Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging
Abstract
1. Introduction
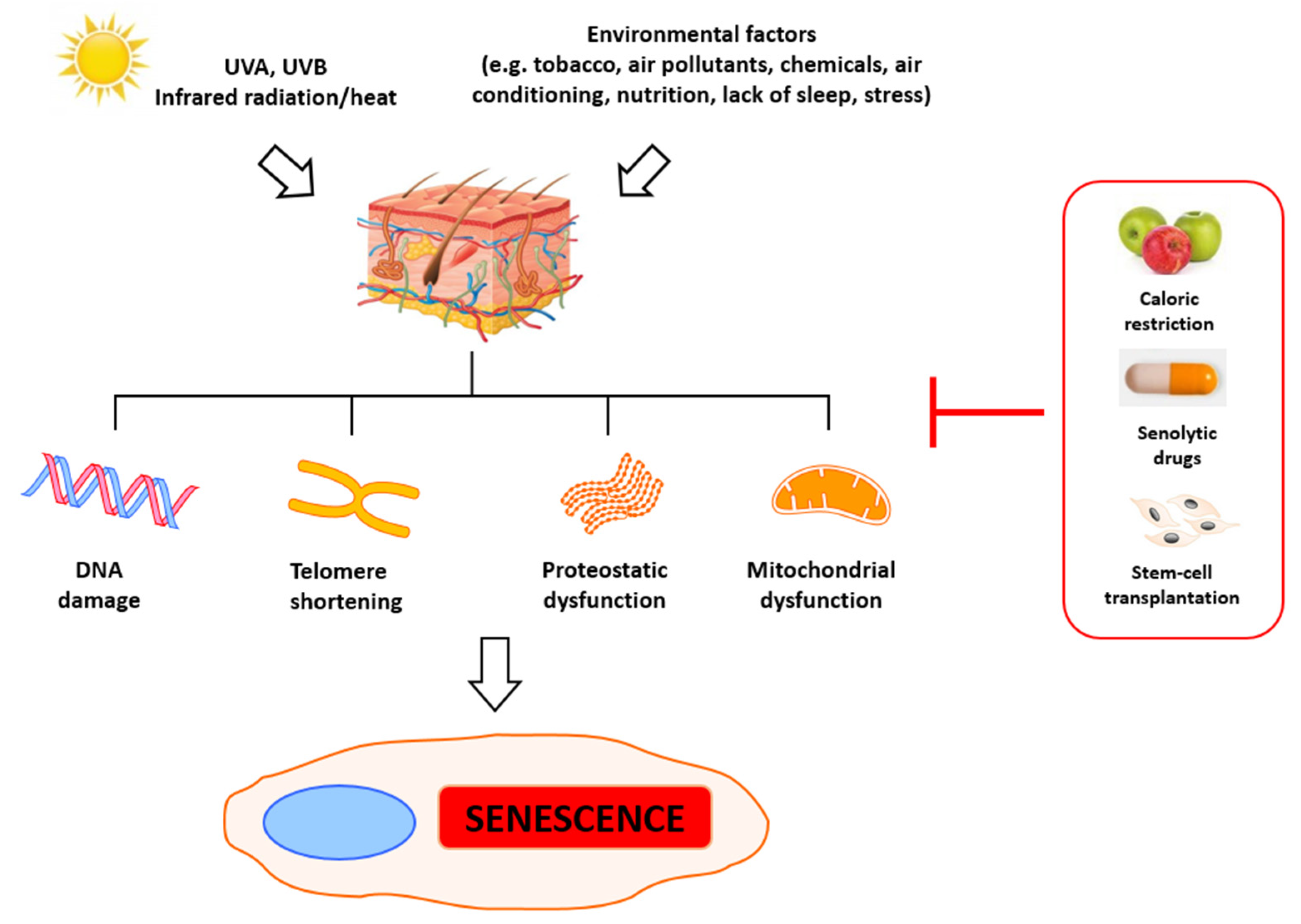
2. Molecular Basis of Senescence and Aging
3. Skin Structure and Telomerase
4. Aging in Skin
5. Roles of Telomeres in Skin Aging
5.1. Oxidative Stress
5.2. Inflammaging
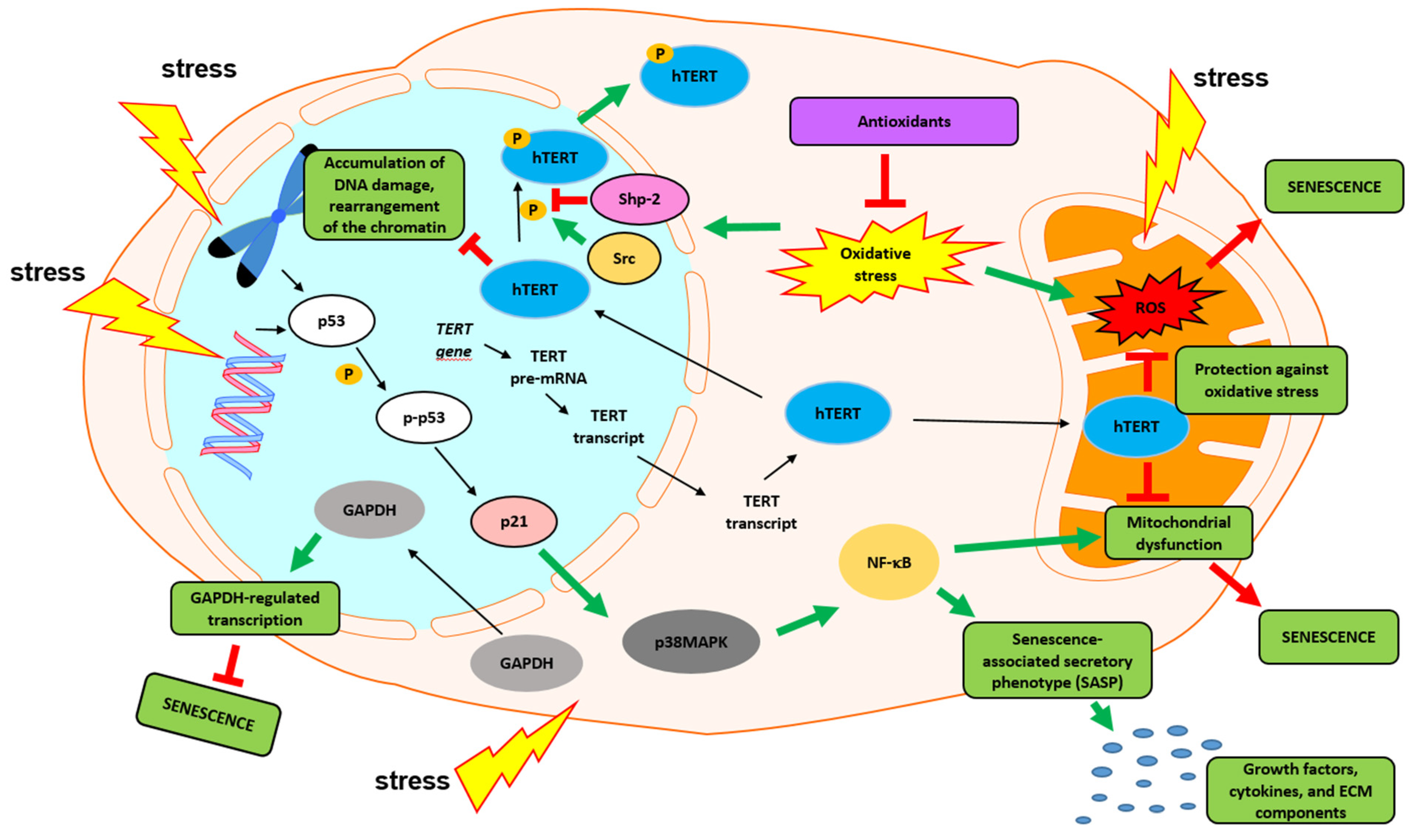
6. Nontelomeric Functions of Telomerase
7. Telomerase Restoration and Potential Threats
8. Telomerase- and Telomere-Based Anti-Aging Strategies in Skin
8.1. Telomeres and Telomerase Modulation—Food
8.2. Polyphenols
8.3. Fatty Acids
8.4. Polysaccharides
8.5. Keeping the Balance
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGE | advanced glycation end products |
| ATM | ATM serine/threonine kinase |
| ATP | adenosine triphosphate |
| BRG1 | transcription activator BRG1 |
| DCs | dendritic cells |
| DSCs | dermal sheath cells |
| ECM | extracellular matrix |
| ETA | eicosatrienoic acid |
| FFO | fermented fish oil |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HFSC | hair follicles stem cells |
| hTERT | human telomerase reverse transcriptase |
| IL 1/6 | interleukin 1/6 |
| KSCs | keratinocytes stem cells |
| LHCs | Langerhans cells |
| MAPKs | mitogen-activated protein kinases |
| MHC | main histocompatibility complex |
| MMPs | matrix metalloproteinases |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor κB2 |
| PUFA | polyunsaturated fatty acid |
| p21 | cyclin-dependent kinase inhibitor 1 |
| p53 | tumor protein |
| SASP | senescence-associated secretory phenotype |
| Shp-2 | Src homology region 2 domain-containing phosphatase-2 |
| SOD | superoxide dismutase |
| Src | proto-oncogene tyrosine-protein kinase |
| TERC | Telomerase RNA component |
| TERF1 | elomeric repeat binding factor 1 |
| mTERT | mice TERT |
| TNF-α | tumor necrosis factor α |
| TRF | telomere restriction fragment |
| RNP | ribonucleoprotein |
| ROS | reactive oxygen species |
| RTKs | receptor tyrosine kinases |
| UVA | ultraviolet radiation A |
| UVB | ultraviolet radiation B |
| UVR | ultraviolet radiation |
References
- Quan, C.; Cho, M.K.; Perry, D.; Quan, T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J. Biomed. Sci. 2015, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Attia, E.A.S.; Seada, L.S.; El-Sayed, M.H.; El-Shiemy, S.M. Study of telomerase reverse transcriptase (hTERT) expression in normal, aged, and photo-aged skin. Int. J. Dermatol. 2010, 49, 886–893. [Google Scholar] [CrossRef]
- Liu, J.-P.; Wang, L.; Wang, Z. Roles of Telomere Biology in Cell Senescence, Replicative and Chronological Ageing. Cells 2019, 8, 54. [Google Scholar] [CrossRef]
- Haendeler, J.; Dröse, S.; Büchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial Telomerase Reverse Transcriptase Binds to and Protects Mitochondrial DNA and Function From Damage. Arter. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; De Cabo, R. Measuring biological aging in humans: A quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Campisi, J.; Daddadifagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Lo’pez-Otı’, C. The Hallmarks of Aging. Cell 2013, 153, 2013. [Google Scholar]
- Hodes, R. Disease drivers of aging. Ann. N. Y. Acad. Sci. 2016, 1386, 45–68. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Damiani, E.; Brugè, F.; Cirilli, I.; Marcheggiani, F.; Olivieri, F.; Armeni, T.; Cianfruglia, L.; Giuliani, A.; Orlando, P.; Tiano, L. Modulation of Oxidative Status by Normoxia and Hypoxia on Cultures of Human Dermal Fibroblasts: How Does It Affect Cell Aging? Hindawi Oxidative Med. Cell. Longev. 2018, 2018, 5469159. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.D.P.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Rufini, A.; Tucci, P.; Celardo, I.; Melino, G. Senescence and aging: The critical roles of p53. Oncogene. 2013, 32, 5129–5143. [Google Scholar] [CrossRef]
- Loughery, J.; Cox, M.; Smith, L.M.; Meek, D.W. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic. Acids Res. 2014, 42, 7666–7680. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Ohtani, N.; Yamakoshi, K.; Takahashi, A.; Hara, E. The p16INK4a-RB pathway: Molecular link between cellular senescence and tumor suppression. J. Med. Investig. 2004, 51, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Tsai, S.Y.; Leone, G. Emerging roles of E2Fs in cancer: An exit from cell cycle control. Nat. Rev. Cancer 2009, 9, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Rossiello, F.; Mondello, C.; d’Adda di Fagagna, F. Stable Cellular Senescence Is Associated with Persistent DDR Activation. PLoS ONE 2014, 9, e110969. [Google Scholar] [CrossRef] [PubMed]
- di Fagagna, F.D. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Sarin, K.; Cheung, P.; Gilison, D.; Lee, E.; Tennen, R.I.; Wang, E.; Artandi, M.K.; Oro, A.E.; Artandi, S.E. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nat. Cell Biol. 2005, 436, 1048–1052. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Rajeswari, R.; Venugopal, J.; Sundarrajan, S.; Sridhar, R.; Shayanti, M.; Ramakrishna, S. Polysaccharide nanofibrous sca olds as a model for in vitro skin tissue regeneration. J. Mater. Sci. Mater. Electron. 2012, 23, 1511–1519. [Google Scholar]
- Kang, S.; Amagai, M.; Anna, L.; Bruckner, A.H.E.; Margolis, D.J.; McMichael, A.J.; Orringer, J.S. (Eds.) Fitzpatrick’s Dermatology; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Richmond, J.; Harris, E. Immunology and Skin in Health and Disease. Cold Spring Harb. Perspect. Med. 2014, 4, 15339. [Google Scholar] [CrossRef]
