Correlations between the Type of Aggregates in the Bulk Phase and the Functionality and Safety of All-Purpose Cleaners
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of Formulations and Technologies for the Production of APCs
2.2. Appearance and Stability
2.3. The Expected Detergency, Based on the Assessment of the Ability to Reduce Surface Tension, Wettability, and Ability to Emulsify Fatty Dirt
2.4. Foaming Properties
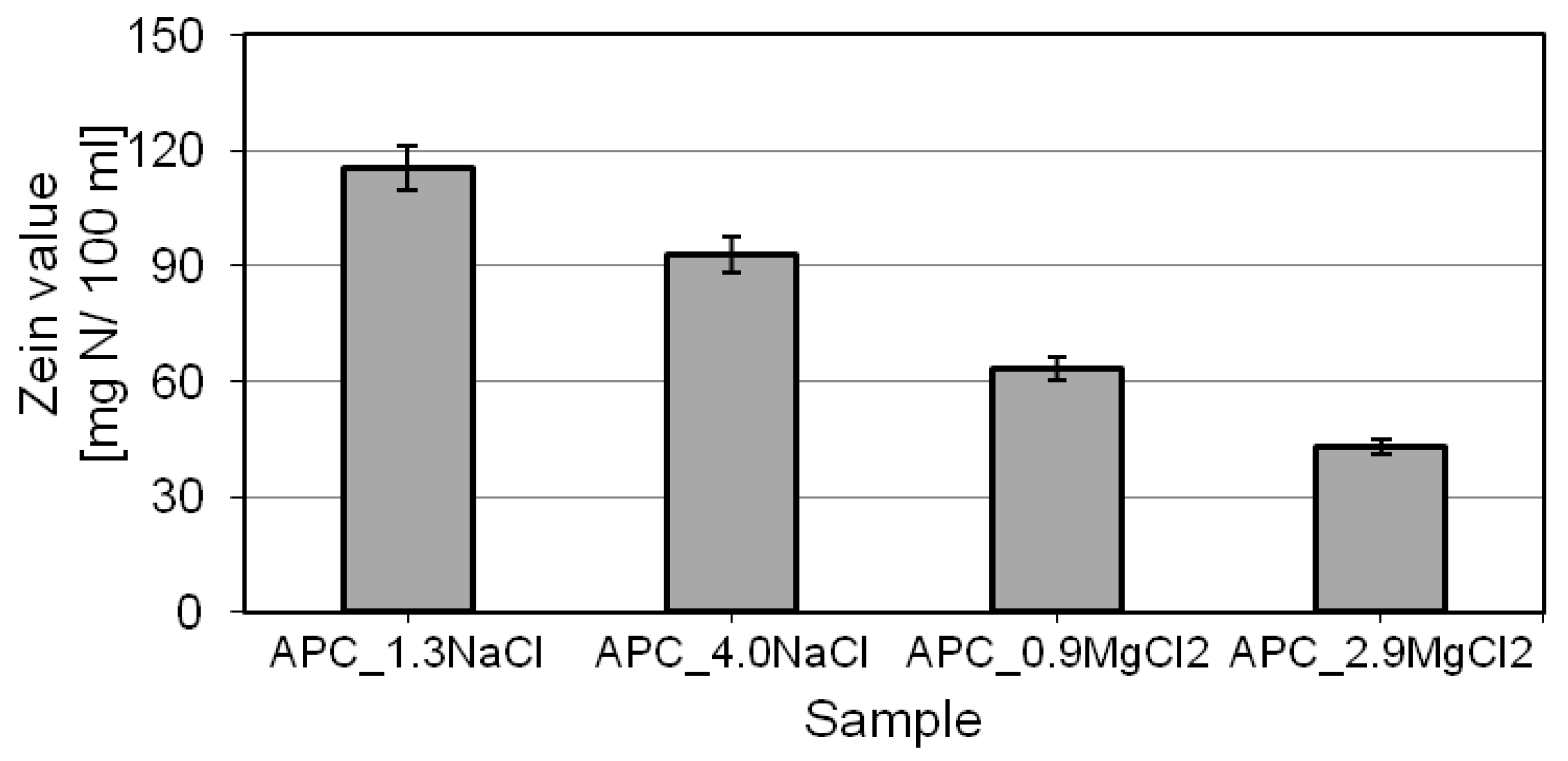
2.5. Determination of Irritant Potential—Zein Value (ZV)
3. Materials and Methods
3.1. Materials
3.2. Viscosity Measurements
3.3. Determination of Turbidity
3.4. Measurement of Particle-Size Distributions
3.5. Evaluation of the Ability to Emulsify Fatty Soil
3.6. Surface Tension Measurements
3.7. Contact Angle
3.8. Evaluation of Foaming Properties
3.9. Determination of Irritant Potential—Zein Value (ZV)
3.10. Error Analysis
4. Conclusions
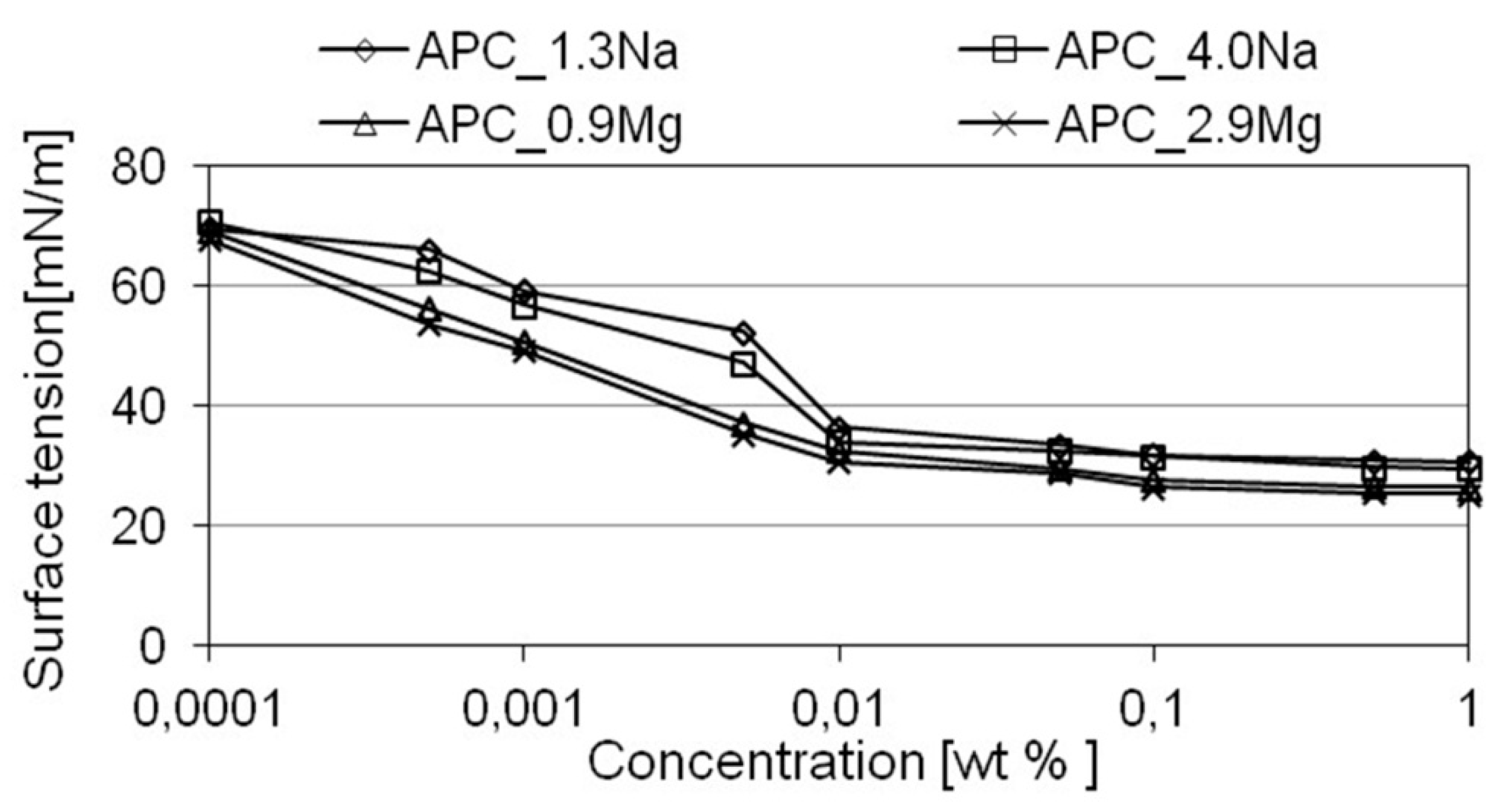
- electrolyte type has an effect on the surface activity of formulations in aqueous solutions. Addition of magnesium chloride into the compositions reduced the surface tension of their aqueous solutions. The effect of the specific salt type concentration on the parameter in question was not determined;
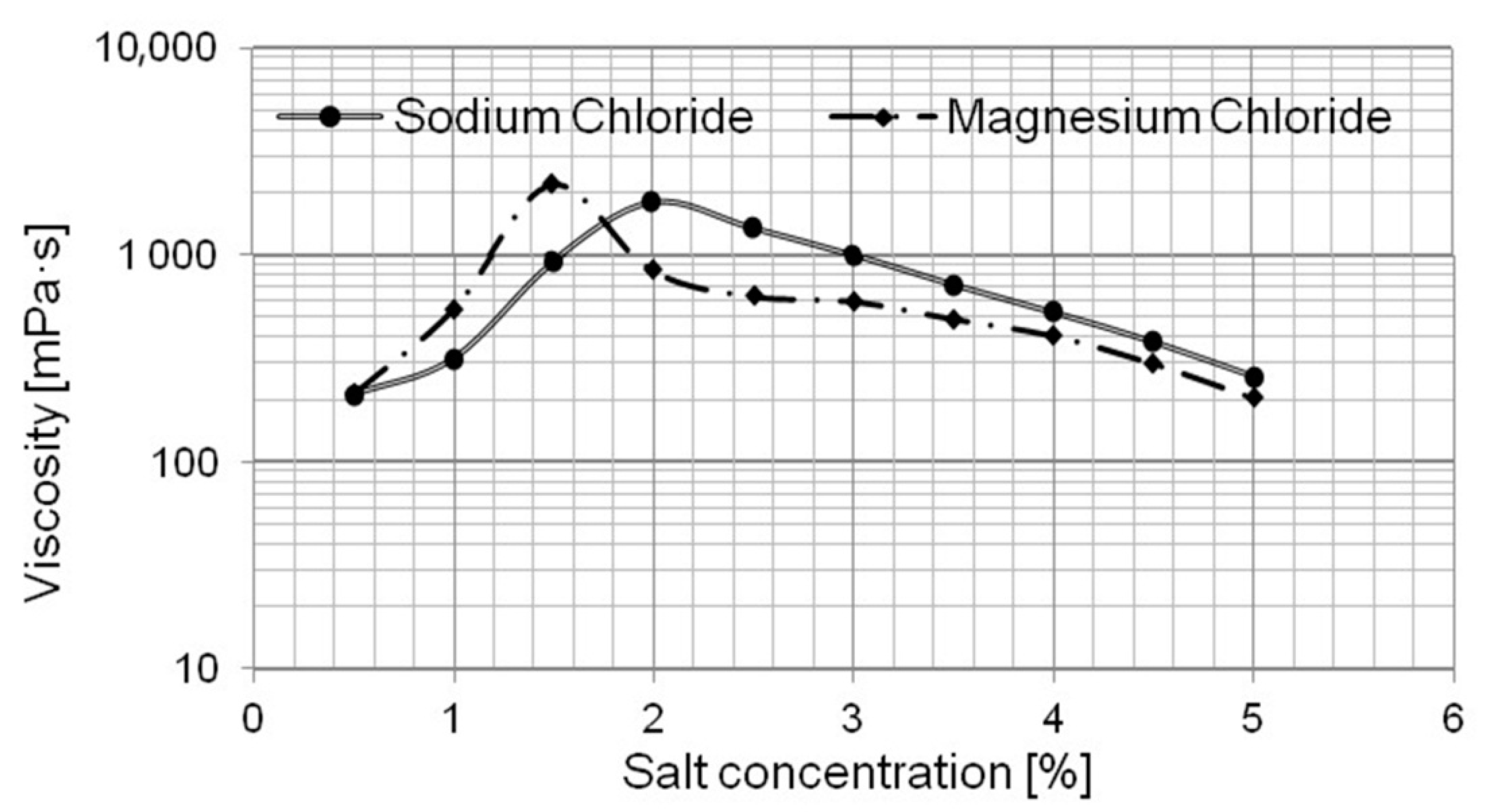
- particle-size distribution analyses indicate that the salt type and concentration had an effect on the size and type of aggregates being formed in the solution. The systems with magnesium chloride were shown to form large aggregates in comparison with those containing sodium chloride. The results correspond to those obtained in the turbidity tests;
- the formulations are characterized by sufficiently high wettability with respect to hydrophobic surfaces. Measurements of the wetting angle indicate that the composition with magnesium chloride has higher wettability in comparison with the formulations with sodium chloride. The effect of concentration of the specific chloride on the investigated parameter was not determined. The results correspond to those obtained in surface tension measurements for aqueous solutions of the formulations;
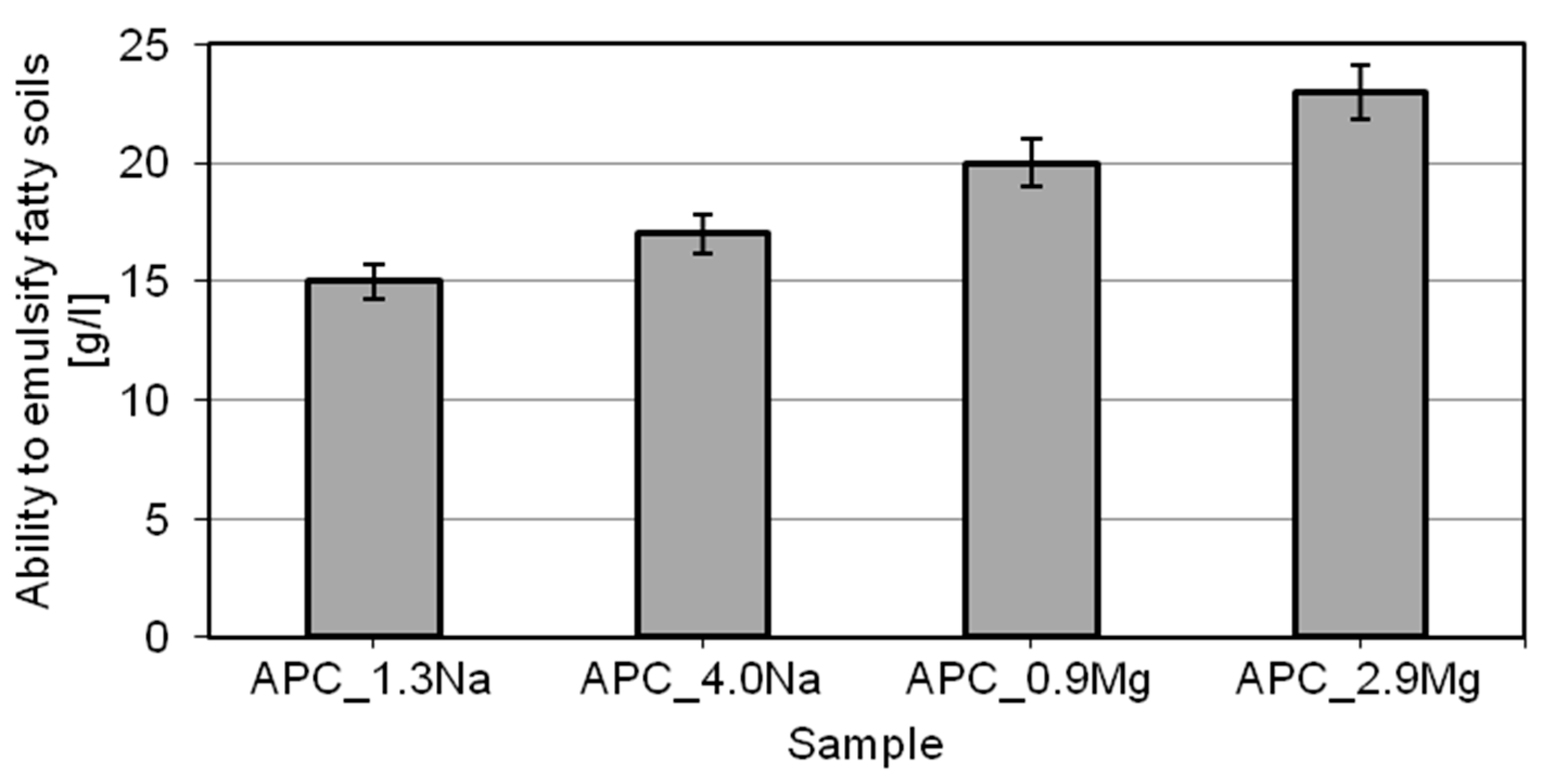
- emulsification properties of the model products increase with increasing concentrations of the chloride type used. Moreover, much better characteristics relating to the ability to emulsify hydrophobic dirt was recorded in the presence of magnesium chloride;
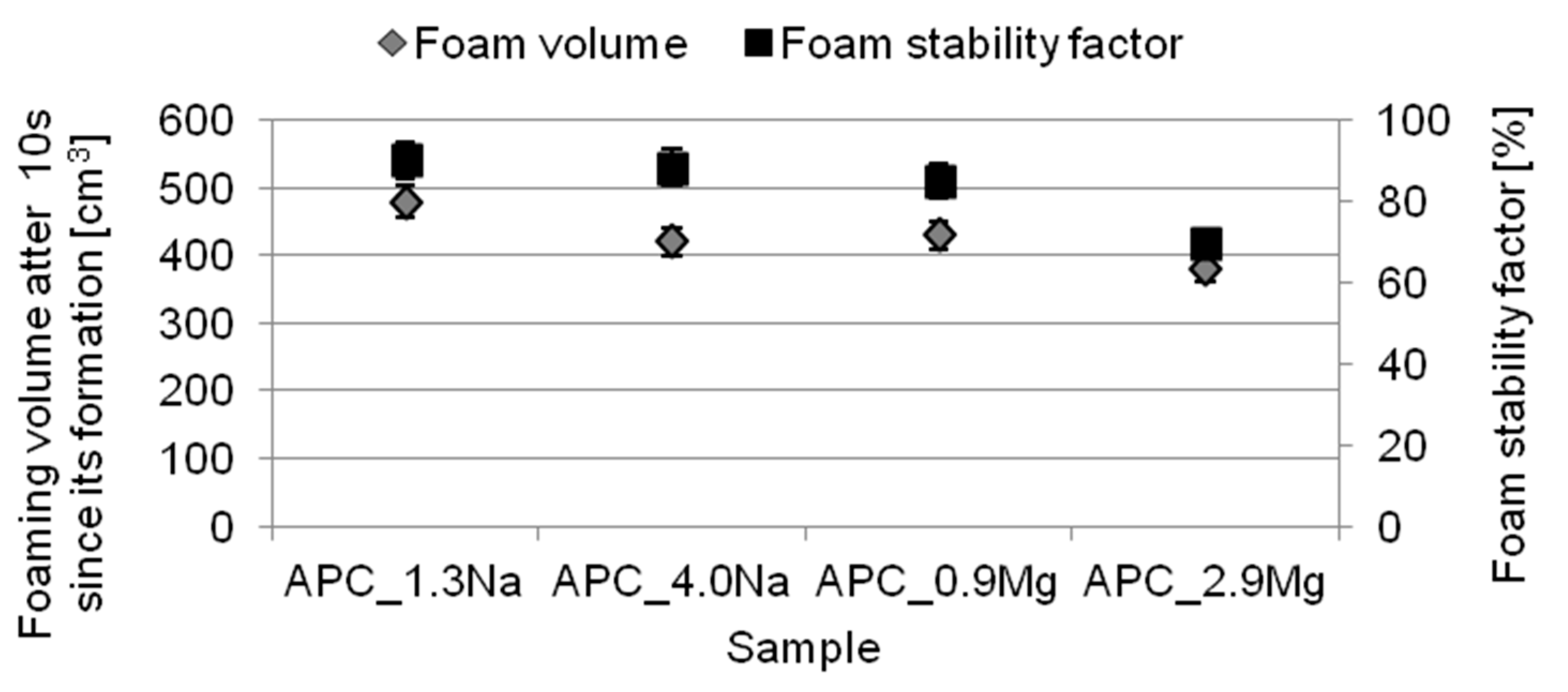
- the formulations revealed good foaming properties. Moreover, for increasing concentrations of the specific chloride type, the solutions were characterized by worse foaming properties and foam stability. The compositions with magnesium chloride showed worse foaming properties in comparison with the systems containing sodium chloride;
