Metallacarborane Derivatives Effective against Pseudomonas aeruginosa and Yersinia enterocolitica
Abstract
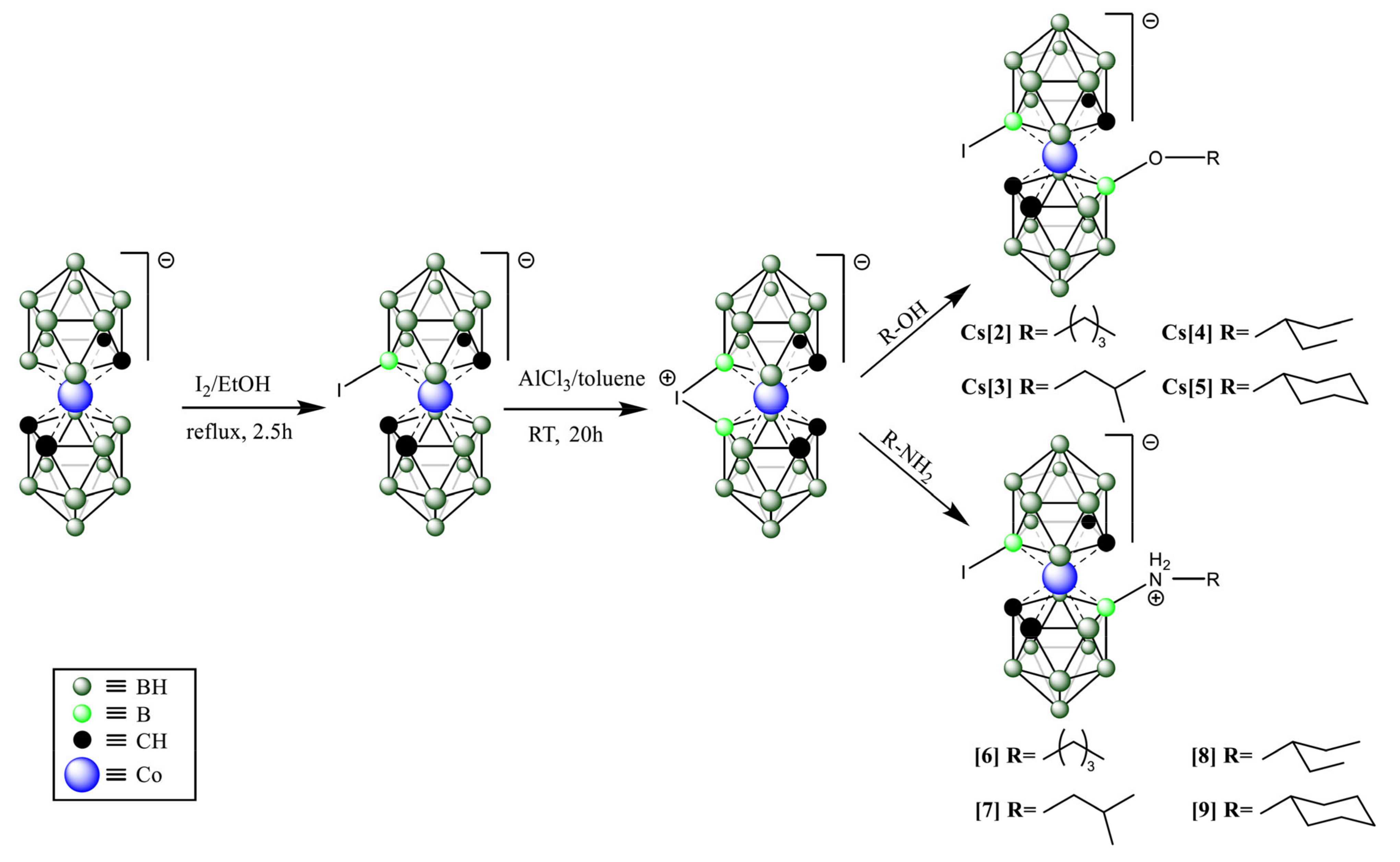
1. Introduction
2. Results
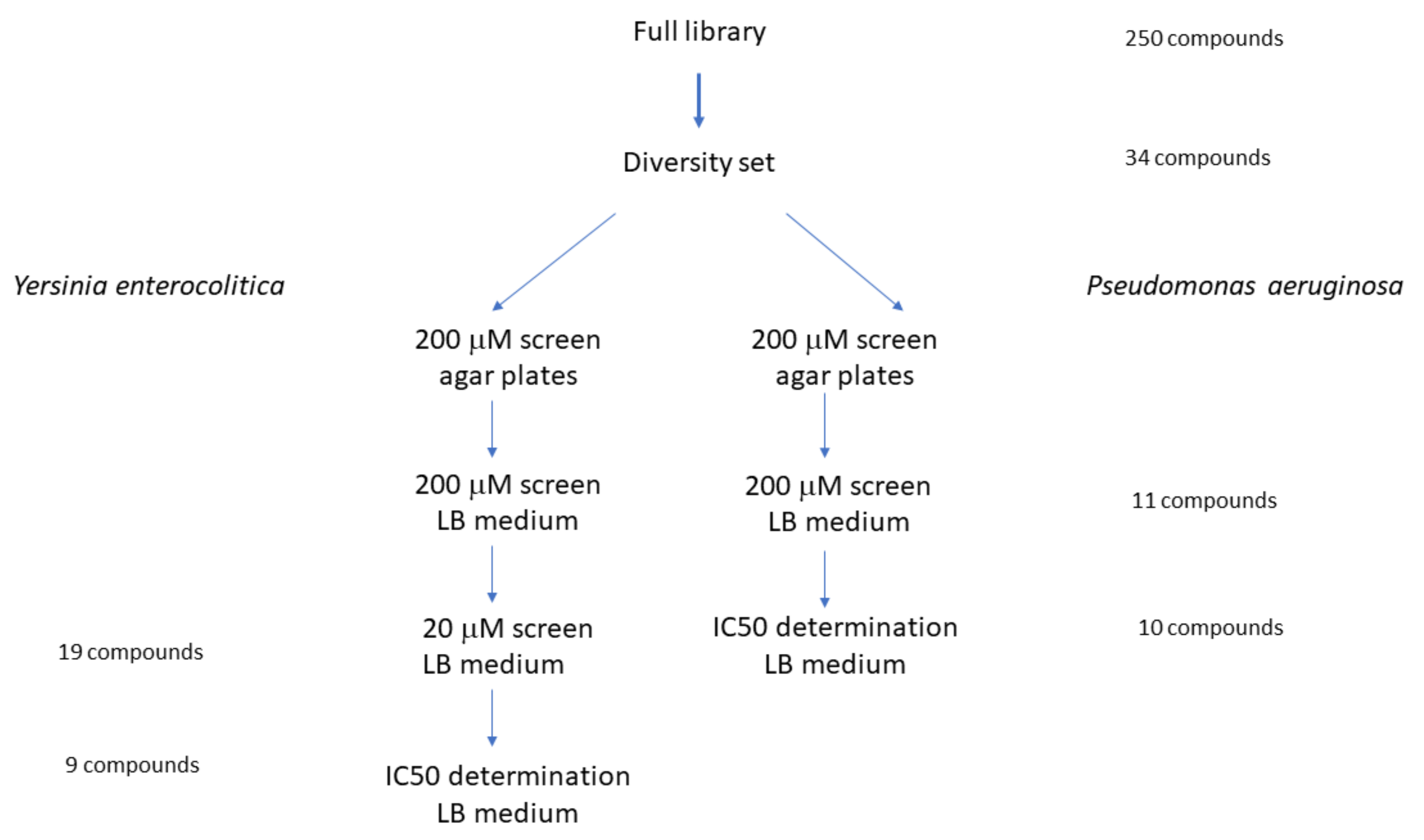
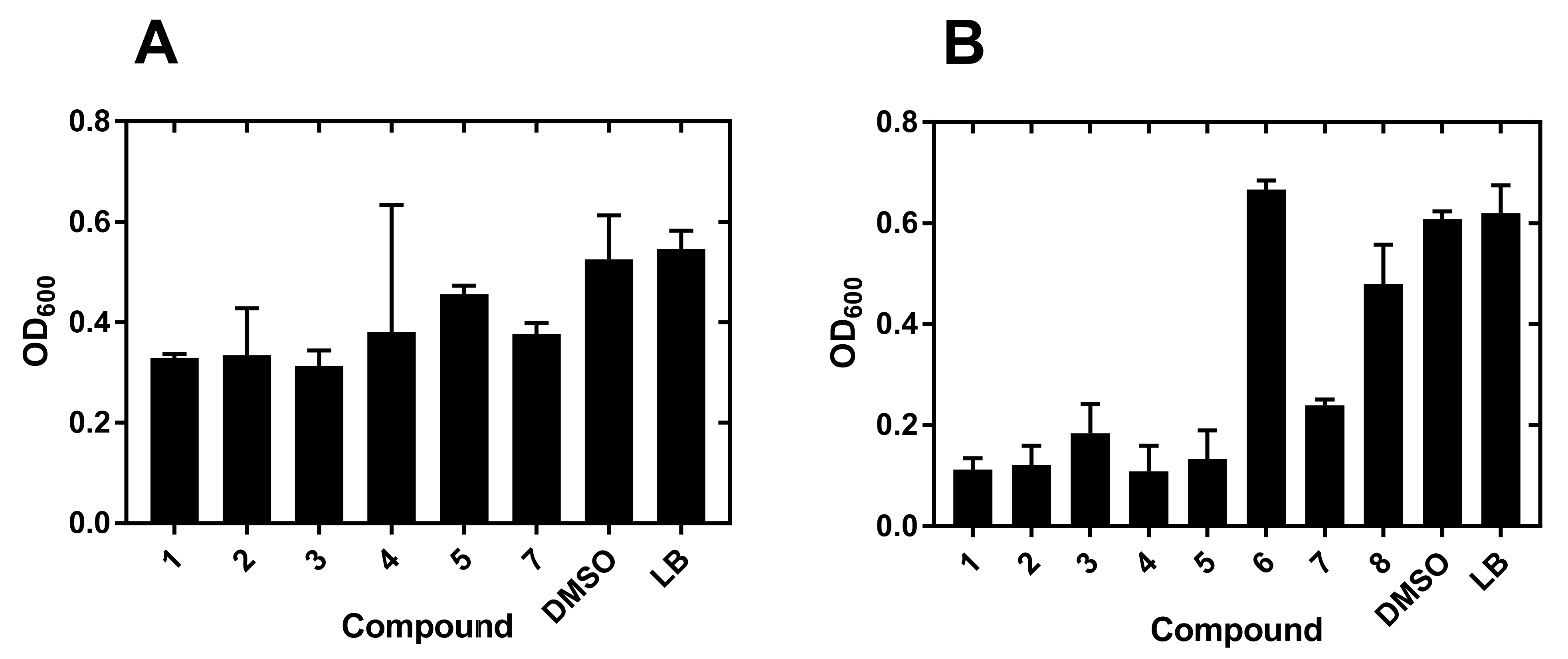
2.1. Compound Screening and Selection
2.2. IC50 Determination
2.3. Metallacarborane Resistance Generation
2.4. SEM Investigation of Resistant Bacteria
2.5. Mammalian Cell Toxicity Studies
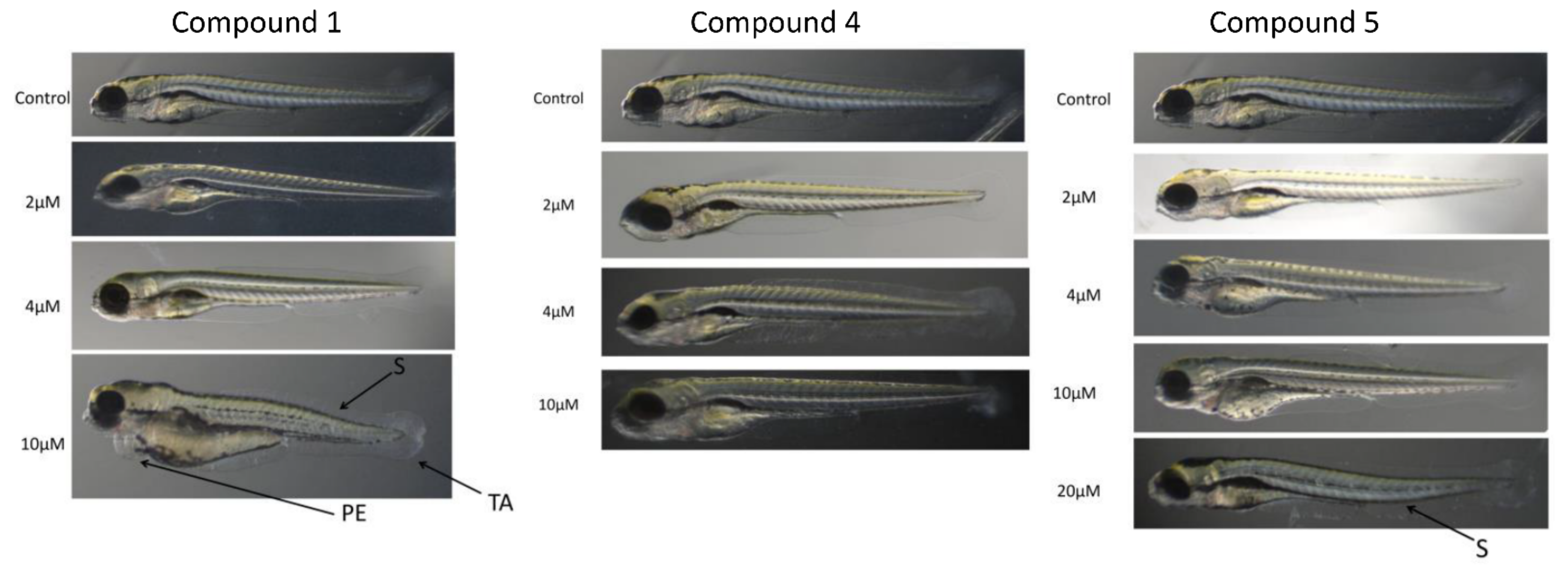
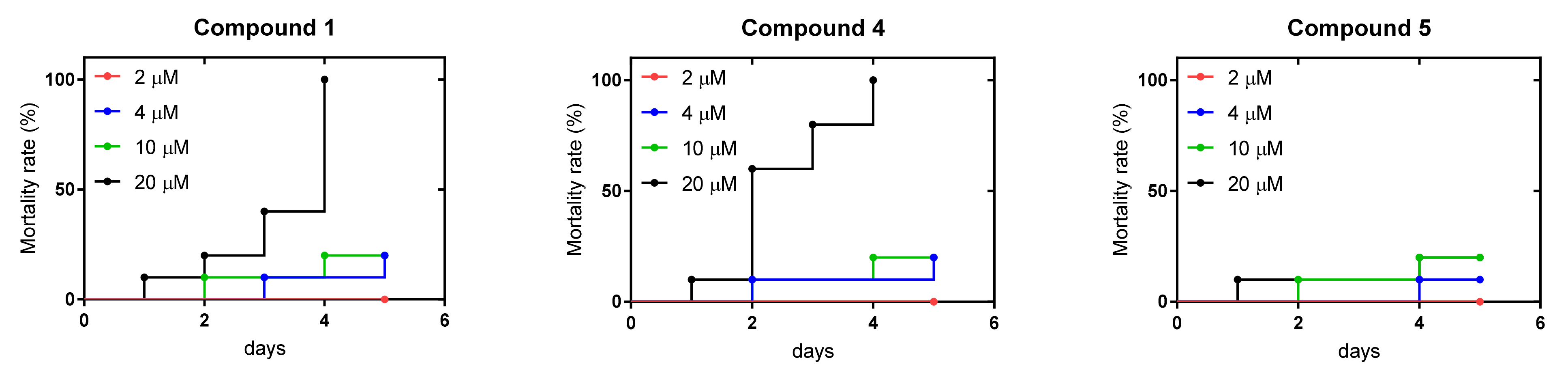
2.6. Zebrafish Toxicity Studies
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Media
4.2. Cell Culture
4.3. Antiproliferative Assay
4.4. Danio rerio Toxicity Assay
4.5. Chemical Reagents and Common Supplies
4.6. LC-MS Measurements
4.7. NMR Spectroscopy
4.8. Chemical Synthesis
4.9. Library Screening for Bacteria Growth Inhibition
4.10. Bacterial Growth Inhibition IC50 Measurements
4.11. Compound Resistance Evolution
4.12. Scanning Electron Microscopy (SEM)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, Y.; Kondo, A.; Hoshino, K.; Yano, H.; Arai, K.; Hirotani, A.; Kunishima, H.; Yamamoto, N.; Hatta, M.; Kitagawa, M.; et al. Efflux pump inhibitors reduce the invasiveness of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2009, 34, 343–346. [Google Scholar] [CrossRef]
- Anantharajah, A.; Mingeot-Leclercq, M.P.; Van Bambeke, F. Targeting the Type Three Secretion System in Pseudomonas aeruginosa. Trends Pharmacol. Sci. 2016, 37, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Berni, B.; Soscia, C.; Djermoun, S.; Ize, B.; Bleves, S. A Type VI Secretion System Trans-Kingdom Effector Is Required for the Delivery of a Novel Antibacterial Toxin in Pseudomonas aeruginosa. Front. Microbiol. 2019, 10, 1218. [Google Scholar] [CrossRef]
- Wood, T.E.; Howard, S.A.; Forster, A.; Nolan, L.M.; Manoli, E.; Bullen, N.P.; Yau, H.C.L.; Hachani, A.; Hayward, R.D.; Whitney, J.C.; et al. The Pseudomonas aeruginosa T6SS Delivers a Periplasmic Toxin that Disrupts Bacterial Cell Morphology. Cell Rep. 2019, 29, 187–201.e7. [Google Scholar] [CrossRef]
- Trunk, K.; Peltier, J.; Liu, Y.C.; Dill, B.D.; Walker, L.; Gow, N.A.R.; Stark, M.J.R.; Quinn, J.; Strahl, H.; Trost, M.; et al. The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 2018, 3, 920–931. [Google Scholar] [CrossRef]
- Chen, H.; Yang, D.; Han, F.; Tan, J.; Zhang, L.; Xiao, J.; Zhang, Y.; Liu, Q. The Bacterial T6SS Effector EvpP Prevents NLRP3 Inflammasome Activation by Inhibiting the Ca(2+)-Dependent MAPK-Jnk Pathway. Cell Host Microbe 2017, 21, 47–58. [Google Scholar] [CrossRef]
- Ratner, D.; Orning, M.P.; Lien, E. Bacterial secretion systems and regulation of inflammasome activation. J. Leukoc. Biol. 2017, 101, 165–181. [Google Scholar] [CrossRef]
- Lee, E.J.; Cowell, B.A.; Evans, D.J.; Fleiszig, S.M. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3892–3898. [Google Scholar] [CrossRef]
