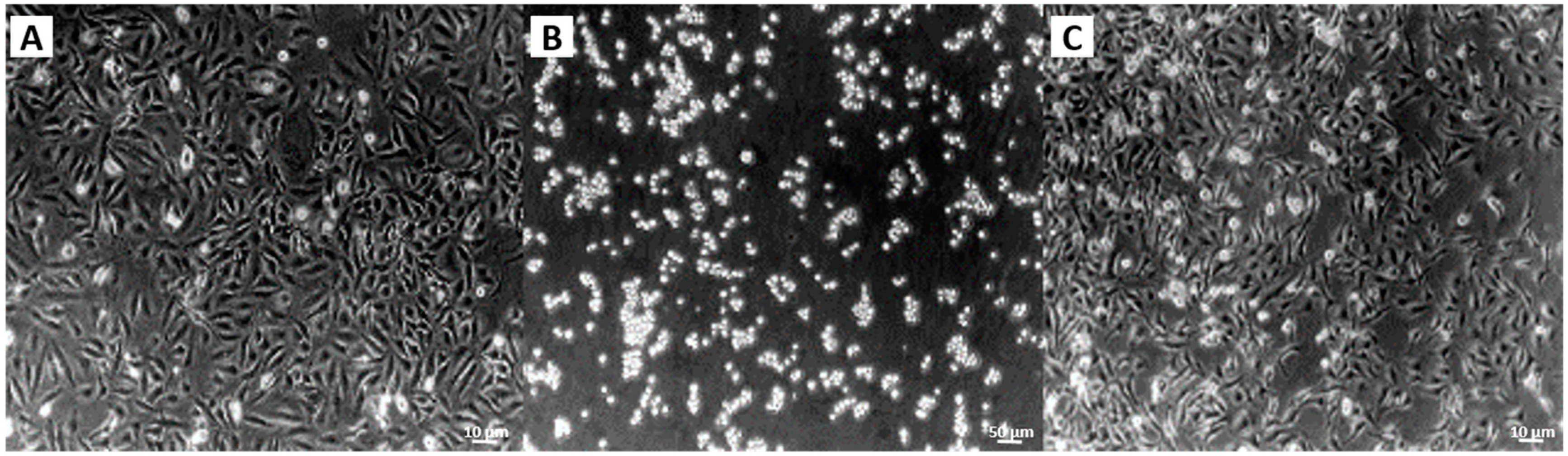
2. Results
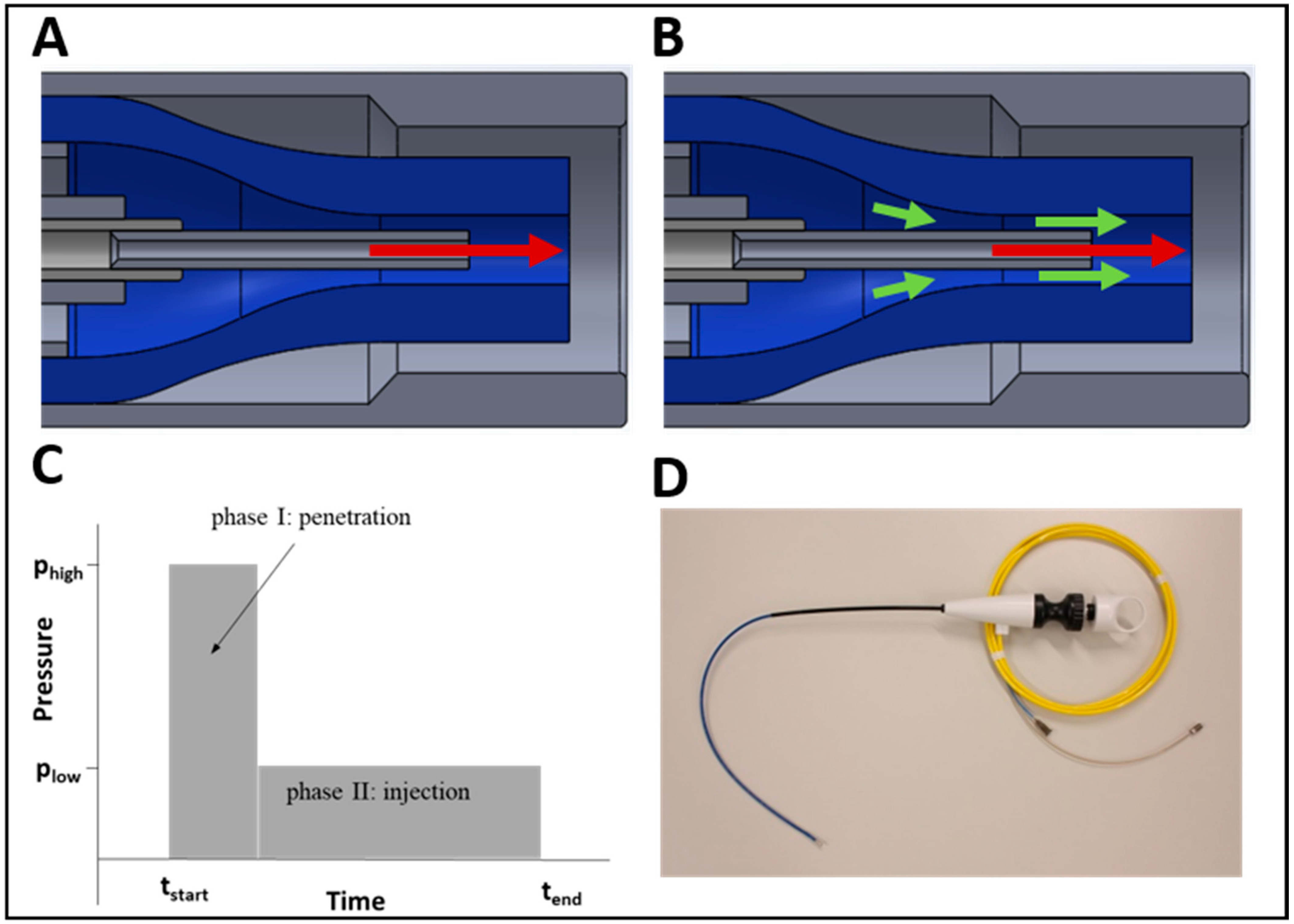
2.1. Design of the Injection System and Working Principle
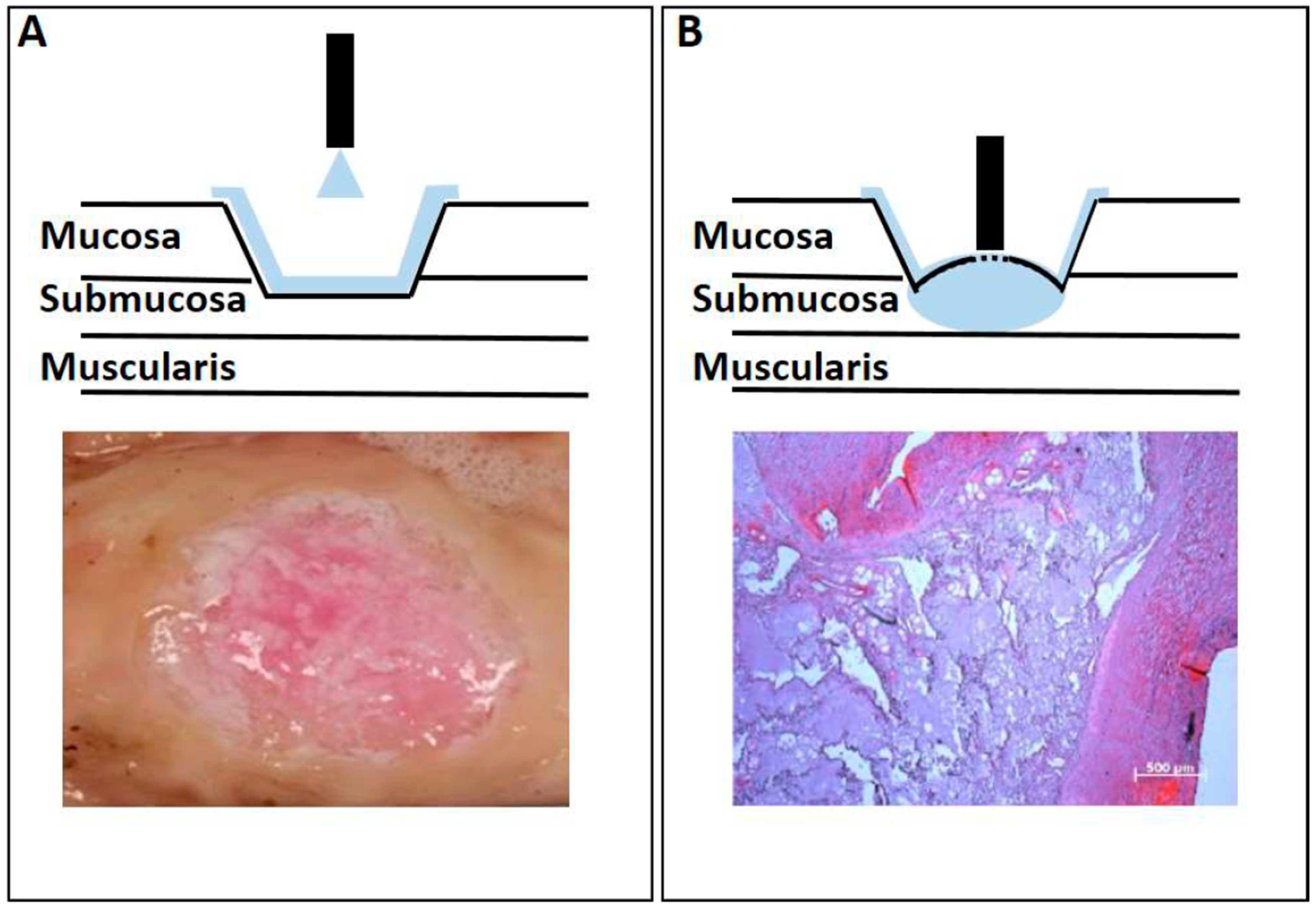
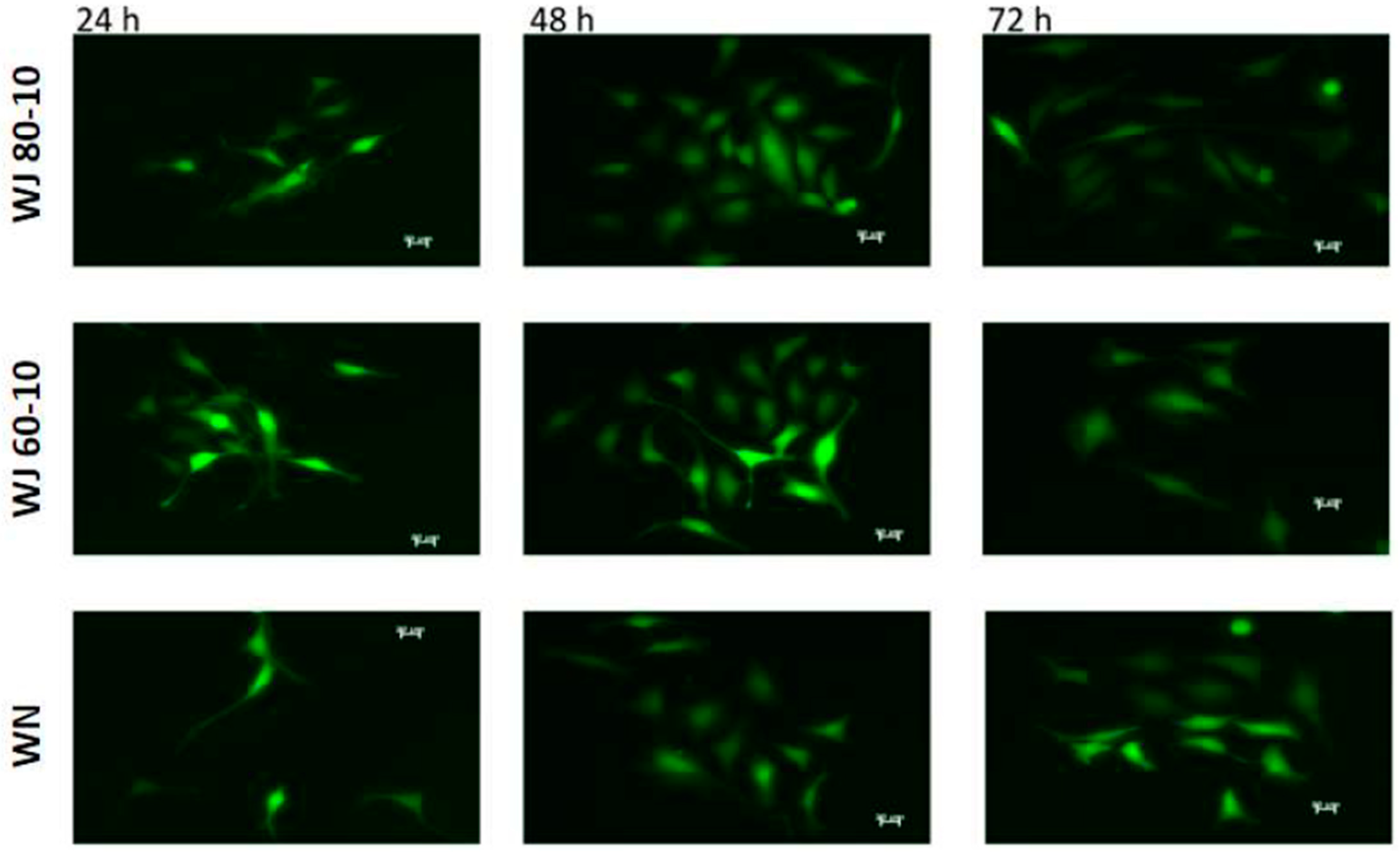
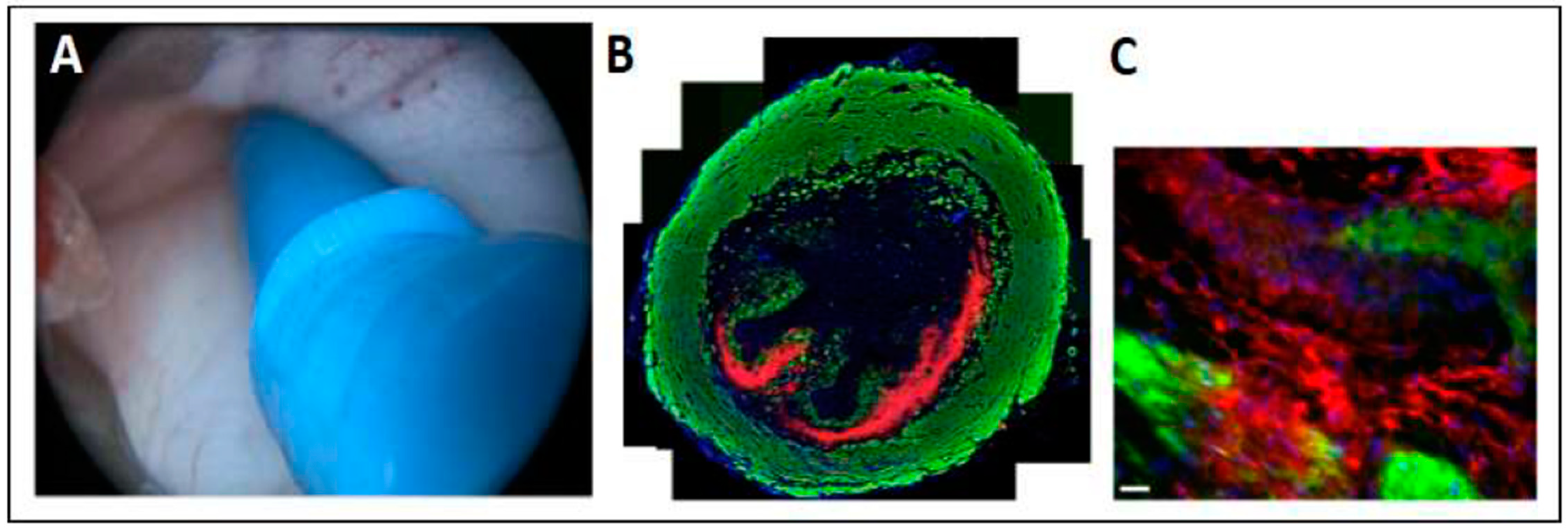
2.2. Adapting the Water-Jet Technology to Urology for Transurethral Applications
3. Discussion
4. Materials and Methods
4.1. Waterjet Technology
4.2. Production of Cells for Injections
4.3. Labeling Cells
4.4. Cell Injections
4.5. Histology
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keiner, D.; Gaab, M.R.; Backhaus, V.; Piek, J.; Oertel, J. Water jet dissection in neurosurgery: An update after 208 procedures with special reference to surgical technique and complications. Neurosurgery 2010, 67, ons342–ons354. [Google Scholar] [CrossRef]
- Gakis, G.; Karl, A.; Bertz, S.; Burger, M.; Fritsche, H.-M.; Hartmann, A.; Jokisch, F.; Kempkensteffen, C.; Miller, K.; Mundhenk, J.; et al. Transurethral en bloc submucosal hydrodissection versus conventional resection for resection of non-muscle invasive bladder cancer (HYBRIDBLUE): A randomized, multicentre trial. BJU Int. 2020, 126, 209–519. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Ning, Y.; Cui, X.; Yin, L.; Chen, J.; Wang, J.; Shao, B.; Xu, D. Hydro-Jet-assisted laparoscopic partial nephrectomy with no renal arterial clamping: A preliminary study in a single center. Int. Urol. Nephrol. 2014, 46, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Honl, M.; Dierk, O.; Kuster, J.R.; Muller, G.; Muller, V.; Hille, E.; Morlock, M. Water jet discotomy with microinvasive approach--in vitro testing and initial clinical aspects of a new procedure. Z. Orthop. Ihre Grenzgeb. 2001, 139, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bahls, T.; Fröhlich, F.A.; Hellings, A.; Deutschmann, B.; Albu-Schäffer, A. Extending the Capability of Using a Waterjet in Surgical Interventions by the Use of Robotics. IEEE Trans. Biomed. Eng. 2017, 64, 284–294. [Google Scholar] [CrossRef]
