CYLD Inhibits the Development of Skin Squamous Cell Tumors in Immunocompetent Mice
Abstract
:1. Introduction
2. Results
2.1. Expression of the Transgene in the Skin of K5-CYLDwt Mice
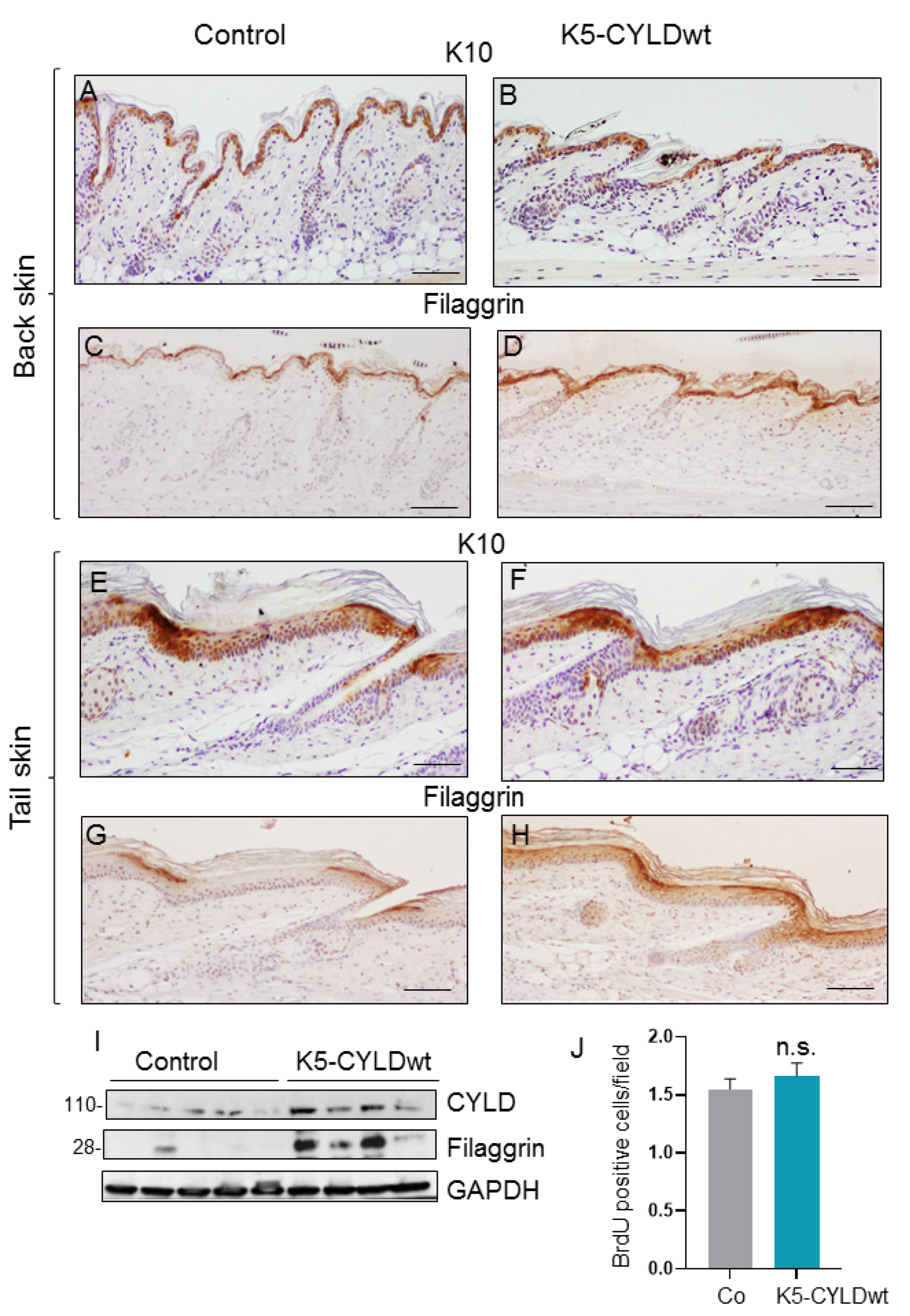
2.2. CYLDwt Transgene Expression Favors Epidermal Differentiation in K5-CYLDwt Mice
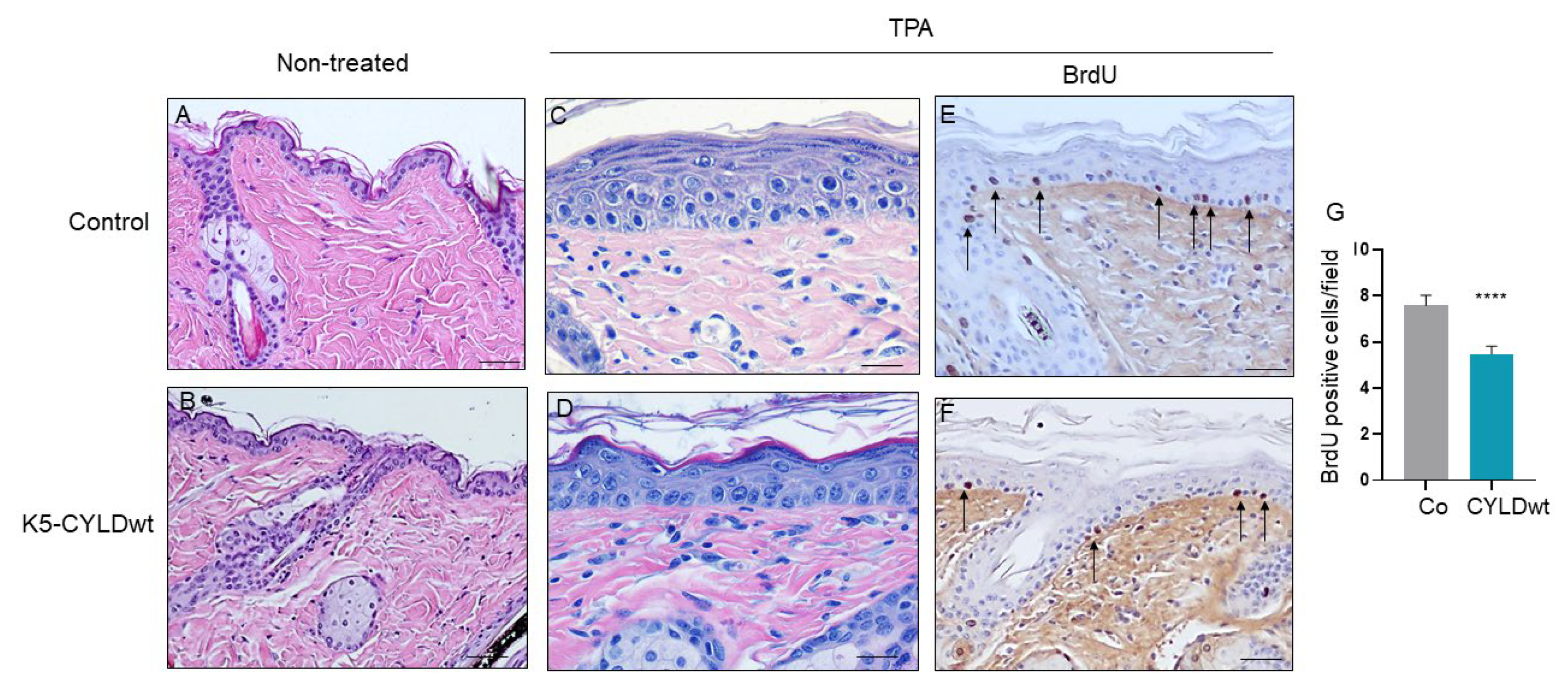
2.3. The Skin of K5-CYLDwt Mice Shows Decreased NF-kB Activation and Increased Protection Against Harmful External Stimuli
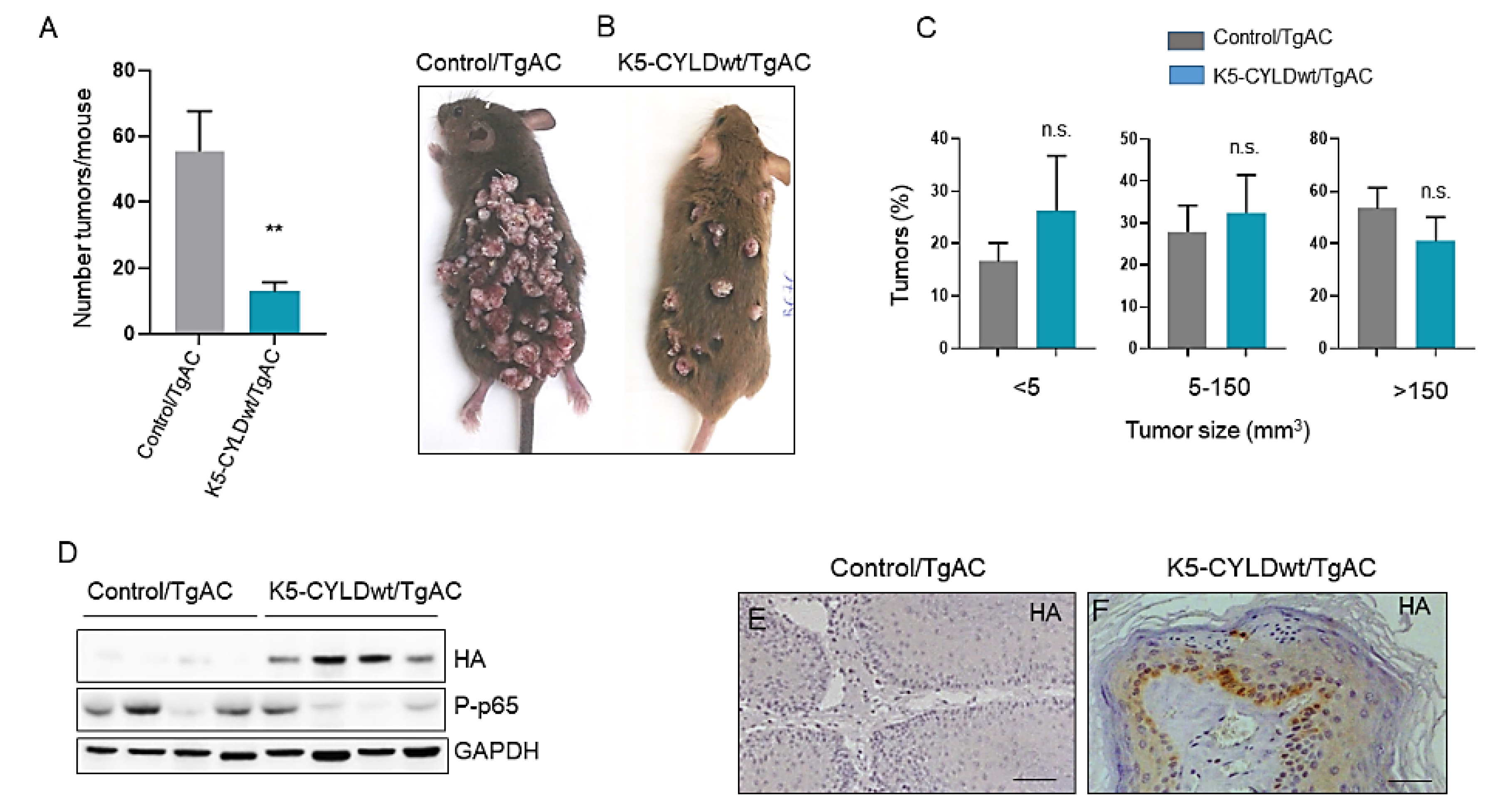
2.4. Inhibition of Skin Squamous Cell Tumor Development in K5-CYLDwt Mice
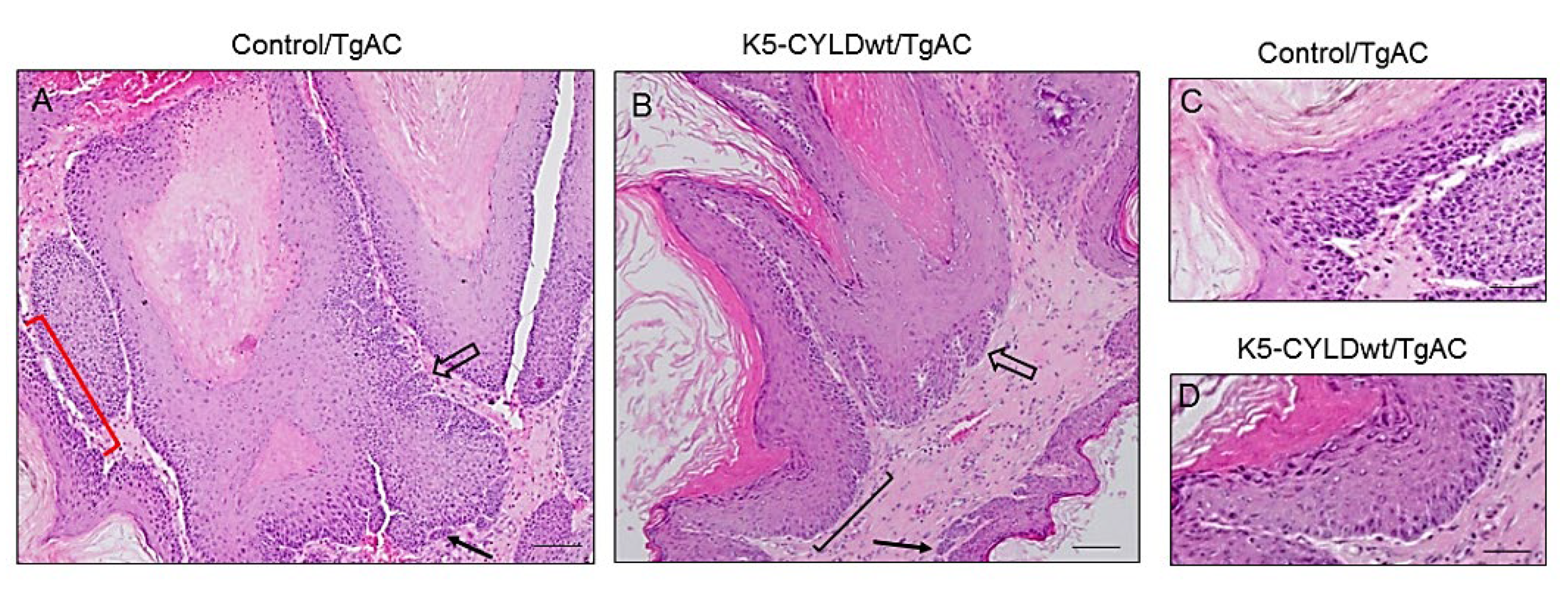
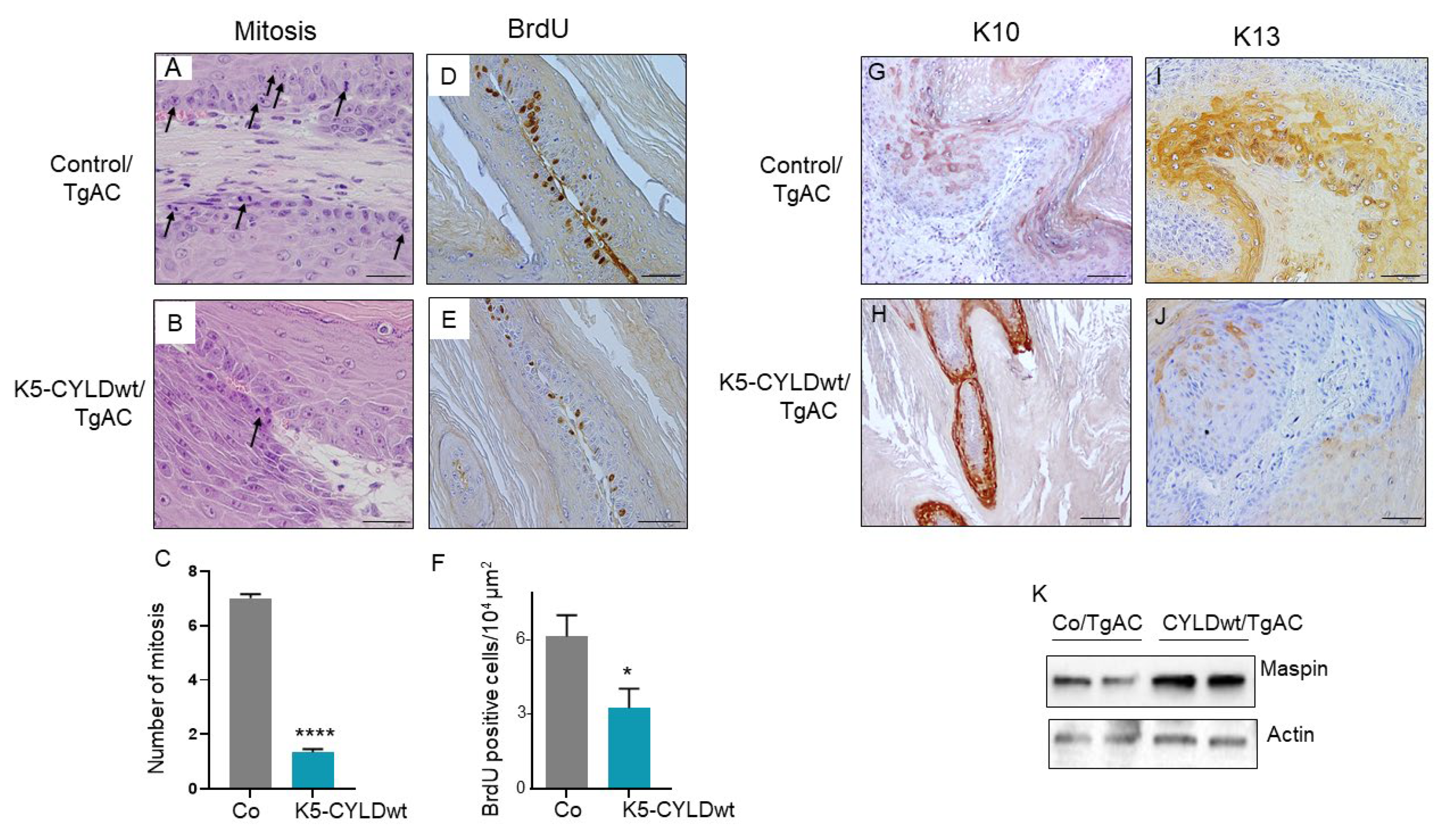
2.5. Skin Squamous K5-CYLDwt/TgAC Cell Tumors Show Reduced Proliferation and Increased Differentiation
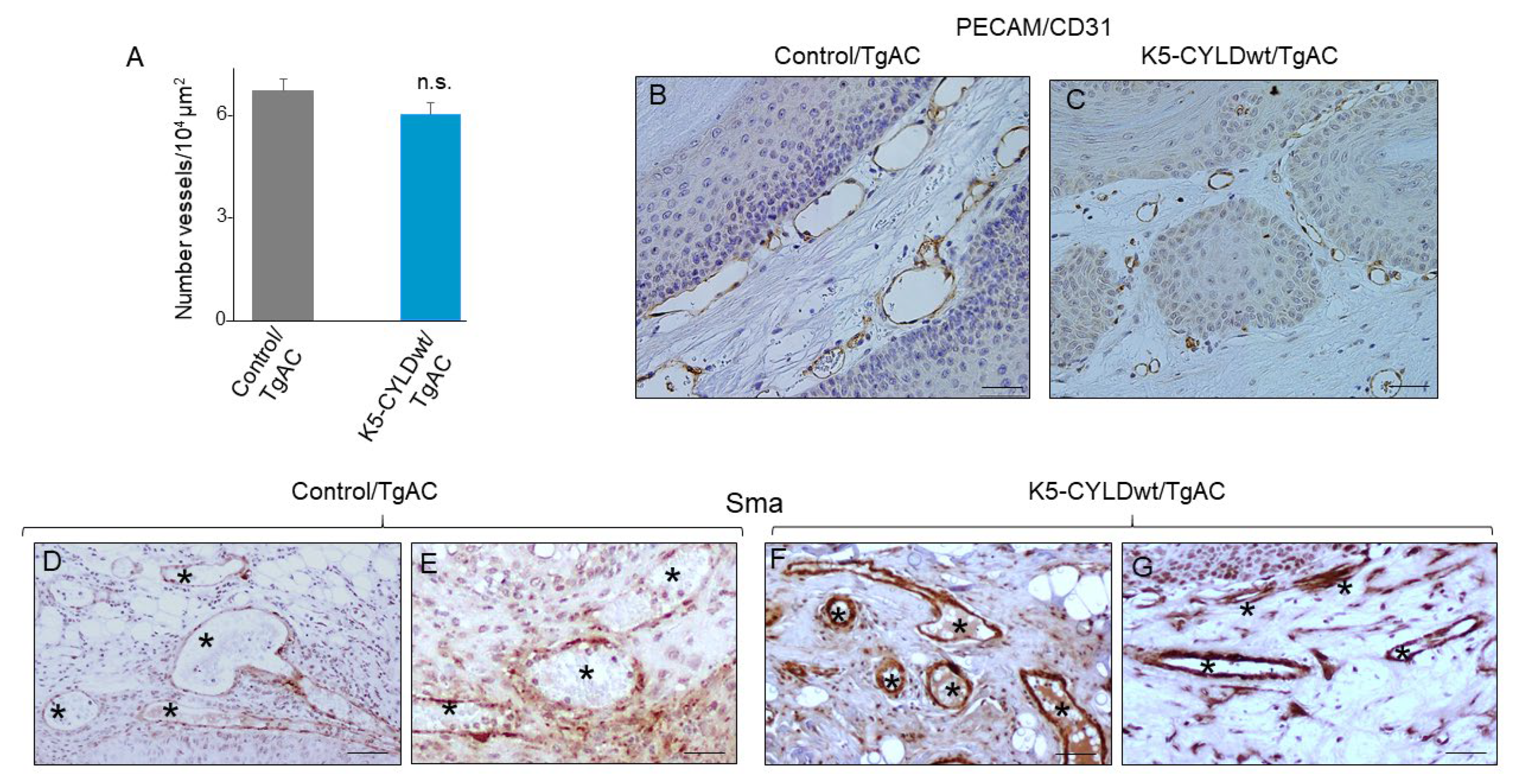
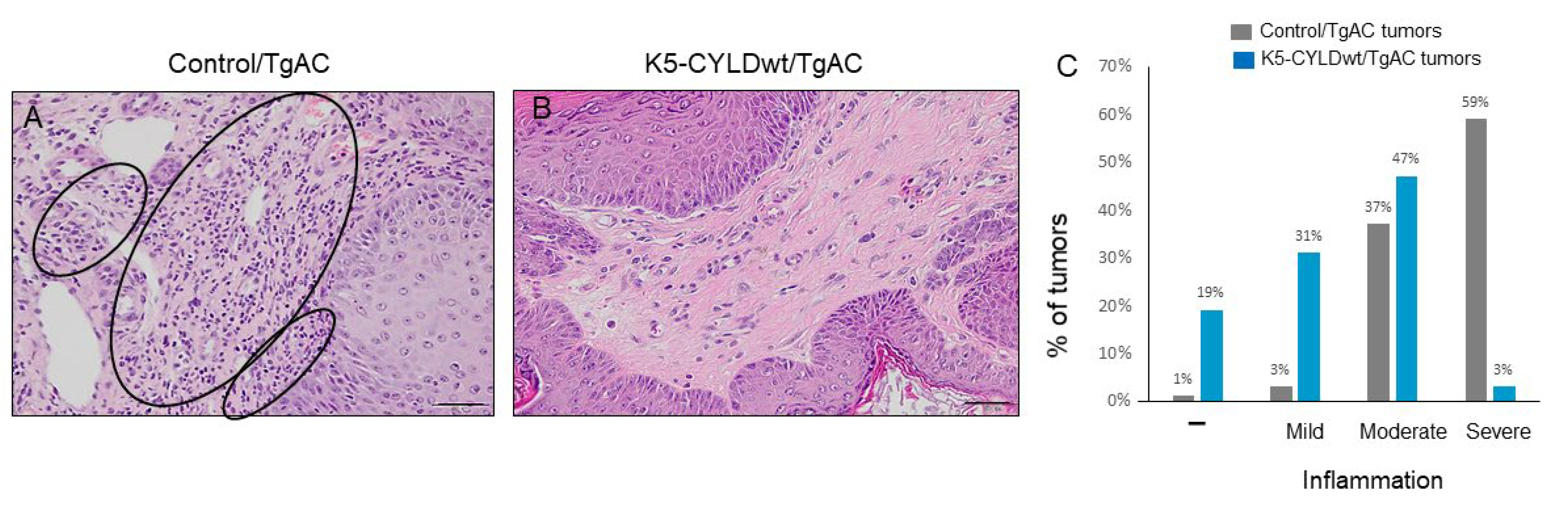
2.6. Skin Squamous Cell Tumors Developed in K5-CYLDwt/TgAC Mice Show Less Tumor Angiogenesis and Lower Inflammation
3. Discussion
3.1. Inhibition of NF-kB Activation in Keratinocytes and Skin Squamous Cell Tumors of K5-CYLDwt/TgAC Mice
3.2. More Differentiated and Less Proliferative Phenotype of K5-CYLDwt/TgAC Tumors
3.3. Diminished Tumor Inflammation and Angiogenesis in K5-CYLDwt/TgAC-Derived Tumors
3.4. Relevance of Wild Type CYLD Expression for Clinical Management of Skin SCCs
4. Materials and Methods
4.1. Generation of Transgenic Mice
4.2. Histology and Immunohistochemical Staining
4.3. Western Blot Analysis
4.4. TNF-α In Vivo Treatment
4.5. TPA Hyperplastic Treatment
4.6. Primary Keratinocyte Cultures
4.7. Apoptosis Assays in Primary Keratinocytes
4.8. Carcinogenesis Assays
4.9. BrdU Labeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCC | basal cell carcinomas |
| BrdU | 5-bromo-2-desoxiuridina |
| Chx | cycloheximide |
| DMBA | 7, 12-dimethylbenzanthracene |
