Interplay between Coumarin Accumulation, Iron Deficiency and Plant Resistance to Dickeya spp.
Abstract
:1. Introduction
2. Results
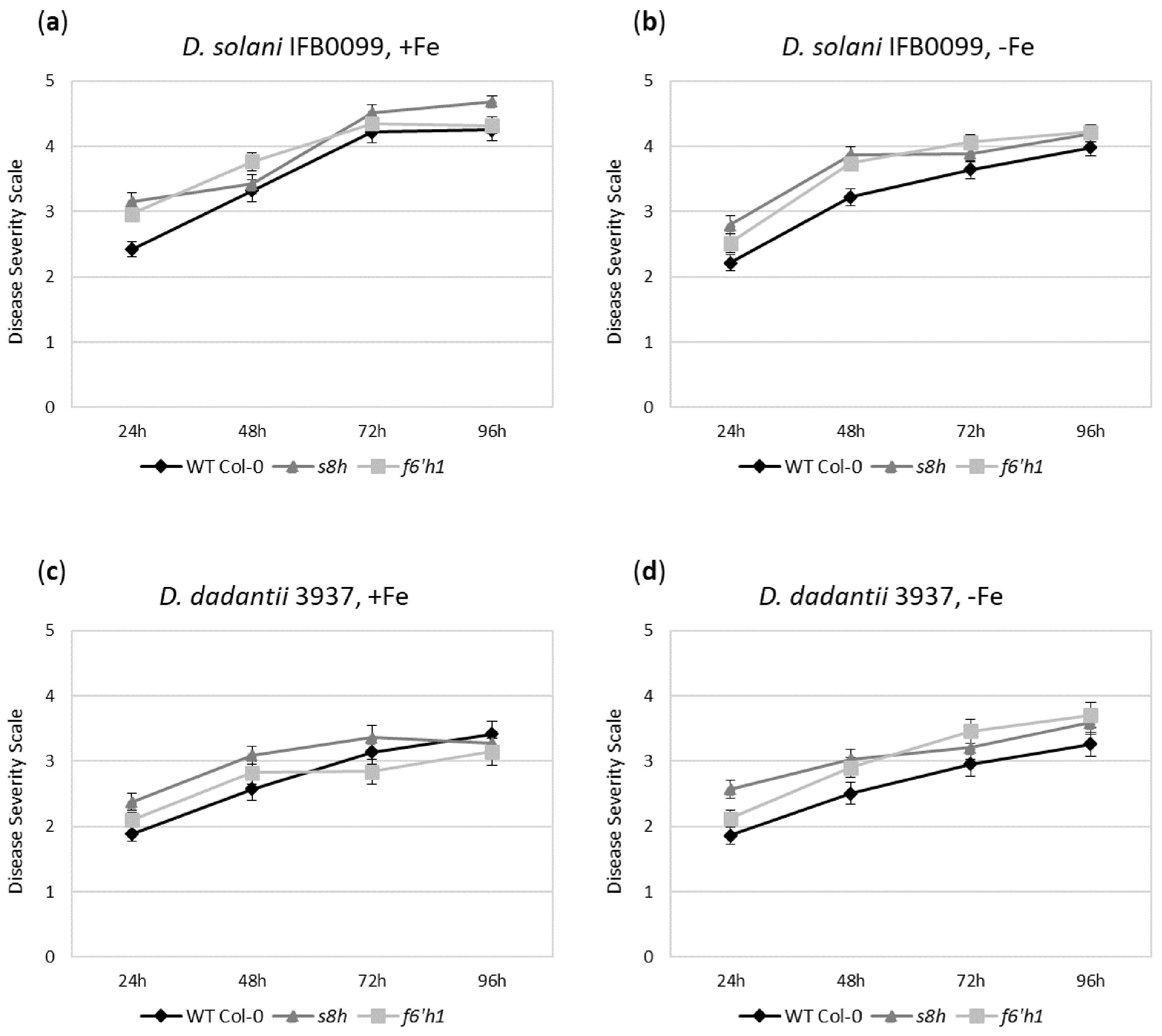
2.1. Differential Susceptibility of Arabidopsis Plants Grown in Fe-Deficient Hydroponics to Tested Dickeya spp. Strains
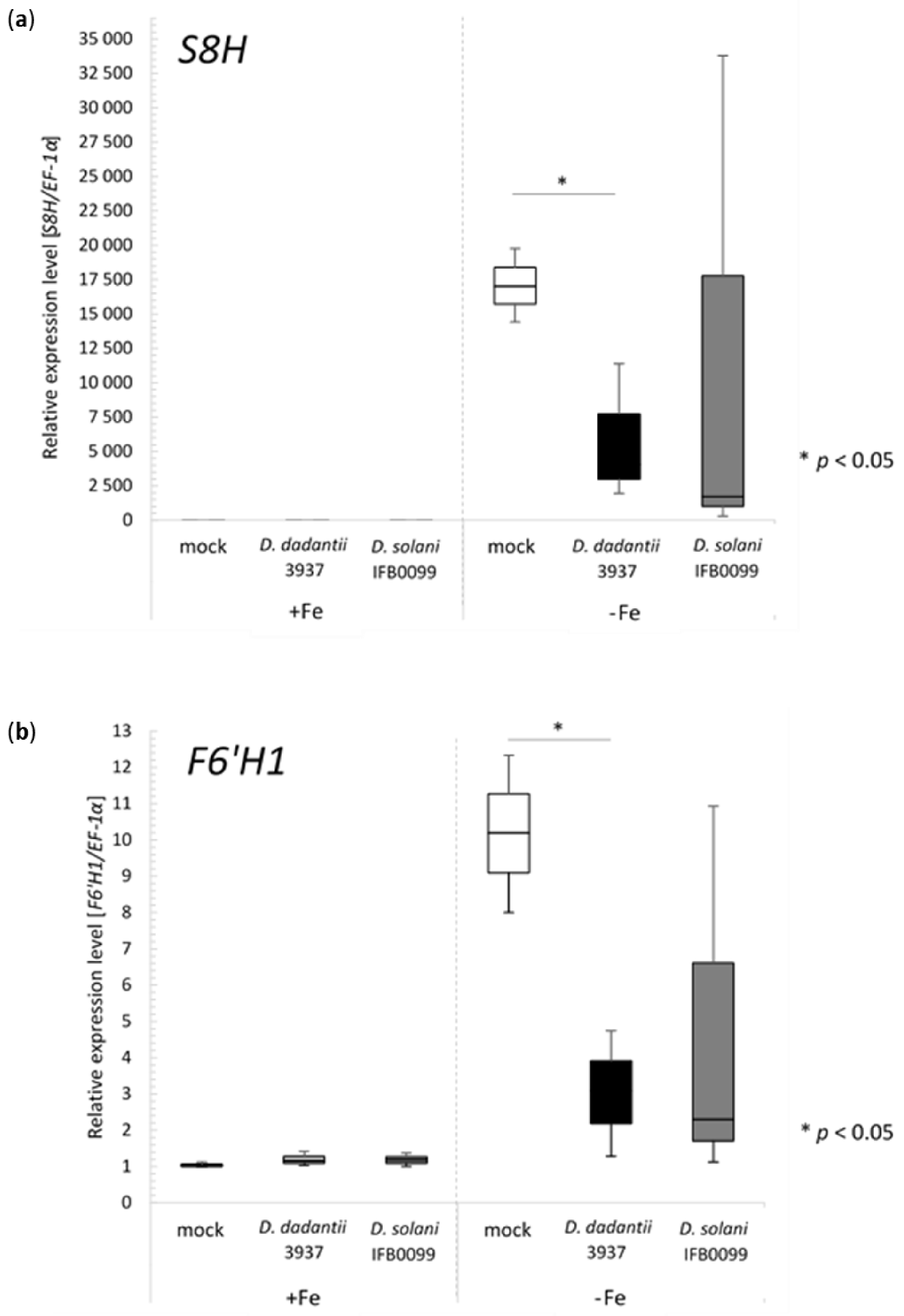
2.2. Inoculation of Arabidopsis WT Col-0 Grown in Fe-Deficient Hydroponics with Dickeya spp. Cause Decrease in the Expression of S8H and F6′H1 Genes Involved in Coumarin Biosynthesis
2.3. Fe-Chelation in CAS Agar Plate Assay
2.4. Differential Susceptibility of Soil-Grown Arabidopsis Plants to Tested Dickeya spp. Strains
2.5. Expression of Selected Plant Stress-Response Genes Is Strongly Induced in Arabidopsis Mutants Defective in Coumarin Accumulation Inoculated with D. solani IFB0099
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Bacterial Strains, Media, Growth Conditions
4.3. Hydroponic Cultures
4.4. Soil Cultures
4.5. Siderophore Production in the Presence of Plant Extracts
4.6. Plant Inoculation with Bacterial Strains
4.7. Quantification of Arabidopsis Plants Susceptibility to Dickeya spp. Strains by Visual Symptom Scoring (Disease Severity Scale, DSS)
4.8. RNA Extraction and Expression Analysis by qRT-PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemens, S.; Weber, M. The Essential Role of Coumarin Secretion for Fe Acquisition from Alkaline Soil. Plant Signal. Behav. 2016, 11, e1114197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourcroy, P.; Sisó-Terraza, P.; Sudre, D.; Savirón, M.; Reyt, G.; Gaymard, F.; Abadía, A.; Abadia, J.; Alvarez-Fernández, A.; Briat, J.-F. Involvement of the ABCG37 Transporter in Secretion of Scopoletin and Derivatives by Arabidopsis Roots in Response to Iron Deficiency. New Phytol. 2014, 201, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Rajniak, J.; Giehl, R.F.H.; Chang, E.; Murgia, I.; von Wirén, N.; Sattely, E.S. Biosynthesis of Redox-Active Metabolites in Response to Iron Deficiency in Plants. Nat. Chem. Biol. 2018, 14, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Celma, J.; Lin, W.-D.; Fu, G.-M.; Abadía, J.; López-Millán, A.-F.; Schmidt, W. Mutually Exclusive Alterations in Secondary Metabolism Are Critical for the Uptake of Insoluble Iron Compounds by Arabidopsis and Medicago Truncatula. Plant Physiol. 2013, 162, 1473–1485. [Google Scholar] [CrossRef]
