Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives
Abstract
1. Introduction
2. σ1 Receptors
Overview on σ1 Receptors Ligands: Functional Activity, Proposed Endogenous Ligands, and Reference Compounds
- (+)-Benzomorphans as agonists: This class of ligands has a great historical value, as (+)-N-allylnormetazocine ((+)-SKF-10047) led to distinguishing a new group of receptors different from opioid receptors [40] that were named σ, after the first letter of the compound code [41]. Moreover, (+)-Pentazocine is still a σ1 reference compound, and its tritium radiolabeled form, which shed light on the existence of two classes of σ receptors [4], is still used for σ1 receptor radioligand binding assays.
- Antidepressants as agonists: Imipramine, Fluoxetine, Fluvoxamine, and Sertraline showed moderate to high affinities for σ1 receptors (Ki values ranging from 30 to 300 nM) [44], and several σ1 receptor agonists have shown antidepressant-like effects in animal models of depression, proving the involvement of the protein in depression [45,46].
- Neurosteroids as agonists/antagonists: This class contains both agonists and antagonists. Progesterone in fact is a σ1 receptor antagonist, while deoxycorticosterone, testosterone, pregnenolone–sulfate, and dehydroepiandrosterone (DHEA) can produce agonist activities. All these ligands have unconventional structures because of the absence of a basic nitrogen that determines weak affinities for σ1 receptors. Neurosteroids have been proposed as endogenous ligands for σ1 receptors, as their effect seems also to be mediated by σ1 receptor binding [39,47].
- Others: A number of other ligands have been synthesized with the aim to better understand the role of these receptors in different pathologies. The most representative examples are as follows:
- -
- -
- -
- -
- -
- -
- AC915: a highly selective ligand with an antagonist profile [62];
- -
- Pridopidine: initially considered a dopaminergic D2 receptor antagonist, it was later repositioned as a selective σ1 receptor agonist (see Section 3.5) [63];
- -
- -
- -
| Name | Structure | σ1 R Ki nM | σ2 R Ki nM | Reference for Binding Values |
|---|---|---|---|---|
| (+)-Benzomorphans: | ||||
| (+)-SKF-10047 |  | 153 | 154335 | [9] |
| (+)-pentazocine |  | 15.4 | 3475 | [9] |
| Antipsychotics: | ||||
| Haloperidol |  | 1.09 | 41.9 | (σ1 R) [71] (σ2 R) [9] |
| Antidepressants: | ||||
| Imipramine |  | 343 | 2107 | [44] |
| Fluoxetine |  | 240 | 16100 | [44] |
| Fluvoxamine |  | 36 | 8439 | [44] |
| Sertraline |  | 57 | 5297 | [44] |
| Neurosteroids: | ||||
| Progesterone |  | 239 | 441 | [38] |
| Deoxycorticosterone |  | 938 | n.d. | [39] |
| Testosterone |  | 1014 | n.d. | [39] |
| Pregnenolone-sulphate |  | 3196 | n.d. | [39] |
| DHEA |  | 2.959 | n.d. | [47] |
| Others: | ||||
| SA4503 |  | 4.63 | 63.09 | [72] |
| PB190 |  | 0.42 | 36.3 | [53] |
| PRE-084 |  | 53.2 | 32.100 | [73] |
| NE-100 |  | 1.1 | 170 | [74] |
| PB212 |  | 0.030 | 17.9 | [53] |
| AC915 |  | 4.89 | >10000 | [62] |
| Pridopidine |  | 57 | 5450 | [75] |
| FTC-146 |  | 0.0025 | 364 | [64] |
| S1RA |  | 23.5 | >1000 | [76] |
| BD1063 |  | 9.15 | 449 | [70] |
3. σ1 Receptor Ligands in a MTDLs Approach
3.1. MTDLs Acting at σ1 Receptor and Cholinergic System
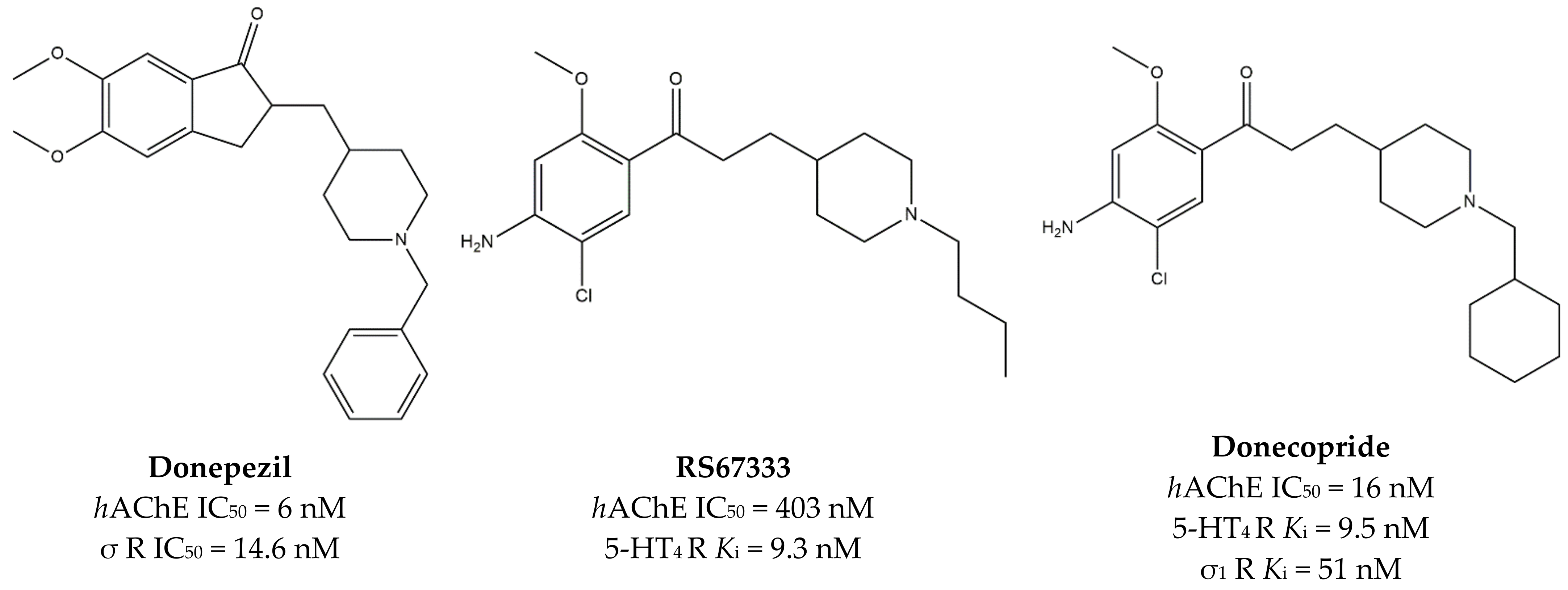
3.2. MTDLs Acting at σ1, 5-HT4 Receptors and AChE
3.3. MTDLs Acting at σ1 Receptor, AChE, BuChE, BACE1, MAO-A, MAO-B, 5-LOX, ROS, and Stem Cells
3.4. MTDLs Acting at σ1, NMDA Receptors, and VGCC
3.5. MTDLs Acting at σ1 Receptor/Dopaminergic Transmission
3.6. MTDLs Acting at σ1 and μ-Opioid Receptors
3.7. σ1. Receptor and Cancer in a MTDLs Approach
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bansal, Y.; Silakari, O. Multifunctional Compounds: Smart Molecules for Multifactorial Diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Korcsmáros, T.; Szalay, M.S.; Böde, C.; Kovács, I.A.; Csermelyt, P. How to Design Multi-Target Drugs: Target Search Options in Cellular Networks. Expert Opin. Drug Discov. 2007, 2, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Bowen, W.D.; Walker, F.O.; Matsumoto, R.R.; De Costa, B.; Rice, K.C. Sigma Receptors: Biology and Function. Pharmacol. Rev. 1990, 42, 355–402. [Google Scholar]
- Hellewell, S.B.; Bowen, W.D. A Sigma-like Binding Site in Rat Pheochromocytoma (PC12) Cells: Decreased Affinity for (+)-Benzomorphans and Lower Molecular Weight Suggest a Different Sigma Receptor Form from That of Guinea Pig Brain. Brain Res. 1990, 527, 244–253. [Google Scholar] [CrossRef]
- Alonso, G.; Phan, V.; Guillemain, I.; Saunier, M.; Legrand, A.; Anoal, M.; Maurice, T. Immunocytochemical Localization of the Sigma(1) Receptor in the Adult Rat Central Nervous System. Neuroscience 2000, 97, 155–170. [Google Scholar] [CrossRef]
- Roman, F.; Pascaud, X.; Chomette, G.; Bueno, L.; Junien, J.L. Autoradiographic Localization of Sigma Opioid Receptors in the Gastrointestinal Tract of the Guinea Pig. Gastroenterology 1989, 97, 76–82. [Google Scholar] [CrossRef]
- Su, T.P.; Wu, X.Z. Guinea Pig Vas Deferens Contains ρ but Not Phencyclidine Receptors. Neurosci. Lett. 1990, 108, 341–345. [Google Scholar] [CrossRef]
- Hellewell, S.B.; Bruce, A.; Feinstein, G.; Orringer, J.; Williams, W.; Bowen, W.D. Rat Liver and Kidney Contain High Densities of Sigma 1 and Sigma 2 Receptors: Characterization by Ligand Binding and Photoaffinity Labeling. Eur. J. Pharmacol. 1994, 268, 9–18. [Google Scholar] [CrossRef]