- Rubinstein, M.; Moinzadeh, A.; Colombo, J.R.; Favorito, L.A.; Sampaio, F.J.; Gill, I.S. Energy sources for laparoscopic partial nephrectomy—Critical appraisal. Int. Braz. J. Urol. 2007, 33, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shekarriz, B. Hydro-Jet technology in urologic surgery. Expert Rev. Med. Devices 2005, 2, 287–291. [Google Scholar] [CrossRef]
- Basting, R.; Djakovic, N.; Widmann, P. Use of water jet resection in organ-sparing kidney surgery. J. Endourol. 2000, 14, 501–505. [Google Scholar] [CrossRef]
- Aragon, R.J.; Solomon, N.L. Techniques of hepatic resection. J. Gastrointest. Oncol. 2012, 3, 28–40. [Google Scholar]
- Romano, F.; Garancini, M.; Uggeri, F.; Degrate, L.; Nespoli, L.; Gianotti, L.; Nespoli, A.; Uggeri, F. Bleeding in Hepatic Surgery: Sorting through Methods to Prevent It. HPB Surg. 2012, 2012, 169351. [Google Scholar] [CrossRef]
- Schurr, M.O.; Wehrmann, M.; Kunert, W.; Melzer, A.; Lirici, M.M.; Trapp, R.; Kanehira, E.; Buess, G. Histologic effects of different technologies for dissection in endoscopic surgery: Nd:YAG laser, high frequency and water-jet. Endosc. Surg. Allied Technol. 1994, 2, 195. [Google Scholar]
- Farin, G. Technology of water-jet dissection. Minim. Invasive Ther. Allied Technol. 2002, 11, 249–255. [Google Scholar] [CrossRef]
- Shekarriz, B.; Upadhyay, J.; Jewett, M.A. Nerve-sparing retroperitoneal lymphadenectomy using hydro-jet dissection: Initial experience. J. Endourol. 2004, 18, 273–276. [Google Scholar] [CrossRef]
- Poon, R.T.P. Current techniques of liver transection. HPB 2007, 9, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.M.; Dixon, E.; Sahajpal, A.; Cattral, M.S.; Grant, D.R.; Gallinger, S.; Taylor, B.R.; Greig, P.D. Water-jet dissection for parenchymal division during hepatectomy. HPB 2006, 8, 377–385. [Google Scholar] [CrossRef]
- Hamaoka, M.; Kobayashi, T.; Kuroda, S.; Okimoto, S.; Honmyo, N.; Yamaguchi, M.; Yamamoto, M.; Ohdan, H. Experience and outcomes in living donor liver procurement using the water jet scalpel. J. Hepato Biliary Pancreat. Sci. 2019, 26, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Draganov, P.V.; Gotoda, T.; Chavalitdhamrong, D.; Wallace, M.B. Techniques of endoscopic submucosal dissection: Application for the Western endoscopist? Gastrointest. Endosc. 