A Novel Method Facilitating the Simple and Low-Cost Preparation of Human Osteochondral Slice Explants for Large-Scale Native Tissue Analysis
Abstract
:1. Introduction
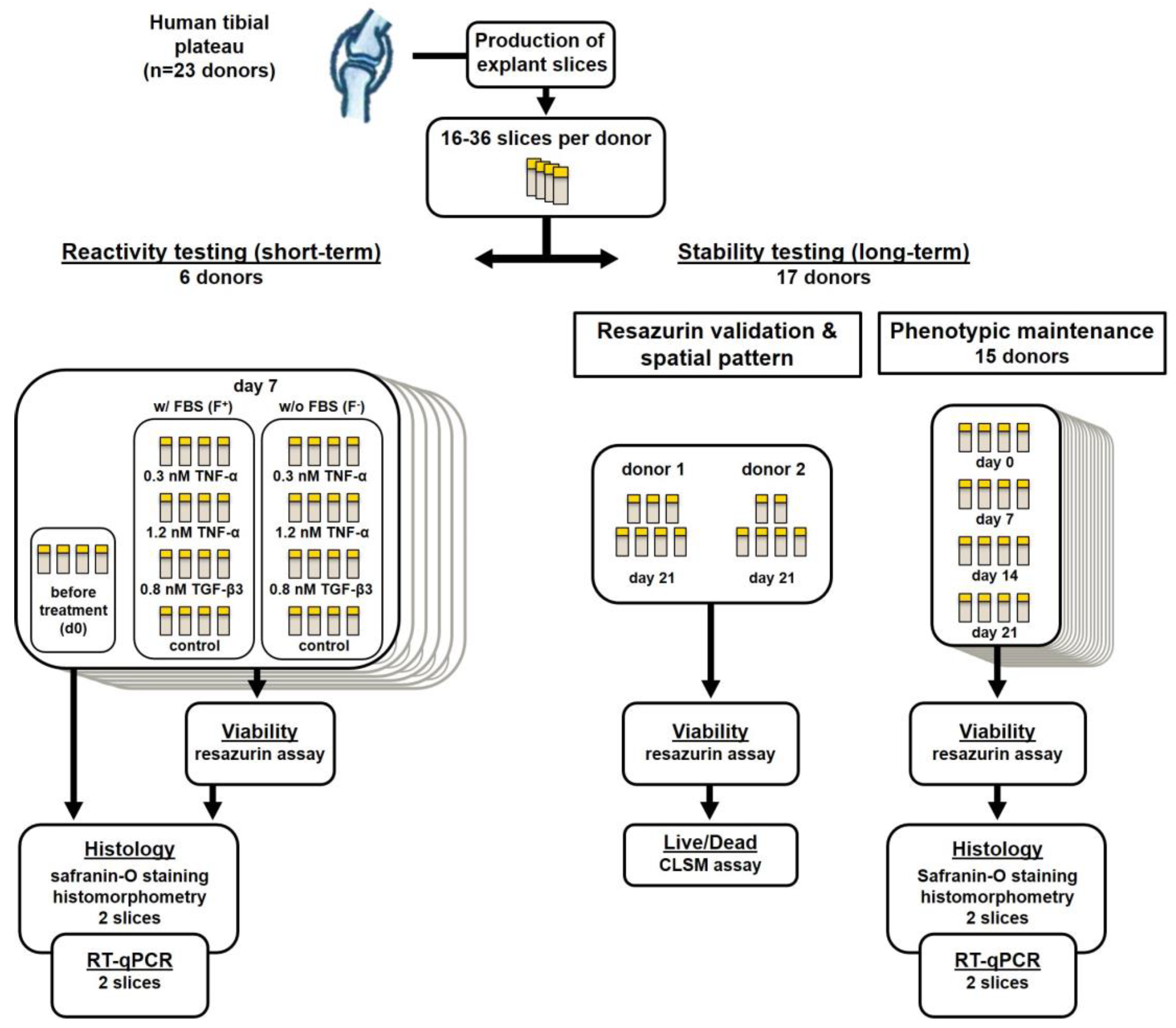
2. Results
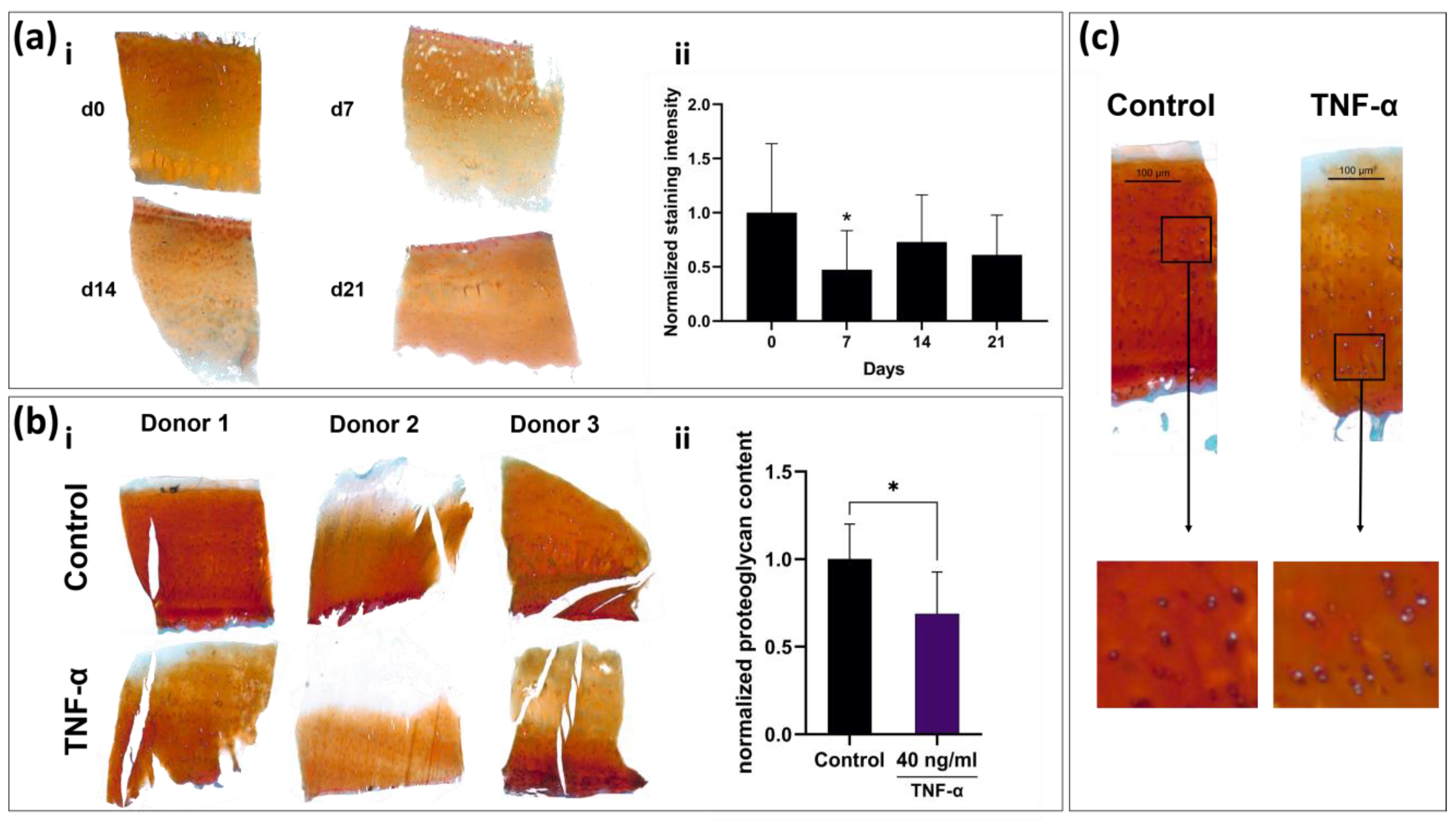
2.1. Suitability for Slicing Varies with the Degree of Subchondral Bone Sclerosis
2.2. Confocal Laser Scanning Microscopy Shows Highly Conserved Spatial Cell Order
2.3. Resazurin Assay Correlates with Optically Determined Viability
2.4. Cell Viability Is Maintained in F− and Increased in F+ Groups over 21 Days
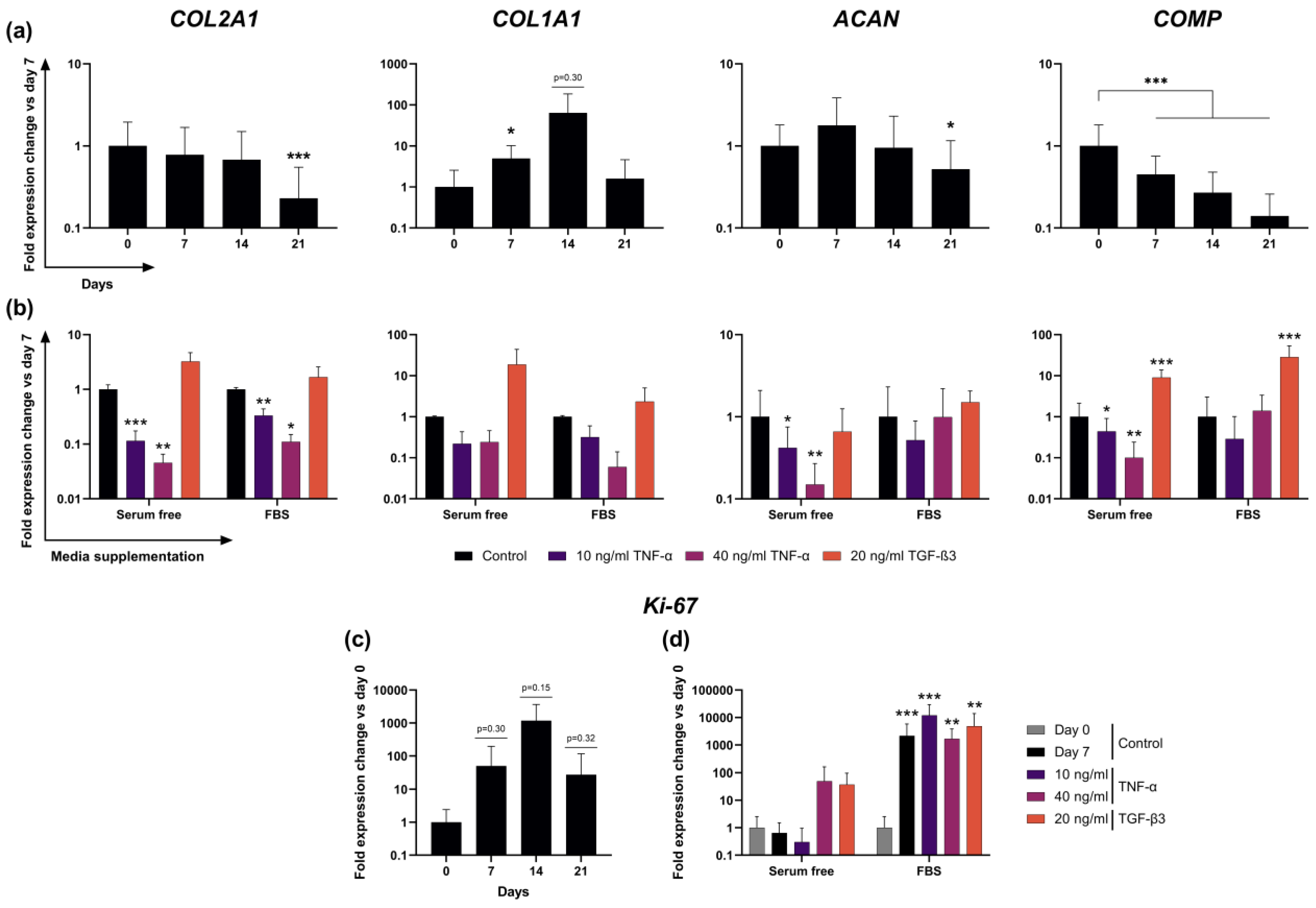
2.5. Expression of Cartilage-Typical Markers ACAN and COL2A1 Is Maintained for 14 Days in Unstimulated Slice Culture
2.6. Transcription of ECM Proteins Remains Highly Reactive to External Stimuli in F− Culture
2.7. Addition of FBS to Culture Media Causes Dominant Effects on Proliferation Marker Ki-67 Expression
2.8. Tissue Slices React to TNF-α Stimulation with Observable Remodeling of ECM and Cellular Swelling
3. Discussion
4. Materials and Methods
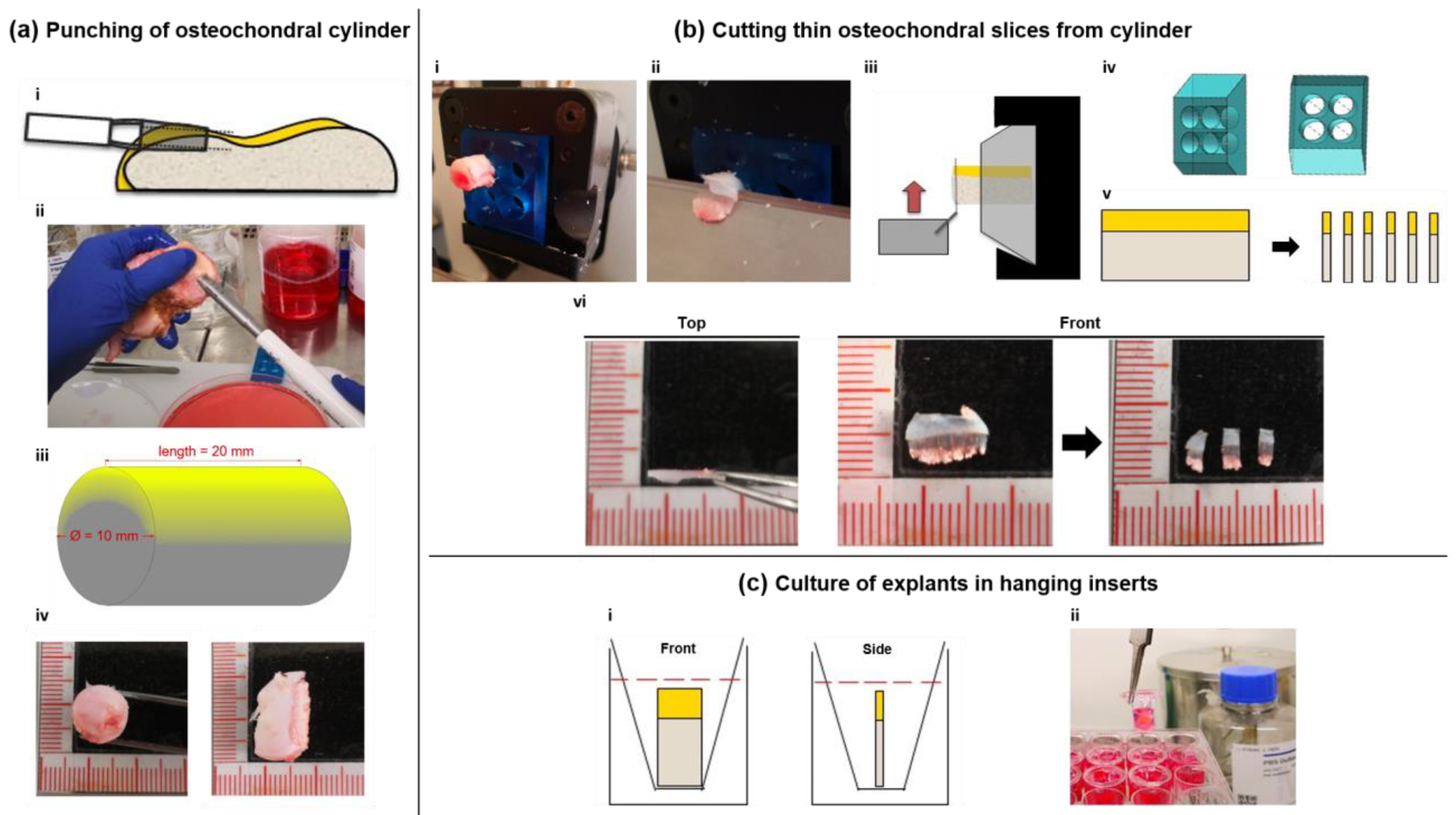
4.1. Sample Acquisition and Slice Production
4.2. Maintenance and Stimulation
4.3. Resazurin Viability Analysis
4.4. Confocal Laser Scanning Microscopy
4.5. RNA Extraction and Real-Time Quantitative PCR