- Cryz, S.J., Jr.; Lang, A.; Rudeberg, A.; Wedgwood, J.; Que, J.U.; Furer, E.; Schaad, U. Immunization of cystic fibrosis patients with a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Behring Inst. Mitt. 1997, 98, 345–349. [Google Scholar]
- Behrouz, B.; Hashemi, F.B.; Fatemi, M.J.; Naghavi, S.; Irajian, G.; Halabian, R.; Imani Fooladi, A.A. Immunization with Bivalent Flagellin Protects Mice against Fatal Pseudomonas aeruginosa Pneumonia. J. Immunol. Res. 2017, 2017, 5689709. [Google Scholar] [CrossRef]
- Hegerle, N.; Choi, M.; Sinclair, J.; Amin, M.N.; Ollivault-Shiflett, M.; Curtis, B.; Laufer, R.S.; Shridhar, S.; Brammer, J.; Toapanta, F.R.; et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0203143. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.B.; Behrouz, B.; Irajian, G.; Laghaei, P.; Korpi, F.; Fatemi, M.J. A trivalent vaccine consisting of “flagellin A+B and pilin” protects against Pseudomonas aeruginosa infection in a murine burn model. Microb. Pathog. 2020, 138, 103697. [Google Scholar] [CrossRef] [PubMed]
- Doring, G.; Meisner, C.; Stern, M.; Flagella Vaccine Trial Study Group. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2007, 104, 11020–11025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, C.; Gu, J.; Yan, X.; Wang, B.; Cui, Z.; Sun, X.; Tong, C.; Feng, X.; Lei, L.; et al. Salmonella Typhimurium strain expressing OprF-OprI protects mice against fatal infection by Pseudomonas aeruginosa. Microbiol. Immunol. 2015, 59, 533–544. [Google Scholar] [CrossRef]
- Fakoor, M.H.; Mousavi Gargari, S.L.; Owlia, P.; Sabokbar, A. Protective Efficacy of the OprF/OprI/PcrV Recombinant Chimeric Protein Against Pseudomonas aeruginosa in the Burned BALB/c Mouse Model. Infect. Drug Resist. 2020, 13, 1651–1661. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Wurm, R.; Depuydt, P.; Spapen, H.; Lorente, J.A.; Staudinger, T.; Creteur, J.; Zauner, C.; Meier-Hellmann, A.; Eller, P.; et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial. Crit. Care 2020, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Zhang, X.; Zou, J.; Yuan, Y.; Chen, Z.; Liu, D.; Wu, W.; Yang, F.; Lu, D.; Zou, Q.; et al. Oligomerization of IC43 resulted in improved immunogenicity and protective efficacy against Pseudomonas aeruginosa lung infection. Int. J. Biol. Macromol. 2020, 159, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Holder, I.A.; Neely, A.N.; Frank, D.W. PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect. Immun. 2001, 69, 5908–5910. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, J.; Zhao, L.; Cheng, X.; Gao, C.; Wang, Y.; Xu, W.; Zou, Q.; Gu, J. Rational Design of a Chimeric Derivative of PcrV as a Subunit Vaccine Against Pseudomonas aeruginosa. Front. Immunol. 2019, 10, 781. [Google Scholar] [CrossRef]
- Hamaoka, S.; Naito, Y.; Katoh, H.; Shimizu, M.; Kinoshita, M.; Akiyama, K.; Kainuma, A.; Moriyama, K.; Ishii, K.J.; Sawa, T. Efficacy comparison of adjuvants in PcrV vaccine against Pseudomonas aeruginosa pneumonia. Microbiol. Immunol. 2017, 61, 64–74. [Google Scholar] [CrossRef]