- the type and concentration of the electrolyte used determine the zein value. For increasing concentrations of the given salt, the parameter was observed to decrease. In the presence of double-charged cations (Mg2+), the test compositions proved to be definitely better in the aspect of irritation potential. For such formulations, the zein value was nearly 60% lower than that of the compositions containing sodium chloride.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wisniewski, K. Specialty Liquid Household Surface Cleaners. In Liquid Detergent, 2nd ed.; Lai, Y.-K., Ed.; CRC Press: New York, NY, USA, 2006; pp. 555–621. [Google Scholar]
- Wisniewski, K. All–purpose cleaners and their formulation. In Handbook of Detergents, Part E Applications; Zoller, U., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 5–39. [Google Scholar]
- Scialla, S. The Formulation of Liquid Household Cleaners. In Handbook of Detergents, Part D—Formulations; Showell, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 153–178. [Google Scholar]
- Franklin, V.J. Cleaning efficacy of single-purpose surfactant cleaners and multi-purpose solutions. Cont. Lens Anterior Eye 1997, 20, 63–68. [Google Scholar] [CrossRef]
- Berkholz, P.; Kobersky, V.; Stamminger, R. Comparative analysis of global consumer behaviour in the context of different manual dishwashing methods. Int. J. Consum. Stud. 2013, 37, 46–58. [Google Scholar] [CrossRef]
- Geetha, D.; Tyagi, R. Consumer behavior and fascinating challenges on household laundry and dishwashing. Tenside Surfactants Deterg. 2016, 53, 568–575. [Google Scholar] [CrossRef]
- Karsa, D.R. The development of household laundry detergents in Western Europe. Coloration Technol. 1990, 20, 70–76. [Google Scholar] [CrossRef]
- Järvi, P.; Paloviita, A. Product-related information for sustainable use of laundry detergents in Finnish households. J. Clean. Prod. 2007, 15, 681–689. [Google Scholar] [CrossRef]
- Subramanian, V.; Golden, J.S. Patching life cycle inventory (LCI) data gaps through expert elicitation: Case study of laundry detergents. J. Clean. Prod. 2016, 115, 354–361. [Google Scholar] [CrossRef]
- Nitschke, L.; Malcomber, I.; Tibazarwa, C.; Steber, J. Is the EU ecolabel DID list a useful environmental evaluation tool for detergent-like consumer products? Tenside Surfactants Deterg. 2007, 44, 155–159. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin–Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Blagojević, S.N.; Blagojević, M.S.; Pejić, N.D. Performance and Efficiency of Anionic Dishwashing Liquids with Amphoteric and Nonionic Surfactants. J. Surfactants Deterg. 2016, 19, 363–372. [Google Scholar] [CrossRef]
- Seweryn, A.; Klimaszewska, E.; Ogorzałek, M. Improvement in the Safety of Use of Hand Dishwashing Liquids through the Addition of Sulfonic Derivatives of Alkyl Polyglucosides. J. Surfactants Deterg. 2019, 22, 743–750. [Google Scholar] [CrossRef]
- Seweryn, A.; Wasilewski, T.; Bujak, T. Effect of salt on the manufacturing and properties of hand dishwashing liquids in the coacervate form. Ind. Eng. Chem. Res. 2016, 55, 1134–1141. [Google Scholar] [CrossRef]
- Wasilewski, T.; Bujak, T. Effect of the Type of Nonionic Surfactant on the Manufacture and Properties of Hand Dishwashing Liquids in the Coacervate Form. Ind. Eng. Chem. Res. 2014, 53, 13356–13361. [Google Scholar] [CrossRef]
- Wasilewski, T. Coacervates as a Modern Delivery System of Hand Dishwashing Liquids. J. Surfactants Deterg. 2010, 13, 513–520. [Google Scholar] [CrossRef]
- Wasilewski, T.; Seweryn, A.; Krajewski, M. Improvement in the safety of use of hand dishwashing liquids through the addition of hydrophobic plant extracts. J. Surfactants Deterg. 2016, 19, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasilewski, T.; Seweryn, A.; Bujak, T. Supercritical carbon dioxide blackcurrant seed extract as an anti-irritant additive for hand dishwashing liquids. Green Chem. Lett. Rev. 2016, 9, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T.; Czerwonka, D.; Piotrowska, U. Effect of the Concentration of Hop Cone Extract on the Antibacterial, Physico-Chemical and Functional Properties of Adhesive Toilet Cleaners. Tenside Surfactants Deterg. 2016, 53, 368–374. [Google Scholar] [CrossRef]
- Bocho-Janiszewska, A.; Wasilewski, T. Application of Glycerin in Liquid Laundry Detergents as an Example of Innovation in the Household Chemicals Industry. Tenside Surfactants Deterg. 2017, 54, 372–376. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Christov, N.; Cristobal, G.; Bourgaux, C.; Heux, L.; Boucenna, I.; Berret, J.F. Design of eco-friendly fabric softeners: Structure, rheology and interaction with cellulose nanocrystals. J. Colloid Interface Sci. 2018, 525, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.S. Fabric softener technology: A review. J. Surfactants Deterg. 2015, 18, 199–204. [Google Scholar] [CrossRef]
- Hauthal, H.G.; Wagner, G. Household Cleaning, Care and Maintenance Products; Ziolkowsky GmbH: Thannhausen, Germany, 2004. [Google Scholar]
- Falbe, J. Surfactants in Consumer Products: Theory, Technology and Application; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Toussaint, C.; Andries, N.; Mondin, M. All-Purpose Cleaning Compositions. U.S. Patent 8,618,041, 31 December 2013. [Google Scholar]
- Cate, S.; Garabedian, A.; Cheng, L.; Deleeuw, D. Low Residue Cleaners for Food Contact Surfaces. U.S. Patent 11/424,667, 16 June 2006. [Google Scholar]
- Requejo, L.P.; Keyes, G.B. Solvent, Nonionic or Anionic Surfactant, Builder System Which Includes Polyacrylic Acid or Salt, Fatty Acid Dimer Alkali Salt Hydrotrope. U.S. Patent 4,983,317, 8 January 1991. [Google Scholar]
- Peters, D.S. Multi-Purpose Cleaning Compositions and Method. U.S. Patent 7,592,303, 22 September 2009. [Google Scholar]
- Sein, A.; Engberts, J. Micelle to lamellar aggregate transition of an anionic surfactants in dilute aqueous solution induced by alkali metal chloride and tetraalkylammonium chloride salts. Langmuir 1995, 11, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Sein, A.; Engberts, J.; van der Linden, E.; van de Pas, J.C. Salt induced transition from a micellar to a lamellar liquid crystalline phase in dilute mixtures of anionic and nonionic surfactants in aqueous solutions. Langmuir 1993, 9, 1714–1720. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zheng, C.; Wang, D.; Zhan, H. Effect of temperature on foaming ability and foam stability of typical surfactants used for foaming agent. J. Surfactants Deterg. 2017, 20, 615–622. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutkiewicz, E.; Jakubowska, A. Effect of electrolytes on the physicochemical behaviour of sodium dodecyl sulphate micelles. Colloid Polym. Sci. 2002, 280, 1009–1014. [Google Scholar] [CrossRef]
- Ren, Z.H.; Huang, J.; Luo, Y.; Zheng, Y.C.; Mei, P.; Lai, L.; Chang, Y.L. Micellization behavior of binary mixtures of amino sulfonate amphoteric surfactant with different octylphenol polyoxyethylene ethers in aqueous salt solution: Both cationic and hydrophilic effects. J. Ind. Eng. Chem. 2016, 36, 263–270. [Google Scholar] [CrossRef]
- Ren, Z.H.; Huang, J.; Zheng, Y.C.; Lai, L.; Hu, L.L.; Chang, Y.L. Micellization of binary mixture of amino sulfonate amphoteric surfactant and octylphenol polyoxyethylene ether (10) in aqueous solution: Different electrolyte effect. J. Chem. Eng. Data 2017, 62, 938–946. [Google Scholar] [CrossRef]
- Sood, A.K.; Aggarwal, M. Evaluation of micellar properties of sodium dodecylbenzene sulphonate in the presence of some salts. J. Chem. Sci. 2018, 130, 39. [Google Scholar] [CrossRef] [Green Version]
- Staszak, K.; Wieczorek, D.; Michocka, K. Effect of sodium chloride on the surface and wetting properties of aqueous solutions of cocamidopropyl betaine. J. Surfactants Deterg. 2015, 18, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, P.A. A review of shampoo surfactant technology: Consumer benefits, raw materials and recent developments. Int. J. Cosmet. Sci. 2018, 40, 16–30. [Google Scholar] [CrossRef]
- Gallegos, C.; Franco, J.M. Rheology of food, cosmetics and pharmaceuticals. Curr. Opin. Colloid Interface Sci. 1999, 4, 288–293. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mohameed, H.A.; Bsoul, A. Determination of optimal Dead Sea salt content in a cosmetic emulsion using rheology and stability measurements. J. Cosm. Sci. 2008, 59, 1–14. [Google Scholar]
- Abu-Jdayil, B.; Mohameed, H.A. Rheology of Dead Sea shampoo containing the antidandruff climbazole. Int. J. Cosmet. Sci. 2004, 26, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Czerwonka, D.; Seweryn, A. Use of rapeseed oil fatty acid methyl esters for preparation of self-adhesive formulations for hygiene in toilets. Przem. Chem. 2016, 95, 784–788. [Google Scholar] [CrossRef]
- Seweryn, A.; Wasilewski, T.; Bocho-Janiszewska, A. Correlation between sequestrant type and properties of mild soap-based hand washing products. Ind. Eng. Chem. Res. 2018, 57, 12683–12688. [Google Scholar] [CrossRef]
- Seweryn, A.; Bujak, T. Application of anionic phosphorus derivatives of alkyl polyglucosides for the production of sustainable and mild body wash cosmetics. ACS Sustain. Chem. Eng. 2018, 6, 17294–17301. [Google Scholar] [CrossRef]
- Denda, M.; Katagiri, C.; Hirao, T.; Maruyama, N.; Takahashi, M. Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch. Dermatol. Res. 1999, 291, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.; Costa, P.C.; Bahia, M.F. Effect of São Pedro do Sul thermal water on skin irritation. Int. J. Cosmet. Sci. 2010, 32, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Polefka, T.G.; Bianchini, R.J.; Shapiro, S. Interaction of mineral salts with the skin: A literature survey. Int. J. Cosmet. Sci. 2012, 34, 416–423. [Google Scholar] [CrossRef]
- Penfield, K. A Look behind the Salt Curve: An Examination of Thickening Mechanisms in Shampoo Formulations. In AIP Conference Proceedings; Co, A., Leal, G.L., Colby, R.H., Giacomin, A.J., Eds.; American Institute of Physics: College Park, MD, USA, 2008; Volume 1027, pp. 899–901. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Santos, M.S.; Biscaia, E.C., Jr.; Tavares, F.W. Effect of electrostatic correlations on micelle formation. Colloids Surf. A 2017, 533, 169–178. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P. Micelle–monomer equilibria in solutions of ionic surfactants and in ionic-nonionic mixtures: A generalized phase separation model. Adv. Colloid Interface Sci. 2014, 206, 17–45. [Google Scholar] [CrossRef]
- Khan, A.; Marques, E.F. Synergism and polymorphism in mixed surfactant systems. Curr. Opin. Colloid Interface Sci. 1999, 4, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Geng, T.; Jiang, Y.; Zhao, L.; Ju, H.; Wang, Y. Impact of NaCl concentration on equilibrium and dynamic surface adsorption of cationic surfactants in aqueous solution. J. Mol. Liq. 2017, 238, 423–429. [Google Scholar] [CrossRef]
- Naqvi, A.Z. Clouding Phenomenon in Amphiphilic Systems: A Review of Five Decades. Colloids Surf. B 2018, 165, 325–344. [Google Scholar] [CrossRef]
- Miller, C.A.; Raney, K.H. Solubilization–emulsification mechanism of detergency. Colloids Surf. A 1993, 74, 169–215. [Google Scholar] [CrossRef]
- Bergström, M.; Eriksson, J.C. A theoretical analysis of synergistic effects in mixed surfactant systems. Langmuir 2000, 16, 7173–7181. [Google Scholar] [CrossRef]
- Gharibi, H.; Sohrabi, B.; Javadian, S.; Hashemianzadeh, M. Study of the electrostatic and steric contributions to the free energy of ionic/nonionic mixed micellization. Colloids Surf. A 2004, 244, 187–196. [Google Scholar] [CrossRef]
- Shinoda, K.; Takeda, H. The effect of added salts in water on the hydrophile-lipophile balance of nonionic surfactants: The effect of added salts on the phase inversion temperature of emulsions. J. Colloid Interface Sci. 1970, 32, 642–646. [Google Scholar] [CrossRef]
- Iwanaga, T.; Suzuki, M.; Kunieda, H. Effect of added salts or polyols on the liquid crystalline structures of polyoxyethylene-type nonionic surfactants. Langmuir 1998, 14, 5775–5781. [Google Scholar] [CrossRef]
- Vora, S.; George, A.; Desai, H.; Bahadur, P. Mixed micelles of some anionic-anionic, cationic-cationic, and ionic-nonionic surfactants in aqueous media. J. Surfactants Deterg. 1999, 2, 213–221. [Google Scholar] [CrossRef]
- Friberg, S.E.; Ma, Z. Amphiphilic association structures: Some recent applications. Adv. Colloid Interface Sci. 1992, 41, 45–56. [Google Scholar] [CrossRef]
- Yang, J. Viscoelastic wormlike micelles and their applications. Curr. Opin. Colloid Interface Sci. 2002, 7, 276–281. [Google Scholar] [CrossRef]
- Cates, M.E.; Candau, S.J. Statics and dynamics of worm-like surfactant micelles. J. Phys. Condens. Matter 1990, 2, 6869–6892. [Google Scholar] [CrossRef]
- Schubert, B.A.; Kaler, E.W.; Wagner, N.J. The microstructure and rheology of mixed cationic/anionic wormlike micelles. Langmuir 2003, 19, 4079–4089. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M. Influence of surfactant and electrolyte concentrations on surfactant Adsorption and foaming characteristics. J. Pet. Sci. Eng. 2017, 149, 612–622. [Google Scholar] [CrossRef]
- Oko, M.U.; Venable, R.L. The effects of divalent metal ions on the micellar properties of sodium dodecyl sulfate. J. Colloid Interface Sci. 1971, 35, 53–59. [Google Scholar] [CrossRef]
- Ladanyi, B.M. Computer simulation studies of counterion effects on the properties of surfactant systems. Curr. Opin. Colloid Interface Sci. 2013, 18, 15–25. [Google Scholar] [CrossRef]
- Mu, J.H.; Li, G.Z.; Jia, X.L.; Wang, H.X.; Zhang, G.Y. Rheological properties and microstructures of anionic micellar solutions in the presence of different inorganic salts. J. Phys. Chem. B 2002, 106, 11685–11693. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, G.; Yuan, S.; Chen, Y.; Yan, H. Molecular dynamics study of alkyl benzene sulfonate at air/water interface: Effect of inorganic salts. J. Phys. Chem. B 2010, 114, 5025–5033. [Google Scholar] [CrossRef]
- Homendra, N.; Devi, C.I. Turbidity studies on mixed surfactant systems in hard water: A new method for estimation of water hardness. Indian J. Chem. Technol. 2004, 11, 783–786. [Google Scholar]
- Alargova, R.G.; Petkov, J.T.; Petsev, D.N. Micellization and interfacial properties of alkyloxyethylene sulfate surfactants in the presence of multivalent counterions. J. Colloid Interface Sci. 2003, 261, 1–11. [Google Scholar] [CrossRef]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry, 4th ed.; Butterworth–Heinemann: London, UK, 1991. [Google Scholar]
- Raney, K.A.; Benton, W.J.; Miller, C.A. Optimum detergency conditions with nonionic surfactants. J. Colloid Interface Sci. 1987, 117, 282–290. [Google Scholar] [CrossRef]
- Urum, K.; Pekdemir, T.; Gopur, M. Optimum conditions for washing of crude oil contaminated soil with biosurfactant solutions. Process Saf. Environ. Prot. 2003, 81, 203–209. [Google Scholar] [CrossRef]
- Weerawardena, A.; Boyd, B.J.; Drummond, C.J.; Furlong, D.N. Removal of a solid organic soil from a hard surface by glucose-derived surfactants: Effect of surfactant chain length, headgroup polymerisation and anomeric configuration. Colloids Surf. A 2000, 169, 317–328. [Google Scholar] [CrossRef]
- Urum, K.; Grigson, S.; Pekdemir, T.; McMenamy, S. A comparison of the efficiency of different surfactants for removal of crude oil from contaminates soils. Chemosphere 2006, 62, 1403–1410. [Google Scholar] [CrossRef]
- Nikolov, A.D.; Wasan, D.T.; Chengara, A.; Koczo, K.; Policello, G.A.; Kolossvary, I. Superspreading driven by Marangoni flow. Adv. Colloid Interface Sci. 2002, 96, 325–338. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Starov, V.M. Wetting of low free energy surfaces by aqueous surfactant solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 285–291. [Google Scholar] [CrossRef]