4.6. Histological Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puig-Junoy, J.; Ruiz Zamora, A. Socio-economic costs of osteoarthritis: A systematic review of cost-of-illness studies. Semin. Arthritis Rheum. 2015, 44, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Knecht, S.; Erggelet, C.; Endres, M.; Sittinger, M.; Kaps, C.; Stüssi, E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83B, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.H.; Li, Y.S.; Gao, X.; Lei, G.H.; Huard, J. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthr. Cartil. 2018, 26, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foldager, C.B. Advances in autologous chondrocyte implantation and related techniques for cartilage repair. Dan. Med. J. 2013, 60, B4600. [Google Scholar] [PubMed]
- Jimenez, P.A.; Glasson, S.S.; Trubetskoy, O.V.; Haimes, H.B. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: Histologic, radiologic, and biochemical changes. Lab. Anim. Sci. 1997, 47, 598–601. [Google Scholar]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Schlichting, N.; Dehne, T.; Mans, K.; Endres, M.; Stuhlmüller, B.; Sittinger, M.; Kaps, C.; Ringe, J. Suitability of Porcine Chondrocyte Micromass Culture To Model Osteoarthritis In Vitro. Mol. Pharm. 2014, 11, 2092–2105. [Google Scholar] [CrossRef]
- Jorgensen, C.; Simon, M. In Vitro Human Joint Models Combining Advanced 3D Cell Culture and Cutting-Edge 3D Bioprinting Technologies. Cells 2021, 10, 596. [Google Scholar] [CrossRef]