- Ela, C.; Barg, J.; Vogel, Z.; Hasin, Y.; Eilam, Y. Sigma Receptor Ligands Modulate Contractility, Ca++ Influx and Beating Rate in Cultured Cardiac Myocytes. J. Pharmacol. Exp. Ther. 1994, 269, 1300–1309. [Google Scholar]
- Wolfe, S.A.J.; Culp, S.G.; De Souza, E.B. Sigma-Receptors in Endocrine Organs: Identification, Characterization, and Autoradiographic Localization in Rat Pituitary, Adrenal, Testis, and Ovary. Endocrinology 1989, 124, 1160–1172. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Gasparre, G.; Abate, C.; Carlucci, R.; Berardi, F.; Cassano, G. The σ1 Receptor Agonist (+)-Pentazocine Increases Store-Operated Ca2+ Entry in MCF7σ1 and SK-N-SH Cell Lines. Pharmacol. Rep. 2017, 69, 542–545. [Google Scholar] [CrossRef]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.P. Sigma-1 Receptor Chaperone at the ER-Mitochondrion Interface Mediates the Mitochondrion-ER-Nucleus Signaling for Cellular Survival. PLoS ONE 2013, 8, e76941. [Google Scholar] [CrossRef]
- Weng, T.-Y.; Tsai, S.-Y.A.; Su, T.-P. Roles of Sigma-1 Receptors on Mitochondrial Functions Relevant to Neurodegenerative Diseases. J. Biomed. Sci. 2017, 24, 74. [Google Scholar] [CrossRef]
- Su, T.P.; Su, T.C.; Nakamura, Y.; Tsai, S.Y. The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Kruse, A.C. The Molecular Function of σ Receptors: Past, Present, and Future. Trends Pharmacol. Sci. 2019, 40, 636–654. [Google Scholar] [CrossRef]
- Vela, J.M. Repurposing Sigma-1 Receptor Ligands for COVID-19 Therapy? Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Gordon, D.E.; Gordon, D.E.; Hiatt, J.; Bouhaddou, M.; Rezelj, V.V.; Ulferts, S. Comparative Host-Coronavirus Protein Interaction Networks Reveal Pan-Viral Disease Mechanisms. Science 2020, 9403, eabe9403. [Google Scholar] [CrossRef]
- Mishina, M.; Ohyama, M.; Ishii, K.; Kitamura, S.; Kimura, Y.; Oda, K.I.; Kawamura, K.; Sasaki, T.; Kobayashi, S.; Katayama, Y.; et al. Low Density of Sigma1 Receptors in Early Alzheimer’s Disease. Ann. Nucl. Med. 2008, 22, 151–156. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, L.; Halliday, G.; Dobson-Stone, C.; Wang, Y.; Tang, H.-D.; Cao, L.; Deng, Y.-L.; Wang, G.; Zhang, Y.-M.; et al. Genetic Polymorphisms in Sigma-1 Receptor and Apolipoprotein E Interact to Influence the Severity of Alzheimers Disease. Curr. Alzheimer Res. 2011, 8, 765–770. [Google Scholar] [CrossRef]
- Fehér, Á.; Juhász, A.; László, A.; Kálmán, J.; Pákáski, M.; Kálmán, J.; Janka, Z. Association between a Variant of the Sigma-1 Receptor Gene and Alzheimer’s Disease. Neurosci. Lett. 2012, 517, 136–139. [Google Scholar] [CrossRef]
- Christ, M.G.; Clement, A.M.; Behl, C. The Sigma-1 Receptor at the Crossroad of Proteostasis, Neurodegeneration, and Autophagy. Trends Neurosci. 2020, 43, 79–81. [Google Scholar] [CrossRef]
- Hanner, M.; Moebius, F.F.; Flandorfer, A.; Knaus, H.G.; Striessnig, J.; Kempner, E.; Glossmann, H. Purification, Molecular Cloning, and Expression of the Mammalian Sigma1-Binding Site. Proc. Natl. Acad. Sci. USA 1996, 93, 8072–8077. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal Structure of the Human σ1 Receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Betz, R.M.; Dror, R.O.; Kruse, A.C. Structural Basis for σ(1) Receptor Ligand Recognition. Nat. Struct. Mol. Biol. 2018, 25, 981–987. [Google Scholar] [CrossRef]
- Glennon, R. Pharmacophore Identification for Sigma-1 (σ1) Receptor Binding: Application of the “Deconstruction-Reconstruction-Elaboration” Approach. Mini-Rev. Med. Chem. 2005, 5, 927–940. [Google Scholar] [CrossRef]
- Niso, M.; Mosier, P.D.; Marottoli, R.; Ferorelli, S.; Cassano, G.; Gasparre, G.; Leopoldo, M.; Berardi, F.; Abate, C. High-Affinity Sigma-1 (σ1) Receptor Ligands Based on the σ 1 Antagonist PB212. Future Med Chem. 2019, 1, 2547–2562. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mavlyutov, T.; Singh, D.R.; Biener, G.; Yang, J.; Oliver, J.A.; Ruoho, A.; Raicu, V. The Sigma-1 Receptors Are Present in Monomeric and Oligomeric Forms in Living Cells in the Presence and Absence of Ligands. Biochem. J. 2015, 466, 263–271. [Google Scholar] [CrossRef]
- Albayrak, Y.; Hashimoto, K. Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders. Adv. Exp. Med. Biol. 2017, 964, 153–161. [Google Scholar] [CrossRef]
- Ferris, R.M.; Tang FL, M.; Chang, K.J.; Russell, A. Evidence That the Potential Antipsychotic Agent Rimcazole (BW 234U) Is a Specific, Competitive Antagonist of Sigma Sites in Brain. J. Chem. Inf. Model. 2019, 53, 1689–1699. [Google Scholar] [CrossRef]
- Gilmore, D.L.; Liu, Y.; Matsumoto, R.R. Review of the Pharmacological and Clinical Profile of Rimcazole. CNS Drug Rev. 2004, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Schoenwald, R.D.; Barfknecht, C.F.; Shirolkar, S.; Xia, E. The Effects of Sigma Ligands on Protein Release from Lacrimal Acinar Cells: A Potential Agonist/Antagonist Assay. Life Sci. 1995, 56, 1275–1285. [Google Scholar] [CrossRef]
- Taylor, D.P.; Eison, M.S.; Moon, S.L.; Schlemmer, R.F.J.; Shukla, U.A.; VanderMaelen, C.P.; Yocca, F.D.; Gallant, D.J.; Behling, S.H.; Boissard, C.G. A Role for Sigma Binding in the Antipsychotic Profile of BMY 14802? NIDA Res. Monogr. 1993, 133, 125–157. [Google Scholar] [PubMed]
- Yano, H.; Bonifazi, A.; Xu, M.; Guthrie, D.A.; Schneck, S.N.; Abramyan, A.M.; Fant, A.D.; Hong, W.C.; Newman, A.H.; Shi, L. Pharmacological Profiling of Sigma 1 Receptor Ligands by Novel Receptor Homomer Assays. Neuropharmacology 2018, 133, 264–275. [Google Scholar] [CrossRef]
- Gómez-Soler, M.; Fernández-Dueñas, V.; Portillo-Salido, E.; Pérez, P.; Zamanillo, D.; Vela, J.M.; Burgueño, J.; Ciruela, F. Predicting the Antinociceptive Efficacy of σ1 Receptor Ligands by a Novel Receptor Fluorescence Resonance Energy Transfer (FRET) Based Biosensor. J. Med. Chem. 2014, 57, 238–242. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The Hallucinogen N,N-Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef]
- Johannessen, M.; Fontanilla, D.; Mavlyutov, T.; Ruoho, A.E.; Jackson, M.B. Antagonist Action of Progesterone at σ-Receptors in the Modulation of Voltage-Gated Sodium Channels. Am. J. Physiol. Cell Physiol. 2011, 300, C328–C337. [Google Scholar] [CrossRef]
- Su, T.P.; London, E.D.; Jaffe, J.H. Steroid Binding at σ Receptors Suggests a Link between Endocrine, Nervous, and Immune Systems. Science 1988, 240, 219–221. [Google Scholar] [CrossRef]
- Su, T.P. Evidence for Sigma Opioid Receptor: Binding of [3H]SKF-10047 to Etorphine-Inaccessible Sites in Guinea-Pig Brain. J. Pharmacol. Exp. Ther. 1982, 223, 284–290. [Google Scholar]
- Matsumoto, R.R.; Nguyen, L.; Kaushal, N.; Robson, M.J. Sigma (σ) Receptors as Potential Therapeutic Targets to Mitigate Psychostimulant Effects. Adv. Pharmacol. 2014, 69, 323–386. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Ohtsuki, T.; Toru, M.; Itokawa, M.; Aoki, J.; Shibuya, H.; Kurumaji, A.; Okubo, Y.; Iwawaki, A.; Ota, K.; et al. Association between Polymorphisms in the Type 1 Sigma Receptor Gene and Schizophrenia. Neurosci. Lett. 1998, 257, 45–48. [Google Scholar] [CrossRef]
- Uchida, N.; Ujike, H.; Nakata, K.; Takaki, M.; Nomura, A.; Katsu, T.; Tanaka, Y.; Imamura, T.; Sakai, A.; Kuroda, S. No Association between the Sigma Receptor Type 1 Gene and Schizophrenia: Results of Analysis and Meta-Analysis of Case-Control Studies. BMC Psychiatry 2003, 3, 13. [Google Scholar] [CrossRef]
- Narita, N.; Hashimoto, K.; Tomitaka, S.I.; Minabe, Y. Interactions of Selective Serotonin Reuptake Inhibitors with Subtypes of σ Receptors in Rat Brain. Eur. J. Pharmacol. 1996, 307, 117–119. [Google Scholar] [CrossRef]
- Urani, A.; Roman, F.J.; Phan, V.L.; Su, T.P.; Maurice, T. The Antidepressant-like Effect Induced by Sigma(1)-Receptor Agonists and Neuroactive Steroids in Mice Submitted to the Forced Swimming Test. J. Pharmacol. Exp. Ther. 2001, 298, 1269–1279. [Google Scholar]
- Skuza, G.; Szymańska, M.; Budziszewska, B.; Abate, C.; Berardi, F. Effects of PB190 and PB212, New σ Receptor Ligands, on Glucocorticoid Receptor-Mediated Gene Transcription in LMCAT Cells. Pharmacol. Rep. 2011, 63, 1564–1568. [Google Scholar] [CrossRef]
- Maurice, T.; Roman, F.J.; Privat, A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to σ1 receptors in the mouse forebrain. J. Neurosci. Res. 1996, 46, 734–743. [Google Scholar] [CrossRef]
- Matsuno, K.; Nakazawa, M.; Okamoto, K.; Kawashima, Y.; Mita, S. Binding Properties of SA4503, a Novel and Selective s Receptor Agonist. Eur. J. Pharmacol. 1996, 306, 271–279. [Google Scholar] [CrossRef]
- Senda, T.; Matsuno, K.; Okamoto, K.; Kobayashi, T.; Nakata, K.; Mita, S. Ameliorating Effect of SA4503, a Novel σ1 Receptor Agonist, on Memory Impairments Induced by Cholinergic Dysfunction in Rats. Eur. J. Pharmacol. 1996, 315, 137–146. [Google Scholar] [CrossRef]
- Skuza, G.; Rogóz, Z. A Potential Antidepressant Activity of SA4503, a Selective σ1 Receptor Agonist. Behav. Pharmacol. 2002, 13, 537–543. [Google Scholar] [CrossRef]
- Hirano, K.; Tagashira, H.; Fukunaga, K. Cardioprotective Effect of the Selective Sigma-1 Receptor Agonist, SA4503. Yakugaku Zasshi 2014, 134, 707–713. [Google Scholar] [CrossRef]
- Yamashita, D.; Sun, G.-W.; Cui, Y.; Mita, S.; Otsuki, N.; Kanzaki, S.; Nibu, K.I.; Ogawa, K.; Matsunaga, T. Neuroprotective Effects of Cutamesine, a Ligand of the Sigma-1 Receptor Chaperone, against Noise-Induced Hearing Loss. J. Neurosci. Res. 2015, 93, 788–795. [Google Scholar] [CrossRef]
- Berardi, F.; Ferorelli, S.; Abate, C.; Pedone, M.P.; Colabufo, N.A.; Contino, M.; Perrone, R. Methyl Substitution on the Piperidine Ring of N-[ω-(6- Methoxynaphthalen-1-Yl)Alkyl] Derivatives as a Probe for Selective Binding and Activity at the σ1 Receptor. J. Med. Chem. 2005, 48, 8237–8244. [Google Scholar] [CrossRef]
- Moritz, C.; Berardi, F.; Abate, C.; Peri, F. Live Imaging Reveals a New Role for the Sigma-1 (σ1) Receptor in Allowing Microglia to Leave Brain Injuries. Neurosci. Lett. 2015, 591, 13–18. [Google Scholar] [CrossRef]
- Skuza, G.; Sadaj, W.; Kabziński, M.; Cassano, G.; Gasparre, G.; Abate, C.; Berardi, F. The Effects of New Sigma (σ) Receptor Ligands, PB190 and PB212, in the Models Predictive of Antidepressant Activity. Pharmacol. Rep. 2014, 66, 320–324. [Google Scholar] [CrossRef]
- Su, T.P.; Wu, X.Z.; Cone, E.J.; Shukla, K.; Gund, T.M.; Dodge, A.L.; Parish, D.W. Sigma Compounds Derived from Phencyclidine: Identification of PRE-084, a New, Selective Sigma Ligand. J. Pharmacol. Exp. Ther. 1991, 259, 543–550. [Google Scholar]
- Maurice, T.; Su, T.P.; Parish, D.W.; Nabeshima, T.; Privat, A. PRE-084, a σ Selective PCP Derivative, Attenuates MK-801-Induced Impairment of Learning in Mice. Pharmacol. Biochem. Behav. 1994, 49, 859–869. [Google Scholar] [CrossRef]
- Maurice, T. Beneficial Effect of the σ1 Receptor Agonist PRE-084 against the Spatial Learning Deficits in Aged Rats. Eur. J. Pharmacol. 2001, 431, 223–227. [Google Scholar] [CrossRef]
- Okuyama, S.; Nakazato, A. NE-100: A Novel Sigma Receptor Antagonist. CNS Drug Rev. 1996, 2, 226–237. [Google Scholar] [CrossRef]
- Chaki, S.; Tanaka, M.; Muramatsu, M.; Otomo, S. NE-100, a Novel Potent σ Ligand, Preferentially Binds to Σ1 Binding Sites in Guinea Pig Brain. Eur. J. Pharmacol. 1994, 251, R1–R2. [Google Scholar] [CrossRef]
- Spinelli, F.; Haider, A.; Toscano, A.; Pati, M.L.; Keller, C.; Berardi, F.; Colabufo, N.A.; Abate, C.; Ametamey, S.M. Synthesis, Radiolabelling, and Evaluation of [(11)C]PB212 as a Radioligand for Imaging Sigma-1 Receptors Using PET. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 32–40. [Google Scholar] [PubMed]
- Maeda, D.Y.; Williams, W.; Bowen, W.D.; Coop, A. A Sigma-1 Receptor Selective Analogue of BD1008. A Potential Substitute for (+)-Opioids in Sigma Receptor Binding Assays. Bioorganic Med. Chem. Lett. 2000, 10, 17–18. [Google Scholar] [CrossRef]
- Sahlholm, K.; Århem, P.; Fuxe, K.; Marcellino, D. The Dopamine Stabilizers ACR16 and ()-OSU6162 Display Nanomolar Affinities at the σ-1 Receptor. Mol. Psychiatry 2013, 18, 12–14. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Shen, B.; Zavaleta, C.L.; Nielsen, C.H.; Mesangeau, C.; Vuppala, P.K.; Chan, C.; Avery, B.A.; Fishback, J.A.; Matsumoto, R.R.; et al. New Positron Emission Tomography (PET) Radioligand for Imaging σ-1 Receptors in Living Subjects. J. Med. Chem. 2012, 55, 8272–8282. [Google Scholar] [CrossRef]
- James, M.L.; Shen, B.; Nielsen, C.H.; Behera, D.; Buckmaster, C.L.; Mesangeau, C.; Zavaleta, C.; Vuppala, P.K.; Jamalapuram, S.; Avery, B.A.; et al. Evaluation of σ-1 Receptor Radioligand 18F-FTC-146 in Rats and Squirrel Monkeys Using PET. J. Nucl. Med. 2014, 55, 147–153. [Google Scholar] [CrossRef]
- Shen, B.; Park, J.H.; Hjørnevik, T.; Cipriano, P.W.; Yoon, D.; Gulaka, P.K.; Holly, D.; Behera, D.; Avery, B.A.; Gambhir, S.S.; et al. Radiosynthesis and First-In-Human PET/MRI Evaluation with Clinical-Grade [18F]FTC-146. Mol. Imaging Biol. 2017, 19, 779–786. [Google Scholar] [CrossRef]
- Díaz, J.L.; Zamanillo, D.; Corbera, J.; Baeyens, J.M.; Maldonado, R.; Pericàs, M.A.; Vela, J.M.; Torrens, A. Selective Sigma-1 (Sigma1) Receptor Antagonists: Emerging Target for the Treatment of Neuropathic Pain. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 172–183. [Google Scholar] [CrossRef]
- Abadias, M.; Escriche, M.; Vaqué, A.; Sust, M.; Encina, G. Safety, Tolerability and Pharmacokinetics of Single and Multiple Doses of a Novel Sigma-1 Receptor Antagonist in Three Randomized Phase I Studies. Br. J. Clin. Pharmacol. 2013, 75, 103–117. [Google Scholar] [CrossRef]
- Matsumoto, R.R.; Bowen, W.D.; Tom, M.A.; Vo, V.N.; Truong, D.D.; De Costa, B.R. Characterization of Two Novel σ Receptor Ligands: Antidystonic Effects in Rats Suggest σ Receptor Antagonism. Eur. J. Pharmacol. 1995, 280, 301–310. [Google Scholar] [CrossRef]
- Matsumoto, R.R.; McCracken, K.A.; Friedman, M.J.; Pouw, B.; De Costa, B.R.; Bowen, W.D. Conformationally Restricted Analogs of BD1008 and an Antisense Oligodeoxynucleotide Targeting σ1 Receptors Produce Anti-Cocaine Effects in Mice. Eur. J. Pharmacol. 2001, 419, 163–174. [Google Scholar] [CrossRef]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Leopoldo, M.; Abate, C.; Tortorella, V. N-Aryl or N-Alkylpiperazine Derivatives: The Role of N-Substituent on σ1, σ2, 5-HT1A and D2 Receptor Affinity. Med. Chem. Res. 2000, 10, 201–207. [Google Scholar]
- Lever, J.R.; Gustafson, J.L.; Xu, R.; Allmon, R.L.; Lever, S.Z. σ1 and σ2 Receptor Binding Affinity and Selectivity of SA4503 and Fluoroethyl SA4503. Synapse 2006, 59, 350–358. [Google Scholar] [CrossRef]
- Garces-Ramirez, L.; Green, J.L.; Hiranita, T.; Kopajtic, T.A.; Mereu, M.; Thomas, A.M.; Mesangeau, C.; Narayanan, S.; McCurdy, C.R.; Katz, J.L.; et al. Sigma Receptor Agonists: Receptor Binding and Effects on Mesolimbic Dopamine Neurotransmission Assessed by Microdialysis. Biol. Psychiatry 2011, 69, 208–217. [Google Scholar] [CrossRef]
- Colabufo, N.A.; Berardi, F.; Contino, M.; Perrone, R.; Tortorella, V. A New Method for Evaluating σ2 Ligand Activity in the Isolated Guinea-Pig Bladder. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003, 368, 106–112. [Google Scholar] [CrossRef]
- Johnston, T.H.; Geva, M.; Steiner, L.; Orbach, A.; Papapetropoulos, S.; Savola, J.M.; Reynolds, I.J.; Ravenscroft, P.; Hill, M.; Fox, S.H.; et al. Pridopidine, a Clinic-Ready Compound, Reduces 3,4-Dihydroxyphenylalanine-Induced Dyskinesia in Parkinsonian Macaques. Mov. Disord. 2019, 34, 708–716. [Google Scholar] [CrossRef]
- Romero, L.; Zamanillo, D.; Nadal, X.; Sánchez-Arroyos, R.; Rivera-Arconada, I.; Dordal, A.; Montero, A.; Muro, A.; Bura, A.; Segalés, C.; et al. Pharmacological Properties of S1RA, a New Sigma-1 Receptor Antagonist That Inhibits Neuropathic Pain and Activity-Induced Spinal Sensitization. Br. J. Pharmacol. 2012, 166, 2289–2306. [Google Scholar] [CrossRef]
- Lecanu, L.; Tillement, L.; McCourty, A.; Rammouz, G.; Yao, W.; Greeson, J.; Papadopoulos, V. Dimethyl-Carbamic Acid 2,3-Bis-Dimethylcarbamoyloxy-6-(4-Ethyl-Piperazine- 1-Carbonyl)-Phenyl Ester: A Novel Multi-Target Therapeutic Approach to Neuroprotection. Med. Chem. 2012, 6, 123–140. [Google Scholar] [CrossRef]
- Caccamo, A.; Oddo, S.; Billings, L.M.; Green, K.N.; Martinez-Coria, H.; Fisher, A.; LaFerla, F.M. M1 Receptors Play a Central Role in Modulating AD-like Pathology in Transgenic Mice. Neuron 2006, 49, 671–682. [Google Scholar] [CrossRef]
- Medeiros, R.; Kitazawa, M.; Caccamo, A.; Baglietto-Vargas, D.; Estrada-Hernandez, T.; Cribbs, D.H.; Fisher, A.; Laferla, F.M. Loss of Muscarinic M1 Receptor Exacerbates Alzheimer’s Disease-like Pathology and Cognitive Decline. Am. J. Pathol. 2011, 179, 980–991. [Google Scholar] [CrossRef]
- Vamvakidès, A. [Anticonvulsant and forced swim anti-immobility effects of tetrahydro-N, N-dimethyl-2,2-diphenyl-3-furanemethanamine (AE37): Common action mechanism?]. Ann. Pharm. Fr. 2002, 60, 88–92. [Google Scholar]
- Vamvakidès, A. [Mechanism of action of tetrahydro-N, N-dimethyl-5, 5-diphenyl-3-furanemethanamine, a putative nootropic, anti-epileptic and antidepressant compound]. Ann. Pharm. Fr. 2002, 60, 415–422. [Google Scholar]
- Fisher, A.; Bezprozvanny, I.; Wu, L.; Ryskamp, D.A.; Bar-Ner, N.; Natan, N.; Brandeis, R.; Elkon, H.; Nahum, V.; Gershonov, E.; et al. AF710B, a Novel M1/σ1 Agonist with Therapeutic Efficacy in Animal Models of Alzheimer’s Disease. Neurodegener. Dis. 2016, 16, 95–110. [Google Scholar] [CrossRef]
- Chow, H.M.; Guo, D.; Zhou, J.C.; Zhang, G.Y.; Li, H.F.; Herrup, K.; Zhang, J. CDK5 Activator Protein P25 Preferentially Binds and Activates GSK3β. Proc. Natl. Acad. Sci. USA 2014, 111, E4887–E4895. [Google Scholar] [CrossRef]
- Lahmy, V.; Meunier, J.; Malmström, S.; Naert, G.; Givalois, L.; Kim, S.H.; Villard, V.; Vamvakides, A.; Maurice, T. Blockade of Tau Hyperphosphorylation and Aβ 1-42 Generation by the Aminotetrahydrofuran Derivative ANAVEX2-73, a Mixed Muscarinic and σ 1 Receptor Agonist, in a Nontransgenic Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2013, 38, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Espallergues, J.; Lapalud, P.; Christopoulos, A.; Avlani, V.A.; Sexton, P.M.; Vamvakides, A.; Maurice, T. Involvement of the Sigma1 (σ1) Receptor in the Anti-Amnesic, but Not Antidepressant-like, Effects of the Aminotetrahydrofuran Derivative ANAVEX1-41. Br. J. Pharmacol. 2007, 152, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Behensky, A.A.; Yasny, I.E.; Shuster, A.M.; Seredenin, S.B.; Petrov, A.V.; Cuevas, J. Stimulation of Sigma Receptors with Afobazole Blocks Activation of Microglia and Reduces Toxicity Caused by Amyloid-Β25-35. J. Pharmacol. Exp. Ther. 2013, 347, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Behensky, A.A.; Katnik, C.; Yin, H.; Cuevas, J. Activation of Sigma Receptors with Afobazole Modulates Microglial, but Not Neuronal, Apoptotic Gene Expression in Response to Long-Term Ischemia Exposure. Front. Neurosci. 2019, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Iulita, M.F.; Gubert, P.; Flores Aguilar, L.; Ducatenzeiler, A.; Fisher, A.; Cuello, A.C. AF710B, an M1/Sigma-1 Receptor Agonist with Long-Lasting Disease-Modifying Properties in a Transgenic Rat Model of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 811–823. [Google Scholar] [CrossRef]
- Rochais, C.; Lecoutey, C.; Gaven, F.; Giannoni, P.; Hamidouche, K.; Hedou, D.; Dubost, E.; Genest, D.; Yahiaoui, S.; Freret, T.; et al. Novel Multitarget-Directed Ligands (MTDLs) with Acetylcholinesterase (AChE) Inhibitory and Serotonergic Subtype 4 Receptor (5-HT4R) Agonist Activities As Potential Agents against Alzheimer’s Disease: The Design of Donecopride. J. Med. Chem. 2015, 58, 3172–3187. [Google Scholar] [CrossRef]
- Lalut, J.; Santoni, G.; Karila, D.; Lecoutey, C.; Davis, A.; Nachon, F.; Silman, I.; Sussman, J.; Weik, M.; Maurice, T.; et al. Novel Multitarget-Directed Ligands Targeting Acetylcholinesterase and σ1 Receptors as Lead Compounds for Treatment of Alzheimer’s Disease: Synthesis, Evaluation, and Structural Characterization of Their Complexes with Acetylcholinesterase. Eur. J. Med. Chem. 2019, 162, 234–248. [Google Scholar] [CrossRef]
- Kato, K.; Hayako, H.; Ishihara, Y.; Marui, S.; Iwane, M.; Miyamoto, M. TAK-147, an Acetylcholinesterase Inhibitor, Increases Choline Acetyltransferase Activity in Cultured Rat Septal Cholinergic Neurons. Neurosci. Lett. 1999, 260, 5–8. [Google Scholar] [CrossRef]
- Maurice, T.; Meunier, J.; Feng, B.; Ieni, J.; Monaghan, D.T. Interaction with σ1 Protein, but Not N-Methyl-D-Aspartate Receptor, Is Involved in the Pharmacological Activity of Donepezil. J. Pharmacol. Exp. Ther. 2006, 317, 606–614. [Google Scholar] [CrossRef]
- Meunier, J.; Ieni, J.; Maurice, T. The Anti-Amnesic and Neuroprotective Effects of Donepezil against Amyloid Β 25-35 Peptide-Induced Toxicity in Mice Involve an Interaction with the σ1 Receptor. Br. J. Pharmacol. 2006, 149, 998–1012. [Google Scholar] [CrossRef]
- Ramakrishnan, N.K.; Visser, A.K.D.; Schepers, M.; Luurtsema, G.; Nyakas, C.J.; Elsinga, P.H.; Ishiwata, K.; Dierckx, R.A.J.O.; Van Waarde, A. Dose-Dependent Sigma-1 Receptor Occupancy by Donepezil in Rat Brain Can Be Assessed with 11C-SA4503 and MicroPET. Psychopharmacology 2014, 231, 3997–4006. [Google Scholar] [CrossRef]
- Maurice, T. Protection by Sigma-1 Receptor Agonists Is Synergic with Donepezil, but Not with Memantine, in a Mouse Model of Amyloid-Induced Memory Impairments. Behav. Brain Res. 2016, 296, 270–278. [Google Scholar] [CrossRef]
- Ganapathy, M.E.; Prasad, P.D.; Huang, W.; Seth, P.; Leibach, F.H.; Ganapathy, V. Molecular and Ligand-Binding Characterization of the Sigma-Receptor in the Jurkat Human T Lymphocyte Cell Line. J. Pharmacol. Exp. Ther. 1999, 289, 251–260. [Google Scholar]
- Castañeda-Arriaga, R.; Alvarez-Idaboy, J.R. Lipoic Acid and Dihydrolipoic Acid. A Comprehensive Theoretical Study of Their Antioxidant Activity Supported by Available Experimental Kinetic Data. J. Chem. Inf. Model. 2014, 54, 1642–1652. [Google Scholar] [CrossRef]
- Fava, A.; Pirritano, D.; Plastino, M.; Cristiano, D.; Puccio, G.; Colica, C.; Ermio, C.; De Bartolo, M.; Mauro, G.; Bosco, D. The Effect of Lipoic Acid Therapy on Cognitive Functioning in Patients with Alzheimer’s Disease. J. Neurodegener. Dis. 2013, 2013, 454253. [Google Scholar] [CrossRef]
- Estrada, M.; Pérez, C.; Soriano, E.; Laurini, E.; Romano, M.; Pricl, S.; Morales-García, J.A.; Pérez-Castillo, A.; Rodríguez-Franco, M.I. New Neurogenic Lipoic-Based Hybrids as Innovative Alzheimer’s Drugs with σ-1 Agonism and β-Secretase Inhibition. Future Med. Chem. 2016, 8, 1191–1207. [Google Scholar] [CrossRef]
- Estrada Valencia, M.; Herrera-Arozamena, C.; de Andrés, L.; Pérez, C.; Morales-García, J.A.; Pérez-Castillo, A.; Ramos, E.; Romero, A.; Viña, D.; Yáñez, M.; et al. Neurogenic and Neuroprotective Donepezil-Flavonoid Hybrids with Sigma-1 Affinity and Inhibition of Key Enzymes in Alzheimer’s Disease. Eur. J. Med. Chem. 2018, 156, 534–553. [Google Scholar] [CrossRef]
- Estrada-Valencia, M.; Herrera-Arozamena, C.; Pérez, C.; Viña, D.; Morales-García, J.A.; Pérez-Castillo, A.; Ramos, E.; Romero, A.; Laurini, E.; Pricl, S.; et al. New Flavonoid–N,N-Dibenzyl(N-Methyl)Amine Hybrids: Multi-Target-Directed Agents for Alzheimer’s Disease Endowed with Neurogenic Properties. J. Enzym. Inhib. Med. Chem. 2019, 34, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Quirion, R.; Chicheportiche, R.; Contreras, P.C.; Johnson, K.M.; Lodge, D.; William Tam, S.; Woods, J.H.; Zukin, S.R. Classification and Nomenclature of Phencyclidine and Sigma Receptor Sites. Trends Neurosci. 1987, 10, 444–446. [Google Scholar] [CrossRef]
- Quirion, R.; Bowen, W.D.; Itzhak, Y.; Junien, J.L.; Musacchio, J.M.; Rothman, R.B.; Su, T.P.; Tam, S.W.; Taylor, D.P. A Proposal for the Classification of Sigma Binding Sites. Trends Pharmacol. Sci. 1992, 13, 85–86. [Google Scholar] [CrossRef]
- Pabba, M.; Sibille, E. Sigma-1 and N-Methyl-D-Aspartate Receptors: A Partnership with Beneficial Outcomes. Mol. Neuropsychiatry 2015, 1, 47–51. [Google Scholar] [CrossRef]
- Bergeron, R.; de Montigny, C.; Debonnel, G. Biphasic Effects of Sigma Ligands on the Neuronal Response to N-Methyl-D-Aspartate. Naunyn. Schmiedebergs. Arch. Pharmacol. 1995, 351, 252–260. [Google Scholar] [CrossRef]
- Liang, X.; Wang, R.Y. Biphasic Modulatory Action of the Selective Sigma Receptor Ligand SR 31742A on N-Methyl-D-Aspartate-Induced Neuronal Responses in the Frontal Cortex. Brain Res. 1998, 807, 208–213. [Google Scholar] [CrossRef]
- Fletcher, E.J.; Church, J.; Abdel-Hamid, K.; MacDonald, J.F. Blockade by Sigma Site Ligands of N-methyl-D-aspartate-evoked Responses in Rat and Mouse Cultured Hippocampal Pyramidal Neurones. Br. J. Pharmacol. 1995, 116, 2791–2800. [Google Scholar] [CrossRef]
- Martina, M.; Turcotte, M.E.B.; Halman, S.; Bergeron, R. The Sigma-1 Receptor Modulates NMDA Receptor Synaptic Transmission and Plasticity via SK Channels in Rat Hippocampus. J. Physiol. 2007, 578, 143–157. [Google Scholar] [CrossRef]
- Van der Schyf, C.J.; Squier, G.J.; Coetzee, W.A. Characterization of NGP 1-01, an Aromatic Polycyclic Amine, as a Calcium Antagonist. Pharmacol. Res. Commun. 1986, 18, 407–417. [Google Scholar] [CrossRef]
- Geldenhuys, J.W.; Van der Schyf, C.J. Rationally Designed Multi-Targeted Agents Against Neurodegenerative Diseases. Curr. Med. Chem. 2013, 20, 1662–1672. [Google Scholar] [CrossRef]
- Kassiou, M.; Nguyen, V.H.; Knott, R.; Christie, M.J.; Hambley, T.W. Trishomocubanes, a New Class of Selective and High Affinity Ligands for the Sigma Binding Site. Bioorganic Med. Chem. Lett. 1996, 6, 595–600. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Kassiou, M.; Johnston, G.A.R.; Christie, M.J. Comparison of Binding Parameters of σ1 and σ2 Binding Sites in Rat and Guinea Pig Brain Membranes: Novel Subtype-Selective Trishomocubanes. Eur. J. Pharmacol. 1996, 311, 233–240. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Malan, S.F.; Bloomquist, J.R.; Marchand, A.P.; Van Der Schyf, C.J. Pharmacology and Structure-Activity Relationships of Bioactive Polycyclic Cage Compounds: A Focus on Pentacycloundecane Derivatives. Med. Res. Rev. 2005, 25, 21–48. [Google Scholar] [CrossRef]
- Kiewert, C.; Hartmann, J.; Stoll, J.; Thekkumkara, T.J.; Van Der Schyf, C.J.; Klein, J. NGP1-01 Is a Brain-Permeable Dual Blocker of Neuronal Voltage- and Ligand-Operated Calcium Channels. Neurochem. Res. 2006, 31, 395–399. [Google Scholar] [CrossRef]
- Kornhuber, J.; Schoppmeyer, K.; Riederer, P. Affinity of 1-Aminoadamantanes for the σ Binding Site in Post-Mortem Human Frontal Cortex. Neurosci. Lett. 1993, 163, 129–131. [Google Scholar] [CrossRef]
- Kornhuber, J.; Bormann, J.; Hübers, M.; Rusche, K.; Riederer, P. Effects of the 1-Amino-Adamantanes at the MK-801-Binding Site of the NMDA-Receptor-Gated Ion Channel: A Human Postmortem Brain Study. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 206, 297–300. [Google Scholar] [CrossRef]
- Peeters, M.; Romieu, P.; Maurice, T.; Su, T.P.; Maloteaux, J.M.; Hermans, E. Involvement of the Sigma1 Receptor in the Modulation of Dopaminergic Transmission by Amantadine. Eur. J. Neurosci. 2004, 19, 2212–2220. [Google Scholar] [CrossRef]
- Moreno, E.; Moreno-Delgado, D.; Navarro, G.; Hoffmann, H.M.; Fuentes, S.; Rosell-Vilar, S.; Gasperini, P.; Rodríguez-Ruiz, M.; Medrano, M.; Mallol, J.; et al. Cocaine Disrupts Histamine H3 Receptor Modulation of Dopamine D1 Receptor Signaling: σ1-D1-H3 Receptor Complexes as Key Targets for Reducing Cocaine’s Effects. J. Neurosci. 2014, 34, 3545–3558. [Google Scholar] [CrossRef]
- Navarro, G.; Moreno, E.; Bonaventura, J.; Brugarolas, M.; Farré, D.; Aguinaga, D.; Mallol, J.; Cortés, A.; Casadó, V.; Lluís, C.; et al. Cocaine Inhibits Dopamine D2 Receptor Signaling via Sigma-1-D2 Receptor Heteromers. PLoS ONE 2013, 8, e61245. [Google Scholar] [CrossRef]
- Sambo, D.O.; Lin, M.; Owens, A.; Lebowitz, J.J.; Richardson, B.; Jagnarine, D.A.; Shetty, M.; Rodriquez, M.; Alonge, T.; Ali, M.; et al. The Sigma-1 Receptor Modulates Methamphetamine Dysregulation of Dopamine Neurotransmission. Nat. Commun. 2017, 8, 2228. [Google Scholar] [CrossRef]
- Sahlholm, K.; Sijbesma, J.W.A.; Maas, B.; Kwizera, C.; Marcellino, D.; Ramakrishnan, N.K.; Dierckx, R.A.J.O.; Elsinga, P.H.; Van Waarde, A. Pridopidine Selectively Occupies Sigma-1 Rather than Dopamine D2 Receptors at Behaviorally Active Doses. Psychopharmacology 2015, 232, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Francardo, V.; Geva, M.; Bez, F.; Denis, Q.; Steiner, L.; Hayden, M.R.; Cenci, M.A. Pridopidine Induces Functional Neurorestoration Via the Sigma-1 Receptor in a Mouse Model of Parkinson’s Disease. Neurotherapeutics 2019, 16, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Ryskamp, D.; Wu, L.; Wu, J.; Kim, D.; Rammes, G.; Geva, M.; Hayden, M.; Bezprozvanny, I. Pridopidine Stabilizes Mushroom Spines in Mouse Models of Alzheimer’s Disease by Acting on the Sigma-1 Receptor. Neurobiol. Dis. 2019, 124, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Kadnikov, I.A.; Voronin, M.V.; Seredenin, S.B. Cytoprotective Effect of Afobazole and Its Main Metabolite M-11. Bull. Exp. Biol. Med. 2015, 159, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Voronin, M.V.; Kadnikov, I.A. Contribution of Sigma-1 Receptor to Cytoprotective Effect of Afobazole. Pharmacol. Res. Perspect. 2016, 4, e00273. [Google Scholar] [CrossRef]
- Voronin, M.V.; Kadnikov, I.A.; Voronkov, D.N.; Seredenin, S.B. Chaperone Sigma1R Mediates the Neuroprotective Action of Afobazole in the 6-OHDA Model of Parkinson’s Disease. Sci. Rep. 2019, 9, 17020. [Google Scholar] [CrossRef]
- Matsumoto, R.R.; Pouw, B. Correlation between Neuroleptic Binding to σ1 and σ2 Receptors and Acute Dystonic Reactions. Eur. J. Pharmacol. 2000, 401, 155–160. [Google Scholar] [CrossRef]
- Cobos, E.J.; Del Pozo, E.; Baeyens, J.M. Irreversible Blockade of Sigma-1 Receptors by Haloperidol and Its Metabolites in Guinea Pig Brain and SH-SY5Y Human Neuroblastoma Cells. J. Neurochem. 2007, 102, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Sozio, P.; Fiorito, J.; Di Giacomo, V.; Di Stefano, A.; Marinelli, L.; Cacciatore, I.; Cataldi, A.; Pacella, S.; Turkez, H.; Parenti, C.; et al. Haloperidol Metabolite II Prodrug: Asymmetric Synthesis and Biological Evaluation on Rat C6 Glioma Cells. Eur. J. Med. Chem. 2015, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Korpi, E.R.; Costakos, D.T.; Wyatt, R.J. Interconversions of Haloperidol and Reduced Haloperidol in Guinea Pig and Rat Liver Microsomes. Biochem. Pharmacol. 1985, 34, 2923–2927. [Google Scholar] [CrossRef]
- Usuki, E.; Van Der Schyf, C.J.; Castagnoli, N. Metabolism of Haloperidol and Its Tetrahydrophyridine Dehydration Product HPTP. Drug Metab. Rev. 1998, 30, 809–826. [Google Scholar] [CrossRef]
- Maurice, T.; Martin-Fardon, R.; Romieu, P.; Matsumoto, R.R. Sigma1 (σ1) Receptor Antagonists Represent a New Strategy against Cocaine Addiction and Toxicity. Neurosci. Biobehav. Rev. 2002, 26, 499–527. [Google Scholar] [CrossRef]
- Guitart, X.; Codony, X.; Monroy, X. Sigma Receptors: Biology and Therapeutic Potential. Psychopharmacology 2004, 174, 301–319. [Google Scholar] [CrossRef]
- Marrazzo, A.; Fiorito, J.; Zappal, L.; Prezzavento, O.; Ronsisvalle, S.; Pasquinucci, L.; Scoto, G.M.; Bernardini, R.; Ronsisvalle, G. Antiproliferative Activity of Phenylbutyrate Ester of Haloperidol Metabolite II [(±)-MRJF4] in Prostate Cancer Cells. Eur. J. Med. Chem. 2011, 46, 433–438. [Google Scholar] [CrossRef]
- Vidal-Torres, A.; De La Puente, B.; Rocasalbas, M.; Touriño, C.; Andreea Bura, S.; Fernández-Pastor, B.; Romero, L.; Codony, X.; Zamanillo, D.; Buschmann, H.; et al. Sigma-1 Receptor Antagonism as Opioid Adjuvant Strategy: Enhancement of Opioid Antinociception without Increasing Adverse Effects. Eur. J. Pharmacol. 2013, 711, 63–72. [Google Scholar] [CrossRef]
- Bruna, J.; Videla, S.; Argyriou, A.A.; Velasco, R.; Villoria, J.; Santos, C.; Nadal, C.; Cavaletti, G.; Alberti, P.; Briani, C.; et al. Efficacy of a Novel Sigma-1 Receptor Antagonist for Oxaliplatin-Induced Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Phase IIa Clinical Trial. Neurotherapeutics 2018, 15, 178–189. [Google Scholar] [CrossRef]
- Discovery Studio 16; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2016.
- Laggner, C.; Schieferer, C.; Fiechtner, B.; Poles, G.; Hoffmann, R.D.; Glossmann, H.; Langer, T.; Moebius, F.F. Discovery of High-Affinity Ligands of σ1 Receptor, ERG2, and Emopamil Binding Protein by Pharmacophore Modeling and Virtual Screening. J. Med. Chem. 2005, 48, 4754–4764. [Google Scholar] [CrossRef]
- García, M.; Virgili, M.; Alonso, M.; Alegret, C.; Fernández, B.; Port, A.; Pascual, R.; Monroy, X.; Vidal-Torres, A.; Serafini, M.T.; et al. 4-Aryl-1-Oxa-4,9-Diazaspiro[5.5]Undecane Derivatives as Dual μ-Opioid Receptor Agonists and σ1 Receptor Antagonists for the Treatment of Pain. J. Med. Chem. 2020, 63, 2434–2454. [Google Scholar] [CrossRef]
- García, M.; Virgili, M.; Alonso, M.; Alegret, C.; Farran, J.; Fernández, B.; Bordas, M.; Pascual, R.; Burgueño, J.; Vidal-Torres, A.; et al. Discovery of EST73502, a Dual μ-Opioid Receptor Agonist and σ1 Receptor Antagonist Clinical Candidate for the Treatment of Pain. J. Med. Chem. 2020, 63, 15508–15526. [Google Scholar] [CrossRef]
- Fulgenzi, G.; Graciotti, L.; Faronato, M.; Soldovieri, M.V.; Miceli, F.; Amoroso, S.; Annunziato, L.; Procopio, A.; Taglialatela, M. Human Neoplastic Mesothelial Cells Express Voltage-Gated Sodium Channels Involved in Cell Motility. Int. J. Biochem. Cell Biol. 2006, 38, 1146–1159. [Google Scholar] [CrossRef]
- Kim, F.J.; Maher, C.M. Sigma1 Pharmacology in the Context of Cancer. Handb. Exp. Pharmacol. 2017, 244, 237–308. [Google Scholar] [CrossRef] [PubMed]
- Brackenbury, W.J.; Djamgoz, M.B.A.; Isom, L.L. An Emerging Role for Voltage-Gated Na+ Channels in Cellular Migration: Regulation of Central Nervous System Development and Potentiation of Invasive Cancers. Neuroscientist 2008, 14, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Niso, M.; Abatematteo, F.S.; Contino, M.; Colabufo, N.A.; Berardi, F. PB28, the Sigma-1 and Sigma-2 Receptors Modulator With Potent Anti–SARS-CoV-2 Activity: A Review About Its Pharmacological Properties and Structure Affinity Relationships. Front. Pharmacol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Riganas, S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Dimas, K.; Kourafalos, V.N.; Eleutheriades, A.; Moutsos, V.I.; Khan, H.; Margarita, P.; et al. New Adamantane Derivatives with Sigma Affinity and Antiproliferative Activity. Med. Chem. 2012, 8, 569–586. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Berardi, F.; Ferorelli, S.; Abate, C.; Colabufo, N.A.; Contino, M.; Perrone, R.; Tortorella, V. 4-(Tetralin-1-Yl)- and 4-(Naphthalen-1-Yl)Alkyl Derivatives of 1-Cyclohexylpiperazine as σ Receptor Ligands with Agonist σ 2 Activity. J. Med. Chem. 2004, 47, 2308–2317. [Google Scholar] [CrossRef]
- Pati, M.L.; Hornick, J.R.; Niso, M.; Berardi, F.; Spitzer, D.; Abate, C.; Hawkins, W. Sigma-2 Receptor Agonist Derivatives of 1-Cyclohexyl-4-[3-(5-Methoxy-1,2,3,4-Tetrahydronaphthalen-1-Yl)Propyl]Piperazine (PB28) Induce Cell Death via Mitochondrial Superoxide Production and Caspase Activation in Pancreatic Cancer. BMC Cancer 2017, 17, 51. [Google Scholar] [CrossRef]
- Liu, C.C.; Yu, C.F.; Wang, S.C.; Li, H.Y.; Lin, C.M.; Wang, H.H.; Abate, C.; Chiang, C.S. Sigma-2 Receptor/TMEM97 Agonist PB221 as an Alternative Drug for Brain Tumor. BMC Cancer 2019, 19, 473. [Google Scholar] [CrossRef]
- Niso, M.; Abate, C.; Contino, M.; Ferorelli, S.; Azzariti, A.; Perrone, R.; Colabufo, N.A.; Berardi, F. Sigma-2 Receptor Agonists as Possible Antitumor Agents in Resistant Tumors: Hints for Collateral Sensitivity. ChemMedChem 2013, 8, 2026–2035. [Google Scholar] [CrossRef]
- Pati, M.L.; Niso, M.; Ferorelli, S.; Abate, C.; Berardi, F. Novel Metal Chelators Thiosemicarbazones with Activity at the σ2 Receptors and P-Glycoprotein: An Innovative Strategy for Resistant Tumor Treatment. RSC Adv. 2015, 5, 103131–103146. [Google Scholar] [CrossRef]
- Pati, M.L.; Niso, M.; Spitzer, D.; Berardi, F.; Contino, M.; Riganti, C.; Hawkins, W.G.; Abate, C. Multifunctional Thiosemicarbazones and Deconstructed Analogues as a Strategy to Study the Involvement of Metal Chelation, Sigma-2 (σ2) Receptor and P-Gp Protein in the Cytotoxic Action: In Vitro and in Vivo Activity in Pancreatic Tumors. Eur. J. Med. Chem. 2018, 144, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Niso, M.; Lacivita, E.; Mosier, P.D.; Toscano, A.; Perrone, R. Analogues of σ Receptor Ligand 1-Cyclohexyl-4-[3-(5-Methoxy-1,2,3,4- Tetrahydronaphthalen-1-Yl)Propyl]Piperazine (PB28) with Added Polar Functionality and Reduced Lipophilicity for Potential Use as Positron Emission Tomography Radiotracers. J. Med. Chem. 2011, 54, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Berardi, F.; Abate, C.; Ferorelli, S.; Uricchio, V.; Colabufo, N.A.; Niso, M.; Perrone, R. Exploring the Importance of Piperazine N-Atoms for σ2 Receptor Affinity and Activity in a Series of Analogs of 1-Cyclohexyl-4-[3-(5-Methoxy-1,2,3,4-Tetrahydronaphthalen-1-Yl)-Propyl]Piperazine (PB28). J. Med. Chem. 2009, 52, 7817–7828. [Google Scholar] [CrossRef] [PubMed]
- Brent, P.J.; Pang, G.; Little, G.; Dosen, P.J.; Van Helden, D.F. The Sigma Receptor Ligand, Reduced Haloperidol, Induces Apoptosis and Increases Intracellular-Free Calcium Levels [Ca2+]i in Colon and Mammary Adenocarcinoma Cells. Biochem. Biophys. Res. Commun. 1996, 219, 219–226. [Google Scholar] [CrossRef]
- Nieto, F.R.; Cendán, C.M.; Sánchez-Fernández, C.; Cobos, E.J.; Entrena, J.M.; Tejada, M.A.; Zamanillo, D.; Vela, J.M.; Baeyens, J.M. Role of Sigma-1 Receptors in Paclitaxel-Induced Neuropathic Pain in Mice. J. Pain 2012, 13, 1107–1121. [Google Scholar] [CrossRef]
- Nieto, F.R.; Cendán, C.M.; Cañizares, F.J.; Cubero, M.A.; Vela, J.M.; Fernández-Segura, E.; Baeyens, J.M. Genetic Inactivation and Pharmacological Blockade of Sigma-1 Receptors Prevent Paclitaxel-Induced Sensory-Nerve Mitochondrial Abnormalities and Neuropathic Pain in Mice. Mol. Pain 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Hashimoto, K. Potential Role of the Sigma-1 Receptor Chaperone in the Beneficial Effects of Donepezil in Dementia with Lewy Bodies. Clin. Psychopharmacol. Neurosci. 2013, 11, 43–44. [Google Scholar] [CrossRef]
- Mangiatordi, G.F.; Intranuovo, F.; Delre, P.; Abatematteo, F.S.; Abate, C.; Niso, M.; Creanza, T.M.; Ancona, N.; Stefanachi, A.; Contino, M. Cannabinoid Receptor Subtype 2 (CB2R) in a Multitarget Approach: Perspective of an Innovative Strategy in Cancer and Neurodegeneration. J. Med. Chem. 2020, 63, 14448–14469. [Google Scholar] [CrossRef]
- Abate, C.; Ferorelli, S.; Contino, M.; Marottoli, R.; Colabufo, N.A.; Perrone, R.; Berardi, F. Arylamides Hybrids of Two High-Affinity σ 2 Receptor Ligands as Tools for the Development of PET Radiotracers. Eur. J. Med. Chem. 2011, 46, 4733–4741. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abatematteo, F.S.; Niso, M.; Contino, M.; Leopoldo, M.; Abate, C. Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives. Int. J. Mol. Sci. 2021, 22, 6359. https://doi.org/10.3390/ijms22126359
Abatematteo FS, Niso M, Contino M, Leopoldo M, Abate C. Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives. International Journal of Molecular Sciences. 2021; 22(12):6359. https://doi.org/10.3390/ijms22126359
Chicago/Turabian StyleAbatematteo, Francesca Serena, Mauro Niso, Marialessandra Contino, Marcello Leopoldo, and Carmen Abate. 2021. "Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives" International Journal of Molecular Sciences 22, no. 12: 6359. https://doi.org/10.3390/ijms22126359
APA StyleAbatematteo, F. S., Niso, M., Contino, M., Leopoldo, M., & Abate, C. (2021). Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives. International Journal of Molecular Sciences, 22(12), 6359. https://doi.org/10.3390/ijms22126359