2013, 78, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Abe, S.; Kumaravel, A.; Vargo, J.; Saito, Y. Indications and Techniques for Endoscopic Submucosal Dissection. Am. J. Gastroenterol. 2015, 110, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Hassan, C.; Pagano, N.; Rando, G.; Romeo, F.; Spaggiari, P.; Roncalli, M.; Ferrara, E.; Malesci, A. High efficacy of endoscopic submucosal dissection for rectal laterally spreading tumors larger than 3 cm. Gastrointest. Endosc. 2013, 77, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-Y.; Zhou, P.-H.; Yao, L.-Q.; Xu, M.-D.; Zhong, Y.-S.; Li, Q.-L.; Chen, W.-F.; Hu, J.-W.; Cui, Z.; Zhu, B.-Q. Peroral endoscopic myotomy for idiopathic achalasia: Randomized comparison of water-jet assisted versus conventional dissection technique. Surg. Endosc. 2014, 28, 1158–1165. [Google Scholar] [CrossRef]
- Tang, X.; Gong, W.; Deng, Z.; Zhou, J.; Ren, Y.; Zhang, Q.; Chen, Z.; Jiang, B. Comparison of conventional versus Hybrid knife peroral endoscopic myotomy methods for esophageal achalasia: A case-control study. Scand. J. Gastroenterol. 2016, 51, 494–500. [Google Scholar] [CrossRef]
- Zhou, P.-H.; Cai, M.-Y.; Yao, L.-Q.; Zhong, Y.-S.; Ren, Z.; Xu, M.-D.; Qin, X.-Y. Peroral Endoscopic Myotomy for Esophageal Achalasia by HybridKnife: A Case Report. Case Rep. Gastrointest. Med. 2012, 2012, 325479. [Google Scholar] [CrossRef] [PubMed]
- Terheggen, G.; Horn, E.M.; Vieth, M.; Gabbert, H.; Enderle, M.; Neugebauer, A.; Schumacher, B.; Neuhaus, H. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut 2016, 66, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zong, Y.; Zhao, H.-Y.; Wu, Y.-D.; Ji, M. Complete excision of esophageal bronchogenic cyst by endoscopic submucosal tunnel dissection: A case presentation. BMC Gastroenterol. 2019, 19, 155. [Google Scholar] [CrossRef]