- Rothbauer, M.; Schobesberger, S.; Byrne, R.; Kiener, H.P.; Tögel, S.; Ertl, P. A human joint-on-a-chip as alternative to animal models in osteoarthritis. Osteoarthr. Cartil. 2020, 28, S89. [Google Scholar] [CrossRef]
- Pretzel, D.; Pohlers, D.; Weinert, S.; Kinne, R.W. In vitro model for the analysis of synovial fibroblast-mediated degradation of intact cartilage. Arthritis Res. Ther. 2009, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Haltmayer, E.; Ribitsch, I.; Gabner, S.; Rosser, J.; Gueltekin, S.; Peham, J.; Giese, U.; Dolezal, M.; Egerbacher, M.; Jenner, F. Co-culture of osteochondral explants and synovial membrane as in vitro model for osteoarthritis. PLoS ONE 2019, 14, e0214709. [Google Scholar] [CrossRef]
- Vainieri, M.L.; Wahl, D.; Alini, M.; Van Osch, G.; Grad, S. Mechanically stimulated osteochondral organ culture for evaluation of biomaterials in cartilage repair studies. Acta Biomater. 2018, 81, 256–266. [Google Scholar] [CrossRef]
- Maroudas, A. Transport of solutes through cartilage: Permeability to large molecules. J. Anat. 1976, 122, 335–347. [Google Scholar]
- Schwarz, N.; Uysal, B.; Welzer, M.; Bahr, J.C.; Layer, N.; Löffler, H.; Stanaitis, K.; Pa, H.; Weber, Y.G.; Hedrich, U.; et al. Long-term adult human brain slice cultures as a model system to study human CNS circuitry and disease. eLife 2019, 8, e48417. [Google Scholar] [CrossRef] [PubMed]
- Zwerina, J.; Redlich, K.; Polzer, K.; Joosten, L.; Krönke, G.; Distler, J.; Hess, A.; Pundt, N.; Pap, T.; Hoffmann, O.; et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl. Acad. Sci. USA 2007, 104, 11742–11747. [Google Scholar] [CrossRef] [Green Version]