- Aguilera-Herce, J.; Garcia-Quintanilla, M.; Romero-Flores, R.; McConnell, M.J.; Ramos-Morales, F. A Live Salmonella Vaccine Delivering PcrV through the Type III Secretion System Protects against Pseudomonas aeruginosa. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Naito, Y.; Hamaoka, S.; Kinoshita, M.; Kainuma, A.; Shimizu, M.; Katoh, H.; Moriyama, K.; Ishii, K.J.; Sawa, T. The protective effects of nasal PcrV-CpG oligonucleotide vaccination against Pseudomonas aeruginosa pneumonia. Microbiol. Immunol. 2018, 62, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Golpasha, I.D.; Mousavi, S.F.; Owlia, P.; Siadat, S.D.; Irani, S. Immunization with 3-oxododecanoyl-L-homoserine lactone-r-PcrV conjugate enhances survival of mice against lethal burn infections caused by Pseudomonas aeruginosa. Bosn. J. Basic Med. Sci. 2015, 15, 15–24. [Google Scholar] [CrossRef]
- Sawa, T.; Yahr, T.L.; Ohara, M.; Kurahashi, K.; Gropper, M.A.; Wiener-Kronish, J.P.; Frank, D.W. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 1999, 5, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Howlader, D.R.; Zheng, Q.; Ratnakaram, S.S.K.; Whittier, S.K.; Lu, T.; Keith, J.D.; Picking, W.D.; Birket, S.E.; Picking, W.L. Development of a Broadly Protective, Self-Adjuvanting Subunit Vaccine to Prevent Infections by Pseudomonas aeruginosa. Front. Immunol. 2020, 11, 583008. [Google Scholar] [CrossRef]
- Schaefers, M.M.; Duan, B.; Mizrahi, B.; Lu, R.; Reznor, G.; Kohane, D.S.; Priebe, G.P. PLGA-encapsulation of the Pseudomonas aeruginosa PopB vaccine antigen improves Th17 responses and confers protection against experimental acute pneumonia. Vaccine 2018, 36, 6926–6932. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, L.; Wen, X.; Liu, Q.; Liu, Y.; Wang, X.; Lei, L.; Chen, Q.; Liu, L. Construction of Genomic Library and High-Throughput Screening of Pseudomonas aeruginosa Novel Antigens for Potential Vaccines. Biol. Pharm. Bull. 2020, 43, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, F.; Zou, J.; Wu, W.; Jing, H.; Gou, Q.; Li, H.; Gu, J.; Zou, Q.; Zhang, J. Immunization with Pseudomonas aeruginosa outer membrane vesicles stimulates protective immunity in mice. Vaccine 2018, 36, 1047–1054. [Google Scholar] [CrossRef]
- Francois, B.; Luyt, C.E.; Dugard, A.; Wolff, M.; Diehl, J.L.; Jaber, S.; Forel, J.M.; Garot, D.; Kipnis, E.; Mebazaa, A.; et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: A randomized, double-blind, placebo-controlled trial. Crit. Care Med. 2012, 40, 2320–2326. [Google Scholar] [CrossRef]