- Zhou, J.-Q.; Tang, X.-W.; Ren, Y.-T.; Wei, Z.-J.; Huang, S.-L.; Gao, Q.-P.; Zhang, X.-F.; Yang, J.-F.; Gong, W.; Jiang, B. Endoscopic submucosal tunnel dissection of upper gastrointestinal submucosal tumors: A comparative study of hook knife vs. hybrid knife. World J. Gastroenterol. 2017, 23, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Libânio, D.; Pita, I.; Dinis-Ribeiro, M. Solutions for submucosal injection: What to choose and how to do it. World J. Gastroenterol. 2019, 25, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Kerever, S.; Carrier, P.; Couquet, C.-Y.; Debette-Gratien, M.; Tabouret, T.; Lepetit, H.; Geyl, S.; Loustaud-Ratti, V.; Sautereau, D.; et al. HybridKnife high-pressure glycerol jet injection for endoscopic submucosal dissection increases procedural ease and speed: A randomised study in pigs and a human case series. Surg. Endosc. 2016, 30, 3152–3159. [Google Scholar] [CrossRef]
- Repici, A.; Maselli, R.; Carrara, S.; Anderloni, A.; Enderle, M.; Hassan, C. Standard needle versus needleless injection modality: Animal study on different fluids for submucosal elevation. Gastrointest. Endosc. 2017, 86, 553–558. [Google Scholar] [CrossRef]
- Neuhaus, H.; Costamagna, G.; Devière, J.; Fockens, P.; Ponchon, T.; Rösch, T. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”). Endoscopy 2006, 38, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; López, J. Methods of stem cell delivery in cardiac diseases. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, S110–S113. [Google Scholar] [CrossRef]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef]
- O’Cearbhaill, E.D.; Ng, K.S.; Karp, J.M. Emerging medical devices for minimally invasive cell therapy. Mayo Clin. Proc. 2014, 89, 259–273. [Google Scholar] [CrossRef]
- Amer, M.; Rose, F.R.A.J.; Shakesheff, K.M.; Modo, M.; White, L.J. Translational considerations in injectable cell-based therapeutics for neurological applications: Concepts, progress and challenges. NPJ Regen. Med. 2017, 2, 23. [Google Scholar] [CrossRef]