- Kierszenbaum, L.A.; Tres, L. Histology and Cell Biology: An Introduction to Pathology E-Book, 4th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2016. [Google Scholar]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The Role of microRNAs in Organismal and Skin Aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef]
- Elder, D.E.; Elenitsas, R.; Rosenbach, M.; Murphy, G.F.; Rubin, A.I.; Xu, X. Lever’s Histopathology of the Skin, 11th ed.; Elder, D.E., Rosenbach, M., Murphy, G.F., Adam, I., Rubin, X.X., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2015. [Google Scholar]
- Cavinato, M.; Koziel, R.; Romani, N.; Weinmüllner, R.; Jenewein, B.; Hermann, M.; Dubrac, S.; Ratzinger, G.; Grillari, J.; Schmuth, M.; et al. UVB-Induced Senescence of Human Dermal Fibroblasts Involves Impairment of Proteasome and Enhanced Autophagic Activity. J. Gerontol. Ser. Boil. Sci. Med. Sci. 2016, 72, 150–639. [Google Scholar] [CrossRef][Green Version]
- Horsburgh, S.; Fullard, N.; Roger, M.; Degnan, A.; Todryk, S.; Przyborski, S.; O’Reilly, S. MicroRNAs in the skin: Role in development, homoeostasis and regeneration. Clin. Sci. 2017, 131, 1923–1940. [Google Scholar] [CrossRef]
- Boukamp, P. Skin aging: A role for telomerase and telomere dynamics? Curr. Mol. Med. 2005, 5, 171–177. [Google Scholar] [CrossRef]
- Taylor, R.S.; Ramirez, R.D.; Ogoshi, M.; Chaffins, M.; Piatyszek, M.A.; Shay, J.W. Detection of telomerase activity in malignant and nonmalignant skin conditions. J. Investig. Dermatol. 1996, 106, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, D.; Kirin, I.; Rabic, D.; Dojcinovic, B.; Coklo, M.; Zamolo, G. The role of telomerase activity in psoriatic skin lesions. Med. Hypotheses 2007, 68, 1093–1095. [Google Scholar] [CrossRef]
- Krunic, D.; Moshir, S.; Greulich-Bode, K.M.; Figueroa, R.; Cerezo, A.; Stammer, H.; Stark, H.J.; Gray, S.G.; Nielsen, K.V. Tissue context-activated telomerase in human epidermis correlates with little age-dependent telomere loss. Biochim. Biophys. Acta 2009, 1792, 297–308. [Google Scholar] [CrossRef]
- da Costa, C.E.T.; Egeler, R.M.; Hoogeboom, M.; Szuhai, K.; Forsyth, R.G.; Niesters, M.; de Krijger, R.R.; Tazi, A.; Hogendoorn, P.C.W.; Annels, N.E. Differences in telomerase expression by the CD1a+ cells in Langerhans cell histiocytosis reflect the diverse clinical presentation of the disease. J. Pathol. 2007, 212, 188–197. [Google Scholar] [CrossRef]
- Blasco, M.A.; Lee, H.W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere Shortening and Tumor Formation by Mouse Cells Lacking Telomerase RNA. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef]
- Kumar, A.; Mohanty, S.; Airan, B. Hair & skin derived progenitor cells: In search of a candidate cell for regenerative medicine. Indian J. Med. Res. 2016, 143, 175–183. [Google Scholar] [PubMed]
- Engelhardt, M.; Kumar, R.; Albanell, J.; Pettengell, R.; Han, W.; Moore, M.A. Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood 1997, 90, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.D.; Wright, W.E.; Shay, J.W.; Taylor, R.S. Telomerase activity concentrates in the mitotically active segments of human hair follicles. J. Investig. Dermatol. 1997, 108, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. Degenerative Changes in Aging Skin. In Textbook of Aging Skin; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 15–30. [Google Scholar]
- Li, L.; Fukunaga-Kalabis, M.; Herlyn, M. Isolation and cultivation of dermal stem cells that differentiate into functional epidermal melanocytes. Methods Mol. Biol. 2012, 806, 15–29. [Google Scholar] [PubMed]
- Li, L.; Fukunaga-Kalabis, M.; Yu, H.; Xu, X.; Kong, J.; Lee, J.T.; Herlyn, M. Human dermal stem cells differentiate into functional epidermal melanocytes. J. Cell Sci. 2010, 123, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Toma, J.G.; McKenzie, I.A.; Bagli, D.; Miller, F.D. Isolation and Characterization of Multipotent Skin-Derived Precursors from Human Skin. Stem Cells 2005, 23, 727–737. [Google Scholar] [CrossRef]
- Fernandes, K.J.L.; McKenzie, I.A.; Mill, P.; Smith, K.M.; Akhavan, M.; Barnabé-Heider, F.; Biernaskie, J.; Junek, A.; Kobayashi, N.R.; Toma, J.G.; et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004, 6, 1082–1093. [Google Scholar] [CrossRef]
- Zabierowski, S.E.; Fukunaga-Kalabis, M.; Li, L.; Herlyn, M. Dermis-derived stem cells: A source of epidermal melanocytes and melanoma? Pigment. Cell Melanoma Res. 2011, 24, 422–429. [Google Scholar] [CrossRef]
- Toma, J.G.; Akhavan, M.; Fernandes, K.J.L.; Barnabé-Heider, F.; Sadikot, A.; Kaplan, D.R.; Miller, F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001, 3, 778–784. [Google Scholar] [CrossRef]
- Biernaskie, J.; Paris, M.; Morozova, O.; Fagan, B.M.; Marra, M.; Pevny, L.; Miller, F.D. SKPs Derive from Hair Follicle Precursors and Exhibit Properties of Adult Dermal Stem Cells. Cell Stem Cell 2009, 5, 610–623. [Google Scholar] [CrossRef]
- Morrison, S.J.; Prowse, K.R.; Ho, P.; Weissman, I.L. Telomerase Activity in Hematopoietic Cells Is Associated with Self-Renewal Potential. Immunity 1996, 5, 207–216. [Google Scholar] [CrossRef]
- Yui, J.; Chiu, C.P.; Lansdorp, P.M. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood 1998, 91, 3255–3262. [Google Scholar] [CrossRef]