- Ranjbar, M.; Behrouz, B.; Norouzi, F.; Mousavi Gargari, S.L. Anti-PcrV IgY antibodies protect against Pseudomonas aeruginosa infection in both acute pneumonia and burn wound models. Mol. Immunol. 2019, 116, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sheremet, A.B.; Zigangirova, N.A.; Zayakin, E.S.; Luyksaar, S.I.; Kapotina, L.N.; Nesterenko, L.N.; Kobets, N.V.; Gintsburg, A.L. Small Molecule Inhibitor of Type Three Secretion System Belonging to a Class 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-ones Improves Survival and Decreases Bacterial Loads in an Airway Pseudomonas aeruginosa Infection in Mice. Biomed. Res. Int. 2018, 2018, 5810767. [Google Scholar] [CrossRef]
- Uusitalo, P.; Hagglund, U.; Rhoos, E.; Scherman Norberg, H.; Elofsson, M.; Sundin, C. The salicylidene acylhydrazide INP0341 attenuates Pseudomonas aeruginosa virulence in vitro and in vivo. J. Antibiot. 2017, 70, 937–943. [Google Scholar] [CrossRef]
- Feng, C.; Huang, Y.; He, W.; Cheng, X.; Liu, H.; Huang, Y.; Ma, B.; Zhang, W.; Liao, C.; Wu, W.; et al. Tanshinones: First-in-Class Inhibitors of the Biogenesis of the Type 3 Secretion System Needle of Pseudomonas aeruginosa for Antibiotic Therapy. ACS Cent. Sci. 2019, 5, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Massai, F.; Saleeb, M.; Doruk, T.; Elofsson, M.; Forsberg, A. Development, Optimization, and Validation of a High Throughput Screening Assay for Identification of Tat and Type II Secretion Inhibitors of Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 2019, 9, 250. [Google Scholar] [CrossRef]
- Swietnicki, W.; Czarny, A.; Antkowiak, L.; Zaczynska, E.; Kolodziejczak, M.; Sycz, J.; Stachowicz, L.; Alicka, M.; Marycz, K. Identification of a potent inhibitor of type II secretion system from Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2019, 513, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Pullen, J.K.; Anderson, G.W., Jr.; Welkos, S.L.; Friedlander, A.M. Analysis of the Yersinia pestis V protein for the presence of linear antibody epitopes. Infect. Immun. 1998, 66, 521–527. [Google Scholar] [CrossRef]
- Cabanel, N.; Bouchier, C.; Rajerison, M.; Carniel, E. Plasmid-mediated doxycycline resistance in a Yersinia pestis strain isolated from a rat. Int. J. Antimicrob. Agents 2018, 51, 249–254. [Google Scholar] [CrossRef]
- Rasoamanana, B.; Coulanges, P.; Michel, P.; Rasolofonirina, N. [Sensitivity of Yersinia pestis to antibiotics: 277 strains isolated in Madagascar between 1926 and 1989]. Arch. Inst. Pasteur Madag. 1989, 56, 37–53. [Google Scholar]
- Galimand, M.; Guiyoule, A.; Gerbaud, G.; Rasoamanana, B.; Chanteau, S.; Carniel, E.; Courvalin, P. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 1997, 337, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.; Williams, P. Yersinia virulence factors—A sophisticated arsenal for combating host defences. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Swietnicki, W.; Carmany, D.; Retford, M.; Guelta, M.; Dorsey, R.; Bozue, J.; Lee, M.S.; Olson, M.A. Identification of small-molecule inhibitors of Yersinia pestis Type III secretion system YscN ATPase. PLoS ONE 2011, 6, e19716. [Google Scholar] [CrossRef]