- Amer, M.; White, L.J.; Shakesheff, K.M. The effect of injection using narrow-bore needles on mammalian cells: Administration and formulation considerations for cell therapies. J. Pharm. Pharmacol. 2015, 67, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.M.; Schnabel, L.V.; Cassano, J.M.; Fortier, L.A. Effect of needle diameter on the viability of equine bone marrow derived mesenchymal stem cells. Vet. Surg. 2017, 46, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Current status and future prospects of needle-free liquid jet injectors. Nat. Rev. Drug Discov. 2006, 5, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.; Mitragotri, S. Needle-free liquid jet injections: Mechanisms and applications. Expert Rev. Med. Devices 2006, 3, 565–574. [Google Scholar] [CrossRef]
- Beck, F. Penetration Depth and Distribution of Nanoparticles Sfter Injection into the Porcine Urethra Using a New Water Jet Technology. Ph.D. Thesis, University of Tuebingen, Tuebingen, Germany, 2020. (In German). [Google Scholar]
- Schreiber, J. Investigation of the Penetration Depth of Microparticles by Water Jet Application in Porcine Urethra. Ph.D. Thesis, University of Tuebingen, Tuebingen, Germany, 2020. (In German). [Google Scholar]
- Egorov, V.I.; Schastlivtsev, I.V.; Prut, E.V.; Baranov, A.O.; Turusov, R.A. Mechanical properties of the human gastrointestinal tract. J. Biomech. 2002, 35, 1417–1425. [Google Scholar] [CrossRef]
- Jäger, L.; Linzenbold, W.; Fech, A.; Enderle, M.; Abruzzese, T.; Stenzl, A.; Aicher, W.K. A novel waterjet technology for transurethral cystoscopic injection of viable cells in the urethral sphincter complex. Neurourol. Urodyn. 2020, 39, 594–602. [Google Scholar] [CrossRef]
- Linzenbold, W.; Jäger, L.; Stoll, H.; Abruzzese, T.; Harland, N.; Bézière, N.; Fech, A.; Enderle, M.; Amend, B.; Stenzl, A.; et al. Rapid and precise delivery of cells in the urethral sphincter complex by a novel needle-free waterjet technology. BJU Int. 2020, 127, 463–472. [Google Scholar] [CrossRef]
- Danalache, M.; Knoll, J.; Linzenbold, W.; Enderle, M.; Abruzzese, T.; Stenzl, A.; Aicher, W. Injection of Porcine Adipose Tissue-Derived Stromal Cells by a Novel Waterjet Technology. Int. J. Mol. Sci. 2021, 22, 3958. [Google Scholar] [CrossRef]