- Forsyth, N.R.; Wright, W.E.; Shay, J.W. Telomerase and differentiation in multicellular organisms: Turn it off, turn it on, and turn it off again. Differ. 2002, 69, 188–197. [Google Scholar] [CrossRef]
- Lee, H.-W.; Blasco, M.A.; Gottlieb, G.J.; Ii, J.W.H.; Greider, C.W.; DePinho, R.A. Essential role of mouse telomerase in highly proliferative organs. Nat. Cell Biol. 1998, 392, 569–574. [Google Scholar] [CrossRef]
- Rudolph, K.L.; Chang, S.; Lee, H.-W.; Blasco, M.; Gottlieb, G.J.; Greider, C.; DePinho, R.A. Longevity, Stress Response, and Cancer in Aging Telomerase-Deficient Mice. Cell 1999, 96, 701–712. [Google Scholar] [CrossRef]
- Lämmermann, I.; Terlecki-Zaniewicz, L.; Weinmüllner, R.; Schosserer, M.; Dellago, H.; Branco, A.D.D.M.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. NPI Aging Mech. Dis. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Mancini, M.; Lena, A.M.; Saintigny, G.; Mahé, C.; Di Daniele, N.; Melino, G.; Candi, E. MicroRNAs in human skin ageing. Ageing Res. Rev. 2014, 17, 9–15. [Google Scholar] [CrossRef]
- Dudonné, S.; Coutière, P.; Woillez, M.; Mérillon, J.-M.; Vitrac, X. DNA macroarray study of skin aging-related genes expression modulation by antioxidant plant extracts on a replicative senescence model of human dermal fibroblasts. Phytotherapy Res. 2010, 25, 686–693. [Google Scholar] [CrossRef]
- Hönigsmann, H.; Schuler, G.; Aberer, W.; Romani, N.; Wolff, K. Immediate Pigment Darkening Phenomenon. A Reevaluation of Its Mechanisms. J. Investig. Dermatol. 1986, 87, 648–652. [Google Scholar] [CrossRef]
- Kang, S.M.; Han, S.; Oh, J.-H.; Lee, Y.M.; Park, C.-H.; Shin, C.-Y.; Lee, D.H.; Chung, J.H. A synthetic peptide blocking TRPV1 activation inhibits UV-induced skin responses. J. Dermatol. Sci. 2017, 88, 126–133. [Google Scholar] [CrossRef][Green Version]
- Aubert, G.; Lansdorp, P.M. Telomeres and Aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef]
- Pani, B.; Nudler, E. Mechanistic Insights into Transcription Coupled DNA Repair. Author manuscript; available in PMC 2018 August 01. DNA Repair 2017, 56, 42–50. [Google Scholar] [CrossRef]
- van Deursen, J.M. The role of senescent cells in ageing. Nat. Cell Biol. 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Cannan, W.J.; Pederson, D.S. Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J. Cell. Physiol. 2016, 231, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Fisher, G.J.; Shao, Y.; He, T.; Qin, Z.; Perry, D.; Voorhees, J.J.; Quan, T. Reduction of fibroblast size/mechanical force down-regulates tgf-beta type ii receptor: Implications for human skin aging. Aging Cell 2016, 15, 67–76. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen Fragmentation Promotes Oxidative Stress and Elevates Matrix Metalloproteinase-1 in Fibroblasts in Aged Human Skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Fiedler, J.; Grönniger, E.; Pfanne, A.; Brönneke, S.; Schmidt, K.; Falk, C.S.; Wenck, H.; Terstegen, L.; Thum, T.; Winnefeld, M. Identification of miR-126 as a new regulator of skin ageing. Exp. Dermatol. 2017, 26, 284–286. [Google Scholar] [CrossRef]
- Henle, E.S.; Han, Z.; Tang, N.; Rai, P.; Luo, Y.; Linn, S. Sequence-specific DNA Cleavage by Fe2+-mediated Fenton Reactions Has Possible Biological Implications. J. Biol. Chem. 1999, 274, 962–971. [Google Scholar] [CrossRef]
- Petersen, S.; Saretzki, G.; von Zglinicki, T. Preferential Accumulation of Single-Stranded Regions in Telomeres of Human Fibroblasts. Exp. Cell Res. 1998, 239, 152–160. [Google Scholar] [CrossRef]
- Yin, B.; Jiang, X. Telomere shortening in cultured human dermal fibroblasts is associated with acute photodamage induced by UVA irradiation. Adv. Dermatol. Allergol. 2013, 1, 13–18. [Google Scholar] [CrossRef]
- Fouquerel, E.; Barnes, R.P.; Uttam, S.; Watkins, S.C.; Bruchez, M.P.; Opresko, P.L. Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis. Mol. Cell 2019, 75, 117–130. [Google Scholar] [CrossRef]
- Kruk, P.A.; Rampino, N.J.; Bohr, V.A. DNA damage and repair in telomeres: Relation to aging. Proc. Natl. Acad. Sci. USA 1995, 92, 258–262. [Google Scholar] [CrossRef]
- Bombarde, O.; Boby, C.; Gomez, D.; Frit, P.; Giraud-Panis, M.-J.; Gilson, E.; Salles, B.; Calsou, P. TRF2/RAP1 and DNA–PK mediate a double protection against joining at telomeric ends. EMBO J. 2010, 29, 1573–1584. [Google Scholar] [CrossRef]
- Hewitt, G.M.; Jurk, D.; Marques, F.D.; Correia-Melo, C.; Hardy, T.L.D.; Gackowska, A.; Anderson, R.; Taschuk, M.T.; Mann, J.; Passos, J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef]
- Toutfaire, M.; Bauwens, E.; Debacq-Chainiaux, F. The impact of cellular senescence in skin ageing: A notion of mosaic and therapeutic strategies. Biochem. Pharmacol. 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Shoubing, Z.; Enkui, D. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar]
- Rubio, M.A.; Davalos, A.R.; Campisi, J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp. Cell Res. 2004, 298, 17–27. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- He, T.; Quan, T.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative exposure impairs TGF-β pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. AGE 2014, 36, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Fougère, B.; Boulanger, E.; Nourhashémi, F.; Guyonnet, S.; Cesari, M. RETRACTED: Chronic Inflammation: Accelerator of Biological Aging. J. Gerontol. Ser. Boil. Sci. Med. Sci. 2016, 72, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Kang, K.; Liu, L.; Yoshida, Y.; McCormick, T.S.; Cooper, K.D. iC3b Arrests Monocytic Cell Differentiation Into CD1c-Expressing Dendritic Cell Precursors: A Mechanism for Transiently Decreased Dendritic Cells in vivo After Human Skin Injury by Ultraviolet B. J. Investig. Dermatol. 2003, 120, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kang, K.; Berger, M.; Chen, G.; Gilliam, A.C.; Moser, A.; Wu, L.; Hammerberg, C.; Cooper, K.D. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J. Immunol. 1998, 161, 5873–5879. [Google Scholar]