- Schmid, N.B.; Giehl, R.F.H.; Döll, S.; Mock, H.-P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wirén, N. Feruloyl-CoA 6′-Hydroxylase1-Dependent Coumarins Mediate Iron Acquisition from Alkaline Substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Sisó-Terraza, P.; Luis-Villarroya, A.; Fourcroy, P.; Briat, J.-F.; Abadía, A.; Gaymard, F.; Abadía, J.; Álvarez-Fernández, A. Accumulation and Secretion of Coumarinolignans and Other Coumarins in Arabidopsis Thaliana Roots in Response to Iron Deficiency at High PH. Front. Plant Sci. 2016, 7, 1711. [Google Scholar] [CrossRef] [Green Version]
- Siwinska, J.; Siatkowska, K.; Olry, A.; Grosjean, J.; Hehn, A.; Bourgaud, F.; Meharg, A.A.; Carey, M.; Lojkowska, E.; Ihnatowicz, A. Scopoletin 8-Hydroxylase: A Novel Enzyme Involved in Coumarin Biosynthesis and Iron-Deficiency Responses in Arabidopsis. J. Exp. Bot. 2018, 69, 1735–1748. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-H.; Rodríguez-Celma, J.; Lan, P.; Wu, Y.-C.; Vélez-Bermúdez, I.C.; Schmidt, W. Scopoletin 8-Hydroxylase-Mediated Fraxetin Production Is Crucial for Iron Mobilization. Plant Physiol. 2018, 177, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Vanholme, R.; Sundin, L.; Seetso, K.C.; Kim, H.; Liu, X.; Li, J.; De Meester, B.; Hoengenaert, L.; Goeminne, G.; Morreel, K.; et al. COSY Catalyses Trans-Cis Isomerization and Lactonization in the Biosynthesis of Coumarins. Nat. Plants 2019, 5, 1066–1075. [Google Scholar] [CrossRef]
- Kai, K.; Mizutani, M.; Kawamura, N.; Yamamoto, R.; Tamai, M.; Yamaguchi, H.; Sakata, K.; Shimizu, B. Scopoletin Is Biosynthesized via Ortho-Hydroxylation of Feruloyl CoA by a 2-Oxoglutarate-Dependent Dioxygenase in Arabidopsis Thaliana. Plant J. 2008, 55, 989–999. [Google Scholar] [CrossRef]
- Curie, C.; Briat, J.-F. Iron Transport and Signaling in Plants. Annu. Rev. Plant Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef]
- Verbon, E.H.; Trapet, P.L.; Stringlis, I.A.; Kruijs, S.; Bakker, P.A.H.M.; Pieterse, C.M.J. Iron and Immunity. Annu. Rev. Phytopathol. 2017, 55, 355–375. [Google Scholar] [CrossRef]
- Grillet, L.; Schmidt, W. Iron Acquisition Strategies in Land Plants: Not so Different after All. New Phytol. 2019, 224, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Siwinska, J.; Kadzinski, L.; Banasiuk, R.; Gwizdek-Wisniewska, A.; Olry, A.; Banecki, B.; Lojkowska, E.; Ihnatowicz, A. Identification of QTLs Affecting Scopolin and Scopoletin Biosynthesis in Arabidopsis Thaliana. BMC Plant Biol. 2014, 14, 280. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Ding, W.; Xu, Y.; Wu, D.; Li, S.; Chen, J.; Guo, B. New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia Solanacearum. Molecules 2016, 21, 468. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Zhang, B.; Ma, J.; Hettenhausen, C.; Cao, G.; Sun, G.; Wu, J.; Wu, J. Scopoletin Is a Phytoalexin against Alternaria Alternata in Wild Tobacco Dependent on Jasmonate Signalling. J. Exp. Bot. 2014, 65, 4305–4315. [Google Scholar] [CrossRef]