| CYLD | cylindromatosis |
| DUB | deubiquitinase |
| HA | hemagglutinin A |
| H&E | hematoxylin & eosin |
| HF | hair follicles |
| K5 | keratin 5 |
| NMSC | non melanoma skin cancer |
| ORS | outer root sheath |
| SRF | Serum Response Factor |
| SCC | squamous cell carcinomas |
| Sma | α-smooth muscle actin |
| SEM | standard error of the mean |
| TNF-α | tumor necrosis factor-α |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| WB | Western blot |
References
- Mathis, J.B.; Lai, Y.; Qu, C.; Janicki, S.J.; Cui, T. CYLD-mediated signaling and diseases. Curr. Drug Targets 2015, 16, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brummelkamp, T.R.; Nijman, S.M.; Dirac, A.M.; Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 2003, 424, 797–801. [Google Scholar] [CrossRef]
- Kovalenko, A.; Chable-Bessia, C.; Cantarella, G.; Israel, A.; Wallach, D.; Courtois, G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 2003, 424, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Trompouki, E.; Hatzivassiliou, E.; Tsichritzis, T.; Farmer, H.; Ashworth, A.; Mosialos, G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 2003, 424, 793–796. [Google Scholar] [CrossRef] [PubMed]
- AACR Project Genie Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [Green Version]
- Hellerbrand, C.; Bumes, E.; Bataille, F.; Dietmaier, W.; Massoumi, R.; Bosserhoff, A.K. Reduced expression of CYLD in human colon and hepatocellular carcinomas. Carcinogenesis 2007, 28, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, H.; Okabe, H.; Beppu, T.; Chikamoto, A.; Hayashi, H.; Imai, K.; Mima, K.; Nakagawa, S.; Yokoyama, N.; Ishiko, T.; et al. CYLD downregulation is correlated with tumor development in patients with hepatocellular carcinoma Down-regulation of the Tumor Suppressor CYLD Enhances the Transformed Phenotype of Human Breast Cancer Cells. Mol. Clin. Oncol. 