- Finnson, K.W.; Chi, Y.; Bou-Gharios, G.; Leask, A.; Philip, A. TGF-b signaling in cartilage homeostasis and osteoarthritis. Front. Biosci. 2012, 4, 251–268. [Google Scholar] [CrossRef]
- Secretan, C.; Bagnall, K.M.; Jomha, N.M. Effects of introducing cultured human chondrocytes into a human articular cartilage explant model. Cell Tissue Res. 2010, 339, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gavénis, K.; Andereya, S.; Schmidt-Rohlfing, B.; Mueller-Rath, R.; Silny, J.; Schneider, U. Millicurrent stimulation of human articular chondrocytes cultivated in a collagen type-I gel and of human osteochondral explants. BMC Complement. Altern Med. 2010, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyman, J.R.; Chappell, J.D.; Morales, T.I.; Kelley, S.S.; Lee, G.M. Response of Chondrocytes to Local Mechanical Injury in an Ex Vivo Model. Cartilage 2012, 3, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Lima, E.; Angione, S.; Ng, K.; Williams, D.; Xu, D.; Stoker, A.; Cook, J.; Ateshian, G.; Hung, C. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J. Biomech. 2008, 41, 1153–1159. [Google Scholar] [CrossRef] [Green Version]
- Ragan, P.M.; Badger, A.M.; Cook, M.; Chin, V.I.; Gowen, M.; Grodzinsky, A.J.; Lark, M.W. Down-regulation of Chondrocyte Aggrecan and Type-II Collagen Gene Expression Correlates with Increases in Static Compression Magnitude and Duration. J. Bone Jt. Surg.-Am. Vol. 2000, 82, 32. [Google Scholar] [CrossRef]