- Pan, N.J.; Brady, M.J.; Leong, J.M.; Goguen, J.D. Targeting type III secretion in Yersinia pestis. Antimicrob. Agents Chemother. 2009, 53, 385–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bzdzion, L.; Krezel, H.; Wrzeszcz, K.; Grzegorek, I.; Nowinska, K.; Chodaczek, G.; Swietnicki, W. Design of small molecule inhibitors of type III secretion system ATPase EscN from enteropathogenic Escherichia coli. Acta Biochim. Pol. 2017, 64, 49–63. [Google Scholar] [CrossRef]
- Heesemann, J.; Gaede, K.; Autenrieth, I.B. Experimental Yersinia enterocolitica infection in rodents: A model for human yersiniosis. APMIS 1993, 101, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Bosio, C.F.; Jarrett, C.O.; Gardner, D.; Hinnebusch, B.J. Kinetics of innate immune response to Yersinia pestis after intradermal infection in a mouse model. Infect. Immun. 2012, 80, 4034–4045. [Google Scholar] [CrossRef]
- Giamarellou, H.; Kanellakopoulou, K. Current therapies for Pseudomonas aeruginosa. Crit. Care Clin. 2008, 24, 261–278. [Google Scholar] [CrossRef]
- Wendte, J.M.; Ponnusamy, D.; Reiber, D.; Blair, J.L.; Clinkenbeard, K.D. In vitro efficacy of antibiotics commonly used to treat human plague against intracellular Yersinia pestis. Antimicrob. Agents Chemother. 2011, 55, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef]
- Baker, S.J.; Ding, C.Z.; Akama, T.; Zhang, Y.K.; Hernandez, V.; Xia, Y. Therapeutic potential of boron-containing compounds. Future Med. Chem. 2009, 1, 1275–1288. [Google Scholar] [CrossRef]
- Celenza, G.; Vicario, M.; Bellio, P.; Linciano, P.; Perilli, M.; Oliver, A.; Blazquez, J.; Cendron, L.; Tondi, D. Phenylboronic Acid Derivatives as Validated Leads Active in Clinical Strains Overexpressing KPC-2: A Step against Bacterial Resistance. ChemMedChem 2018, 13, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, A.; Brem, J.; Hinchliffe, P.; Calvopina, K.; Panduwawala, T.D.; Lang, P.A.; Kamps, J.; Tyrrell, J.M.; Widlake, E.; Saward, B.G.; et al. Bicyclic Boronate VNRX-5133 Inhibits Metallo- and Serine-beta-Lactamases. J. Med. Chem. 2019, 62, 8544–8556. [Google Scholar] [CrossRef] [PubMed]
- Parkova, A.; Lucic, A.; Krajnc, A.; Brem, J.; Calvopina, K.; Langley, G.W.; McDonough, M.A.; Trapencieris, P.; Schofield, C.J. Broad Spectrum beta-Lactamase Inhibition by a Thioether Substituted Bicyclic Boronate. ACS Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Ke, W.; Bethel, C.R.; Papp-Wallace, K.M.; Pagadala, S.R.; Nottingham, M.; Fernandez, D.; Buynak, J.D.; Bonomo, R.A.; van den Akker, F. Crystal structures of KPC-2 beta-lactamase in complex with 3-nitrophenyl boronic acid and the penam sulfone PSR-3-226. Antimicrob. Agents Chemother. 2012, 56, 2713–2718. [Google Scholar] [CrossRef][Green Version]
- Obuobi, S.; Voo, Z.X.; Low, M.W.; Czarny, B.; Selvarajan, V.; Ibrahim, N.L.; Yang, Y.Y.; Ee, P.L.R. Phenylboronic Acid Functionalized Polycarbonate Hydrogels for Controlled Release of Polymyxin B in Pseudomonas aeruginosa Infected Burn Wounds. Adv. Healthc. Mater. 2018, 7, e1701388. [Google Scholar] [CrossRef]