- Amend, B.; Kelp, A.; Vaegler, M.; Klünder, M.; Frajs, V.; Klein, G.; Sievert, K.-D.; Sawodny, O.; Stenzl, A.; Aicher, W.K. Precise injection of human mesenchymal stromal cells in the urethral sphincter complex of Göttingen minipigs without unspecific bulking effects. Neurourol. Urodyn. 2017, 36, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Fech, A.; Jäger, L.; Steinle, H.; Bühler, L.; Perl, R.M.; Martirosian, P.; Mehling, R.; Sonanini, D.; Aicher, W.K.; et al. Hydrojet-based delivery of footprint-free iPSC-derived cardiomyocytes into porcine myocardium. Sci. Rep. 2020, 10, 16787. [Google Scholar] [CrossRef] [PubMed]
- Burdzinska, A.; Dybowski, B.; Zarychta-Wiśniewska, W.; Kulesza, A.; Hawryluk, J.; Graczyk-Jarzynka, A.; Kaupa, P.; Gajewski, Z.; Paczek, L. Limited accuracy of transurethral and periurethral intrasphincteric injections of cellular suspension. Neurourol. Urodyn. 2018, 37, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; Hamdoon, M.; Sriprasad, S. Stem cell therapy for stress urinary incontinence. J. Clin. Urol. 2019, 13, 62–69. [Google Scholar] [CrossRef]
- Wallner, C.; Dabhoiwala, N.F.; DeRuiter, M.C.; Lamers, W.H. The anatomical components of urinary continence. Eur. Urol. 2009, 55, 932–944. [Google Scholar] [CrossRef]
- Blaganje, M.; Lukanović, A. Ultrasound-guided autologous myoblast injections into the extrinsic urethral sphincter: Tissue engineering for the treatment of stress urinary incontinence. Int. Urogynecol. J. 2013, 24, 533–535. [Google Scholar] [CrossRef]
- Burdzinska, A.; Dybowski, B.; Zarychta-Wiśniewska, W.; Kulesza, A.; Butrym, M.; Zagozdzon, R.; Graczyk-Jarzynka, A.; Radziszewski, P.; Gajewski, Z.; Paczek, L. Intraurethral co-transplantation of bone marrow mesenchymal stem cells and muscle-derived cells improves the urethral closure. Stem Cell Res. Ther. 2018, 9, 239. [Google Scholar] [CrossRef]
- Strasser, H.; Marksteiner, R.; Margreiter, E.; Pinggera, G.M.; Mitterberger, M.; Frauscher, F.; Hering, S.; Bartsch, G. 328: Transurethral Ultrasound Guided Stem Cell Therapy of Urinary Incontinence. J. Urol. 2006, 175, 107. [Google Scholar] [CrossRef]