- Zaucke, F.; Dinser, R.; Maurer, P.; Paulsson, M. Cartilage oligomeric matrix protein (COMP) and collagen IX are sensitive markers for the differentiation state of articular primary chondrocytes. Biochem. J. 2001, 358, 17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jagga, S.; Lee, S.-S.; Nam, J.-S. Interplay between Cartilage and Subchondral Bone Contributing to Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2013, 14, 19805–19830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manicourt, D.-H.; Poilvache, P.; Van Egeren, A.; Devogelaer, J.-P.; Lenz, M.-E.; Thonar, E.J.-M.A. Synovial fluid levels of tumor necrosis factor α and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum. 2000, 43, 281. [Google Scholar] [CrossRef]
- Hui, W.; Rowan, A.D.; Richards, C.D.; Cawston, T.E. Oncostatin M in combination with tumor necrosis factor α induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 2003, 48, 3404–3418. [Google Scholar] [CrossRef] [PubMed]
- Thudium, C.S.; Engstrom, A.; Groen, S.S.; Karsdal, M.A.; Bay-Jensen, A.-C. An Ex Vivo Tissue Culture Model of Cartilage Remodeling in Bovine Knee Explants. J. Vis. Exp. 2019, e59467. [Google Scholar] [CrossRef]
- Clutterbuck, A.L.; Mobasheri, A.; Shakibaei, M.; Allaway, D.; Harris, P. Interleukin-1β-Induced Extracellular Matrix Degradation and Glycosaminoglycan Release Is Inhibited by Curcumin in an Explant Model of Cartilage Inflammation. Ann. N. Y. Acad. Sci. 2009, 1171, 428–435. [Google Scholar] [CrossRef]
- Theodoropoulos, J.S.; De Croos, A.J.N.; Petrera, M.; Park, S.; Kandel, R.A. Mechanical stimulation enhances integration in an in vitro model of cartilage repair. Knee Surgery Sport. Traumatol. Arthrosc. 2016, 24, 2055–2064. [Google Scholar] [CrossRef]
- Spitters, T.W.; Leijten, J.C.; Deus, F.D.; Costa, I.B.; Van Apeldoorn, A.A.; Van Blitterswijk, C.A.; Karperien, M. A Dual Flow Bioreactor with Controlled Mechanical Stimulation for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2013, 19, 774–783. [Google Scholar] [CrossRef]
- Qu, P.; Qi, J.; Han, Y.; Zhou, L.; Xie, D.; Song, H.; Geng, C.; Zhang, K.; Wang, G. Effects of Rolling-Sliding Mechanical Stimulation on Cartilage Preserved In Vitro. Cell Mol. Bioeng. 2019, 12, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Elder, B.D.; Athanasiou, K.A. Synergistic and Additive Effects of Hydrostatic Pressure and Growth Factors on Tissue Formation. PLoS ONE 2008, 3, e2341. [Google Scholar] [CrossRef] [PubMed]
- Lüderitz, L.; Dehne, T.; Sittinger, M.; Ringe, J. Dose-Dependent Effect of Mesenchymal Stromal Cell Recruiting Chemokine CCL25 on Porcine Tissue-Engineered Healthy and Osteoarthritic Cartilage. Int. J. Mol. Sci. 2018, 20, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinnen, J.; Shopperly, L.K.; Rendenbach, C.; Kühl, A.A.; Sentürk, U.; Kendoff, D.; Hemmati-Sadeghi, S.; Sittinger, M.; Dehne, T. A Novel Method Facilitating the Simple and Low-Cost Preparation of Human Osteochondral Slice Explants for Large-Scale Native Tissue Analysis. Int. J. Mol. Sci. 2021, 22, 6394. https://doi.org/10.3390/ijms22126394
Spinnen J, Shopperly LK, Rendenbach C, Kühl AA, Sentürk U, Kendoff D, Hemmati-Sadeghi S, Sittinger M, Dehne T. A Novel Method Facilitating the Simple and Low-Cost Preparation of Human Osteochondral Slice Explants for Large-Scale Native Tissue Analysis. International Journal of Molecular Sciences. 2021; 22(12):6394. https://doi.org/10.3390/ijms22126394
Chicago/Turabian StyleSpinnen, Jacob, Lennard K. Shopperly, Carsten Rendenbach, Anja A. Kühl, Ufuk Sentürk, Daniel Kendoff, Shabnam Hemmati-Sadeghi, Michael Sittinger, and Tilo Dehne. 2021. "A Novel Method Facilitating the Simple and Low-Cost Preparation of Human Osteochondral Slice Explants for Large-Scale Native Tissue Analysis" International Journal of Molecular Sciences 22, no. 12: 6394. https://doi.org/10.3390/ijms22126394
APA StyleSpinnen, J., Shopperly, L. K., Rendenbach, C., Kühl, A. A., Sentürk, U., Kendoff, D., Hemmati-Sadeghi, S., Sittinger, M., & Dehne, T. (2021). A Novel Method Facilitating the Simple and Low-Cost Preparation of Human Osteochondral Slice Explants for Large-Scale Native Tissue Analysis. International Journal of Molecular Sciences, 22(12), 6394. https://doi.org/10.3390/ijms22126394