- Purnapatre, K.P.; Rao, M.; Pandya, M.; Khanna, A.; Chaira, T.; Bambal, R.; Upadhyay, D.J.; Masuda, N. In Vitro and In Vivo Activities of DS86760016, a Novel Leucyl-tRNA Synthetase Inhibitor for Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Reynolds, R.C.; Campbell, S.R.; Fairchild, R.G.; Kisliuk, R.L.; Micca, P.L.; Queener, S.F.; Riordan, J.M.; Sedwick, W.D.; Waud, W.R.; Leung, A.K.; et al. Novel boron-containing, nonclassical antifolates: Synthesis and preliminary biological and structural evaluation. J. Med. Chem. 2007, 50, 3283–3289. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhang, Y.; Liu, J.; van der Veen, S.; Duttwyler, S. The closo-Dodecaborate Dianion Fused with Oxazoles Provides 3D Diboraheterocycles with Selective Antimicrobial Activity. Chemistry 2018, 24, 10364–10371. [Google Scholar] [CrossRef]
- Gozzi, M.; Schwarze, B.; Hey-Hawkins, E. Preparing (Metalla)carboranes for Nanomedicine. ChemMedChem 2021. [Google Scholar] [CrossRef] [PubMed]
- Goszczynski, T.M.; Fink, K.; Boratynski, J. Icosahedral boron clusters as modifying entities for biomolecules. Expert Opin. Biol. Ther. 2018, 18, 205–213. [Google Scholar] [CrossRef]
- Lesnikowski, Z.J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef]
- Popova, T.; Zaulet, A.; Teixidor, F.; Alexandrova, R.; Vinas, C. Investigations on antimicrobial activity of cobaltabisdicarbollides. J. Organomet. Chem. 2013, 747, 229–234. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Liu, W.W.; Chen, Y.; Jiang, H.; Yan, H.; Kosenko, I.; Chekulaeva, L.; Sivaev, I.; Bregadze, V.; Wang, X.M. A Highly Potent Antibacterial Agent Targeting Methicillin-Resistant Staphylococcus aureus Based on Cobalt Bis(1,2-Dicarbollide) Alkoxy Derivative. Organometallics 2017, 36, 3484–3490. [Google Scholar] [CrossRef]
- Totani, T.; Aono, K.; Yamamoto, K.; Tawara, K. Synthesis and in vitro antimicrobial property of o-carborane derivatives. J. Med. Chem. 1981, 24, 1492–1499. [Google Scholar] [CrossRef]
- Kvasnickova, E.; Masak, J.; Cejka, J.; Matatkova, O.; Sicha, V. Preparation, characterization, and the selective antimicrobial activity of N-alkylammonium 8-diethyleneglycol cobalt bis-dicarbollide derivatives. J. Organomet. Chem. 2017, 827, 23–31. [Google Scholar] [CrossRef]
- Vankova, E.; Lokocova, K.; Matatkova, O.; Krizova, I.; Masak, J.; Gruner, B.; Kaule, P.; Cermak, J.; Sicha, V. Cobalt bis-dicarbollide and its ammonium derivatives are effective antimicrobial and antibiofilm agents. J. Organomet. Chem. 2019, 899. [Google Scholar] [CrossRef]
- Tarres, M.; Canetta, E.; Paul, E.; Forbes, J.; Azzouni, K.; Vinas, C.; Teixidor, F.; Harwood, A.J. Biological interaction of living cells with COSAN-based synthetic vesicles. Sci. Rep. 2015, 5, 7804. [Google Scholar] [CrossRef]