- Schumacher, B.; Charton, J.-P.; Nordmann, T.; Vieth, M.; Enderle, M.; Neuhaus, H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: A Western, single-center experience. Gastrointest. Endosc. 2012, 75, 1166–1174. [Google Scholar] [CrossRef]
- Kaehler, G.F.B.A.; Sold, M.G.; Fischer, K.; Post, S.; Enderle, M. Selective fluid cushion in the submucosal layer by water jet: Advantage for endoscopic mucosal resection. Eur. Surg. Res. 2007, 39, 93–97. [Google Scholar] [CrossRef]
- Neuhaus, H.; Terheggen, G.; Rutz, E.M.; Vieth, M.; Schumacher, B. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett’s esophagus. Endoscopy 2012, 44, 1105–1113. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Qiu, W.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. The Achievements and Challenges of Mesenchymal Stem Cell-Based Therapy in Inflammatory Bowel Disease and Its Associated Colorectal Cancer. Stem Cells Int. 2020, 2020, 7819824. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, R.; Aicher, W.K. Allogenic Use of Human Placenta-Derived Stromal Cells as a Highly Active Subtype of Mesenchymal Stromal Cells for Cell-Based Therapies. Int. J. Mol. Sci. 2021, 22, 5302. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, D.; Iizuka, T.; Makino, S.; Hayasaka, J.; Odagiri, H.; Ochiai, Y.; Suzuki, Y.; Nomura, K.; Ohkura, Y.; Okamoto, Y.; et al. Utility of autologous fibrin glue and polyglycolic acid sheet for preventing delayed bleeding associated with antithrombotic therapy after gastric ESD. Endosc. Int. Open 2019, 7, E1542–E1548. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, K.; Hagiwara, A. Filling and shielding for postoperative gastric perforations of endoscopic submucosal dissection using polyglycolic acid sheets and fibrin glue. Endosc. Int. Open 2016, 4, E661–E664. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.S.; Wang, H.; Lua, G.W.; Liu, F.; Shi, X.G.; Li, Z.S. Fibrin Glue Spray as a Simple and Promising Method to Prevent Bleeding after Gastric Endoscopic Submucosal Dissection. Dig. Surg. 2016, 33, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Hsu, S.H.; Cowan, C.M.; Liu, A.; Tai, J.; Chan, Y.; Sherman, W.; Basu, S. Transcatheter injection-induced changes in human bone marrow-derived mesenchymal stem cells. Cell Transplant. 2009, 18, 1111–1121. [Google Scholar] [CrossRef]