- Bochkov, V.N.; Oskolkova, O.V.; Birukov, K.G.; Levonen, A.-L.; Binder, C.J.; Stöckl, J. Generation and Biological Activities of Oxidized Phospholipids. Antioxid. Redox Signal. 2010, 12, 1009–1059. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Molecular Mechanisms of Skin Aging: State of the Art. Ann. N. Y. Acad. Sci. 2007, 1119, 40–50. [Google Scholar] [CrossRef]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.; Hsueh, W.-C.; Satterfield, S.; et al. Cumulative Inflammatory Load Is Associated with Short Leukocyte Telomere Length in the Health, Aging and Body Composition Study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef]
- Aviv, A.; Valdes, A.; Gardner, J.P.; Swaminathan, R.; Kimura, M.; Spector, T.D. Menopause Modifies the Association of Leukocyte Telomere Length with Insulin Resistance and Inflammation. J. Clin. Endocrinol. Metab. 2006, 91, 635–640. [Google Scholar] [CrossRef]
- Rentoukas, E.; Tsarouhas, K.; Kaplanis, I.; Korou, E.; Nikolaou, M.; Marathonitis, G.; Kokkinou, S.; Haliassos, A.; Mamalaki, A.; Kouretas, D.; et al. Connection between Telomerase Activity in PBMC and Markers of Inflammation and Endothelial Dysfunction in Patients with Metabolic Syndrome. PLoS ONE 2012, 7, e35739. [Google Scholar] [CrossRef]
- Tedone, E.; Huang, E.; O’Hara, R.; Batten, K.; Ludlow, A.T.; Lai, T.-P.; Arosio, B.; Mari, D.; Wright, W.E.; Shay, J.W. Telomere length and telomerase activity in T cells are biomarkers of high-performing centenarians. Aging Cell 2018, 18, e12859. [Google Scholar] [CrossRef]
- La, S.H.; Kim, S.J.; Kang, H.G.; Lee, H.W.; Chun, K.H. Ablation of human telomerase reverse transcriptase (hTERT) induces cellular senescence in gastric cancer through a galectin-3 dependent mechanism. Oncotarget 2016, 7, 57117–57130. [Google Scholar] [CrossRef]
- De Vitis, M.; Berardinelli, F.; Sgura, A. Telomere Length Maintenance in Cancer: At the Crossroad between Telomerase and Alternative Lengthening of Telomeres (ALT). Int. J. Mol. Sci. 2018, 19, 606. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef]
- Martínez, P.; Blasco, M.A. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer 2011, 11, 161–176. [Google Scholar] [CrossRef]
- Chiodi, I.; Mondello, C. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front. Oncol. 2012, 2, 133. [Google Scholar] [CrossRef]
- Li, Y.; Tergaonkar, V. Noncanonical Functions of Telomerase: Implications in Telomerase-Targeted Cancer Therapies. Cancer Res. 2014, 74, 1639–1644. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, D.; Wang, M.; Cong, Y.-S. Telomerase reverse transcriptase in the regulation of gene expression. BMB Rep. 2014, 47, 8–14. [Google Scholar] [CrossRef]
- Masutomi, K.; Yu, E.Y.; Khurts, S.; Ben-Porath, I.; Currier, J.L.; Metz, G.B.; Brooks, M.W.; Kaneko, S.; Murakami, S.; DeCaprio, J.A.; et al. Telomerase Maintains Telomere Structure in Normal Human Cells. Cell 2003, 114, 241–253. [Google Scholar] [CrossRef]
- Masutomi, K.; Possemato, R.; Wong, J.; Currier, J.L.; Tothova, Z.; Manola, J.B.; Ganesan, S.; Lansdorp, P.M.; Collins, K.; Hahn, W.C. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. USA 2005, 102, 8222–8227. [Google Scholar] [CrossRef]
- Armstrong, L.; Saretzki, G.; Peters, H.; Wappler, I.; Evans, J.; Hole, N.; Von Zglinicki, T.; Lako, M. Overexpression of Telomerase Confers Growth Advantage, Stress Resistance, and Enhanced Differentiation of ESCs Toward the Hematopoietic Lineage. Stem Cells 2005, 23, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Klotz, L.-O. Highlight: Oxidative Stress and Senescence. Biol. Chem. 2008, 389, 201. [Google Scholar] [CrossRef] [PubMed]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. hTERT Overexpression Alleviates Intracellular ROS Production, Improves Mitochondrial Function, and Inhibits ROS-Mediated Apoptosis in Cancer Cells. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef]
- Mattiussi, M.; Tilman, G.; Lenglez, S.; Decottignies, A. Human telomerase represses ROS-dependent cellular responses to Tumor Necrosis Factor-α without affecting NF-κB activation. Cell. Signal. 2012, 24, 708–717. [Google Scholar] [CrossRef]
- Spilsbury, A.; Miwa, S.; Attems, J.; Saretzki, G. The Role of Telomerase Protein TERT in Alzheimer’s Disease and in Tau-Related Pathology In Vitro. J. Neurosci. 2015, 35, 1659–1674. [Google Scholar] [CrossRef]
- Santos, J.H.; Meyer, J.N.; Van Houten, B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum. Mol. Genet. 2006, 15, 1757–1768. [Google Scholar] [CrossRef]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Müller, F.L.; Guo, M.; Cooper, M.; Kotton, D.N.; Fabian, A.J.; Walkley, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nat. Cell Biol. 2011, 470, 359–365. [Google Scholar] [CrossRef]
- Haendeler, J.; Hoffmann, J.; Diehl, J.F.; Vasa, M.; Spyridopoulos, I.; Zeiher, A.M.; Dimmeler, S. Antioxidants Inhibit Nuclear Export of Telomerase Reverse Transcriptase and Delay Replicative Senescence of Endothelial Cells. Circ. Res. 2004, 94, 768–775. [Google Scholar] [CrossRef]