- El Oirdi, M.; Trapani, A.; Bouarab, K. The Nature of Tobacco Resistance against Botrytis Cinerea Depends on the Infection Structures of the Pathogen. Environ. Microbiol. 2010, 12, 239–253. [Google Scholar] [CrossRef]
- Shimizu, B.; Miyagawa, H.; Ueno, T.; Sakata, K.; Watanabe, K.; Ogawa, K. Morning Glory Systemically Accumulates Scopoletin and Scopolin after Interaction with Fusarium Oxysporum. Z. Naturforsch. C J. Biosci. 2005, 60, 83–90. [Google Scholar] [CrossRef]
- Prats, E.; Bazzalo, M.E.; León, A.; Jorrín, J.V. Fungitoxic Effect of Scopolin and Related Coumarins on Sclerotinia Sclerotiorum. A Way to Overcome Sunflower Head Rot. Euphytica 2006, 147, 451–460. [Google Scholar] [CrossRef]
- Ndam, Y.N.; Nyegue, M.A.; Mounjouenpou, P.; Kansci, G.; Kenfack, M.J.; Eugène, E.E. LC-MS Quantification of Scopoletin in Cassava(Manihot Esculenta Crantz) Varieties, Local Derived Foods, and Activity on Some Food Spoilage Fungi. J. Food Process. Preserv. 2020, 44, e14387. [Google Scholar] [CrossRef]
- Modafar, C.E.; Clérivet, A.; Vigouroux, A.; Macheix, J.J. Accumulation of Phytoalexins in Leaves of Plane Tree(Platanus Spp.) Expressing Susceptibility or Resistance ToCeratocystis Fimbriata f. Sp.Platani. Eur. J. Plant Pathol. 1995, 101, 503–509. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin—A Coumarin Phytoalexin with Medicinal Properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Napiroon, T.; Bacher, M.; Balslev, H.; Tawaitakham, K.; Santimaleeworagun, W.; Vajrodaya, S. Scopoletin from Lasianthus Lucidus Blume(Rubiaceae): A Potential Antimicrobial against Multidrug-Resistant Pseudomonas Aeruginosa. J. Appl. Pharm. Sci. 2018, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Acharya, D.; Bogati, B.; Risal, P. Scopoletin reduces intracellular survival of Salmonella typhi within U937 human macrophage cell line in vitro. Afr. J. Microbiol. Res. 2013, 1, 47–51. [Google Scholar]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial Activities of the Extracts, Fractions and Isolated Compounds from Canarium Patentinervium Miq. against Bacterial Clinical Isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-Dependent Coumarin Exudation Shapes Root Microbiome Assembly to Promote Plant Health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.-R.; Wu, X.-M.; Xu, J.-Y. Current Natural Products with Antihypertensive Activity. Chin. J. Nat. Med. 2015, 13, 721–729. [Google Scholar] [CrossRef]