2013, 1, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Orfanidou, T.; Xanthopoulos, K.; Dafou, D.; Pseftogas, A.; Hadweh, P.; Psyllaki, C.; Hatzivassiliou, E.; Mosialos, G. Down-regulation of the Tumor Suppressor CYLD Enhances the Transformed Phenotype of Human Breast Cancer Cells. Anticancer Res. 2017, 37, 3493–3503. [Google Scholar]
- Fuchs, E. Epidermal differentiation: The bare essentials. J. Cell Biol. 1990, 111, 2807–2814. [Google Scholar] [CrossRef]
- Alameda, J.P.; Moreno-Maldonado, R.; Navarro, M.; Bravo, A.; Ramirez, A.; Page, A.; Jorcano, J.L.; Fernandez-Acenero, M.J.; Casanova, M.L. An inactivating CYLD mutation promotes skin tumor progression by conferring enhanced proliferative, survival and angiogenic properties to epidermal cancer cells. Oncogene 2010, 29, 6522–6532. [Google Scholar] [CrossRef] [Green Version]
- Alameda, J.P.; Ramirez, A.; Garcia-Fernandez, R.A.; Navarro, M.; Page, A.; Segovia, J.C.; Sanchez, R.; Suarez-Cabrera, C.; Paramio, J.M.; Bravo, A.; et al. Premature aging and cancer development in transgenic mice lacking functional CYLD. Aging 2019, 11, 127–159. [Google Scholar] [CrossRef]
- de Marval, P.M.; Lutfeali, S.; Jin, J.Y.; Leshin, B.; Selim, M.A.; Zhang, J.Y. CYLD Inhibits Tumorigenesis and Metastasis by Blocking JNK/AP1 Signaling at Multiple Levels. Cancer Prev. Res. 2011, 4, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Masoumi, K.C.; Shaw-Hallgren, G.; Massoumi, R. Tumor Suppressor Function of CYLD in Nonmelanoma Skin Cancer. J. Skin Cancer 2011, 2011, 614097. [Google Scholar] [CrossRef] [PubMed]
- Limmer, B.L. Nonmelanoma skin cancer: Today’s epidemic. Tex. Med. 2001, 97, 56–58. [Google Scholar] [PubMed]
- Karia, P.S.; Han, J.; Schmults, C.D. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef]
- Christenson, L.J.; Borrowman, T.A.; Vachon, C.M.; Tollefson, M.M.; Otley, C.C.; Weaver, A.L.; Roenigk, R.K. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA 2005, 294, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, T.; Roth, J.A.; Maxwell, S.A. Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene 1995, 11, 999–1003. [Google Scholar]
- Nair, A.; Venkatraman, M.; Maliekal, T.T.; Nair, B.; Karunagaran, D. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene 2003, 22, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Neri, A.; Chang, C.C.; Lombardi, L.; Salina, M.; Corradini, P.; Maiolo, A.T.; Chaganti, R.S.; Dalla-Favera, R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell 1991, 67, 1075–1087. [Google Scholar] [CrossRef]
- Sau, A.; Lau, R.; Cabrita, M.A.; Nolan, E.; Crooks, P.A.; Visvader, J.E.; Pratt, M.A. Persistent Activation of NF-κB in BRCA1-Deficient Mammary Progenitors Drives Aberrant Proliferation and Accumulation of DNA Damage. Cell Stem Cell 2016, 19, 52–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornburg, N.J.; Pathmanathan, R.; Raab-Traub, N. Activation of nuclear factor-kappaB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res. 2003, 63, 8293–8301. [Google Scholar] [PubMed]
- Walther, W.; Kobelt, D.; Bauer, L.; Aumann, J.; Stein, U. Chemosensitization by diverging modulation by short-term and long-term TNF-α action on ABCB1 expression and NF-κB signaling in colon cancer. Int. J. Oncol. 2015, 47, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budunova, I.V.; Perez, P.; Vaden, V.R.; Spiegelman, V.S.; Slaga, T.J.; Jorcano, J.L. Increased expression of p50-NF-kappaB and constitutive activation of NF-kappaB transcription factors during mouse skin carcinogenesis. Oncogene 1999, 18, 7423–7431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Pasparakis, M. Epidermal p65/NF-kappaB signalling is essential for skin carcinogenesis. EMBO Mol. Med. 2014, 6, 970–983. [Google Scholar] [CrossRef]