- Plesek, J.; Stibr, B.; Hermanek, S. A [8,8′-Mu-I-3-Co(1,2-C2b9h10)2] Metallacarborane Complex with a Iodonium Bridge—Evidence for a Bromonium Analog. Collect. Czechoslov. Chem. Commun. 1984, 49, 1492–1496. [Google Scholar] [CrossRef]
- Kosenko, I.D.; Lobanova, I.A.; Ananyev, I.V.; Godovikov, I.A.; Chekulaeva, L.A.; Starikova, Z.A.; Qi, S.C.; Bregadze, V.I. Novel alkoxy derivatives of cobalt bis(1,2-dicarbollide). J. Organomet. Chem. 2014, 769, 72–79. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Kosenko, I.D.; Lobanova, I.A.; Starikova, Z.A.; Godovikov, I.A.; Sivaev, I.B. C-H Bond Activation of Arenes by [8,8′-mu-I-3,3′-Co(1,2-C2B9H10)(2)] in the Presence of Sterically Hindered Lewis Bases. Organometallics 2010, 29, 5366–5372. [Google Scholar] [CrossRef]
- Sayin, Z.; Ucan, U.S.; Sakmanoglu, A. Antibacterial and Antibiofilm Effects of Boron on Different Bacteria. Biol. Trace Elem. Res. 2016, 173, 241–246. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Wei, Y.; Wu, C.; Gao, S.; Jiang, H.; Zhao, X.; Yan, H.; Wang, X. Antimicrobial activity of a ferrocene-substituted carborane derivative targeting multidrug-resistant infection. Biomaterials 2013, 34, 902–911. [Google Scholar] [CrossRef]
- Vicente, M.G.; Edwards, B.F.; Shetty, S.J.; Hou, Y.; Boggan, J.E. Syntheses and preliminary biological studies of four meso-Tetra[(nido-carboranylmethyl)phenyl]porphyrins. Bioorg. Med. Chem. 2002, 10, 481–492. [Google Scholar] [CrossRef]
- Cigler, P.; Kozisek, M.; Rezacova, P.; Brynda, J.; Otwinowski, Z.; Pokorna, J.; Plesek, J.; Gruner, B.; Doleckova-Maresova, L.; Masa, M.; et al. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. Proc. Natl. Acad. Sci. USA 2005, 102, 15394–15399. [Google Scholar] [CrossRef]
- Gruner, B.; Brynda, J.; Das, V.; Sicha, V.; Stepankova, J.; Nekvinda, J.; Holub, J.; Pospisilova, K.; Fabry, M.; Pachl, P.; et al. Metallacarborane Sulfamides: Unconventional, Specific, and Highly Selective Inhibitors of Carbonic Anhydrase IX. J. Med. Chem. 2019, 62, 9560–9575. [Google Scholar] [CrossRef] [PubMed]
- Vinas, C.; Fuentes, I.; Garcia-Mendiola, T.; Sato, S.; Pita, M.; Nakamura, H.; Lorenzo, E.; Teixidor, F.; Marques, F. Metallacarboranes on the road to anticancer therapies: Cellular uptake, DNA interaction and biological evaluation of cobaltabisdicarbollide ([COSAN]-). Chemistry 2018. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Setyawati, M.I.; Lim, T.P.; Leong, D.T.; Xie, J.P. Antimicrobial Cluster Bombs: Silver Nanoclusters Packed with Daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef] [PubMed]
- Natalio, F.; Andre, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.Y.; Tian, Y.; Zhang, W.; Lu, X.Y.; Jiang, X.Y. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Rojo, I.; Teixidor, F.; Vinas, C.; Kivekas, R.; Sillanpaa, R. Relevance of the electronegativity of boron in eta(5)-coordinating ligands: Regioselective monoalkylation and monoarylation in cobaltabisdicarbollide [3,3′-Co(1,2-C2B9H11)(2)](-) clusters. Chem. Eur. J. 2003, 9, 4311–4323. [Google Scholar] [CrossRef]