- Ale-Agha, N.; Dyballa-Rukes, N.; Jakob, S.; Altschmied, J.; Haendeler, J. Cellular functions of the dual-targeted catalytic subunit of telomerase, telomerase reverse transcriptase—Potential role in senescence and aging. Exp. Gerontol. 2014, 56, 189–193. [Google Scholar] [CrossRef]
- Santos, J.H.; Meyer, J.N.; Skorvaga, M.; Annab, L.A.; Van Houten, B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 2004, 3, 399–411. [Google Scholar] [CrossRef]
- Ghosh, A.; Saginc, G.; Leow, S.C.; Khattar, E.; Shin, E.M.; Yan, T.D.; Wong, M.; Zhang, Z.; Li, G.; Sung, W.-K.; et al. Telomerase directly regulates NF-κB-dependent transcription. Nat. Cell Biol. 2012, 14, 1270–1281. [Google Scholar] [CrossRef]
- Ding, D.; Zhou, J.; Wang, M.; Cong, Y.-S. Implications of telomere-independent activities of telomerase reverse transcriptase in human cancer. FEBS J. 2013, 280, 3205–3211. [Google Scholar] [CrossRef]
- Ségal-Bendirdjian, E.; Geli, V. Non-canonical Roles of Telomerase: Unraveling the Imbroglio. Front. Cell Dev. Biol. 2019, 7, 332. [Google Scholar] [CrossRef]
- Flores, I.; Cayuela, M.L.; Blasco, M.A. Eects of telomerase and telomere length on epidermal stem cell behavior. Science 2005, 309, 1253–1256. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef]
- Hatada, I.; Fukasawa, M.; Kimura, M.; Morita, S.; Yamada, K.; Yoshikawa, T.; Yamanaka, S.; Endo, C.; Sakurada, A.; Sato, M.; et al. Genome-wide profiling of promoter methylation in human. Oncogene 2006, 25, 3059–3064. [Google Scholar] [CrossRef]
- Park, J.-I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nat. Cell Biol. 2009, 460, 66–72. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, D. Telomerase Reverse Transcriptase (TERT) in Action: Cross-Talking with Epigenetics. Int. J. Mol. Sci. 2019, 20, 3338. [Google Scholar] [CrossRef]
- Shin, H.-S.; Park, S.-Y.; Hwang, E.-S.; Lee, D.-G.; Song, H.-G.; Mavlonov, G.T.; Yi, T.-H. The inductive effect of ginsenoside F2 on hair growth by altering the WNT signal pathway in telogen mouse skin. Eur. J. Pharmacol. 2014, 730, 82–89. [Google Scholar] [CrossRef]
- Saretzki, G. Extra-telomeric Functions of Human Telomerase: Cancer, Mitochondria and Oxidative Stress. Curr. Pharm. Des. 2014, 20, 6386–6403. [Google Scholar] [CrossRef] [PubMed]
- Avilion, A.A.; Piatyszek, M.A.; Gupta, J.; Shay, J.W.; Bacchetti, S.; Greider, C.W. Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res. 1996, 56, 645–650. [Google Scholar] [PubMed]
- Yi, X.; Tesmer, V.M.; Savre-Train, I.; Shay, J.W.; Wright, W.E. Both Transcriptional and Posttranscriptional Mechanisms Regulate Human Telomerase Template RNA Levels. Mol. Cell. Biol. 1999, 19, 3989–3997. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.S.; Wright, W.E.; Shay, J.W. Human Telomerase and Its Regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Valls-Bautista, C.; Bougel, S.; Piñol-Felis, C.; Viñas-Salas, J.; Benhattar, J. hTERT methylation is necessary but not sufficient for telomerase activity in colorectal cells. Oncol. Lett. 2011, 2, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Takakura, M.; Kyo, S.; Sowa, Y.; Wang, Z.; Yatabe, N.; Maida, Y.; Tanaka, M.; Inoue, M. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001, 29, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Kaul, R.; Murakami, M.; Robertson, E.S. Tumor viruses and cancer biology. Cancer Biol. Ther. 2010, 10, 961–978. [Google Scholar] [CrossRef]
- Nomikos, N.N.; Nikolaidis, P.T.; Sousa, C.V.; Papalois, A.E.; Rosemann, T.; Knechtle, B. Exercise, Telomeres, and Cancer: “The Exercise-Telomere Hypothesis”. Front. Physiol. 2018, 9, 1798. [Google Scholar] [CrossRef]
- Schagen, S.; Zampeli, V.; Makrantonaki, E.; Zouboulis, C. Discovering the link between nutrition and skin aging. Derm. Endocrinol. 2012, 4, 298–307. [Google Scholar] [CrossRef]
- Changwei, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar]
- Dearlove, R.P.; Greenspan, P.; Hartle, D.K.; Swanson, R.B.; Hargrove, J.L. Inhibition of Protein Glycation by Extracts of Culinary Herbs and Spices. J. Med. Food 2008, 11, 275–281. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Katta, R. Sugar Sag: Glycation and the Role of Diet in Aging Skin. Ski. Ther. Lett. 2015, 20, 1–5. [Google Scholar]
- Thirunavukkarasu, V.; Nandhini, A.T.A.; Anuradha, C.V. Fructose Diet-Induced Skin Collagen Abnormalities Are Prevented by Lipoic Acid. Exp. Diabesity Res. 2004, 5, 237–244. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2018, 19, 13. [Google Scholar] [CrossRef]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere shortening during aging: Attenuation by antioxidants and anti-inflammatory agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Theodosiou, M.; Laudet, V.; Schubert, M. From carrot to clinic: An overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci. 2010, 67, 1423–1445. [Google Scholar] [CrossRef]
- Sharma, H.W.; Sokoloski, J.A.; Perez, J.R.; Maltese, J.Y.; Sartorelli, A.C.; Stein, C.A.; Nichols, G.; Khaled, Z.; Telang, N.T.; Narayanan, R. Differentiation of immortal cells inhibits telomerase activity. Proc. Natl. Acad. Sci. USA 1995, 92, 12343–12346. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-O.; Lee, G.; Min, B.-M. Retinoic Acid Extends the in Vitro Life Span of Normal Human Oral Keratinocytes by Decreasing p16INK4A Expression and Maintaining Telomerase Activity. Biochem. Biophys. Res. Commun. 2000, 268, 268–274. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef]
