Abstract
Trillions of microbes exist in the human body, particularly the gastrointestinal tract, coevolved with the host in a mutually beneficial relationship. The main role of the intestinal microbiome is the fermentation of non-digestible substrates and increased growth of beneficial microbes that produce key antimicrobial metabolites such as short-chain fatty acids, etc., to inhibit the growth of pathogenic microbes besides other functions. Intestinal microbiota can prevent pathogen colonization through the mechanism of colonization resistance. A wide range of resistomes are present in both beneficial and pathogenic microbes. Giving antibiotic exposure to the intestinal microbiome (both beneficial and hostile) can trigger a resistome response, affecting colonization resistance. The following review provides a mechanistic overview of the intestinal microbiome and the impacts of antibiotic therapy on pathogen colonization and diseases. Further, we also discuss the epidemiology of immunocompromised patients who are at high risk for nosocomial infections, colonization and decolonization of multi-drug resistant organisms in the intestine, and the direct and indirect mechanisms that govern colonization resistance to the pathogens.
1. Overview of the Microbiota
Humans and other mammalian gastrointestinal tracts (GIT) are home to trillions of microorganisms, such as bacteria, archaea, fungi, protozoa, helminths, and viruses, collectively named the microbiota [1]. Consequently, there is great diversity in the microbial composition between and within microbiota members. Despite its diversity, most intestinal microbiota are comprised of four phyla, i.e., Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria. The Bacteroidetes and Firmicutes phyla account for the greatest population (more than 90%) in the colon. We will briefly discuss each of these phyla below and highlight important members to understand the intestinal microbiota composition [2].
Phylum Actinobacteria comprises aerobic, anaerobic, Gram-positive bacteria with high G + C content in their genomic DNA. The long-term coexistence of certain bacterial species, such as Bifidobacteria spp. (also known as probiotics), which adhere to the intestinal mucosa, results in a mutually beneficial relationship. The World Health Organization has defined probiotics as live microorganisms that provide a health benefit to the host when administered in sufficient amounts [3]. Probiotics protect the host from pathogens through various activities, including competitive exclusion, bile salt hydrolase activity, nutrient metabolism assistance, and immune and digestive system regulation. On the other hand, the host provides nutrient-rich niches to ensure microbiota survival [4].
Phylum Bacteroidetes comprises aerobic, anaerobic, non-spore-forming, Gram-negative, rod-shaped bacteria that colonize the intestine. Bacteroides is one of the most predominant genera colonizing the intestinal tract [5]. Members of this genus are known to digest complex carbohydrates that are resistant to the host’s digestive enzymes. After the breakdown of complex polysaccharides, the host uses small fatty acids like acetate, propionate, and butyrate for energy [6].
Phylum Firmicutes comprises obligate facultative anaerobic, Gram-positive endospore-forming bacteria. Endospores are dormant, non-reproductive structures that allow bacteria to survive under adverse conditions, such as nutrient deficiency, ultraviolet radiation, desiccation, extreme temperature, and chemical disinfectants, for a long time and become active when the environment becomes favorable. Clostridia within this phylum contain diverse bacteria that live in the intestine and play several beneficial roles. Members of this bacterial class colonize between mucosal folds to establish close relationships with intestinal epithelial cells and produce butyrate as an end product of fermentation. Members of this class also promote host immune homeostasis in the intestine by activating colonic immune cells. The Clostridia class also includes important bacterial pathogens (Clostridium perfringens, Clostridium tetani, and Clostridium difficile) that cause various human diseases. Bacilli members (Enterococcus and Streptococcus spp.) are clinically important, oxygen-tolerant, generally found in low abundance, undergoing pathogenic expansion during intestinal dysbiosis.
Phylum Proteobacteria comprises Gram-negative bacteria that are both obligate and facultative. Lactobacillus spp. and Escherichia coli Nissle adhered to the intestinal mucosa and showed a mutually beneficial relationship. According to some studies, the increased prevalence of Proteobacteria in the microbial community can be diagnosed as dysbiosis and diseases [7]. Pathogenic E. coli strains and Klebsiella are found in low abundance in the Enterobacteriaceae family of Proteobacteria. In addition to probiotics, antibiotics have been shown to improve enteric pathogen colonization in the intestine. For example, changes in the composition of the intestinal microbiome can increase susceptibility to Salmonella enterica subsp. Typhimurium and C. difficile infections [6].
2. Impact of Antibiotic Therapy on Microbe Colonization and Diseases
Intestinal microbes are stable under physiological conditions, but antibiotics administration, nutrients availability, physical stress, and host factors can all cause dysbiosis in the microbiota (Figure 1). Dysbiosis is characterized by a reduction in the diversity of microbes and the normal function of the intestinal microbiota in maintaining host wellness. In addition, it may cause loss of specific microbial populations that dysregulate the production of antimicrobial peptides or metabolites against pathogen colonization [8,9].
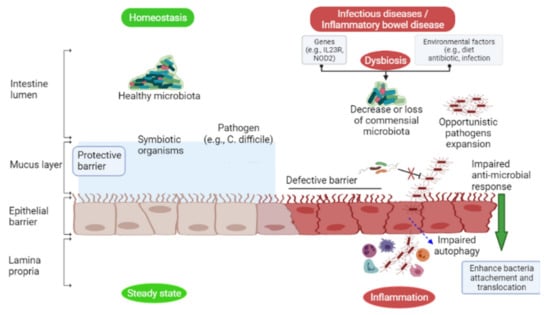
Figure 1.
Under homeostatic conditions (left green), certain Gram-negative commensal bacteria induce IgA and IgG antibody production from B cells, recognizing Gram-negative bacteria surface antigens (flagellin, LPS) in the intestinal lumen, thus contributing to host defense against symbionts and pathogens. Symbiotic organisms stimulate mucus production to prevent enteric pathogens from colonizing the intestinal mucosa. However, during dysbiosis (right red), a decrease or loss of commensal bacteria in the intestine may result in opportunistic pathogens (e.g., C. difficile) infection due to the secretion of virulence factors (toxins) that damage and breach the epithelial layer, causing inflammation.
Antibiotics are used for the treatment of potentially life-threatening bacterial diseases. Previous studies have shown that incorrect or prolonged antibiotics use may lead to the elevation of unanticipated and undesirable microbial communities in the intestines. Theoretically, an antibiotic can only affect the intestinal microbiota composition through direct exposure, which is only possible if the antibiotic reaches the intestinal lumen. Therefore, antibiotics taken orally are delivered directly to the intestinal lumen. Antibiotics circulate in the bloodstream and eventually reach the liver, where they may be modified; depending on the nature of the antibiotic, it may be excreted into the bile or returned to the bloodstream as waste products for renal clearance. Several factors influence antibiotic absorption in the intestines, including antibiotic properties, intestinal membrane integrity, and transport mechanisms. Antibiotics are absorbed in the intestinal lumen, reducing exposure to the microbiota. For example, orally administered metronidazole was entirely absorbed in the small intestine [6], resulting in significantly lower intestinal metronidazole concentrations as it passes through the GIT without disturbing the intestinal microbiota. In contrast, poorly absorbed antibiotics in the intestine, such as vancomycin, remain at high concentrations in the GIT after oral administration, implying that oral metronidazole had a lower impact on the microbiota than oral vancomycin. Thus, antibiotic activity and intestinal absorption determine how antibiotics affect the composition of the microbiota and host susceptibility to pathogens. These key factors, among others, can be considered when selecting antibiotics and determining their effects on the intestinal microbiota.
2.1. Clinical Consequences of Antibiotic Treatment
Antibiotics may alter the composition of the microbiota in the intestines. The restoration of microbial diversity in children after antibiotic treatment has been reported to take about one month [10]. Gentamicin, meropenem, and vancomycin administration increased the prevalence of Enterobacteriaceae and other pathobionts in adults while decreasing the Bifidobacterium species [11]. Although the intestinal microbiota’s baseline composition was restored within 45 days, and several bacteria were undetectable for the remaining 180 days [11]. Antibiotics can also disrupt the balance of the bacterial species in the intestines. For example, reduced bacterial diversity promotes the growth and invasion of enteric pathogens, especially C. difficile [12]. Several previous studies assessing the effect of antibiotic therapy on intestinal microbiota are listed in Table 1.

Table 1.
Studies assessing the impact of antibiotic therapy on intestinal microbiota composition.
2.1.1. Antibiotic-Associated Diarrhea
Some antibiotics thin the mucus layer and disrupt tight junctions, exposing the intestinal epithelium to damage. Antibiotic-associated diarrhea affects approximately 5–35% of patients following antibiotic treatment [13]. Antibiotic-associated diarrhea occurred in 17.5% of adult patients after receiving antibiotics for 5–10 days [14]. Antibiotic-associated diarrhea caused by C. difficile is more severe than antibiotic-associated diarrhea caused by non-C. difficile [15]. Probiotics therapy (Lactobacillus rhamnosus and Saccharomyces boulardii is recommended in the 2016 European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines to prevent antibiotic-associated diarrhea [16].
2.1.2. C. difficile-Associated Diarrhea
C. difficile is an obligate anaerobe, spore-forming bacteria that commonly infects immunocompromised peoples, such as hematopoietic stem cell transplant recipients [17]. These bacterial spores are typically consumed orally and proliferate in the colon of susceptible hosts. Under favorable conditions, these bacteria produce toxins in the host that disrupt the intestinal epithelial barrier and cause diarrhea [18,19]. Antibiotics are the most important risk factor for C. difficile nosocomial infection, with the most commonly associated drugs are ampicillin, amoxicillin, cephalosporin, clindamycin, and fluoroquinolone. The duration and number of antibiotics taken increase the risk of C. difficile infection. Other risk factors include age, weak body immunity, and being hospitalized [20]. Metronidazole 500 mg three times a day for ten days is recommended in mild and moderate cases and vancomycin 125 mg four times a day for ten days in severe cases. Antibiotics that caused C. difficile infection should be discontinued [21].