- Voges, M.J.E.E.E.; Bai, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-Derived Coumarins Shape the Composition of an Arabidopsis Synthetic Root Microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 12558–12565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbort, C.J.; Hashimoto, M.; Inoue, H.; Niu, Y.; Guan, R.; Rombolà, A.D.; Kopriva, S.; Voges, M.J.E.E.E.; Sattely, E.S.; Garrido-Oter, R.; et al. Root-Secreted Coumarins and the Microbiota Interact to Improve Iron Nutrition in Arabidopsis. Cell Host Microbe 2020, 28, 825–837.e6. [Google Scholar] [CrossRef]
- Stassen, M.J.J.; Hsu, S.-H.; Pieterse, C.M.J.; Stringlis, I.A. Coumarin Communication along the Microbiome-Root-Shoot Axis. Trends Plant Sci. 2021, 26, 169–183. [Google Scholar] [CrossRef]
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The Age of Coumarins in Plant-Microbe Interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef] [Green Version]
- Expert, D. WITHHOLDING AND EXCHANGING IRON: Interactions between Erwinia Spp. and Their Plant Hosts. Annu. Rev. Phytopathol. 1999, 37, 307–334. [Google Scholar] [CrossRef]
- Motyka, A.; Zoledowska, S.; Sledz, W.; Lojkowska, E. Molecular Methods as Tools to Control Plant Diseases Caused by Dickeya and Pectobacterium Spp: A Minireview. New Biotechnol. 2017, 39 Pt B, 181–189. [Google Scholar] [CrossRef]
- Potrykus, M.; Golanowska, M.; Sledz, W.; Zoledowska, S.; Motyka, A.; Kolodziejska, A.; Butrymowicz, J.; Lojkowska, E. Biodiversity of Dickeya Spp. Isolated from Potato Plants and Water Sources in Temperate Climate. Plant Dis. 2016, 100, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Hélias, V.; Pirhonen, M.; Tsror (Lahkim), L.; Elphinstone, J.G. Dickeya Species: An Emerging Problem for Potato Production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Dellagi, A.; Rigault, M.; Segond, D.; Roux, C.; Kraepiel, Y.; Cellier, F.; Briat, J.-F.; Gaymard, F.; Expert, D. Siderophore-Mediated Upregulation of Arabidopsis Ferritin Expression in Response to Erwinia Chrysanthemi Infection. Plant J. 2005, 43, 262–272. [Google Scholar] [CrossRef]
- Kieu, N.P.; Aznar, A.; Segond, D.; Rigault, M.; Simond-Côte, E.; Kunz, C.; Soulie, M.-C.; Expert, D.; Dellagi, A. Iron Deficiency Affects Plant Defence Responses and Confers Resistance to Dickeya Dadantii and Botrytis Cinerea. Mol. Plant Pathol. 2012, 13, 816–827. [Google Scholar] [CrossRef]
- Segond, D.; Dellagi, A.; Lanquar, V.; Rigault, M.; Patrit, O.; Thomine, S.; Expert, D. NRAMP Genes Function in Arabidopsis Thaliana Resistance to Erwinia Chrysanthemi Infection. Plant J. 2009, 58, 195–207. [Google Scholar] [CrossRef]
- Golanowska, M.; Potrykus, M.; Motyka-Pomagruk, A.; Kabza, M.; Bacci, G.; Galardini, M.; Bazzicalupo, M.; Makalowska, I.; Smalla, K.; Mengoni, A.; et al. Comparison of Highly and Weakly Virulent Dickeya Solani Strains, With a View on the Pangenome and Panregulon of This Species. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Potrykus, M.; Golanowska, M.; Hugouvieux-Cotte-Pattat, N.; Lojkowska, E. Regulators Involved in Dickeya Solani Virulence, Genetic Conservation, and Functional Variability. Mol. Plant Microbe Interact. 2014, 27, 700–711. [Google Scholar] [CrossRef] [Green Version]
- Sławiak, M.; Łojkowska, E.; Wolf, J.M.V.D. First Report of Bacterial Soft Rot on Potato Caused by Dickeya Sp. (Syn. Erwinia Chrysanthemi) in Poland. Plant Pathol. 2009, 58, 794. [Google Scholar] [CrossRef]
- Ihnatowicz, A.; Siwinska, J.; Meharg, A.A.; Carey, M.; Koornneef, M.; Reymond, M. Conserved Histidine of Metal Transporter AtNRAMP1 Is Crucial for Optimal Plant Growth under Manganese Deficiency at Chilling Temperatures. New Phytol. 2014, 202, 1173–1183. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root Exudation of Phytochemicals in Arabidopsis Follows Specific Patterns That Are Developmentally Programmed and Correlate with Soil Microbial Functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.-R. Genome-Wide Identification and Testing of Superior Reference Genes for Transcript Normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Hassan, J.A.; de la Torre-Roche, R.; White, J.C.; Lewis, J.D. Soil Mixture Composition Alters Arabidopsis Susceptibility to Pseudomonas Syringae Infection. Plant Direct 2018, 2, e00044. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fan, B.; Fedoseyenko, D.; Kierul, K.; Becker, A.; von Wiren, N.; Borriss, R. Linking Plant Nutritional Status to Plant-Microbe Interactions. PLoS ONE 2013, 8, e68555. [Google Scholar] [CrossRef] [PubMed]
- Lemanceau, P.; Expert, D.; Gaymard, F.; Bakker, P.A.H.M.; Briat, J.-F. Chapter 12 Role of Iron in Plant–Microbe Interactions. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; Volume 51, pp. 491–549. [Google Scholar] [CrossRef]
- Marschner, P. Plant-Microbe Interactions in the Rhizosphere and Nutrient Cycling. In Nutrient Cycling in Terrestrial Ecosystems. Soil Biology; Marschner, P., Rengel, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 10, pp. 159–182. [Google Scholar] [CrossRef]
- Masclaux, C.; Expert, D. Signalling Potential of Iron in Plant—Microbe Interactions: The Pathogenic Switch of Iron Transport in Erwinia Chrysanthemi. Plant J. 1995, 7, 121–128. [Google Scholar] [CrossRef]
- Harris, J.M.; Balint-Kurti, P.; Bede, J.C.; Day, B.; Gold, S.; Goss, E.M.; Grenville-Briggs, L.J.; Jones, K.M.; Wang, A.; Wang, Y.; et al. What Are the Top 10 Unanswered Questions in Molecular Plant-Microbe Interactions? MPMI 2020, 33, 1354–1365. [Google Scholar] [CrossRef]
- Motyka-Pomagruk, A.; Zoledowska, S.; Sledz, W.; Lojkowska, E. The Occurrence of Bacteria from Different Species of Pectobacteriaceae on Seed Potato Plantations in Poland. Eur. J. Plant Pathol. 2021, 159, 309–325. [Google Scholar] [CrossRef]
- Van der Wolf, J.M.; Nijhuis, E.H.; Kowalewska, M.J.; Saddler, G.S.; Parkinson, N.; Elphinstone, J.G.; Pritchard, L.; Toth, I.K.; Lojkowska, E.; Potrykus, M.; et al. Dickeya Solani Sp. Nov., a Pectinolytic Plant-Pathogenic Bacterium Isolated from Potato(Solanum Tuberosum). Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 3, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Degefu, Y.; Potrykus, M.; Golanowska, M.; Virtanen, E.; Lojkowska, E. A New Clade of Dickeya Spp. Plays a Major Role in Potato Blackleg Outbreaks in North Finland. Ann. Appl. Biol. 2013, 162, 231–241. [Google Scholar] [CrossRef]
- Golanowska, M.; Łojkowska, E. A Review on Dickeya Solani, a New Pathogenic Bacterium Causing Loss in Potato Yield in Europe. BioTechnologia 2016, 97, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Hamamouch, N.; Li, C.; Seo, P.J.; Park, C.-M.; Davis, E.L. Expression of Arabidopsis Pathogenesis-Related Genes during Nematode Infection. Mol. Plant Pathol. 2011, 12, 355–364. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.; Pieterse, C.M. Systemic Resistance Induced by Rhizosphere Bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [Green Version]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide Dismutase in Arabidopsis: An Eclectic Enzyme Family with Disparate Regulation and Protein Localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Jiang, H.; Ye, S.; Chen, W.-P.; Liang, W.; Xu, Y.; Sun, B.; Sun, J.; Wang, Q.; Cohen, J.D.; et al. The Arabidopsis P450 Protein CYP82C2 Modulates Jasmonate-Induced Root Growth Inhibition, Defense Gene Expression and Indole Glucosinolate Biosynthesis. Cell Res. 2010, 20, 539–552. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Günther, C.; Weber, M.; Spörlein, C.; Loscher, S.; Böttcher, C.; Schobert, R.; Clemens, S. Metabolome Analysis of Arabidopsis Thaliana Roots Identifies a Key Metabolic Pathway for Iron Acquisition. PLoS ONE 2014, 9, e102444. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-H.; Schmidt, W. One Way. Or Another? Iron Uptake in Plants. New Phytol. 2017, 214, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Zamioudis, C.; Hanson, J.; Pieterse, C.M.J. β-Glucosidase BGLU42 Is a MYB72-Dependent Key Regulator of Rhizobacteria-Induced Systemic Resistance and Modulates Iron Deficiency Responses in Arabidopsis Roots. New Phytol. 2014, 204, 368–379. [Google Scholar] [CrossRef]
- Micallef, S.A.; Shiaris, M.P.; Colón-Carmona, A. Influence of Arabidopsis Thaliana Accessions on Rhizobacterial Communities and Natural Variation in Root Exudates. J. Exp. Bot. 2009, 60, 1729–1742. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Perkowska, I.; Siwinska, J.; Olry, A.; Grosjean, J.; Hehn, A.; Bourgaud, F.; Lojkowska, E.; Ihnatowicz, A. Identification and Quantification of Coumarins by UHPLC-MS in Arabidopsis Thaliana Natural Populations. Molecules 2021, 26, 1804. [Google Scholar] [CrossRef]
- Rigault, M.; Buellet, A.; Masclaux-Daubresse, C.; Fagard, M.; Chardon, F.; Dellagi, A. Quantitative Methods to Assess Differential Susceptibility of Arabidopsis Thaliana Natural Accessions to Dickeya Dadantii. Front. Plant Sci. 2017, 8, 394. [Google Scholar] [CrossRef] [Green Version]
- Austin, S.; Lojkowska, E.; Ehlenfeldt, K.; Kelman, A.; Helgeson, J.P. Fertile Interspecific Somatic Hybrids of Solanum: A Novel Source of Resistance to Erwinia Soft Rot. Phytopathology 1988, 78, 1216–1220. [Google Scholar] [CrossRef]
- Pajerowska, K.M.; Parker, J.E.; Gebhardt, C. Potato Homologs of Arabidopsis Thaliana Genes Functional in Defense Signaling--Identification, Genetic Mapping, and Molecular Cloning. Mol. Plant Microbe Interact. 2005, 18, 1107–1119. [Google Scholar] [CrossRef] [Green Version]
- Zimnoch-Guzowska, E.; Marczewski, W.; Lebecka, R.; Flis, B.; Schäfer-Pregl, R.; Salamini, F.; Gebhardt, C. QTL Analysis of New Sources of Resistance to Erwinia Carotovora Ssp. Atroseptica in Potato Done by AFLP, RFLP, and Resistance-Gene-Like Markers. Crop Sci. 2000, 40, 1156–1167. [Google Scholar] [CrossRef]
- Lennox, E.S.; Luria, S.E.; Benzer, S. On the Mechanism of Photoreactivation of Ultraviolet-Inactivated Bacteriophage. Biochim. Biophys. Acta 1954, 15, 471–474. [Google Scholar] [CrossRef]
- Heeg, C.; Kruse, C.; Jost, R.; Gutensohn, M.; Ruppert, T.; Wirtz, M.; Hell, R. Analysis of the Arabidopsis O-Acetylserine(Thiol)Lyase Gene Family Demonstrates Compartment-Specific Differences in the Regulation of Cysteine Synthesis. Plant Cell 2008, 20, 168–185. [Google Scholar] [CrossRef] [Green Version]
- Conn, S.J.; Hocking, B.; Dayod, M.; Xu, B.; Athman, A.; Henderson, S.; Aukett, L.; Conn, V.; Shearer, M.K.; Fuentes, S.; et al. Protocol: Optimising Hydroponic Growth Systems for Nutritional and Physiological Analysis of Arabidopsis Thaliana and Other Plants. Plant Methods 2013, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]




| (a) | ||||||||
| Soil Mix no. | pH in H2O | NaCl g/dm3 Soil | NO3 | Cl | P | K | Ca | Mg |
| mg/dm3 Soil | ||||||||
| #1 | 6.8 | 2.65 | 224 | 13.6 | 34.6 | 70.1 | 2960 | >400 (548) 1 |
| #2 | 6.9 | 1.59 | 220 | 10.5 | 44.5 | 91.8 | 2509 | >400 (498) 1 |
| (b) | ||||||||
| Soil Mix no. | Cu | Zn | Mn | Fe | B | |||
| mg/dm3 Soil | ||||||||
| #1 | 1.1 | 1.1 | 1.3 | 51.4 | 0.4 | |||
| #2 | 1.0 | 1.3 | 2.3 | 49.2 | 0.4 | |||
| Name | Sequence (5′-3′) | Description |
|---|---|---|
| 3g18780For | CTTGCACCAAGCAGCATGAA | Primer for ACT2 gene 1 |
| 3g18780Rev | CCGATCCAGACACTGTACTTCCTT | Primer for ACT2 gene 1 |
| AT2G14610_F | TTCTTCCCTCGAAAGCTCAAGA | Primer for PR1 gene |
| AT2G14610_R | GTGCCTGGTTGTGAACCCTTA | Primer for PR1 gene |
| AT4G31970_F | GATGGTGAGAATGGTGGCCG | Primer for CYP82C2 gene |
| AT4G31970_R | GCCTCTTCGGCATCTTCAGG | Primer for CYP82C2 gene |
| AT1G08830_F | TCAACCCCGATGGTAAAACAC | Primer for SOD1 gene |
| AT1G08830_R | TCACCAGCATGTCGATTAGCA | Primer for SOD1 gene |
| At5g60390_F | TGAGCACGCTCTTCTTGCTTTCA | Primer for EF-1α gene 1 |
| At5g60390_R | GGTGGTGGCATCCATCTTGTTACA | Primer for EF-1α gene 1 |
| S8HqPCR_F | GCCGAGACACTTGGCTTCTT | Primer for S8H gene |
| S8HqPCR_R | CAGCAGCTCCACCGAAACA | Primer for S8H gene |
| F6H1qPCRf | TGATGAGGACAGAGTCGCTGAA | Primer for F6′H1 gene |
| F6H1qPCRr | CACTTGAAAGAACCCCCATTTC | Primer for F6′H1 gene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkowska, I.; Potrykus, M.; Siwinska, J.; Siudem, D.; Lojkowska, E.; Ihnatowicz, A. Interplay between Coumarin Accumulation, Iron Deficiency and Plant Resistance to Dickeya spp. Int. J. Mol. Sci. 2021, 22, 6449. https://doi.org/10.3390/ijms22126449
Perkowska I, Potrykus M, Siwinska J, Siudem D, Lojkowska E, Ihnatowicz A. Interplay between Coumarin Accumulation, Iron Deficiency and Plant Resistance to Dickeya spp. International Journal of Molecular Sciences. 2021; 22(12):6449. https://doi.org/10.3390/ijms22126449
Chicago/Turabian StylePerkowska, Izabela, Marta Potrykus, Joanna Siwinska, Dominika Siudem, Ewa Lojkowska, and Anna Ihnatowicz. 2021. "Interplay between Coumarin Accumulation, Iron Deficiency and Plant Resistance to Dickeya spp." International Journal of Molecular Sciences 22, no. 12: 6449. https://doi.org/10.3390/ijms22126449