- Lee, K.S.; Ivanova, N.; Starov, V.M.; Hilal, N.; Dutschk, V. Kinetics of wetting and spreading by aqueous surfactant solutions. Adv. Colloid Interface Sci. 2008, 144, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Zisman, W. Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution. In Contact Angle, Wettability, and Adhesion; Johnson, R.E., Dettre, R.H., Eds.; Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1964; Volume 43, pp. 1–51. [Google Scholar]
- Dutschk, V.; Breitzke, B.; Grundke, K. Wetting of aqueous surfactant solutions on polymer surfaces. Tenside Surf. Det. 2003, 40, 250–255. [Google Scholar]
- Dutschk, V.; Breitzke, B. Spreading characteristics of aqueous surfactant solutions on polymer surfaces. Tenside Surf. Det. 2005, 42, 1–6. [Google Scholar] [CrossRef]
- Dutschk, V.; Sabbatovskiy, K.G.; Stolz, M.; Grundke, K.; Rudoy, V.M. Unusual wetting dynamics of aqueous surfactant solutions on polymer surfaces. J. Colloid Interface Sci. 2003, 267, 456–462. [Google Scholar] [CrossRef]
- Rafaï, S.; Sarker, D.; Bergeron, V.; Meunier, J.; Bonn, D. Superspreading: Aqueous surfactant drops spreading on hydrophobic surfaces. Langmuir 2002, 18, 10486–10488. [Google Scholar] [CrossRef]
- Tongcumpou, C.; Acosta, E.J.; Quencer, L.B.; Joseph, A.F.; Scamehorn, J.F.; Sabatini, D.A.; Yanumete, N.; Chavadej, S. Microemulsion formation and detergency with oily soils: III. Performance and mechanisms. J. Surfactants Deterg. 2005, 8, 147–156. [Google Scholar] [CrossRef]
- Márquez, A.L.; Medrano, A.; Panizzolo, L.A.; Wagner, R.J. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Colloid Interface Sci. 2010, 341, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wu, F.; Saito, M.; Tatsumi, E.; Yin, L. Effect of magnesium salt concentration in water-in-oil emulsions on the physical properties and microstructure of tofu. Food Chem. 2016, 201, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, M.R.; Varade, S.R.; Ghosh, P.; Paul, P.; Negi, A.S. Foaming in micellar solutions: Effects of surfactant, salt, and oil concentrations. Ind. Eng. Chem. Res. 2014, 53, 18497–18507. [Google Scholar] [CrossRef]
- Sett, S.; Karakashev, S.I.; Smoukov, S.K.; Yarin, A.L. Ion-specific effects in foams. Adv. Colloid Interface Sci. 2015, 225, 98–113. [Google Scholar] [CrossRef]
- Li, C.; Zhang, T.; Ji, X.; Wang, Z.; Sun, S.; Hu, S. Effect of Ca2+/Mg2+ on the stability of the foam system stabilized by an anionic surfactant: A molecular dynamics study. Colloids Surf. A 2016, 489, 423–432. [Google Scholar] [CrossRef]
- Varade, S.R.; Ghosh, P. Foaming in aqueous solutions of zwitterionic surfactant: Effects of oil and salts. J. Dispers. Sci. Technol. 2017, 38, 1770–1784. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Roy, P.; Okadome, H.; Shiina, T. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. J. Colloid Interface Sci. 2009, 332, 208–214. [Google Scholar] [CrossRef]
- The Dirt on Cleanings–Home Cleaning/Laundry Attitudes and Trends around the World, April 2016, Nielsen Company. Available online: www.nielsen.com/content/dam/nielsenglobal/de/docs/Nielsen%20Global%20Home%20Care%20Report_2016.pdf (accessed on 15 May 2018).
- Jackson, C.T.; Paye, M.; Maibach, H. Mechanism of Skin Irritation by Surfactants and Anti-Irritants for Surfactants Base Products. In Handbook of Cosmetic Science and Technology Fourth Edition; Barel, A., Paye, M., Maibach, H., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 353–365. [Google Scholar]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Challenging the surfactant monomer skin penetration model: Penetration of sodium dodecyl sulfate micelles into the epidermis. J. Cosmet. Sci. 2003, 54, 29–49. [Google Scholar] [PubMed]
- Chen, Y.; Ji, X.; Han, Y.; Wang, Y. Self-Assembly of Oleyl Bis (2-hydroxyethyl) methyl Ammonium Bromide with Sodium Dodecyl Sulfate and Their Interactions with Zein. Langmuir 2016, 32, 8212–8221. [Google Scholar] [CrossRef] [PubMed]
- Pezron, I.; Galet, L.; Clausse, D. Surface Interaction between a Protein Monolayer and Surfactants and Its Correlation with Skin Irritation by Surfactants. J. Colloid Interface Sci. 1996, 180, 285–289. [Google Scholar] [CrossRef]
- Deo, N.; Jockusch, S.; Turro, N.J.; Somasundaran, P. Surfactant interactions with zein protein. Langmuir 2003, 19, 5083–5088. [Google Scholar] [CrossRef]
- Ruso, J.M.; Deo, N.; Somasundaran, P. Complexation between dodecyl sulfate surfactant and zein protein in solution. Langmuir 2004, 20, 8988–8991. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Moreno, A.; Berna, J.L. LAS magnesium salts—A contribution to mildness and performance. Tenside Surf. Det. 1998, 35, 265–269. [Google Scholar]
- Cohen, L.; Martin, M.; Soto, F.; Trujillo, F.; Sanchez, E. The effect of counterions of linear alkylbenzene sulfonate on skin compatibility. J. Surfactants Deterg. 2016, 19, 219–222. [Google Scholar] [CrossRef]
- Adaszyńska, M.; Swarcewicz, M.; Dzięcioł, M.; Dobrowolska, A. Comparison of chemical composition and antibacterial activity of lavender varieties from Poland. Nat. Prod. Res. 2013, 27, 1497–1501. [Google Scholar] [CrossRef]
- Buchbauer, G.; Jirovetz, L.; Jäger, W. Aromatherapy: Evidence for sedative effects of the essential oil of lavender after inhalation. Z. Nat. C 1991, 46, 1067–1072. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Cavanagh, H.M.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, H.M.; Wilkinson, J.M. Lavender essential oil: A review. Aust. Infect. Control 2005, 10, 35–37. [Google Scholar] [CrossRef] [Green Version]
- Akgün, M.; Akgün, N.A.; Dinçer, S. Extraction and modeling of lavender flower essential oil using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2000, 39, 473–477. [Google Scholar] [CrossRef]


| Name according to INCI 1 | Concentration [wt. %] | |||
|---|---|---|---|---|
| APC_1.3 Na | APC_4.0 Na | APC_0.9 Mg | APC_2.9 Mg | |
| Sodium Coco Sulfate | 5.00 | 5.00 | 5.00 | 5.00 |
| Coco Glucoside | 3.00 | 3.00 | 3.00 | 3.00 |
| Cocamidopropyl Betaine | 1.00 | 1.00 | 1.00 | 1.00 |
| Polyglyceryl-4 Laurate/Sebacate (and) Polyglyceryl-6 Caprylate/Caprate | 1.00 | 1.00 | 1.00 | 1.00 |
| Sodium Chloride | 1.30 | 4.00 | - | - |
| Magnesium Chloride | - | - | 0.90 | 2.90 |
| Citric Acid | 0.50 | 0.50 | 0.50 | 0.50 |
| Lavender Flower CO2 Extract | 0.25 | 0.25 | 0.25 | 0.25 |
| Sodium Benzoate (and) Potassium Sorbate | 0.10 | 0.10 | 0.10 | 0.10 |
| Aqua | to 100 | to 100 | to 100 | to 100 |
| Surface | Wetting Angle Ө [deg] | ||||
|---|---|---|---|---|---|
| Water | APC_1.3 Na | APC_4.0 Na | APC_0.9 Mg | APC_2.9 Mg | |
| Steel | 82.1 ± 0.23 | 49.7 ± 0.69 | 41.08 ± 0.54 | 40.9 ± 0.66 | 40.8 ± 0.66 |
| Ceramics | 75.4 ± 0.87 | 51.0 ± 0.26 | 44.3 ± 0.19 | 40.9 ± 0.74 | 36.8 ± 0.88 |
| Glass | 21.5 ± 0.55 | 16.5 ± 0.68 | 13.2 ± 0.40 | 13.4 ± 0.91 | 12.7 ± 0.64 |
| Polymer | 78.5 ± 0.12 | 50.9 ± 0.31 | 48.2 ± 0.56 | 44.7 ± 0.12 | 43.6 ± 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seweryn, A.; Wasilewski, T.; Bocho-Janiszewska, A. Correlations between the Type of Aggregates in the Bulk Phase and the Functionality and Safety of All-Purpose Cleaners. Int. J. Mol. Sci. 2021, 22, 6592. https://doi.org/10.3390/ijms22126592
Seweryn A, Wasilewski T, Bocho-Janiszewska A. Correlations between the Type of Aggregates in the Bulk Phase and the Functionality and Safety of All-Purpose Cleaners. International Journal of Molecular Sciences. 2021; 22(12):6592. https://doi.org/10.3390/ijms22126592
Chicago/Turabian StyleSeweryn, Artur, Tomasz Wasilewski, and Anita Bocho-Janiszewska. 2021. "Correlations between the Type of Aggregates in the Bulk Phase and the Functionality and Safety of All-Purpose Cleaners" International Journal of Molecular Sciences 22, no. 12: 6592. https://doi.org/10.3390/ijms22126592