- Vilsbøll, A.W.; Mouritsen, J.M.; Jensen, L.P.; Bødker, N.; Holst, A.W.; Pennisi, C.P.; Ehlers, L. Cell-based therapy for the treatment of female stress urinary incontinence: An early cost-effectiveness analysis. Regen. Med. 2018, 13, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.; Pinggera, G.M.; Marksteiner, R.; Margreiter, E.; Plattner, R.; Klima, G.; Bartsch, G.; Strasser, H. Functional and histological changes after myoblast injections in the porcine rhabdosphincter. Eur. Urol. 2007, 52, 1736–1743. [Google Scholar] [CrossRef]
- Janssen, K.; Lin, D.L.; Hanzlicek, B.; Deng, K.; Balog, B.M.; Van Der Vaart, C.H.; Damaser, M.S. Multiple doses of stem cells maintain urethral function in a model of neuromuscular injury resulting in stress urinary incontinence. Am. J. Physiol. Physiol. 2019, 317, F1047–F1057. [Google Scholar] [CrossRef] [PubMed]
- Felka, T.; Schäfer, R.; De Zwart, P.; Aicher, W.K. Animal serum-free expansion and differentiation of human mesenchymal stromal cells. Cytotherapy 2010, 12, 143–153. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linzenbold, W.; Fech, A.; Hofmann, M.; Aicher, W.K.; Enderle, M.D. Novel Techniques to Improve Precise Cell Injection. Int. J. Mol. Sci. 2021, 22, 6367. https://doi.org/10.3390/ijms22126367
Linzenbold W, Fech A, Hofmann M, Aicher WK, Enderle MD. Novel Techniques to Improve Precise Cell Injection. International Journal of Molecular Sciences. 2021; 22(12):6367. https://doi.org/10.3390/ijms22126367
Chicago/Turabian StyleLinzenbold, Walter, Andreas Fech, Manuela Hofmann, Wilhelm K. Aicher, and Markus D. Enderle. 2021. "Novel Techniques to Improve Precise Cell Injection" International Journal of Molecular Sciences 22, no. 12: 6367. https://doi.org/10.3390/ijms22126367
APA StyleLinzenbold, W., Fech, A., Hofmann, M., Aicher, W. K., & Enderle, M. D. (2021). Novel Techniques to Improve Precise Cell Injection. International Journal of Molecular Sciences, 22(12), 6367. https://doi.org/10.3390/ijms22126367