- Kobielak, A.; Fuchs, E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc. Natl. Acad. Sci. USA 2006, 103, 2322–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larcher, F.; Murillas, R.; Bolontrade, M.; Conti, C.J.; Jorcano, J.L. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene 1998, 17, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.M. Inflammation in epithelial skin tumours: Old stories and new ideas. Eur. J. Cancer 2006, 42, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Alameda, J.P.; Fernández-Aceñero, M.J.; Moreno-Maldonado, R.; Navarro, M.; Quintana, R.; Page, A.; Ramírez, A.; Bravo, A.; Casanova, M.L. CYLD regulates keratinocyte differentiation and skin cancer progression in humans. Cell Death Dis. 2011, 2, e208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.M.; Zhou, J.J.; Luo, C.L. CYLD suppression enhances the pro-inflammatory effects and hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes by enhancing NF-κB activation. Arthritis Res. Ther. 2018, 20, 219. [Google Scholar] [CrossRef] [Green Version]
- Massoumi, R.; Chmielarska, K.; Hennecke, K.; Pfeifer, A.; Fässler, R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell 2006, 125, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, A.; Bravo, A.; Jorcano, J.L.; Vidal, M. Sequences 5’ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation 1994, 58, 53–64. [Google Scholar] [CrossRef]
- Page, A.; Navarro, M.; Garin, M.; Perez, P.; Casanova, M.L.; Moreno, R.; Jorcano, J.L.; Cascallana, J.L.; Bravo, A.; Ramirez, A. IKKbeta leads to an inflammatory skin disease resembling interface dermatitis. J. Investig. Dermatol. 2010, 130, 1598–1610. [Google Scholar] [CrossRef] [Green Version]
- Alameda, J.P.; Gaspar, M.; Ramirez, A.; Navarro, M.; Page, A.; Suarez-Cabrera, C.; Fernandez, M.G.; Merida, J.R.; Paramio, J.M.; Garcia-Fernandez, R.A.; et al. Deciphering the role of nuclear and cytoplasmic IKKalpha in skin cancer. Oncotarget 2016, 7, 29531–29547. [Google Scholar] [CrossRef]
- Leder, A.; Kuo, A.; Cardiff, R.D.; Sinn, E.; Leder, P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: Effects of phorbol esters and retinoic acid. Proc. Natl. Acad. Sci. USA 1990, 87, 9178–9182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filler, R.B.; Roberts, S.J.; Girardi, M. Cutaneous two-stage chemical carcinogenesis. CSH Protoc. 2007, 2007. [Google Scholar] [CrossRef]
- Alameda, J.P.; Moreno-Maldonado, R.; Fernandez-Acenero, M.J.; Navarro, M.; Page, A.; Jorcano, J.L.; Bravo, A.; Ramirez, A.; Casanova, M.L. Increased IKKalpha Expression in the Basal Layer of the Epidermis of Transgenic Mice Enhances the Malignant Potential of Skin Tumors. PLoS ONE 2011, 6, e21984. [Google Scholar] [CrossRef]
- Toll, A.; Margalef, P.; Masferrer, E.; Ferrandiz-Pulido, C.; Gimeno, J.; Pujol, R.M.; Bigas, A.; Espinosa, L. Active nuclear IKK correlates with metastatic risk in cutaneous squamous cell carcinoma. Arch. Dermatol. Res. 2015, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Alameda, J.P.; Fernandez-Acenero, M.J.; Quintana, R.M.; Page, A.; Ramirez, A.; Navarro, M.; Casanova, M.L. Functional inactivation of CYLD promotes the metastatic potential of tumor epidermal cells. J. Investig. Dermatol. 2013, 133, 1870–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauvar, A.N.; Arpey, C.J.; Hruza, G.; Olbricht, S.M.; Bennett, R.; Mahmoud, B.H. Consensus for Nonmelanoma Skin Cancer Treatment, Part II: Squamous Cell Carcinoma, Including a Cost Analysis of Treatment Methods. Dermatol. Surg. 2015, 41, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Bolontrade, M.F.; Stern, M.C.; Binder, R.L.; Zenklusen, J.C.; Gimenez-Conti, I.B.; Conti, C.J. Angiogenesis is an early event in the development of chemically induced skin tumors. Carcinogenesis 1998, 19, 2107–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, M.M.; Sung, B.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene 2011, 30, 1615–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.L.; Larcher, F.; Casanova, B.; Murillas, R.; Fernandez-Acenero, M.J.; Villanueva, C.; Martinez-Palacio, J.; Ullrich, A.; Conti, C.J.; Jorcano, J.L. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res. 2002, 62, 3402–3407. [Google Scholar]
- O’Donnell, M.A.; Perez-Jimenez, E.; Oberst, A.; Ng, A.; Massoumi, R.; Xavier, R.; Green, D.R.; Ting, A.T. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011, 13, 1437–1442. [Google Scholar] [CrossRef]
- Müller, I.; Strozyk, E.; Schindler, S.; Beissert, S.; Oo, H.Z.; Sauter, T.; Lucarelli, P.; Raeth, S.; Hausser, A.; Al Nakouzi, N.; et al. Cancer Cells Employ Nuclear Caspase-8 to Overcome the p53-Dependent G2/M Checkpoint through Cleavage of USP28. Mol. Cell 2020, 77, 970–984.e7. [Google Scholar] [CrossRef]
- Liang, G.; Ahlqvist, K.; Pannem, R.; Posern, G.; Massoumi, R. Serum response factor controls CYLD expression via MAPK signaling pathway. PLoS ONE 2011, 6, e19613. [Google Scholar] [CrossRef] [Green Version]
- Kiss, A.; Koppel, A.C.; Anders, J.; Cataisson, C.; Yuspa, S.H.; Blumenberg, M.; Efimova, T. Keratinocyte p38δ loss inhibits Ras-induced tumor formation, while systemic p38δ loss enhances skin inflammation in the early phase of chemical carcinogenesis in mouse skin. Mol. Carcinog. 2016, 55, 563–574. [Google Scholar] [CrossRef]
- Kiss, A.; Koppel, A.C.; Murphy, E.; Sall, M.; Barlas, M.; Kissling, G.; Efimova, T. Cell Type-Specific p38δ Targeting Reveals a Context-, Stage-, and Sex-Dependent Regulation of Skin Carcinogenesis. Int. J. Mol. Sci. 2019, 20, 1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, E.M.; Hindes, A.; Gribben, E.L.; Burns, C.J.; Yin, Y.; Lin, M.H.; Owen, R.J.; Longmore, G.D.; Kissling, G.E.; Arthur, J.S.; et al. p38delta Mitogen-activated protein kinase is essential for skin tumor development in mice. Cancer Res. 2009, 69, 4648–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alameda, J.P.; García-García, V.A.; López, S.; Hernando, A.; Page, A.; Navarro, M.; Moreno-Maldonado, R.; Paramio, J.M.; Ramírez, Á.; García-Fernández, R.A.; et al. CYLD Inhibits the Development of Skin Squamous Cell Tumors in Immunocompetent Mice. Int. J. Mol. Sci. 2021, 22, 6736. https://doi.org/10.3390/ijms22136736
Alameda JP, García-García VA, López S, Hernando A, Page A, Navarro M, Moreno-Maldonado R, Paramio JM, Ramírez Á, García-Fernández RA, et al. CYLD Inhibits the Development of Skin Squamous Cell Tumors in Immunocompetent Mice. International Journal of Molecular Sciences. 2021; 22(13):6736. https://doi.org/10.3390/ijms22136736
Chicago/Turabian StyleAlameda, Josefa P., Verónica A. García-García, Silvia López, Ana Hernando, Angustias Page, Manuel Navarro, Rodolfo Moreno-Maldonado, Jesús M. Paramio, Ángel Ramírez, Rosa A. García-Fernández, and et al. 2021. "CYLD Inhibits the Development of Skin Squamous Cell Tumors in Immunocompetent Mice" International Journal of Molecular Sciences 22, no. 13: 6736. https://doi.org/10.3390/ijms22136736
APA StyleAlameda, J. P., García-García, V. A., López, S., Hernando, A., Page, A., Navarro, M., Moreno-Maldonado, R., Paramio, J. M., Ramírez, Á., García-Fernández, R. A., & Casanova, M. L. (2021). CYLD Inhibits the Development of Skin Squamous Cell Tumors in Immunocompetent Mice. International Journal of Molecular Sciences, 22(13), 6736. https://doi.org/10.3390/ijms22136736