2.1.3. Helicobacter Pylori Infection
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that colonizes the human GIT and causes inflammation. In most cases, the inflammatory response is mild and asymptomatic; however, the infection can occasionally cause intestinal metaplasia, gastric cancer, and gastric and duodenal ulcers [15]. Quadruple bismuth is recommended as first-line therapy in areas with high clarithromycin and metronidazole resistance [22]. H. pylori eradication can have both positive and negative effects on the host. It restores microbiota composition [23] and causes changes in the microbiota composition that affect the host [24]. H. pylori eradication was associated with a decrease in the relative abundance of Bacteroidetes and an increase in Firmicutes. The addition of probiotics (S. boulardii) to triple therapy was more effective for H. pylori eradication [25]. Probiotics (Lactobacillus and Bifidobacterium) combined with H. pylori eradication therapy were more effective and safer than therapy without probiotics [26].
2.1.4. Antibiotic Therapy Cause Obesity, Asthma, Allergy, and IBD
The use of antibiotics in infants and children has been linked to several long-term clinical consequences, including obesity, asthma, and allergies [15]. Antibiotic exposure during childhood (particularly between the ages of 6 and 12 months) delayed intestinal microbiota development [27] and decreased Enterobacteriaceae, Lachnospiraceae, and Erysipelotrichaceae species in the intestines [27]. In addition, early antibiotic exposure causes dysbiosis, which aids in the pathogenesis of inflammatory bowel disease (IBD). According to a population-based cohort study, infants who received antibiotics in their first year of life were more likely to be diagnosed with IBD than untreated individuals [28].
2.2. Multi-Drug-Resistant (MDR) Organisms Are Found in the Intestinal Microbiota
Resistomes are microbiota in the intestines that carry multiple antibiotic-resistance genes [29]. There are two types of resistomes in the intestinal microbiota: the resident resistome (commensal bacteria carrying antibiotic-resistance genes) and the transitory resistome (antibiotic-resistance genes carried by bacteria periodically). The transitory resistome can either transfer its resistance gene to commensal bacteria or become a permanent microbiota member [30]. Therefore, characterizing the intestinal microbiota in humans that carry the antibiotic resistance gene and understanding how the antibiotic resistance genes can transfer among different commensal members and opportunistic pathogens is of great interest [31].
The high prevalence of C. difficile opportunistic infection may be due to the emergence of antibiotic-resistant strains [32,33,34]. Antibiotic-induced perturbations of the intestinal microbiota result in the loss of colonization resistance in hosts, making them vulnerable to C. difficile infection [35]. This concern mainly arises in hospitals, where C. difficile can quickly spread from an infected person to a healthy person, making it a leading cause of nosocomial infection in developed countries [18,19]. In addition to C. difficile infection, Klebsiella pneumoniae is an opportunistic pathogen that serves as an infection reservoir. K. pneumoniae is a member of the healthy human intestinal microbiota and a leading cause of hospital-acquired infections. However, the mechanisms that promote colonization to infection are poorly understood. K. pneumoniae GIT carriage was a risk factor for infection in hospitalized patients [36]. In a study, Stercz and his colleagues investigated the effect of antibiotics (ampicillin, ciprofloxacin, ceftazidime) on the intestinal colonization of CTX-M-15 ESBL and OXA-162 carbapenemase-producing K. pneumoniae using the mice model [37]. K. pneumoniae colonization increased after ampicillin and ceftazidime treatments. In contrast, ciprofloxacin treatments decreased colonization. The gene blaOXA-162 was correlated with K. pneumoniae in vivo, whereas the copy numbers of the gene blaCTX-M-15 increased from the first to the fifteenth day after ceftazidime treatment. These findings show that antibiotics have various effects on bacterial colonization, antibiotic resistance genes, and the persistence of K. pneumonia. Furthermore, colonization of MDR bacteria in the intestines has been identified as a risk factor for severe disease in patients undergoing hematopoietic stem cell and liver transplantation [38,39].
3. Nosocomial Infections of the GIT
The intestinal microbiota contains a large number of opportunistic pathogens. The microbiota’s role in protecting immunocompromised patients from opportunistic pathogens is critical in the hospital. These opportunistic pathogens can cross the intestinal barrier (Figure 1) and cause infections after fecal contamination of skin, intravenous lines, or other body sites [40]. Hospitalized patients, such as those receiving chemotherapy or undergoing hematopoietic stem cell transplantation, are at higher risk of C. difficile infection [41]. Antibiotic treatment can prevent these infections; however, antibiotic administration can disrupt the intestinal microbiota and develop antibiotic-resistant bacteria strains [42]. C. difficile colonization causes gastrointestinal infection [43], and other nosocomial pathogenic bacteria can translocate and enter the bloodstream to cause systemic infections [44].
4. Bloodstream Infections Originate from the GIT Colonization
Bloodstream infection in immunocompromised patients originates from the GIT due to changes in the microbiota or damage to the mucosal barrier, mediated by many factors, e.g., exposure to chemotherapy, radiation, or antibiotics that systemically disseminate intestinal bacteria [45]. The most common bacteria translocating are oxygen-tolerant pathobionts such as vancomycin-resistant Enterococcus (VRE), E. coli, Klebsiella, and viridans streptococci [46]. Chemotherapy, which is used to treat cancer, suppresses the immune system. It includes various drugs that inhibit various cellular mitosis steps, affecting rapidly dividing cells (cancer cells). Specialized stem cells in the GIT [47] and hematopoietic stem cells in the bone marrow [48] divide rapidly under healthy homeostatic conditions. These cells become vulnerable to chemotherapy antimitotic effects, which disrupt the normal turnover of these cells. The specialized stem cells in the GIT normally replenish the mucosal epithelial cells to maintain their integrity. On the other hand, chemotherapy prevents the replacement of aging and damaged intestinal epithelial cells lining. Damaged cells produce reactive oxygen species, which initiates a repair response that activates transcription factors in the mucosal epithelial cells. Chemotherapy-induced inflammatory damage cell apoptosis in the GIT mucosa often results in painful lesions and a compromised mucosal barrier [49]. Chemotherapy also affects hematopoietic stem cells in the bone marrow, which give rise to immune blood cells.
Neutrophils are the most abundant among the white blood cells with a short life span that act as a primary defense response to infections [50]. Promyelocytes are the earliest recognizable precursors of neutrophils, which actively synthesize DNA and are susceptible to the antimitotic effects of chemotherapy. Their offspring develop into myelocytes, the numerous proliferating neutrophil precursors, and thus the most severely affected cells by chemotherapy. Chemotherapy-induced neutropenia (low neutrophil count) can predispose a patient to infections. Broad-spectrum antibiotics are commonly used in immunocompromised patients to prevent opportunistic infections. As a result of antibiotic therapy, immunocompromised patients are more susceptible to bacterial infections from the GIT. Infected persons may develop neutropenia, which impairs their immune response to pathogens. Antibiotics treatment in combination with chemotherapy can promote intestinal dysbiosis and antibiotic-resistant bacterial strains. The patients may also develop mucositis, which allows the intestinal bacteria to enter the bloodstream and cause systemic infection.
5. Intestinal Microbiota Modulation by Fecal Microbiota Transplantation (FMT) for the Decolonization of MDR Organisms
MDR organisms in the intestines of hospitalized patients can be opportunistic pathogens due to antibiotic pressure. Patients colonized with MDR organisms from the GIT or transmitted from other individuals are at a higher risk of infection. These pathogens can enter the body through the damaged intestinal barrier and cause local or systemic infections. Intestinal microbiota plays a vital role in preventing these infections, which have gained attention in using FMT as a preventive strategy to reduce MDR organism carriage. FMT is strongly recommended for patients with recurrent C. difficile infection [51] and who have not responded to appropriate antibiotic treatment [52]. Oral capsules containing lyophilized fecal microbiota administered through colonoscopy were equally effective as frozen products [53]. FMT is highly effective [52], although cases of bacteremia following FMT administration have been reported [54]. A rise in the prevalence of C. difficile infection has been observed in pediatric patients [55], particularly in children who received prolonged antibiotic therapy or those with chronic IBD, oncological conditions, or who have recently undergone surgery [56]. According to current evidence, the recipient’s age does not affect FMT efficacy or safety. Disruption of the intestinal microbiota following prolonged antimicrobial therapy contributes to the pathogenesis of graft-versus-host disease and overall mortality in hematopoietic stem cell transplantation recipients [57,58]. Based on these findings, FMT may help to prevent hematopoietic stem cell transplant-related mortality. FMT has been proposed to eradicate drug-resistant bacteria from the intestinal microbiota. Adult patients who received prolonged antibiotic therapy for chronic C. difficile infection showed a higher proportion of antibiotic resistance genes in their intestinal microbiota than healthy adults; however, the number and diversity of antibiotic resistance genes decreased after FMT administration [59,60]. Antimicrobial-resistant genes can also be acquired from FMT donor stool, highlighting the importance of healthy stool donor selection and the need for further standardization [61]. The carbapenem-resistant Enterobacteriaceae and VRE were the most frequently isolated pre-FMT MDR bacteria from MDR-infected patients [62] and immunocompromised patients [63]. Other MDR organisms identified are Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, and Acinetobacter. FMT therapy is commonly given through the nasoduodenal tube, nasogastric tube, oral capsules, or colonoscopy. The donor’s age is specified as being between 6 and 60 years. Patients undergoing FMT must fast for at least 12 h, begin treatment with a proton pump inhibitor twice a day to neutralize gastric acid, and discontinue taking antibiotics. FMT was associated with severe adverse reactions in a hospitalized patient with known liver cirrhosis and recurrent hepatic encephalopathy [64]. Other studies reported mild diarrhea, mild abdominal discomforts, food intolerance, constipation events after FMT administration.
6. The Intestinal Microbiota Showed Colonization Resistance to Pathogens
The concept of colonization resistance refers to the ability of commensal microbiota to prevent the colonization and overgrowth of pathogenic bacteria [65]. Colonization resistance is an intestinal defense mechanism that occurs due to direct competition between commensals and pathogens for the intestinal niche and nutrition. The intestinal microbiota plays a critical role in excluding invading bacteria and inhibiting the growth of enteric pathogens. Several studies elucidated the molecular mechanisms of excluding invading pathogens and inhibiting the growth of enteric bacteria within the GIT using different pathogens and antibiotic regimens. Key members of the bacterial species contributing to colonization resistance against pathogens are C. difficile, VRE, L. monocytogenes, E. coli, and Enterococcus faecalis [66,67,68,69].
Studies have also shown that certain commensal microbiota inhibits the growth of MDR bacteria, although the mechanism by which microbiota influence pathogen colonization is unknown. VRE (obligate anaerobe Barnesiella) did not colonize intestines in mice [70,71]. Mice administered with a specific bacteria consortium (Blautia, Clostridium bolteae prevented VRE colonization and cleared persistent VRE [67]. Four bacterial species (Coprococcus, Desulfovibrio, Oscillospira, and Parabacteroides species) were depleted from the microbiota of adult patients colonized by extended-spectrum β-lactamase (ESBL)-producing bacteria [72]. In contrast, these four bacterial species were present in the microbiomes of patients who were not colonized by ESBL-producing bacteria. Hence, it is clear that beneficial bacteria provide colonization resistance to pathogens by two mechanisms: direct competition between commensals and pathogens for nutrients uptake or physical environment (niches) establishment and indirect mechanisms of colonization resistance, derived from the commensal bacteria stimulated immune system. In this section, we will discuss mechanisms by which intestinal microbiota contribute to pathogens’ colonization resistance.
6.1. Direct Mechanisms of Colonization Resistance
The microbiota promotes direct colonization resistance to pathogens through killing and competition for resources. One mechanism is directly competing for the same niche where intestinal bacteria provide colonization resistance to pathogens (Figure 2a). Some microbiota acquires nutrients more efficiently than pathogens, impeding pathogen replication and colonization in the intestines, while others secrete antimicrobial peptides that directly kill pathogens. These targeted killing mechanisms compete for nutrients and niches between closely related bacterial spp. The following sections discuss the aspects of direct mechanisms of colonization resistance between beneficial and harmful bacteria.
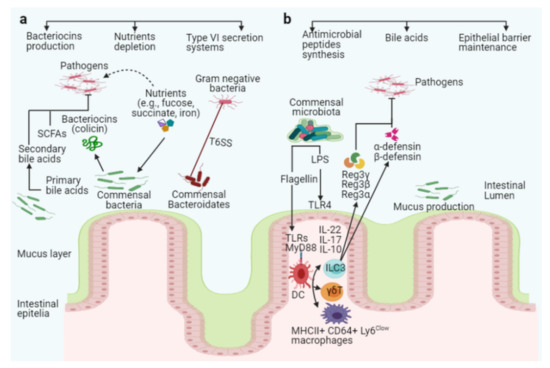
Figure 2.
The intestinal microbiota acts as a barrier against enteric pathogens via direct and indirect colonization resistance mechanisms. Direct colonization resistance mechanisms (a): Several commensal Bacteroidetes prevent pathogens from colonizing the intestinal mucosa, and the commensal E. coli Nissle strain consumes nutrients, limiting nutrients availability to specific pathogens (pink color with flagella). The probiotic E. coli Nissle strain can also absorb iron, limiting its availability to the pathogen S. Typhimurium. Commensal bacteria secrete antimicrobial peptides, such as bacteriocins (colicin), SCFAs, and T6SS, to target and kill invading pathogens. Together with antimicrobial peptides, commensal bacteria produce enzymes that convert conjugated primary bile acids to secondary bile acids (toxic to invading pathogens). Indirect colonization resistance mechanisms (b): Surface antigens such as flagellin or LPS from commensals bacteria stimulate host innate immunity via TLRs and MyD88 on epithelial or dendritic cells (DCs). ILC3, γδT, and Th17 cells can be activated to produce interleukin IL-10, IL-17, and IL-22, which promotes secretion of the antimicrobial peptides Reg3γ, Reg3β, Reg3α, α-defensin, and β-defensin from epithelial cells to inhibit pathogen colonization in the intestines (pink color with flagella).
6.1.1. Killing or Suppression of Pathogens Through Antimicrobial Peptides
Killing or growth suppression can play a significant role in pathogens’ colonization resistance [73]. Bacteriocins are small peptides produced by bacteria in their environment that have antimicrobial activity. Gratia discovered the first bacteriocin, colicin, in 1925 [74]. Since then, many bacteriocins have been isolated and characterized from different bacterial species, including human and animal intestinal bacteria, lactic acid bacteria of fermented foods, and Bifidobacteria [75]. The molecular weight of microcin is less than 10 kDa, whereas the molecular weight of colicin is greater than 20 kDa. Compared to non-producer control strains, bacteriocin (colicin) producing E. coli strains demonstrated improved and prolonged persistence in mice [76]. However, streptomycin pre-treatment resulted in a disrupted E. coli and bacteriocin-producing E. faecalis community in these mice. These bacteria were more capable of colonizing antibiotic-treated mice than mice that had not been pre-treated with antibiotics. Bacteriocin-producing bacteria may also inhibit the colonization of opportunistic pathogens such as E. faecalis and VRE [69].
Microcins are bacteriocins produced by Gram-negative bacteria that have limited antimicrobial activity against other Gram-negative bacteria. Human probiotic E. coli Nissle 1917 produced microcins in mice to protect them from S. Typhimurium infection [77]. In a study, mice administered water supplemented with different bacteriocin-producing bacterial strains inhibited the growth of pathogenic Clostridium and Staphylococcus strains without affecting the beneficial microbiome [78]. Hence, it is clear that bacteriocins from the Enterobacteriaceae play a significant role in colonization resistance to bacterial pathogens without causing significant disruptions in the intestinal microbiota population.
6.1.2. Metabolites from Intestinal Microbiota Inhibit Pathogenic Bacteria
Metabolites produced by intestinal microbiota inhibit the growth of pathogenic bacteria. Short-chain fatty acids (SCFAs) produced by healthy intestinal microbiota are protective against enteric pathogens. Intestinal microbiota ferment unabsorbed starch, soluble dietary lipids, and vitamins. Intestinal microbiota members Bacteroidetes and Clostridia produce SCFAs in adults [73]. Bacteroidetes are the primary fermenters, transforming sugars from complex carbohydrates to organic acids and SCFA, while Clostridia uses the organic acids to generate additional SCFAs. SCFAs have direct antimicrobial activity by diffusing through bacterial membranes and lowering intracellular pH. SCFAs have promising applications in human health and food safety due to their antimicrobial activity [79]. Clostridia SCFAs are important metabolites that inhibit S. Typhimurium growth in mice intestines [73], pathogenic E. coli strain growth in streptomycin-treated mice [80], and C. difficile growth in humans [81,82]. The intestinal microbiota secretes bile acids, which are amphipathic, cholesterol-derived molecules. Bile acids are synthesized in the liver and then conjugated with taurine or glycine before being stored in the gall bladder. Subsequently, the bile acids are secreted into the small intestine, where they emulsify fat and fat-soluble vitamins for absorption. Nearly 95% of bile acids are reabsorbed at the distal ileum in humans, and the remaining 5% converted or deconjugated to secondary bile acids, such as deoxycholic acid and lithocholic acid [83]. Clostridium perfringens and Clostridium scindens secrete bile salt that is reabsorbed in the intestines and transported back to the liver for conjugation [84]. Secondary bile acid that the host has not absorbed is excreted [85]. It has been proposed that bile salt-producing bacterial species (Lactobacilli, Bifidobacteria, Clostridia, Bacteroides) that are resistant to bile acid toxicity have a better chance of survival [86]. Deconjugation by commensals Lactobacillus and Clostridium ensures colonization resistance to pathogenic bacteria [66]. The secretion of deoxycholic acid from B. bifidum reduced the pathogenicity of enterobacteria and Vibrio cholerae by targeting the virulence-associated effectors T6SS and T3SS [87]. Pathogens spread in the absence of deoxycholic acid-producing commensals, as seen in the case of C. difficile enterocolitis [88]. Increased bile acid levels (through therapy or diets) promote the growth of Firmicutes and Clostridium species involved in bile acid deconjugation and inhibit Bacteroidetes and Actinobacteria. Specific bacterial pathogens, such as S. Typhimurium, can survive in the presence of high bile acid concentrations for extended periods [87]. Excess bile acids, such as deoxycholic acid, can lead to cholesterol gallstones and colon cancer, and the intestinal microflora plays an additional role in these complications. Secondary bile acids have antimicrobial properties, as they alter the integrity of the microbial cell membrane, causing spillage of intracellular contents and thus inhibiting the growth of bile acid-intolerant microbes [89,90]. These antimicrobial agents promote the growth of the intestinal microbiota while also protecting the host from various pathogens.
6.1.3. Competition for Shared Niches and Nutrients
Competition between commensals and pathogens for niches and nutrients provides colonization resistance to pathogens in the intestines [9]. The intestinal epithelium monolayer cells are tightly connected, which plays a complex role in mucus production and immune response [91]. Commensals attach to the intestinal mucus from birth, occupying all available space to prevent enteric pathogens colonization [92]. The mucin-rich mucus layer contains O-glycan peptide motifs that act as receptors for commensals like Bifidobacteria and Lactobacilli colonization [93]. Mucin inhibits pathogenic bacterial attachment, biofilm formation, virulence factor detoxification, and commensal survival during competition with pathogens [94]. As a result, mucus protects the natural intestinal environment and acts as the first line of defense against bacterial invasion. Commensals like E. coli and Bifidobacteria secrete mucins to protect themselves from pathogenic E. coli strain and C. difficile invasion [95,96]. Some enteric pathogens, such as S. Typhimurium, C. difficile, pathogenic E. coli strain O157:H7, H. pylori, Campylobacter, and V. cholerae, have evolved specific mechanisms, including flagella motility or lack of adhesins required for bacterial adhesion to avoid mucus [95]. Other virulence mechanisms used to kill commensal competitors include type III or VI secretion systems (T3SS, T6SS) from V. cholerae [97,98], mucin-binding proteins from Listeria monocytogenes [99], and fimbriae adhesins from S. Typhimurium [100]. Hence, it is clear that the mucus layer is essential in preventing pathogenic bacteria and could be a future target for anti-diarrheal drugs. Competition for available nutrients is a mechanism by which commensals provide colonization resistance to pathogens in the human intestines [9]. The use of six sugar molecules by human commensal E. coli HS and seven molecules by probiotic E. coli Nissle limit nutrient availability to pathogenic E. coli O157:H7, thereby inhibiting their colonization in mice [101]. Sometimes pathogenic E. coli O157:H7 can evade this mechanism and start sugar utilization that commensal E. coli failed to utilize [102]. Pathogens use the nutrition provided by commensal bacteria, and those pathogens unable to utilize these resources are eliminated. For example, pathogenic C. difficile survives by metabolizing succinate from the symbiont Bacteroides thetaiotaomicron, whereas C. difficile mutant strains that cannot use this source were eliminated ([103]. The fucosylation of glycan induced by Bacteroides provides a nutrition source to symbionts for their successful colonization. This mechanism is vital for the colonization of Bacteroides and pathogenic bacteria, both of which require fucosis to survive [104,105,106]. Pathogenic bacteria (S. Typhimurium, C. difficile, pathogenic E. coli strain, Campylobacter jejuni) use fucose or other nutrients (sialic acid, succinate) to grow and spread [95,107,108]. Sometimes the source of nutrition available to both commensals and pathogens changes when the intestinal environment is altered by inflammation or antibiotic treatment [109,110,111]. In such conditions, nutrient type and availability promote the growth of specific pathogens capable of utilizing new nutrient sources. For example, S. Typhimurium can use ethanolamine and fructose-asparagine in the inflamed intestine, but the microbiota could not utilize these nutrition sources [112]. The changed microbiota structure and increased specific carbon sources in the mice intestine after antibiotic treatment supported C. difficile growth [109]. Pathogenic E. coli O157:H7 has developed metabolic pathways for distinct sugar resources, some of which are not available to commensal E. coli strains [102]. In the presence of commensal E. coli, pathogenic E. coli strain may fail to colonize the intestines of mice [101]. Commensal bacteria provide complex sugar resources to some pathogens during competition. For example, Citrobacter rodentium requires polysaccharides for successful colonization, whereas monosaccharides alone cannot ensure its survival in competition with other commensals [113]. Therefore, nutrient intake plays a significant role in enteric pathogen pathogenesis. These findings suggest that commensals are well equipped in the healthy intestine to provide colonization resistance to pathogens. The effective colonization of intestinal niches also requires the ability to elude the immune barrier mounted by the mucosal immunity and the antimicrobial metabolites (antimicrobial peptides, secondary bile acids, SCFAs) from microbiota. Similarly, antibiotic treatment induces substantial changes in the intestine microbiota in mice, increasing specific carbon sources that support C. difficile growth [109]. These findings indicate that commensals are best equipped to provide colonization resistance to pathogens in the healthy, unperturbed intestines. Several iron-acquisition systems and genes for siderophore production are encoded in the genome of commensal E. coli Nissle, which contribute to pathogenicity [114,115]. In addition to iron-acquisition and siderophore production, E. coli Nissle developed mechanisms for Curli, Type-1, and F1C fimbriae, which help colonization by increasing adherence and inhibiting other pathogens from colonization [116]. The presence of multiple iron-acquisition systems allows E. coli Nissle to compete successfully with S. Typhimurium and other enteric pathogens for iron utilization and colonization of inflamed intestines [115]. Alfred Nissle isolated E. coli Nissle in 1917 from a soldier stool sample who had not developed diarrhea during the shigellosis outbreak [117]. Currently, probiotic E. coli Nissle is used to treat infectious diarrheal diseases and IBD [118]. It may be possible to genetically engineer probiotic commensal strains to compete for nutrients with pathogenic Enterobacteriaceae in hostile environments, such as the inflamed intestine [118]. Overall, the beneficial E. coli demonstrate how commensals provide colonization resistance to pathogens during the competition for nutrition in the inflamed intestines.
6.2. Indirect Mechanisms of Colonization Resistance
Apart from direct colonization resistance, indirect mechanisms of colonization resistance by commensal microbiota are mediated by enhancing the host mucosal barrier and innate and adaptive immune systems to prevent pathogens from colonizing the intestines. This section will explore indirect mechanisms of colonization resistance in detail (Figure 2b).
6.2.1. Epithelial Barrier Enhancement
The intestinal epithelial barrier is a thick layer of epithelial cells. A thin layer of connective tissue between the epithelial and lamina propria promotes healthy communication between the microbiota and immune cells. Immune cells that reside in the intestinal epithelial layer, such as dendritic cells, B cells, T cells, and macrophages, contribute to the integrity of the intestinal epithelial layer [119]. The overlying Peyer’s patches (M cells) and goblet cells secrete mucus in the small intestine to maintain intestinal integrity [120]. The mucus layer that covers and separates the intestinal epithelium from microbiota is the first line of defense. The absence of this protective layer can result in intestinal inflammation, leading to colitis and colorectal cancer [119]. Therefore, a stable microbiota and a healthy mucus layer are essential for preventing bacterial attachment to intestinal epithelial cells. Commensal microbiota resides and metabolizes nutrients in the outer mucus layer of the mouse intestine [121]. In contrast, the thick inner mucus layer of the intestines in humans is firmly attached to the epithelium, preventing direct contact of commensals with epithelial cells and preventing an inflammatory response. This inner mucus layer that separates bacteria from intestinal epithelial cells is thicker in humans than in rodents. The mucosal barrier is compromised in ulcerative colitis patients, and bacteria are directly attached to the inner epithelium [122].
According to recent research, the presence of bacteria affects mucus layer integrity. A Swedish researcher compared germ-free mouse mucus to conventionally-raised mouse mucus and discovered that the mucus level in the intestine of germ-free mice was equivalent to conventionally-raised mice, and the inner intestinal mucus layer in germ-free mice was more permeable to bacteria-sized beads [123]. Other researchers looked at the mucus barrier in the intestines of different wild-type mice and bred mice in germ-free environments. They discovered that genetically identical mice kept in the same environment could have different microbiota and mucus barrier structures. They also discovered that mice with a thicker impenetrable mucus layer had more Erysipelotrichi cells in their intestines, whereas mice with a thin penetrable mucus layer had more Proteobacteria and candidate division TM7 bacteria [124]. Thus, protein synthesis involved in host responses and mucus production can be increased by stimulating intestinal microbiota. These findings show a complex interaction between the intestinal epithelial barrier, microbiota, and host immune system that aids in pathogen tolerance, balancing, or conferring resistance.
6.2.2. Synthesis of Antimicrobial Peptides
Several studies elucidate that commensal microbiota boosts the host immune response to invading pathogens by triggering antimicrobial peptides secretion. Paneth cells and epithelial cells in the small intestine secrete a diverse group of antimicrobial peptides, such as defensin, lysozymes, phospholipases [125], and C-type lectins family islet-derived 3gamma (Reg3γ), Reg3α, and Reg3β [126] to prevent enteric pathogens from colonizing the epithelial cells. These antimicrobial peptides synthesized in the ribosome target both Gram-positive and Gram-negative bacteria [95,127].
6.2.3. Defensins
Based on six conserved cysteine domains, defensins are classified into two subclasses: α-defensins and β-defensins [128]. Paneth cells in the small intestine are the primary producers of α-defensins [125], and colon epithelial cells produce β-defensins [129]. Defensins are essential components of innate immunity, which produce pores in the membranes of targeted bacteria, resulting in loss of membrane integrity and cell death [128]. Compared to α-defensins, β-defensins were active against Gram-positive and Gram-negative enteric pathogens E. coli, S. aureus, S. pyogenes, and P. aeruginosa [130].
Evidence shows that intestinal microbiota can stimulate α-defensin production to prevent pathogens colonization within the intestines [48,131]. In a study, live E. coli or S. aureus, live or dead S. Typhimurium derived lipopolysaccharide, lipid A, lipoteichoic acid, and liposomes could stimulate intestinal crypts ability of bacteria or bacteria-derived components to promote α-defensins production from Paneth cells [132]. To investigate whether Paneth cells are in direct contact with the intestinal microbiota to prevent pathogen colonization, Vaishnava and colleagues studied Paneth cells lineage in CR2-MyD88 Tg mouse. They found that Paneth cells sense enteric bacteria directly by activating the MyD88-dependent toll-like receptor (TLR) for α-defensins expression [133]. Lactic acid inhibits α-defensin expression in Caco-2 intestinal epithelial cells in vitro, whereas cecal contents promote α-defensin secretion [134]. The transcript of the α-defensin gene Defa was less abundant in the intestinal microbiota of germ-free mice, TLR-deficient mice, and MyD88-deficient mice, but it could be induced by TLR2 or TLR4 stimulation [135]. Miani et al. has reported the relationship between the intestinal microbiome and β-defensin secretion in antibiotic-treated mice and the effect of an aryl hydrocarbon receptor allele on impaired pancreatic β-defensin-14 secretion in non-obese diabetic mice [136]. The findings showed that aryl hydrocarbon receptor ligands, butyrate, and other microbiota-derived compounds were sufficient to induce innate lymphoid cells in the pancreas to secrete interleukin-22 (IL-22), which in turn induced β-defensin-14 secretion by endocrine cells, indicating that dysbiosis and aryl hydrocarbon receptor alleles can influence pancreatic β-defensin-14 secretion in these experimental mice. According to this research study, only live microbiota can promote β-defensin secretion, and specific intestinal microbiota may produce certain metabolites. Aryl hydrocarbon receptor ligands serve as the primary intestinal regulators of β-defensins. However, more in vivo research will be needed to fully understand the mechanisms of β-defensin production in the intestines and how β-defensin prevents pathogen colonization and maintains intestinal homeostasis. Thus, commensal microbiota protects the host by preventing pathogenic bacteria from colonizing the host by activating Paneth cells and colon epithelial cells to produce α-and β-defensin. In addition to defensins, cathelicidins’ antimicrobial peptides with a cathelin domain produced through C-terminal also disrupt the bacterial cell membrane. Cathelicidins, which regulate the microbiota within the colon, are derived from macrophages and colonic epithelial cells in many species, including chickens, fish, humans, mice, and snakes. Researchers have discovered that cathelicidin-WA could improve the intestinal epithelium barrier and protect hosts from enteric pathogens (e.g., E. coli O157:H7) colonization [137].
6.2.4. C-Type Lectins Reg3γ, Reg3α, and Reg3β
The C-type lectins (Reg3γ, Reg3α, Reg3β) are key components of innate immunity, which inhibit enteric pathogens growth in the small intestine [138,139]. Paneth cells are the primary producers of Reg3γ in the small intestine [140]. Reg3β is generally co-regulated with Reg3γ in mice [141]. Reg3γ and Reg3β prevent E. faecalis [142,143], Yersinia pseudotuberculosis [144,145], and Listeria monocytogenes from colonizing the small intestine [142]. Additional evidence suggests that Reg3 prevents pathogen colonization in the presence of healthy intestinal microbiota. For example, metronidazole-treated mice exhibited increased Reg3β and Reg3γ expression, increased E. coli growth, and decreased Turicibacteraceae abundance compared to untreated mice [138]. To further demonstrate how these C-type lectins regulate bacterial composition on the intestinal epithelial surface, Vaishnava et al. reported that Reg3γ deficient mice showed an increased abundance of mucosal-adhered segmented filamentous bacteria without affecting commensals composition in the small intestine [140]. They found no abnormalities in microbial localization within these experimental mice, indicating that Reg3γ can mediate interactions between host tissues and the microbiota. Reg3γ secretion required activation of the MyD88 pathway [146] and recognition of commensal microbiota by TLRs [147]. Earle and colleagues examined the localization of intestinal microbiota and found that removing microbiota-accessible polysaccharides from the diet resulted in distal colonic mucosa thinning, closer microbial proximity to the epithelium, and increased expression level of Reg3β [148]. These findings were consistent with a previous study examining samples from the duodenum, jejunum, ileum, and colon and demonstrated that MyD88 required syncytium endosymbiont-induced colonic epithelial expression of Reg3β and Reg3γ genes. Myd88 deficiency was associated with shaping the microbiota community and increasing the abundance of segmented filamentous bacteria in the small intestine [149].
6.2.5. Interleukins Production can Enhance Pathogens Clearance
Induction of IL-10, IL-17, and IL-22 production by innate immune cells is vital for maintaining mucosal barrier integrity [127,150]. The proinflammatory cytokine IL-22 has been shown to protect the host from a variety of pathogens, including K. pneumoniae [151], C. rodentium [152], VRE [43], and Plasmodium chabaudi [153]. In the presence of antimicrobial peptides, IL-22 can quickly clear the pathogen from the mucosa [154]. Furthermore, evidence showed that specific intestinal microbiota-derived molecules could rapidly induce IL-22 in response to pathogen invasion by activating the host aryl hydrocarbon receptor [155]. For example, Lactobacillus reuteri promotes IL-22 expression by type-3 innate lymphoid cells in the intestines [156,157]. Other studies have shown that supplementation with three commensal Lactobacillus strains with high tryptophan-metabolizing capability was sufficient to restore IL-22 expression in the intestines [158,159]. These findings show that certain Lactobacillus species use tryptophan as an energy source to produce indole-3-aldehyde, which could activate aryl hydrocarbon receptors on intestinal innate lymphoid cells. Other research has shown that Allobaculum, E. coli, Clostridium, and Bacteroides can also use tryptophan to produce indole-3-aldehyde and promote IL-22 production. Thus, we can conclude from the above findings that a specific microbiome may help prevent the pathogen from colonizing the intestines by inducing IL-22 expression.
IL-17 is another proinflammatory cytokine that restricts S. Typhimurium and C. albicans propagation in the intestines by recruiting neutrophils and inducing antimicrobial peptides [127,160]. Studies have shown that commensal microbiota influence both the abundance and activation of IL-17-producing intraepithelial lymphocytes, with enrichment of gamma delta T cells (γδT) being an essential source of IL-17 production [161,162]. Furthermore, a study comparing germ-free mice to specific-pathogen-free mice found that the number of TCRγδ intraepithelial lymphocytes was lower in germ-free mice [127]. Thus, in addition to activating intraepithelial lymphocytes, the intestinal microbiota may also activate TCRγδ intraepithelial lymphocytes in germ-free mice [163]. Moreover, antibiotic treatment of mice revealed a large number of CD62L-γδ T cells (i.e., activated γδ T cells) within the peritonea of specific-pathogen-free mice, whereas germ-free mice showed fewer CD62L-γδ T cells [164]. In conclusion, the intestinal microbiota influences IL-17-producing TCRγδ intraepithelial lymphocytes, which protect the host from pathogen infection and maintain intestinal homeostasis.
The anti-inflammatory cytokine IL-10 regulates the immune response against pathogens and maintains intestinal homeostasis. According to available evidence, macrophages produce IL-10 in the intestines, which plays an essential role in maintaining intestinal homeostasis. Small intestine macrophages have previously been regulated by dietary antigens, whereas the intestinal microbiota regulates colonic macrophages. For example, studies on germ-free mice and specific-pathogen-free mice revealed that colonic lamina propria in germ-free mice produced less IL-10 (by 50%) [165,166]. Morhardt et al. demonstrated that IL-10 is primarily produced by MHCII + CD64 + Ly6Clow macrophages in animal models during early injury and is involved in restoring the intestinal epithelial barrier [167]. They found that a lack of IL-10 secretion by macrophages compromised the recovery of the small intestine epithelial barrier. Because the microbiota does not regulate IL-10 production by MHCII + CD64 + Ly6Clow macrophages in the small intestine, microbiota depletion did not affect epithelial recovery. These findings highlight the importance of IL-10-producing macrophages in the recovery of intestinal epithelial injury caused by non-steroidal anti-inflammatory drugs. Hayashi and his colleagues demonstrated that Clostridium butyricum induces IL-10 production in macrophage-specific IL-10-deficient mice, which helps to prevent acute colitis. C. butyricum treatment, on the other hand, had no effects on IL-10 production from T cells; however, IL-10-producing F4/80 ± CD11b ± CD11cint macrophages accumulated within inflamed mucosa after C. butyricum treatment [166]. Subsequently, it has been suggested that dietary amino acids directly regulate the production of IL-10 by small intestine macrophages [168]. A significant decrease of IL-10-producing macrophages in the small intestine was observed in mice fed total parenteral nutrition, but IL-10-producing CD4+ T cells remained unaffected. Similarly, nutrient deprivation reduced IL-10 production by monocyte-derived F4/80+ macrophages but did not affect non-monocyte precursor-derived CD103+ dendritic cells. Unlike colonic macrophages, small intestinal macrophages replenishment and IL-10 production were not regulated by intestinal microbiota. However, the reasons for the disparities in intestinal microbiota observed in different animal model systems remain unknown. Nonetheless, we hypothesized that local damage-associated molecular proteins might regulate immune cells more rapidly and strongly after intestinal damage, resulting in either a failure of intestinal microbiota to adjust or masking any microbiota-based regulatory effect.
This review article thoroughly discussed the effects of antibiotic treatment on pathogen colonization and antibiotic-resistant microorganisms, whereas previous studies did not address this issue in such detail. We also highlighted the importance of the intestinal microbiota and its role in protecting immunocompromised patients from nosocomial infections. In addition, we discussed the clinical short- and long-term consequences of antibiotic treatment and the detailed mechanisms of direct and indirect colonization resistance.
7. Conclusions and Future Directions
The intestinal microbiota is a complex and stable microbial community whose close relationship with the host is crucial for maintaining intestinal homeostasis and colonization resistance to pathogens. Disruption of this delicate balance will contribute to disease manifestation. The underlying mechanisms of preventing the pathogen from colonizing the intestines by the intestinal microbiota remain debatable due to the lack of detailed mechanisms and direct evidence. A better understanding of how commensal microbiota interacts with the host is necessary to identify pathogenic and pathophysiological aspects of diseases and to develop a more effective therapeutic agent. FMT therapy, which restores the altered intestinal microbiota to a healthy state, can treat various symptomatic diseases. The benefit of microbiome-based therapy is heavily dependent on the role of dysbiosis in contributing to the disease’s nature. FMT is safe, but it does not guarantee long-term safety because of the risks of transferring antibiotic resistance genes or virulent genes among microbiomes. Although severe systemic side effects from FMT therapy have not been reported, minor GI discomfort such as abdominal pain, vomiting, and nausea are common. As a result, it is strongly encouraged to improve the treatment regimen, administration method, and effective communication with patients. In short, the microbiome research field is still relatively new but rapidly expanding, with several preliminary but promising studies on the modulatory role of the intestinal microbiome in host wellness and diseases. Future research projects in the areas of microbiome-based disease diagnosis, prognosis monitoring, prophylaxis, and treatments have the potential to revolutionize current disease prevention and treatment measures.
Author Contributions
All authors contributed to the concept of this study; X.C., X.X., and Z.B, designed the study; T.S., Z.S., and Z.B. wrote the manuscript; all authors reviewed and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by grants from the major science and technology special project of Yunnan Province, (2019F004) and the National Natural Science Foundation of China (Nos. 81760693 and U1702282).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to the risk of compromising the individual privacy of participants but are available from the corresponding author on reasonable request.
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (Nos. 81760693 and U1702282) and the major science and technology special project of Yunnan Province, (2019F004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Walter, J.; Ley, R. The human gut microbiome: Ecology and recent evolutionary changes. Annu. Rev. Microbiol. 2011, 65, 411–429. [Google Scholar] [CrossRef]
- Frei, R.; Akdis, M.; O’Mahony, L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015, 31, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Baghbani, T.; Nikzad, H.; Azadbakht, J.; Izadpanah, F.; Haddad Kashani, H. Dual and mutual interaction between microbiota and viral infections: A possible treat for COVID-19. Microb. Cell Fact. 2020, 19, 217. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343–381. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Ianiro, G.; Mullish, B.H.; Kelly, C.R.; Kassam, Z.; Kuijper, E.J.; Ng, S.C.; Iqbal, T.H.; Allegretti, J.R.; Bibbò, S.; Sokol, H.; et al. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut 2020, 69, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Antibiotic-associated diarrhea: Epidemiology, trends and treatment. Future Microbiol. 2008, 3, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Flahault, A.; Barbut, F.; Atlan, P.; Lalande, V.; Cousin, P.; Cadilhac, M.; Petit, J.C. Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment. Pharmacol. Ther. 2003, 17, 905–912. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Canani, R.B.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolacek, S.; Orel, R.; Shamir, R.; Vandenplas, Y.; van Goudoever, J.B.; et al. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.D.; Marr, K.A. Clostridium difficile infection among hematopoietic stem cell transplant recipients: Beyond colitis. Curr. Opin. Infect. Dis. 2013, 26, 326–331. [Google Scholar] [CrossRef]
- Alyousef, A.A. Clostridium difficile: Epidemiology, Pathogenicity, and an Update on the Limitations of and Challenges in Its Diagnosis. J. AOAC Int. 2018, 101, 1119–1126. [Google Scholar] [CrossRef]
- Ofori, E.; Ramai, D.; Dhawan, M.; Mustafa, F.; Gasperino, J.; Reddy, M. Community-acquired Clostridium difficile: Epidemiology, ribotype, risk factors, hospital and intensive care unit outcomes, and current and emerging therapies. J. Hosp. Infect. 2018, 99, 436–442. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Neut, C.; Mahieux, S.; Dubreuil, L.J. Antibiotic susceptibility of probiotic strains: Is it reasonable to combine probiotics with antibiotics? Med. Mal. Infect. 2017, 47, 477–483. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Li, T.H.; Qin, Y.; Sham, P.C.; Lau, K.S.; Chu, K.M.; Leung, W.K. Alterations in Gastric Microbiota after H. Pylori Eradication and in Different Histological Stages of Gastric Carcinogenesis. Sci. Rep. 2017, 7, 44935. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.W.; Gan, H.M.; Lee, Y.P.; Leow, A.H.; Azmi, A.N.; Francois, F.; Perez-Perez, G.I.; Loke, M.F.; Goh, K.L.; Vadivelu, J. Helicobacter pylori Eradication Causes Perturbation of the Human Gut Microbiome in Young Adults. PLoS ONE 2016, 11, e0151893. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Kołodziej, M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther. 2015, 42, 1149–1157. [Google Scholar] [CrossRef]
- Losurdo, G.; Cubisino, R.; Barone, M.; Principi, M.; Leandro, G.; Ierardi, E.; Di Leo, A. Probiotic monotherapy and Helicobacter pylori eradication: A systematic review with pooled-data analysis. World J. Gastroenterol. 2018, 24, 139–149. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343–382. [Google Scholar] [CrossRef] [PubMed]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef] [PubMed]
- Casals-Pascual, C.; Vergara, A.; Vila, J. Intestinal microbiota and antibiotic resistance: Perspectives and solutions. Hum. Microbiome J. 2018, 9, 11–15. [Google Scholar] [CrossRef]
- Baron, S.A.; Diene, S.M.; Rolain, J.-M. Human microbiomes and antibiotic resistance. Hum. Microbiome J. 2018, 10. [Google Scholar] [CrossRef]
- Penders, J.; Stobberingh, E.E.; Savelkoul, P.H.; Wolffs, P.F. The human microbiome as a reservoir of antimicrobial resistance. Front. Microbiol. 2013, 4, 87. [Google Scholar] [CrossRef]
- Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther. Adv. Infect. Dis. 2016, 3, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cui, L.; Xu, Y.; Xie, L.; Sun, P.; Liu, C.; Xia, W.; Liu, G. The incidence and drug resistance of Clostridium difficile infection in Mainland China: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 37865. [Google Scholar] [CrossRef] [PubMed]
- Isidro, J.; Menezes, J.; Serrano, M.; Borges, V.; Paixão, P.; Mimoso, M.; Martins, F.; Toscano, C.; Santos, A.; Henriques, A.O.; et al. Genomic Study of a Clostridium difficile Multidrug Resistant Outbreak-Related Clone Reveals Novel Determinants of Resistance. Front. Microbiol. 2018, 9, 2994. [Google Scholar] [CrossRef]
- Kachrimanidou, M.; Tsintarakis, E. Insights into the Role of Human Gut Microbiota in Clostridioides difficile Infection. Microorganisms 2020, 8, 200. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal Carriage Is a Major Reservoir of Klebsiella pneumoniae Infection in Intensive Care Patients. Clin. Infect. Dis. 2017, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Stercz, B.; Farkas, F.B.; Tóth, Á.; Gajdács, M.; Domokos, J.; Horváth, V.; Ostorházi, E.; Makra, N.; Kocsis, B.; Juhász, J.; et al. The influence of antibiotics on transitory resistome during gut colonization with CTX-M-15 and OXA-162 producing Klebsiella pneumoniae ST15. Sci. Rep. 2021, 11, 6335. [Google Scholar] [CrossRef]
- Patriarca, F.; Cigana, C.; Massimo, D.; Lazzarotto, D.; Geromin, A.; Isola, M.; Battista, M.L.; Medeot, M.; Cerno, M.; Sperotto, A.; et al. Risk Factors and Outcomes of Infections by Multidrug-Resistant Gram-Negative Bacteria in Patients Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 333–339. [Google Scholar] [CrossRef]
- Macesic, N.; Gomez-Simmonds, A.; Sullivan, S.B.; Giddins, M.J.; Ferguson, S.A.; Korakavi, G.; Leeds, D.; Park, S.; Shim, K.; Sowash, M.G.; et al. Genomic Surveillance Reveals Diversity of Multidrug-Resistant Organism Colonization and Infection: A Prospective Cohort Study in Liver Transplant Recipients. Clin. Infect. Dis. 2018, 67, 905–912. [Google Scholar] [CrossRef]
- Van Schaik, W. The human gut resistome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140087. [Google Scholar] [CrossRef]
- Yu, J.; Sun, H.; Cao, W.; Han, L.; Song, Y.; Wan, D.; Jiang, Z. Applications of gut microbiota in patients with hematopoietic stem-cell transplantation. Exp. Hematol. Oncol. 2020, 9, 35. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, D.C. Facing a new challenge: The adverse effects of antibiotics on gut microbiota and host immunity. Chin. Med. J. 2019, 132, 1135–1138. [Google Scholar] [CrossRef]
- Abt, M.C.; Buffie, C.G.; Sušac, B.; Becattini, S.; Carter, R.A.; Leiner, I.; Keith, J.W.; Artis, D.; Osborne, L.C.; Pamer, E.G. TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant enterococcus. Sci. Transl. Med. 2016, 8, 327ra25. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Dutta, D.; Lim, S.H. Bidirectional interaction between intestinal microbiome and cancer: Opportunities for therapeutic interventions. Biomark. Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Noor, F.; Kaysen, A.; Wilmes, P.; Schneider, J.G. The Gut Microbiota and Hematopoietic Stem Cell Transplantation: Challenges and Potentials. J. Innate Immun. 2019, 11, 405–415. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.; Vermeulen, L. Stem cells in homeostasis and cancer of the gut. Mol. Cancer 2019, 18, 66. [Google Scholar] [CrossRef]
- Kim, D.; Zeng, M.Y.; Núñez, G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Mechanism-based management for mucositis: Option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016, 9, 2007–2016. [Google Scholar] [CrossRef]
- Cassidy, T.; Humphries, A.R.; Craig, M.; Mackey, M.C. Characterizing Chemotherapy-Induced Neutropenia and Monocytopenia through Mathematical Modelling. Bull. Math. Biol. 2020, 82, 104. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. Jama 2017, 318, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Shogbesan, O.; Poudel, D.R.; Victor, S.; Jehangir, A.; Fadahunsi, O.; Shogbesan, G.; Donato, A. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplant for Clostridium difficile Infection in Immunocompromised Patients. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1394379. [Google Scholar] [CrossRef] [PubMed]
- Wendt, J.M.; Cohen, J.A.; Mu, Y.; Dumyati, G.K.; Dunn, J.R.; Holzbauer, S.M.; Winston, L.G.; Johnston, H.L.; Meek, J.I.; Farley, M.M.; et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics 2014, 133, 651–658. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Oliva-Hemker, M.; Hutfless, S. The prevalence of Clostridium difficile infection in pediatric and adult patients with inflammatory bowel disease. Dig. Dis. Sci. 2014, 59, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Jenq, R.R.; Perales, M.A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2017, 23, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Jouhten, H.; Mattila, E.; Arkkila, P.; Satokari, R. Reduction of Antibiotic Resistance Genes in Intestinal Microbiota of Patients with Recurrent Clostridium difficile Infection after Fecal Microbiota Transplantation. Clin. Infect. Dis. 2016, 63, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Millan, B.; Park, H.; Hotte, N.; Mathieu, O.; Burguiere, P.; Tompkins, T.A.; Kao, D.; Madsen, K.L. Fecal Microbial Transplants Reduce Antibiotic-resistant Genes in Patients with Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2016, 62, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Vincent, C.; Edens, T.J.; Miller, M.; Manges, A.R. Antimicrobial Resistance Gene Acquisition and Depletion Following Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2018, 66, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Schmid, H.; Romanos, A.; Schiffl, H.; Lederer, S.R. Persistent nasal methicillin-resistant staphylococcus aureus carriage in hemodialysis outpatients: A predictor of worse outcome. BMC Nephrol. 2013, 14, 93. [Google Scholar] [CrossRef]
- Prematunge, C.; MacDougall, C.; Johnstone, J.; Adomako, K.; Lam, F.; Robertson, J.; Garber, G. VRE and VSE Bacteremia Outcomes in the Era of Effective VRE Therapy: A Systematic Review and Meta-analysis. Infect. Control. Hosp. Epidemiol. 2016, 37, 26–35. [Google Scholar] [CrossRef]
- Huttner, B.; Haustein, T.; Uçkay, I.; Renzi, G.; Stewardson, A.; Schaerrer, D.; Agostinho, A.; Andremont, A.; Schrenzel, J.; Pittet, D.; et al. Decolonization of intestinal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: A randomized, double-blind, placebo-controlled trial. J. Antimicrob. Chemother. 2013, 68, 2375–2382. [Google Scholar] [CrossRef]
- Lawley, T.D.; Walker, A.W. Intestinal colonization resistance. Immunology 2013, 138, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Kim, S.; Carter, R.A.; Leiner, I.M.; Sušac, B.; Miller, L.; Kim, G.J.; Ling, L.; Pamer, E.G. Cooperating Commensals Restore Colonization Resistance to Vancomycin-Resistant Enterococcus faecium. Cell Host Microbe 2017, 21, 592–602.e594. [Google Scholar] [CrossRef]
- Becattini, S.; Littmann, E.R.; Carter, R.A.; Kim, S.G.; Morjaria, S.M.; Ling, L.; Gyaltshen, Y.; Fontana, E.; Taur, Y.; Leiner, I.M.; et al. Commensal microbes provide first line defense against Listeria monocytogenes infection. J. Exp. Med. 2017, 214, 1973–1989. [Google Scholar] [CrossRef]
- Kommineni, S.; Bretl, D.J.; Lam, V.; Chakraborty, R.; Hayward, M.; Simpson, P.; Cao, Y.; Bousounis, P.; Kristich, C.J.; Salzman, N.H. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 2015, 526, 719–722. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef]
- Mahieu, R.; Cassisa, V.; Hilliquin, D.; Coron, N.; Pailhoriès, H.; Kempf, M.; Joly-Guillou, M.L.; Eveillard, M. Impact of faecal microbiota transplantation on mouse digestive colonization with two extensively resistant bacteria. J. Infect. 2017, 75, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Gosalbes, M.J.; Vázquez-Castellanos, J.F.; Angebault, C.; Woerther, P.L.; Ruppé, E.; Ferrús, M.L.; Latorre, A.; Andremont, A.; Moya, A. Carriage of Enterobacteria Producing Extended-Spectrum β-Lactamases and Composition of the Gut Microbiota in an Amerindian Community. Antimicrob. Agents Chemother. 2016, 60, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Núñez, G. Pathogen Colonization Resistance in the Gut and Its Manipulation for Improved Health. Am. J. Pathol. 2019, 189, 1300–1310. [Google Scholar] [CrossRef]
- Cascales, E.; Buchanan, S.K.; Duché, D.; Kleanthous, C.; Lloubès, R.; Postle, K.; Riley, M.; Slatin, S.; Cavard, D. Colicin biology. Microbiol. Mol. Biol. Rev. 2007, 71, 158–229. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Giladi, I.; Riley, M.A. Persistence of colicinogenic Escherichia coli in the mouse gastrointestinal tract. BMC Microbiol. 2009, 9, 165. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Nuccio, S.P.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, R.A.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef]
- Umu, Ö.C.; Bäuerl, C.; Oostindjer, M.; Pope, P.B.; Hernández, P.E.; Pérez-Martínez, G.; Diep, D.B. The Potential of Class II Bacteriocins to Modify Gut Microbiota to Improve Host Health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef]
- Lamas, A.; Regal, P.; Vázquez, B.; Cepeda, A.; Franco, C.M. Short Chain Fatty Acids Commonly Produced by Gut Microbiota Influence Salmonella enterica Motility, Biofilm Formation, and Gene Expression. Antibiotics 2019, 8, 265. [Google Scholar] [CrossRef]
- Leatham, M.P.; Banerjee, S.; Autieri, S.M.; Mercado-Lubo, R.; Conway, T.; Cohen, P.S. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect. Immun. 2009, 77, 2876–2886. [Google Scholar] [CrossRef]
- Brown, J.R.; Flemer, B.; Joyce, S.A.; Zulquernain, A.; Sheehan, D.; Shanahan, F.; O’Toole, P.W. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Manges, A.R. Antimicrobial Use, Human Gut Microbiota and Clostridium difficile Colonization and Infection. Antibiotics 2015, 4, 230–253. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Fonseca, V.A. Bile acids and metabolic regulation: Mechanisms and clinical responses to bile acid sequestration. Diabetes Care 2009, 32, S237–S245. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Sorg, J.A.; Sonenshein, A.L. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 2008, 190, 2505–2512. [Google Scholar] [CrossRef]
- Horáčková, Š.; Plocková, M.; Demnerová, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Kochan, T.J.; Shoshiev, M.S.; Hastie, J.L.; Somers, M.J.; Plotnick, Y.M.; Gutierrez-Munoz, D.F.; Foss, E.D.; Schubert, A.M.; Smith, A.D.; Zimmerman, S.K.; et al. Germinant Synergy Facilitates Clostridium difficile Spore Germination under Physiological Conditions. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Nie, Y.F.; Hu, J.; Yan, X.H. Cross-talk between bile acids and intestinal microbiota in host metabolism and health. J. Zhejiang Univ. Sci. B 2015, 16, 436–446. [Google Scholar] [CrossRef]
- Martin, G.; Kolida, S.; Marchesi, J.R.; Want, E.; Sidaway, J.E.; Swann, J.R. In Vitro Modeling of Bile Acid Processing by the Human Fecal Microbiota. Front. Microbiol. 2018, 9, 1153. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. Biomed. Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef]
- Huang, J.Y.; Lee, S.M.; Mazmanian, S.K. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe 2011, 17, 137–141. [Google Scholar] [CrossRef]
- Kinoshita, H.; Uchida, H.; Kawai, Y.; Kitazawa, H.; Miura, K.; Shiiba, K.; Horii, A.; Saito, T. Quantitative evaluation of adhesion of lactobacilli isolated from human intestinal tissues to human colonic mucin using surface plasmon resonance (BIACORE assay). J. Appl. Microbiol. 2007, 102, 116–123. [Google Scholar] [CrossRef]
- Sicard, J.F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2018, 9, 3328. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.L.; Thomas, J.; Yan, J.; Baker, R.P.; Shields, D.S.; Xavier, J.B.; Hammer, B.K.; Parthasarathy, R. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc. Natl. Acad. Sci. USA 2018, 115, E3779–E3787. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, Z.; Liu, Y.; Zhu, X.; Liu, M.; Luo, P.; Shi, Y. Type III Secretion 1 Effector Gene Diversity Among Vibrio Isolates from Coastal Areas in China. Front. Cell. Infect. Microbiol. 2020, 10, 301. [Google Scholar] [CrossRef]
- Lindén, S.K.; Bierne, H.; Sabet, C.; Png, C.W.; Florin, T.H.; McGuckin, M.A.; Cossart, P. Listeria monocytogenes internalins bind to the human intestinal mucin MUC2. Arch. Microbiol. 2008, 190, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Yin, L.; Latif, M.B.; Chen, J.; Wang, K.; Geng, Y.; Huang, X.; Abaidullah, M.; Guo, H.; Ouyang, P. Adhesive mechanism of different Salmonella fimbrial adhesins. Microb. Pathog. 2019, 137, 103748. [Google Scholar] [CrossRef] [PubMed]
- Maltby, R.; Leatham-Jensen, M.P.; Gibson, T.; Cohen, P.S.; Conway, T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS ONE 2013, 8, e53957. [Google Scholar] [CrossRef]
- Fabich, A.J.; Jones, S.A.; Chowdhury, F.Z.; Cernosek, A.; Anderson, A.; Smalley, D.; McHargue, J.W.; Hightower, G.A.; Smith, J.T.; Autieri, S.M.; et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 2008, 76, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, J.A.; Wu, K.J.; Hryckowian, A.J.; Bouley, D.M.; Weimer, B.C.; Sonnenburg, J.L. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 2014, 16, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M.J.; Reinap, B.; Lee, M.M.; Comstock, L.E. Human symbionts use a host-like pathway for surface fucosylation. Science 2005, 307, 1778–1781. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.R.; Curtis, M.M.; Ritchie, J.M.; Munera, D.; Waldor, M.K.; Moreira, C.G.; Sperandio, V. Fucose sensing regulates bacterial intestinal colonization. Nature 2012, 492, 113–117. [Google Scholar] [CrossRef]
- Wands, A.M.; Fujita, A.; McCombs, J.E.; Cervin, J.; Dedic, B.; Rodriguez, A.C.; Nischan, N.; Bond, M.R.; Mettlen, M.; Trudgian, D.C.; et al. Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife 2015, 4, e09545. [Google Scholar] [CrossRef]
- Dwivedi, R.; Nothaft, H.; Garber, J.; Xin Kin, L.; Stahl, M.; Flint, A.; van Vliet, A.H.; Stintzi, A.; Szymanski, C.M. L-fucose influences chemotaxis and biofilm formation in Campylobacter jejuni. Mol. Microbiol. 2016, 101, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Sicard, J.F.; Vogeleer, P.; Le Bihan, G.; Rodriguez Olivera, Y.; Beaudry, F.; Jacques, M.; Harel, J. N-Acetyl-glucosamine influences the biofilm formation of Escherichia coli. Gut Pathog. 2018, 10, 26. [Google Scholar] [CrossRef]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E., Jr.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M.; et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef]
- Schumann, S.; Alpert, C.; Engst, W.; Loh, G.; Blaut, M. Dextran sodium sulfate-induced inflammation alters the expression of proteins by intestinal Escherichia coli strains in a gnotobiotic mouse model. Appl. Environ. Microbiol. 2012, 78, 1513–1522. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Winter, S.E.; Winter, M.G.; Xavier, M.N.; Tolstikov, V.; Huseby, D.L.; Sterzenbach, T.; Tsolis, R.M.; Roth, J.R.; Bäumler, A.J. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 17480–17485. [Google Scholar] [CrossRef]
- Kamada, N.; Kim, Y.G.; Sham, H.P.; Vallance, B.A.; Puente, J.L.; Martens, E.C.; Núñez, G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 2012, 336, 1325–1329. [Google Scholar] [CrossRef]
- Garcia, E.C.; Brumbaugh, A.R.; Mobley, H.L. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect. Immun. 2011, 79, 1225–1235. [Google Scholar] [CrossRef]
- Massip, C.; Oswald, E. Siderophore-Microcins in Escherichia coli: Determinants of Digestive Colonization, the First Step Toward Virulence. Front. Cell. Infect. Microbiol. 2020, 10, 381. [Google Scholar] [CrossRef]
- Lasaro, M.A.; Salinger, N.; Zhang, J.; Wang, Y.; Zhong, Z.; Goulian, M.; Zhu, J. F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl. Environ. Microbiol. 2009, 75, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Nissle, A. Old and new experiences on therapeutic successes by restoration of the colonic flora with mutaflor in gastrointestinal diseases. Med. Welt 1961, 29-30, 1519–1523. [Google Scholar] [PubMed]
- Jacobi, C.A.; Malfertheiner, P. Escherichia coli Nissle 1917 (Mutaflor): New insights into an old probiotic bacterium. Dig. Dis 2011, 29, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, Y.S. Recent insights into cellular crosstalk in respiratory and gastrointestinal mucosal immune systems. Immune Netw. 2020, 20, e44. [Google Scholar] [CrossRef]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G327–G333. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Johansson, M.E.; Jakobsson, H.E.; Holmén-Larsson, J.; Schütte, A.; Ermund, A.; Rodríguez-Piñeiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef]
- Schuijt, T.J.; van der Poll, T.; de Vos, W.M.; Wiersinga, W.J. The intestinal microbiota and host immune interactions in the critically ill. Trends Microbiol 2013, 21, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Muzaki, A.; Soncin, I.; Setiagani, Y.A.; Sheng, J.; Tetlak, P.; Karjalainen, K.; Ruedl, C. Long-Lived Innate IL-17-Producing γ/δ T Cells Modulate Antimicrobial Epithelial Host Defense in the Colon. J. Immunol. 2017, 199, 3691–3699. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Cobo, E.R.; Chadee, K. Antimicrobial Human β-Defensins in the Colon and Their Role in Infectious and Non-Infectious Diseases. Pathogens 2013, 2, 177–192. [Google Scholar] [CrossRef]
- Dutta, P.; Das, S. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets Against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2016, 16, 99–129. [Google Scholar] [CrossRef]
- Fusco, A.; Savio, V.; Cammarota, M.; Alfano, A.; Schiraldi, C.; Donnarumma, G. Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium. J. Immunol. Res. 2017, 2017, 6976935. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- Sugi, Y.; Takahashi, K.; Kurihara, K.; Nakano, K.; Kobayakawa, T.; Nakata, K.; Tsuda, M.; Hanazawa, S.; Hosono, A.; Kaminogawa, S. α-Defensin 5 gene expression is regulated by gut microbial metabolites. Biosci. Biotechnol. Biochem. 2017, 81, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Menendez, A.; Willing, B.P.; Montero, M.; Wlodarska, M.; So, C.C.; Bhinder, G.; Vallance, B.A.; Finlay, B.B. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J. Innate Immun. 2013, 5, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Miani, M.; Le Naour, J.; Waeckel-Enée, E.; Verma, S.C.; Straube, M.; Emond, P.; Ryffel, B.; van Endert, P.; Sokol, H.; Diana, J. Gut Microbiota-Stimulated Innate Lymphoid Cells Support β-Defensin 14 Expression in Pancreatic Endocrine Cells, Preventing Autoimmune Diabetes. Cell Metab. 2018, 28, 557–572. [Google Scholar] [CrossRef]
- Yi, H.; Hu, W.; Chen, S.; Lu, Z.; Wang, Y. Cathelicidin-WA Improves Intestinal Epithelial Barrier Function and Enhances Host Defense against Enterohemorrhagic Escherichia coli O157:H7 Infection. J. Immunol. 2017, 198, 1696–1705. [Google Scholar] [CrossRef]
- Ju, T.; Shoblak, Y.; Gao, Y.; Yang, K.; Fouhse, J.; Finlay, B.B.; So, Y.W.; Stothard, P.; Willing, B.P. Initial Gut Microbial Composition as a Key Factor Driving Host Response to Antibiotic Treatment, as Exemplified by the Presence or Absence of Commensal Escherichia coli. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Pierson, M.; Uprety, T.; Zhu, L.; Ran, Z.; Sreenivasan, C.C.; Wang, D.; Hause, B.; Francis, D.H.; Li, F.; et al. Comparison of Porcine Airway and Intestinal Epithelial Cell Lines for the Susceptibility and Expression of Pattern Recognition Receptors upon Influenza Virus Infection. Viruses 2018, 10, 312. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; de Sauvage, F.J.; et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hooper, L.V. Antimicrobial defense of the intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Dessein, R.; Gironella, M.; Vignal, C.; Peyrin-Biroulet, L.; Sokol, H.; Secher, T.; Lacas-Gervais, S.; Gratadoux, J.J.; Lafont, F.; Dagorn, J.C.; et al. Toll-like receptor 2 is critical for induction of Reg3 beta expression and intestinal clearance of Yersinia pseudotuberculosis. Gut 2009, 58, 771–776. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Loonen, L.M.; Witte-Bouma, J.; Korteland-van Male, A.M.; de Bruijn, A.C.; van der Sluis, M.; Lu, P.; Van Goudoever, J.B.; Wells, J.M.; Dekker, J.; et al. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3β, Reg3γ and angiogenin-4. PLoS ONE 2012, 7, e38798. [Google Scholar] [CrossRef]
- Bel, S.; Pendse, M.; Wang, Y.; Li, Y.; Ruhn, K.A.; Hassell, B.; Leal, T.; Winter, S.E.; Xavier, R.J.; Hooper, L.V. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science 2017, 357, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Piermattei, A.; Di Sante, G.; Migliara, G.; Delogu, G.; Ria, F. Immunomodulation by gut microbiota: Role of Toll-like receptor expressed by T cells. J. Immunol. Res. 2014, 2014, 586939. [Google Scholar] [CrossRef]
- Earle, K.A.; Billings, G.; Sigal, M.; Lichtman, J.S.; Hansson, G.C.; Elias, J.E.; Amieva, M.R.; Huang, K.C.; Sonnenburg, J.L. Quantitative Imaging of Gut Microbiota Spatial Organization. Cell Host Microbe 2015, 18, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Tremaroli, V.; Lee, Y.S.; Koren, O.; Nookaew, I.; Fricker, A.; Nielsen, J.; Ley, R.E.; Bäckhed, F. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut 2012, 61, 1124–1131. [Google Scholar] [CrossRef]
- Tyler, C.J.; McCarthy, N.E.; Lindsay, J.O.; Stagg, A.J.; Moser, B.; Eberl, M. Antigen-Presenting Human γδ T Cells Promote Intestinal CD4(+) T Cell Expression of IL-22 and Mucosal Release of Calprotectin. J. Immunol. 2017, 198, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Weiss, I.D.; Zhang, H.H.; Singh, S.P.; Wynn, T.A.; Wilson, M.S.; Farber, J.M. Conventional NK cells can produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia. J. Immunol. 2014, 192, 1778–1786. [Google Scholar] [CrossRef]
- Ota, N.; Wong, K.; Valdez, P.A.; Zheng, Y.; Crellin, N.K.; Diehl, L.; Ouyang, W. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat. Immunol. 2011, 12, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Sellau, J.; Alvarado, C.F.; Hoenow, S.; Mackroth, M.S.; Kleinschmidt, D.; Huber, S.; Jacobs, T. IL-22 dampens the T cell response in experimental malaria. Sci. Rep. 2016, 6, 28058. [Google Scholar] [CrossRef]
- Behnsen, J.; Jellbauer, S.; Wong, C.P.; Edwards, R.A.; George, M.D.; Ouyang, W.; Raffatellu, M. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 2014, 40, 262–273. [Google Scholar] [CrossRef]
- Murray, I.A.; Perdew, G.H. Ligand activation of the Ah receptor contributes to gastrointestinal homeostasis. Curr. Opin. Toxicol. 2017, 2, 15–23. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, A.; Taha-Abdelaziz, K.; Hodgins, D.C.; Read, L.; Nagy, É.; Weese, J.S.; Caswell, J.L.; Parkinson, J.; Sharif, S. Gut microbiota-mediated protection against influenza virus subtype H9N2 in chickens is associated with modulation of the innate responses. Sci. Rep. 2018, 8, 13189. [Google Scholar] [CrossRef]
- Etienne-Mesmin, L.; Chassaing, B.; Gewirtz, A.T. Tryptophan: A gut microbiota-derived metabolites regulating inflammation. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e734. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Prise, I.E.; Wemyss, K.; Schenck, L.P.; Bridgeman, H.M.; McClure, F.A.; Zangerle-Murray, T.; O’Boyle, C.; Barbera, T.A.; Mahmood, F.; et al. Amphiregulin-producing γδ T cells are vital for safeguarding oral barrier immune homeostasis. Proc. Natl. Acad. Sci. USA 2018, 115, 10738–10743. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chung, H.; Troy, E.; Kasper, D.L. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 2010, 7, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Kayama, H.; Jeon, S.G.; Kusu, T.; Isaka, Y.; Rakugi, H.; Yamamoto, M.; Takeda, K. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int. Immunol. 2010, 22, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Sato, T.; Kamada, N.; Mikami, Y.; Matsuoka, K.; Hisamatsu, T.; Hibi, T.; Roers, A.; Yagita, H.; Ohteki, T.; et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 2013, 13, 711–722. [Google Scholar] [CrossRef]
- Morhardt, T.L.; Hayashi, A.; Ochi, T.; Quirós, M.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Kao, J.Y.; et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef]
- Ochi, T.; Feng, Y.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Teitelbaum, D.H.; Kamada, N. Diet-dependent, microbiota-independent regulation of IL-10-producing lamina propria macrophages in the small intestine. Sci. Rep. 2016, 6, 27634. [Google Scholar] [CrossRef]
- Pallav, K.; Dowd, S.E.; Villafuerte, J.; Yang, X.; Kabbani, T.; Hansen, J.; Dennis, M.; Leffler, D.A.; Newburg, D.S.; Kelly, C.P. Effects of polysaccharopeptide from Trametes versicolor and amoxicillin on the gut microbiome of healthy volunteers: A randomized clinical trial. Gut Microbes 2014, 5, 458–467. [Google Scholar] [CrossRef]
- Abeles, S.R.; Jones, M.B.; Santiago-Rodriguez, T.M.; Ly, M.; Klitgord, N.; Yooseph, S.; Nelson, K.E.; Pride, D.T. Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome 2016, 4, 39. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; Forslund, K.; Bork, P.; de Vos, W.M. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 2016, 7, 10410. [Google Scholar] [CrossRef]
- Engelbrektson, A.; Korzenik, J.R.; Pittler, A.; Sanders, M.E.; Klaenhammer, T.R.; Leyer, G.; Kitts, C.L. Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy. J. Med. Microbiol. 2009, 58, 663–670. [Google Scholar] [CrossRef]
- Stewardson, A.J.; Gaïa, N.; François, P.; Malhotra-Kumar, S.; Delémont, C.; Martinez de Tejada, B.; Schrenzel, J.; Harbarth, S.; Lazarevic, V. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: A culture-free analysis of gut microbiota. Clin. Microbiol. Infect. 2015, 21, 344.e341. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, J.; Xavier, B.B.; Stewardson, A.; Coenen, S.; Godycki-Cwirko, M.; Adriaenssens, N.; Kowalczyk, A.; Lammens, C.; Harbarth, S.; Goossens, H.; et al. Metagenomic analysis of the impact of nitrofurantoin treatment on the human faecal microbiota. J. Antimicrob. Chemother. 2015, 70, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Mättö, J.; Maukonen, J.; Alakomi, H.L.; Suihko, M.L.; Saarela, M. Influence of oral doxycycline therapy on the diversity and antibiotic susceptibility of human intestinal bifidobacterial population. J. Appl. Microbiol. 2008, 105, 279–289. [Google Scholar] [CrossRef]
- Rashid, M.U.; Panagiotidis, G.; Bäckström, T.; Weintraub, A.; Nord, C.E. Ecological impact of doxycycline at low dose on normal oropharyngeal and intestinal microflora. Int. J. Antimicrob. Agents 2013, 41, 352–357. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).