- Rojo, I.; Teixidor, F.; Kivekas, R.; Sillanpaa, R.; Vinas, C. Methylation and demethylation in cobaltabis(dicarbollide) derivatives. Organometallics 2003, 22, 4642–4646. [Google Scholar] [CrossRef]
- Mátel, Ľ.; Macášek, F.; Rajec, P.; Heřmánek, S.; Plešek, J. B-Halogen derivatives of the bis(1,2-dicarbollyl)cobalt(III) anion. Polyhedron 1982, 1, 511–519. [Google Scholar] [CrossRef]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; De Amicis, C.V.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process. Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Drab, M. Phage Aggregation-Dispersion by Ions: Striving beyond Antibacterial Therapy. Trends Biotechnol. 2018, 36, 875–881. [Google Scholar] [CrossRef]

| Compound No. | Pathogen | |||
|---|---|---|---|---|
| Ye 2080 a | Ye 2090 b | Pa 2058 c | Pa499 d | |
| 1 | 4.8 ± 3.0 | 2.5 ± 1.4 | 28.3 ± 6 | 11.4 ± 1 |
| 2 | 4.3 ± 1.5 | 2.4 ± 0.7 | 28 ± 8 | 12.1 ± 4 |
| 3 | 4.2 ± 1.8 | 3.4 ± 1.0 | 59 ± 30 | 16.9 ± 8 |
| 4 | 6.7 ± 2.3 | 5.3 ± 1 | 72 ± 30 | 52.9 ± 20 |
| 5 | 7.5 ± 4 | 3.3 ± 1 | 52.1 ± 15 | 52.1 ± 10 |
| 7 | 1.8 ± 0.8 | 6.2 ± 0.7 | 24.7 ± 8 | 58.7 ± 20 |
| Compound No. | Resistance a | ||
|---|---|---|---|
| 1 | 3 | 7 | |
| 1 | 2.6 ± 0.2 | 4.7 ± 1 | >37 |
| 3 | 4.0 ± 1 | 10.4 ± 3 | >100 |
| 7 | 3.1 ± 1 | 3.7 ± 1.2 | >100 |
| Compound No. | ||
|---|---|---|
| BALB/3T3 | MCF 10A | |
| 1 | 17.0 (11.4–36.9) | 59.8 (ND) |
| 2 | 3.7 (2.3–6.7) | 16.1 (9.08–239) |
| 3 | 10.2 (8.2–13.3) | 52.5 (30–63) |
| 4 | 11.3 (9.9–14.0) | 50 (ND) |
| 5 | 11.7 (9.3–15.2) | 46.3 (20.0–54.4) |
| 6 | 0.29 (0.17–0.57) | 0,16 (0.098–0,28) |
| 7 | 0.024 (0.017–0.034) | 0.115 (0.084–0.16) |
| 8 | 0.023 (0.016–0.033) | 0.045 (0.041–0.050) |
| 9 | 0.067 (0.043–0.11) | 0.082 (0.068–0.100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swietnicki, W.; Goldeman, W.; Psurski, M.; Nasulewicz-Goldeman, A.; Boguszewska-Czubara, A.; Drab, M.; Sycz, J.; Goszczyński, T.M. Metallacarborane Derivatives Effective against Pseudomonas aeruginosa and Yersinia enterocolitica. Int. J. Mol. Sci. 2021, 22, 6762. https://doi.org/10.3390/ijms22136762
Swietnicki W, Goldeman W, Psurski M, Nasulewicz-Goldeman A, Boguszewska-Czubara A, Drab M, Sycz J, Goszczyński TM. Metallacarborane Derivatives Effective against Pseudomonas aeruginosa and Yersinia enterocolitica. International Journal of Molecular Sciences. 2021; 22(13):6762. https://doi.org/10.3390/ijms22136762
Chicago/Turabian StyleSwietnicki, Wieslaw, Waldemar Goldeman, Mateusz Psurski, Anna Nasulewicz-Goldeman, Anna Boguszewska-Czubara, Marek Drab, Jordan Sycz, and Tomasz M. Goszczyński. 2021. "Metallacarborane Derivatives Effective against Pseudomonas aeruginosa and Yersinia enterocolitica" International Journal of Molecular Sciences 22, no. 13: 6762. https://doi.org/10.3390/ijms22136762
APA StyleSwietnicki, W., Goldeman, W., Psurski, M., