Metformin and Vitamin D Modulate Inflammation and Autophagy during Adipose-Derived Stem Cell Differentiation
Abstract
:1. Introduction
2. Results
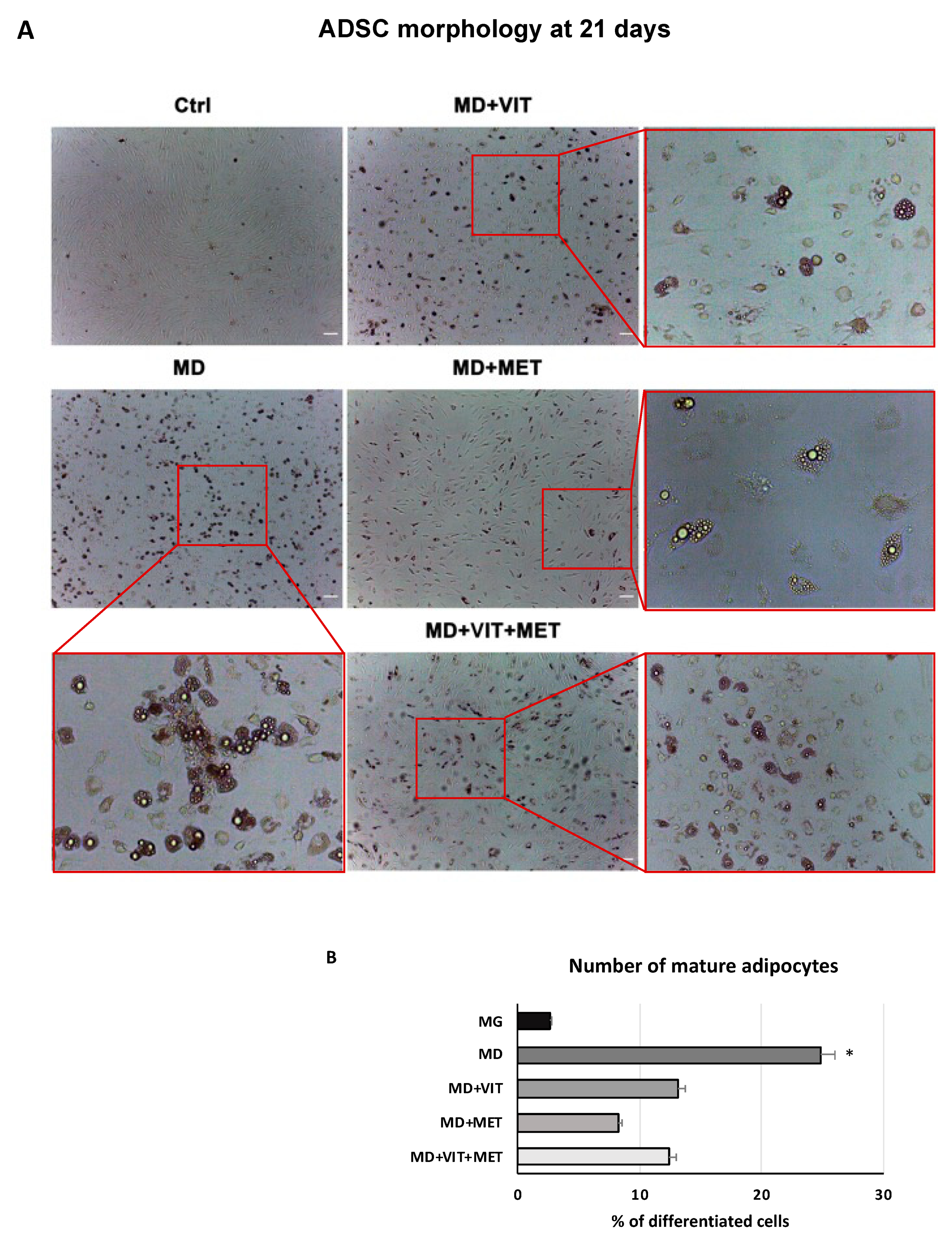
2.1. Metformin and Vitamin D Inhibit ADSC Adipogenic Differentiation
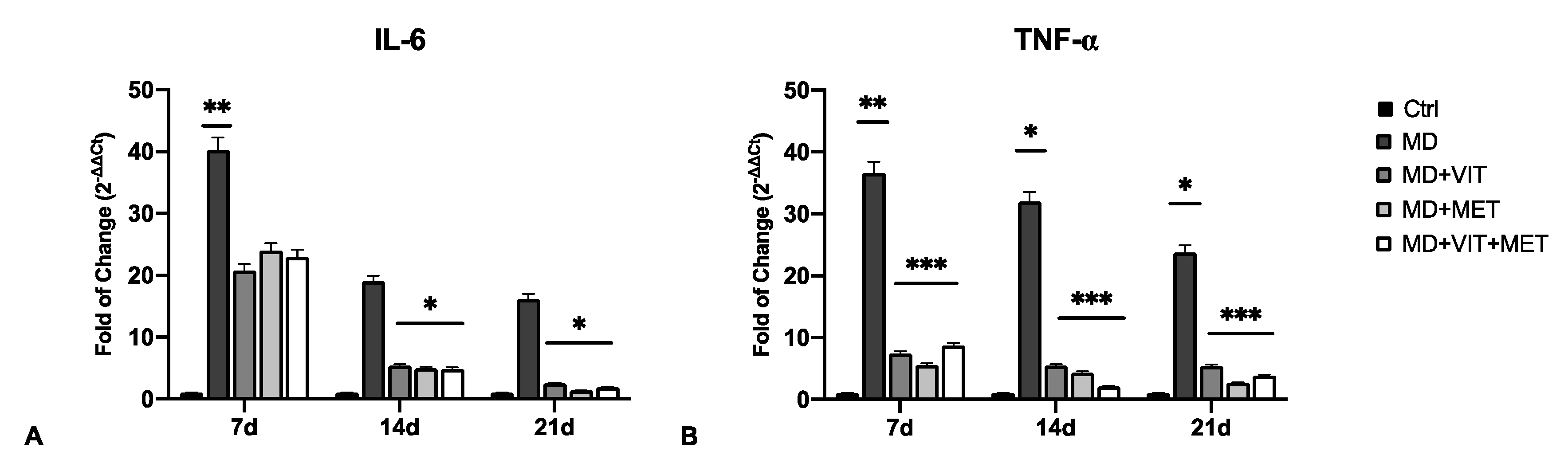
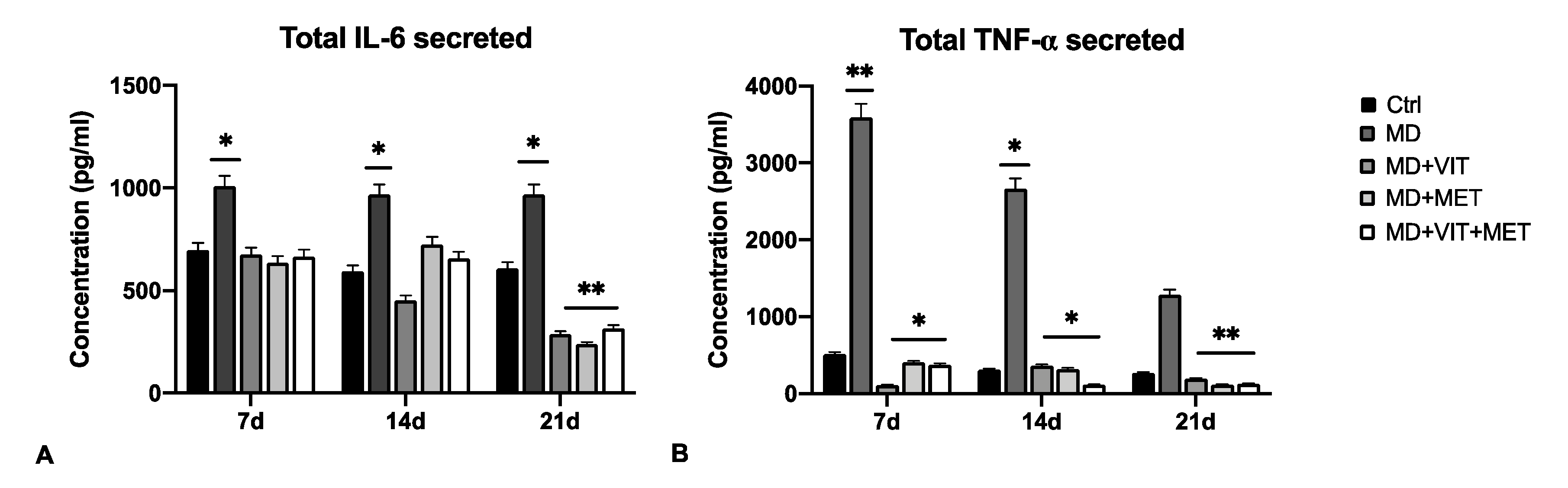
2.2. The Combination of Metformin and Vitamin D Inhibit the Release of Pro-Inflammatory Cytokines
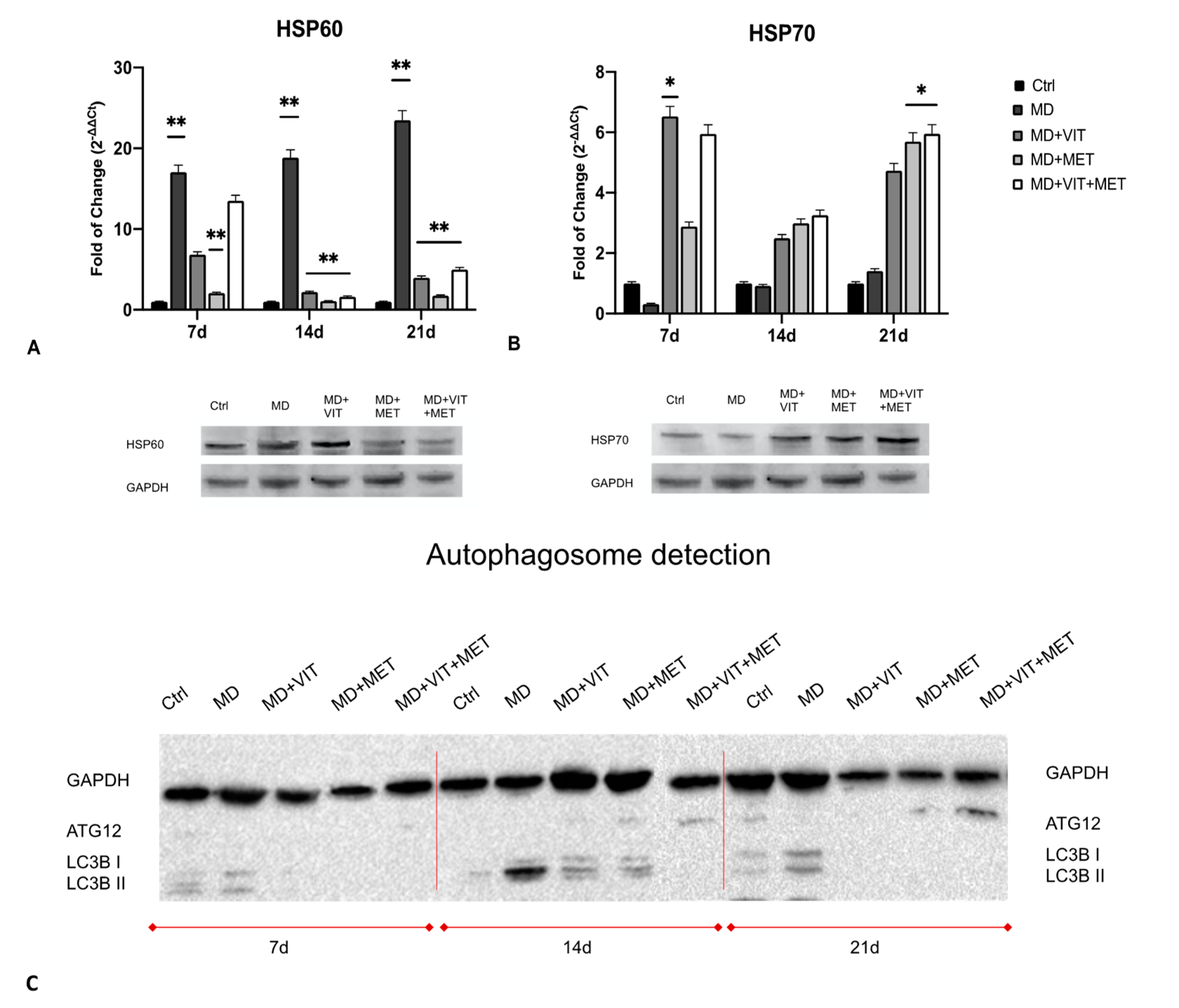
2.3. Exposure to Metformin Alone or Together with Vitamin D Modulates the Expression of HSPs and Autophagy
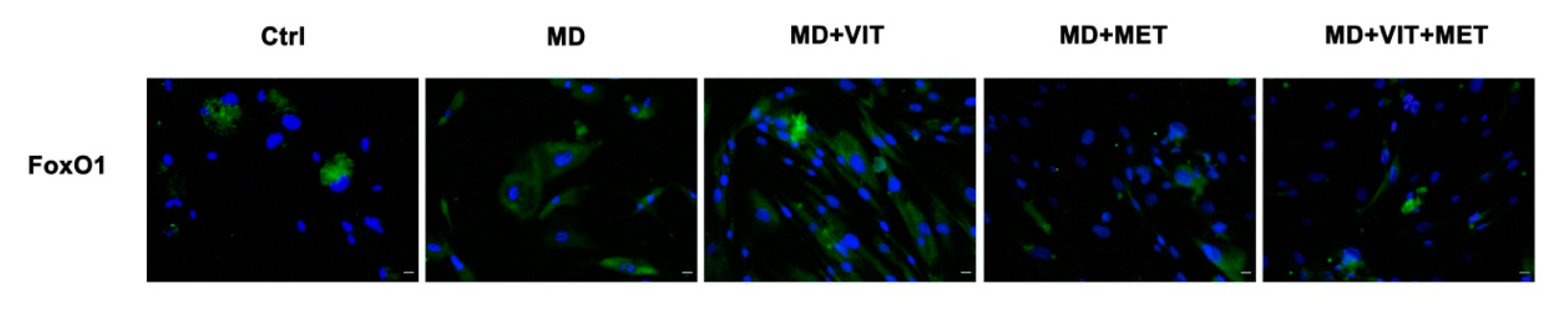
2.4. Exposure to Metformin Alone or Together with Vitamin D Modulates the Expression of FoxO1
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culturing Conditions
4.2. Gene Expression Analysis
4.3. ELISA Assay
4.4. Autophagosome Detection Assays
4.5. Immunostaining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 1–26. [Google Scholar]
- Wankhade, U.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in Adipose-Derived Stem Cells Isolation, Characterization, and Application in Regenerative Tissue Engineering. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Pant, R.; Firmal, P.; Shah, V.K.; Alam, A.; Chattopadhyay, S. Epigenetic Regulation of Adipogenesis in Development of Metabolic Syndrome. Front. Cell Dev. Biol. 2021, 8. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimini, F.A.; Barchetta, I.; Ciccarelli, G.; Leonetti, F.; Silecchia, G.; Chiappetta, C.; Di Cristofano, C.; Capoccia, D.; Bertoccini, L.; Ceccarelli, V.; et al. Adipose tissue remodelling in obese subjects is a determinant of presence and severity of fatty liver disease. Diabetes Metab. Res. Rev. 2021, 37, e3358. [Google Scholar] [CrossRef] [PubMed]
- MLongo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of adipose tissue inflammation to the development of type 2 diabetes HHS Public Access. Physiol. Behav. 2019, 9, 1–58. [Google Scholar]
- Vázquez-Vela, M.E.F.; Torres, N.; Tovar, A.R. White Adipose Tissue as Endocrine Organ and Its Role in Obesity. Arch. Med. Res. 2008, 39, 715–728. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Munekata, K.; Sakamoto, K. Forkhead transcription factor Foxo1 is essential for adipocyte differentiation. Vitr. Cell. Dev. Biol. Anim. 2009, 45, 642–651. [Google Scholar] [CrossRef]
- Ioannilli, L.; Ciccarone, F.; Ciriolo, M.R. Adipose Tissue and FoxO1: Bridging Physiology and Mechanisms. Cells 2020, 9, 849. [Google Scholar] [CrossRef] [Green Version]
- Barbato, D.L.; Ioannilli, L.; Aquilano, K.; Ciccarone, F.; Rosina, M.; Ciriolo, M.R. FoxO1 localizes to mitochondria of adipose tissue and is affected by nutrient stress. Metabolism 2019, 95, 84–92. [Google Scholar] [CrossRef]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef]
- Cui, X.B.; Chen, S.Y. White adipose tissue browning and obesity. J. Biomed. Res. 2017, 31, 1. [Google Scholar]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Gavaldà-Navarro, A.; Giralt, M. Toward an Understanding of How Immune Cells Control Brown and Beige Adipobiology. Cell Metab. 2018, 27, 954–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habich, C.; Sell, H. Heat shock proteins in obesity: Links to cardiovascular disease. Horm. Mol. Biol. Clin. Investig. 2015, 21. [Google Scholar] [CrossRef]
- Gülden, E.; Mollérus, S.; Brüggemann, J.; Burkart, V.; Habich, C. Heat shock protein 60 induces inflammatory mediators in mouse adipocytes. FEBS Lett. 2008, 582, 2731–2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Märker, T.; Sell, H.; Zilleßen, P.; Glöde, A.; Kriebel, J.; Ouwens, D.M.; Pattyn, P.; Ruige, J.; Famulla, S.; Roden, M.; et al. Heat shock protein 60 as a mediator of adipose tissue inflammation and insulin resistance. Diabetes 2012, 61, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Matz, J.M.; LaVoi, K.P.; Blake, M.J. Adrenergic regulation of the heat shock response in brown adipose tissue. J. Pharmacol. Exp. Ther. 1996, 277, 1751–1758. [Google Scholar]
- Di Naso, F.C.; Porto, R.R.; Fillmann, H.S.; Maggioni, L.; Padoin, A.V.; Ramos, R.J.; Mottin, C.C.; Bittencourt, A.; Marroni, N.A.P.; De Bittencourt, P.I.H.; et al. Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity 2014, 23, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.C. Role of heat shock proteins in stem cell behavior. Prog. Mol. Biol. Transl. Sci. 2012, 111, 305–322. [Google Scholar] [PubMed] [Green Version]
- Dokladny, K.; Myers, O.; Moseley, P.L. Heat shock response and Autophagy—Cooperation and control. Autophagy 2015, 11, 200–213. [Google Scholar] [CrossRef] [Green Version]
- Ro, S.H.; Jang, Y.; Bae, J.; Kim, I.M.; Schaecher, C.; Shomo, Z.D. Autophagy in adipocyte browning: Emerging drug target for intervention in obesity. Front. Physiol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, J.; Kern, M.; Klöting, N.; Paeschke, S.; Rudich, A.; Haim, Y.; Gericke, M.; Serke, H.; Stumvoll, M.; Bechmann, I.; et al. Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol. Cell. Endocrinol. 2015, 409, 21–32. [Google Scholar] [CrossRef]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Investig. 2009, 119, 3329–3339. [Google Scholar] [CrossRef] [Green Version]
- Clemente-Postigo, M.; Tinahones, A.; Bekay, R.E.; Malagón, M.M.; Tinahones, F.J. The role of Autophagy in white adipose tissue function: Implications for metabolic health. Metabolites 2020, 10, 179. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.-H.; Kang, Y.-H.; Kim, J.S.; Yun, S.-C.; Kang, S.-W.; Song, Y. Suppression of Brown Adipocyte Autophagy Improves Energy Metabolism by Regulating Mitochondrial Turnover. Int. J. Mol. Sci. 2019, 20, 3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basoli, V.; Santaniello, S.; Cruciani, S.; Ginesu, G.C.; Cossu, M.L.; Delitala, A.P.; Serra, P.A.; Ventura, C.; Maioli, M. Melatonin and Vitamin D Interfere with the Adipogenic Fate of Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2017, 18, 981. [Google Scholar] [CrossRef] [Green Version]
- Santaniello, S.; Cruciani, S.; Basoli, V.; Balzano, F.; Bellu, E.; Garroni, G.; Ginesu, G.C.; Cossu, M.L.; Facchin, F.; Delitala, A.P.; et al. Melatonin and Vitamin D Orchestrate Adipose Derived Stem Cell Fate by Modulating Epigenetic Regulatory Genes. Int. J. Med. Sci. 2018, 15, 1631. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, S.; Garroni, G.; Balzano, F.; Pala, R.; Bellu, E.; Cossu, M.L.; Ginesu, G.C.; Ventura, C.; Maioli, M. Tuning Adipogenic Differentiation in ADSCs by Metformin and Vitamin D: Involvement of miRNAs. Int. J. Mol. Sci. 2020, 21, 6181. [Google Scholar] [CrossRef]
- Rastaldo, R.; Vitale, E.; Giachino, C. Dual Role of Autophagy in Regulation of Mesenchymal Stem Cell Senescence. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Panina, Y.; Yakimov, A.S.; Komleva, Y.K.; Morgun, A.V.; Lopatina, O.L.; Malinovskaya, N.A.; Shuvaev, A.N.; Salmin, V.V.; Taranushenko, T.E.; Salmina, A.B. Plasticity of Adipose Tissue-Derived Stem Cells and Regulation of Angiogenesis. Front. Physiol. 2018, 9, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, L.; Yuan, Y.; Shayan, R.; Greening, D.W.; Karnezis, T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front. Pharmacol. 2020, 11, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruciani, S.; Santaniello, S.; Montella, A.; Ventura, C.; Maioli, M. Orchestrating stem cell fate: Novel tools for regenerative medicine. World J. Stem Cells 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-L.; Liu, L. Effect of metformin on stem cells: Molecular mechanism and clinical prospect. World J. Stem Cells 2020, 12, 1455–1473. [Google Scholar] [CrossRef]
- Hyun, B.; Shin, S.; Lee, A.; Lee, S.; Song, Y.; Ha, N.-J.; Cho, K.-H.; Kim, K. Metformin Down-regulates TNF-α Secretion via Suppression of Scavenger Receptors in Macrophages. Immune Netw. 2013, 13, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, D.C.; Whitham, M.; Febbraio, M.A. Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol. Metab. 2014, 3, 781–793. [Google Scholar] [CrossRef]
- Sell, H.; Poitou, C.; Habich, C.; Bouillot, J.-L.; Eckel, J.; Clément, K. Heat Shock Protein 60 in Obesity: Effect of Bariatric Surgery and its Relation to Inflammation and Cardiovascular Risk. Obesity 2017, 25, 2108–2114. [Google Scholar] [CrossRef]
- Qu, B.; Jia, Y.; Liu, Y.; Wang, H.; Ren, G.; Wang, H. The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: A literature review. Cell Stress Chaperon. 2015, 20, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Xing, B.; Wang, L.; Li, Q.; Cao, Y.; Dong, X.; Liang, J.; Wu, X. Hsp70 plays an important role in high-fat diet induced gestational hyperglycemia in mice. J. Physiol. Biochem. 2015, 71, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Kanninen, T.T.; Sisti, G. The Role of Hsp70 in the Regulation of Autophagy in Gametogenesis, Pregnancy, and Parturition. Role Heat Shock. Proteins Reprod. Syst. Dev. Funct. 2017, 222, 117–127. [Google Scholar]
- Stienstra, R.; Haim, Y.; Riahi, Y.; Netea, M.; Rudich, A.; Leibowitz, G. Autophagy in adipose tissue and the beta cell: Implications for obesity and diabetes. Diabetologia 2014, 57, 1505–1516. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.; Zorzano, A. Role of autophagy in the regulation of adipose tissue biology. Cell Cycle 2019, 18, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Karise, I.; Bargut, T.C.; Del Sol, M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed. Pharmacother. 2019, 111, 1156–1165. [Google Scholar] [CrossRef]
- Maixner, N.; Kovsan, J.; Harman-Boehm, I.; Blüher, M.; Bashan, N.; Rudich, A. Autophagy in Adipose Tissue. Obes. Facts 2012, 5, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, Z.; Germič, N.; Ademi, H.; Frangež, Ž.; Felser, A.; Peng, S.; Riether, C.; Djonov, V.; Nuoffer, J.M.; et al. ATG12 deficiency leads to tumor cell oncosis owing to diminished mitochondrial biogenesis and reduced cellular bioenergetics. Cell Death Differ. 2020, 27, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kitamura, T.; Kitamura, Y.; Biggs, W.H., III; Arden, K.C.; Accili, D. The forkhead transcription factor Fox01 regulates adipocyte differentiation. Dev. Cell 2003, 4, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Zhang, Y.; Xin, N.; Yuan, Y.; Zhang, Q.; Gong, P.; Wu, Y. 1α,25-Dihydroxyvitamin D3 promotes bone formation by promoting nuclear exclusion of the FoxO1 transcription factor in diabetic mice. J. Biol. Chem. 2017, 292, 20270–20280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Tao, Z.; Zheng, L.D.; Brooke, J.P.; Smith, C.M.; Liu, N.; Long, Y.C.; Cheng, Z. FoxO1 interacts with transcription factor EB and differentially regulates mitochondrial uncoupling proteins via autophagy in adipocytes. Cell Death Discov. 2016, 2, 16066. [Google Scholar] [CrossRef] [Green Version]
| Primer Name | Forward | Reverse |
|---|---|---|
| hGAPDH | GAGTCAACGGAATTTGGTCGT | GACAAGCTTCCCGTTCTCAG |
| IL-6 | TCTCAACCCCAATAA | GCCGTCGAGGATGTA |
| TNF-α | CCTCAGACGCCACAT | GAGGGCTGATTAGAGAGA |
| HSP60 | GGGCATCTGTAACTCTGTCTT | TAAAAGGAAAAGGTGACAAGG |
| HSP70 | CACAGCGACGTAGCAGCTCT | ATGTCGGTGGTGGGCATAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruciani, S.; Garroni, G.; Pala, R.; Cossu, M.L.; Ginesu, G.C.; Ventura, C.; Maioli, M. Metformin and Vitamin D Modulate Inflammation and Autophagy during Adipose-Derived Stem Cell Differentiation. Int. J. Mol. Sci. 2021, 22, 6686. https://doi.org/10.3390/ijms22136686
Cruciani S, Garroni G, Pala R, Cossu ML, Ginesu GC, Ventura C, Maioli M. Metformin and Vitamin D Modulate Inflammation and Autophagy during Adipose-Derived Stem Cell Differentiation. International Journal of Molecular Sciences. 2021; 22(13):6686. https://doi.org/10.3390/ijms22136686
Chicago/Turabian StyleCruciani, Sara, Giuseppe Garroni, Renzo Pala, Maria Laura Cossu, Giorgio Carlo Ginesu, Carlo Ventura, and Margherita Maioli. 2021. "Metformin and Vitamin D Modulate Inflammation and Autophagy during Adipose-Derived Stem Cell Differentiation" International Journal of Molecular Sciences 22, no. 13: 6686. https://doi.org/10.3390/ijms22136686
APA StyleCruciani, S., Garroni, G., Pala, R., Cossu, M. L., Ginesu, G. C., Ventura, C., & Maioli, M. (2021). Metformin and Vitamin D Modulate Inflammation and Autophagy during Adipose-Derived Stem Cell Differentiation. International Journal of Molecular Sciences, 22(13), 6686. https://doi.org/10.3390/ijms22136686