- Zarei, M.; Zarezadeh, M.; Kalajahi, F.H.; Javanbakht, M.H. The Relationship Between Vitamin D and Telomere/Telomerase: A comprehensive review. J. Frailty Aging 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.N.; El Touny, L.H.; Jagadeesh, S.; Banerjee, P.P. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinog. 2007, 28, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.P.; Miller, S.L. A kinetic estimate of the free aldehyde content of aldoses. Carbohydr. Res. 2000, 329, 359–365. [Google Scholar] [CrossRef]
- Tarwadi, K.V.; Agte, V.V. Effect of Micronutrients on Methylglyoxal-Mediated In Vitro Glycation of Albumin. Biol. Trace Element Res. 2010, 143, 717–725. [Google Scholar] [CrossRef]
- Davinelli, S.; Bertoglio, J.C.; Polimeni, A.; Scapagnini, G. Cytoprotective Polyphenols against Chronological Skin Aging and Cutaneous Photodamage. Curr. Pharm. Des. 2018, 24, 99–105. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Lin, Y.-K.; Lin, C.-F.; Wang, P.-W.; Chen, E.-L.; Fang, J.-Y. Elucidating the Skin Delivery of Aglycone and Glycoside Flavonoids: How the Structures Affect Cutaneous Absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef]
- Udroiu, I.; Marinaccio, J.; Sgura, A. Epigallocatechin-3-gallate induces telomere shortening and clastogenic damage in glioblastoma cells. Environ. Mol. Mutagen. 2019, 60, 683–692. [Google Scholar] [CrossRef]
- Kwon, K.-R.; Alam, B.; Park, J.-H.; Kim, T.-H.; Lee, S.-H. Attenuation of UVB-Induced Photo-Aging by Polyphenolic-Rich Spatholobus Suberectus Stem Extract via Modulation of MAPK/AP-1/MMPs Signaling in Human Keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, R.; Yang, B.; Yao, X.; Wang, P.; Liu, D.; Wang, Y. Changes of telomere and telomerase in effect of ginsenoside Rg1 to delay hematopoietic stem cell senescence. China J. Chin. Mater. Medica 2011, 36, 3172–3175. [Google Scholar]
- Zhao, C.H.; Chen, X.C.; Zhu, Y.G.; Huang, C.; Shi, G.B.; Zeng, Y.Q.; Li, Y.; Peng, X.; Jin, J.; Peng, X. Roles of telomere and telomerase in the process of ginseno-side Rg1 protection against tert-butylhydroperoxide-induced senescence in W I-38 cells. Chin. Pharm. Bull. 2005, 1, 61–66. [Google Scholar]
- Gadecka, A.; Bielak-Zmijewska, A. Slowing Down Ageing: The Role of Nutrients and Microbiota in Modulation of the Epigenome. Nutrients 2019, 11, 1251. [Google Scholar] [CrossRef]
- François, M.; Leifert, W.; Tellam, R.; Fenech, M. G-quadruplexes: A possible epigenetic target for nutrition. Mutat. Res. Rev. 2015, 764, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kubo, C.; Ogawa, M.; Uehara, N.; Katakura, Y. Fisetin Promotes Hair Growth by Augmenting TERT Expression. Front. Cell Dev. Biol. 2020, 8, 566617. [Google Scholar] [CrossRef]
- Horrobin, D.F. Essential fatty acids in clinical dermatology. J. Am. Acad. Dermatol. 1989, 20, 1045–1053. [Google Scholar] [CrossRef]
- Meksiarun, P.; Maeda, Y.; Hiroi, T.; Andriana, B.B.; Sato, H. Analysis of the effects of dietary fat on body and skin lipids of hamsters by Raman spectroscopy. Anal. 2015, 140, 4238–4244. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Zhen, A.X.; Fernando, P.D.S.M.; Kang, H.K.; Ahn, Y.S.; Hyun, J.W. Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging. Mar. Drugs 2019, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Nain, P.; Chauhan, A.; Sharma, N.; Gupta, A.; Ranjan, R.; Varshney, A. Super Critical Fluid Extracted Fatty Acids from Withania somnifera Seeds Repair Psoriasis-Like Skin Lesions and Attenuate Pro-Inflammatory Cytokines (TNF-α and IL-6) Release. Biomolecules 2020, 10, 185. [Google Scholar] [CrossRef]
- Ding, D.; Xi, P.; Zhou, J.; Wang, M.; Cong, Y. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-κB-dependent transcription. FASEB J. 2013, 27, 4375–4383. [Google Scholar] [CrossRef]
- Vidacek, N.Š.; Nanic, L.; Ravlic, S.; Sopta, M.; Geric, M.; Gajski, G.; Garaj-Vrhovac, V.; Rubelj, I. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J. Gerontol. Biol. Sci. 2018, 73, 1. [Google Scholar] [CrossRef]
- Millner, A.; Atilla-Gokcumen, G.E. Lipid Players of Cellular Senescence. Metabolites 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yuan, Z.; He, X. Research progress on pharmacological effects of astragalus polysaccharides. Chin. J. Biochem. Med. 2012, 5, 692–694. [Google Scholar]
- Li, H.; Ma, F.; Hu, M.; Ma, C.W.; Xiao, L.; Zhang, J.; Xiang, Y.; Huang, Z. Polysaccharides from medicinal herbs as potential therapeutics for aging and age-related neurodegeneration. Rejuvenation Res. 2014, 17, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Song, Y. Effect of polysaccharides of Cistanche deserticola on immune cells and telomerase activity in aging mice. Chin. Pharmaceut. J. 2011, 46, 1081–1083. [Google Scholar]
- Xia, G.; Han, X.; Qi, J.; Liu, W.; Song, J.; Qin, J.; Liu, L. The effects of astragalus polysaccharide on zebrafish cell apoptosis and senescence. Am. J. Mol. Biol. 2012, 2, 103–109. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Vijayalakshmi, M.; Sambasivarao, K.R. Regulation Studies of Telomerase Gene in Cancer Cells by Lentinan. Avicenna J. Med. Biotechnol. 2010, 2, 181–185. [Google Scholar]
- Zhang, X.L.; Liu, X.Z. Effects of catalpol on memory and antioxidative enzyme activity in D-galactose induced sub-acute senescent mice. Chin. J. Biochem. Pharm. 2011, 2, 103–106. [Google Scholar]
- Ma, L.J.; Chen, G.L.; Jia, H.Y.; Xie, J. Anti-senescence effect of Cynomorium songaricum polysaccharide on D-galactose-induced aging mice. Chin. J. Hosp. Pharm. 2009, 14, 1186–1189. [Google Scholar]
- Zhu, G.M.; Jiang, X.D.; Wang, D.D.; Ou, Q.; Zhang, L.; Sun, J. Effect of Astragalus membranaeus polysaccharide on chromosome terminal restriction fragment length of the aged HDF cell. Chin. J. Gerontol. 2012, 8, 1635–1637. [Google Scholar]
- Zhao, L.X.; Yu, L. Pine pollen delays cell senescence and its effects on telomerase activity. Sichuan J Tradit. Chin. Med. 2004, 4, 11–13. [Google Scholar]
- Cao, R.Z.; Wei, Y.C.; Zhang, G.W.; Sun, L.J. Effect of total flavonoids of herba euphorbiae humifusae on the expression of telomerase activity in aged mice. West China J. Pharm. Sci. 2011, 2, 189–190. [Google Scholar]
- Zhang, Y.J.; Wu, T.; Zhou, X.J.; Liu, L.M. Glucocorticoid-induced skin aging and the effects of salvia miltiorrhiza and salvianolic acid B in vivo. Chin. J. Hosp. Pharm. 2008, 20, 1767–1770. [Google Scholar]
- Guo, L.; Wei, X.D.; Ou, Q.; Wang, S.; Zhu, G.M. Effect of astragaloside on the expression of telomerase activity and klotho gene in aged HELF cells. Chin. J. Gerontol. 2010, 13, 1819–1822. [Google Scholar]
- Zhang, S.X.; Li, X.; Yin, J.L.; Chen, L.L.; Zhang, H.Q. Study on antiaging effect of C21 steroidal glycoside from the root of Cynanchum auriculatum planted in Jiangsu. Pract. Geriatr. 2007, 2, 104–107. [Google Scholar]
- Ke, G.A.; You, C.H.; Liu, B.L.; Zhang, H.; Wang, M.M. Study of mechanism of telomere and cell cycle in the process of allicin protection against senescence in fibroblast cells 1. Nrf2: Friend or foe for chemoprevention? Carcinogenesis 2006, 31, 90–99. [Google Scholar]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.O.; Alegakis, A.K.; Sarandi, E.; Thanasoula, M.; Spandidos, D.A.; Tsatsakis, A.; Razgonova, M.P.; Calina, D. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 2019, 20, 3701–3708. [Google Scholar] [CrossRef]
- Sondhi, N.; Bhardwaj, R.; Kaur, S.; Chandel, M.; Kumar, N.; Singh, B. Inhibition of H2O2-induced DNA damage in single cell gel electrophoresis assay (comet assay) by castasterone isolated from leaves of centella asiatica. Health 2010, 2, 595–602. [Google Scholar] [CrossRef][Green Version]
- Raguraman, V.; Subramaniam, J.R. Withania somnifera Root Extract Enhances Telomerase Activity in the Human HeLa Cell Line. Adv. Biosci. Biotechnol. 2016, 7, 199–204. [Google Scholar] [CrossRef]
- Kumar, N.; Yadav, A.; Gupta, R.; Aggarwal, N. Antigenotoxic Effect of Withania somnifera (Ashwagandha) Extract Against DNA Damage Induced by Hydrogen Peroxide in Cultured Human Peripheral Blood Lymphocytes. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 713–719. [Google Scholar] [CrossRef]
- Plant, J. Effects of essential oils on telomere length in human cells. Med. Aromat. Plants 2016, 5, 1–6. [Google Scholar]
- Eitsuka, T.; Nakagawa, K.; Miyazawa, T. Dual mechanisms for telomerase inhibition in DLD-1 human colorectal adenocarcinoma cells by polyunsaturated fatty acids. BioFactors 2004, 21, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, Y.; Chen, X.; Jiao, J.; Zhang, Y. Polyunsaturated fatty acids ameliorate aging via redox telomere-antioncogene axis. Oncotarget 2017, 8, 7301–7314. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int. J. Cancer 2009, 125, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Pearce, V.P.; Sherrell, J.; Lou, Z.; Kopelovich, L.; Wright, W.E.; Shay, J.W. Immortalization of epithelial progenitor cells mediated by resveratrol. Oncogene 2008, 27, 2365–2374. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.X.; Hu, X.S.; Guo, X.G.; Shang, Y.P.; Chen, H.J.; Zeng, C.L.; Zhang, F.R.; Chen, J.Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008, 155, 387–394. [Google Scholar] [CrossRef]
- Lanzilli, G.; Fuggetta, M.P.; Tricarico, M.; Cottarelli, A.; Serafino, A.; Falchetti, R.; Ravagnan, G.; Turriziani, M.; Adamo, R.; Franzese, O.; et al. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int. J. Oncol. 2006, 28, 641–648. [Google Scholar] [CrossRef]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Ysunaga, M.; Yamasaki, F.; Iwasaka, T. Antiproliferative effects of the major tea polyphenol, (-)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol. Oncol. 2008, 108, 326–331. [Google Scholar] [CrossRef]
- Thelen, P.; Wuttke, W.; Jarry, H.; Grzmil, M.; Ringert, R.H. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J. Urol. 2004, 171, 1934–1938. [Google Scholar] [CrossRef]
- Freitas-Simoes, T.-M.; Ros, E.; Sala-Vila, A. Nutrients, foods, dietary patterns and telomere length: Update of epidemiological studies and randomized trials. Metabolism 2016, 65, 406–415. [Google Scholar] [CrossRef]
- Trichopoulou, A. Traditional Mediterranean diet and longevity in the elderly: A review. Public Heal. Nutr. 2004, 7, 943–947. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, B.S.; Blackburn, E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav. Immun. 2013, 28, 16–24. [Google Scholar] [CrossRef]
- Marín, C.; Yubero-Serrano, E.M.; López-Miranda, J.; Jiménez, F.P. Endothelial Aging Associated with Oxidative Stress Can Be Modulated by a Healthy Mediterranean Diet. Int. J. Mol. Sci. 2013, 14, 8869. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; De Vivo, I. Mediterranean diet and telomere length in Nurses’ Health Study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef]
- Holian, O.; Walter, R.J. Resveratrol inhibits the proliferation of normal human keratinocytes in vitro. J. Cell. Biochem. Suppl. 2001, 81, 55–62. [Google Scholar] [CrossRef]

| Skin Cells | Relative hTERT/Telomerase Expression | Reference |
|---|---|---|
| Epidermis | ||
| Keratinocytes | + | [35] |
| Melanocytes | + | [37] |
| Langerhan cells | + | [38] |
| Dermis | ||
| Mast cells | low | [39] |
| Fibroblasts | - | [35] |
| Dermal stem cells | low | [40] |
| Hair follicle stem cells | + | [41] |
| Bulge component of the hair follicle | low | [42] |
| Hypodermis | ||
| Fat cells | low | [43] |
| Nuclear | Cytoplasmic | Mitochondrial |
|---|---|---|
| Maintenance of telomeres and genomic stability | Interaction with signaling pathways | Decrease of mitochondrial ROS and protection from stress |
| Interaction with signaling pathways | Redox balancing and cell adaptation to stress | Decrease of apoptosis |
| Regulation of chromatin structure, gene expression and modulation of DNA damage response | Telomerase complex maturation | Binding to mtDNA and protection against mtDNA damage |
| Active Ingredients/Source | Mechanism | Reference |
|---|---|---|
| Polyphenols tea curcumin red grapes | Inhibition of collagen degradation by blocking the development of inflammation | [131,154,155] |
| Flavonoids: catechins green tea | Inhibition of AGE formation | [147,148,149] |
| Herbs and spices ginger garlic cinnamon cloves oregano allspice | Inhibition of fructose—induces glycation | [136] |
| Active Ingredients/Source | Mechanism | Experimental Model | Reference |
|---|---|---|---|
| Polysaccharide Cistanche deserticola Cinamorium songarium Astragalus membranaeus | Increased telomerase activity by reducing free radicals Increased telomerase activity in testicles Telomerase activity and telomere-binding protein modification Reduction of shortening rate of telomere restriction fragment (TRF) | subacute aging model mice aging mouse model human embryonic lung diploid fibroblasts | [167] [171] [172] [173] |
| Pine pollen Pinus massoniana | Modulation of telomerase activity, increased cell population | human embryonic lung fibroblasts | [174] |
| Flavonoid Euphorbia humifusa Willd. | Regulation of telomerase activity via antioxidant effect (enhanced SOD activity) | aging mouse model | [175] |
| Acteoside Cistanche tubulosa | Increased telomerase activity, antioxidant function | aging mouse model | [176] |
| Astragaloside Astragalus membranaeus | Increased telomerase activity | human embryonic lung diploid fibroblasts | [177] |
| Steroidal glycoside Cynanchum bungei | Increased telomerase activity, antioxidant protection via the increase of SOD activity | aging mouse model | [178] |
| Ginsenoside Rg1 Panax ginseng | Decreased of telomere shortening via increased telomerase expression and restored telomerase activity | hemopoietic stem-cell ageing in mice | [154,155] |
| Allicin Allium sativum Linn. | Restored telomerase activity | fibroblast cells | [179] |
| Triterpenoid saponins Centella asiatica (L.) Urban | Nine-fold increase of telomerase activity, inhibition of the negative effects of H2O2 on DNA | peripheral blood mononuclear cells | [180,181] |
| Withanolide Withania somnifera (L.) Dunal | Increased telomerase activity Decreased effects of H2O2-induced damage on DNA | human HeLa cell | [182,183] |
| Basil oil Ocimum basilicum L. | Downregulation of the telomeric repeat binding factor 1 (TERF–1), which is a telomere length suppressor | K562 cells (chronic myelogenous leukemia) | [184] |
| Polyunsaturated fatty acids (PUFA) 11,14,17—eicosapentaenoic acid (ETA) | Suppression of telomerase activity and TERT miRNA-mediated antioxidant effect via promotion of SOD activity | mouse model | [185,186] |
| Flavonoid—Genistein Soiae semen | Bilateral effect on telomerase activity: Reduced hTERT transcription and reduced telomerase activity in higher concentrations (50 µM), and activation of telomerase in lower concentration (0.5 − 1.0 µM) | MCF-7 cell line (human breast cancer) | [146,187] |
| Resveratrol Red Grape | Bilateral effect on telomerase activity: activation of telomerase via the upregulation of SIRT 1 in epithelial and endothelial progenitor cells and telomerase activity inhibition in cancer cells | epithelial and endothelial progenitor cells cancer cells | [188,189,190,191] |
| Epigallocatechin gallate Green Tea | Reduction of hTERT expression | cervical adenocarcinoma | [192] |
| Silibinin Milk Thistle | Reduced TERT expression and telomerase activity | LNCaP cells (human prostate carcinoma) | [193] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacczak, B.; Rubiś, B.; Totoń, E. Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. Int. J. Mol. Sci. 2021, 22, 6381. https://doi.org/10.3390/ijms22126381
Jacczak B, Rubiś B, Totoń E. Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. International Journal of Molecular Sciences. 2021; 22(12):6381. https://doi.org/10.3390/ijms22126381
Chicago/Turabian StyleJacczak, Barbara, Błażej Rubiś, and Ewa Totoń. 2021. "Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging" International Journal of Molecular Sciences 22, no. 12: 6381. https://doi.org/10.3390/ijms22126381
APA StyleJacczak, B., Rubiś, B., & Totoń, E. (2021). Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. International Journal of Molecular Sciences, 22(12), 6381. https://doi.org/10.3390/ijms22126381







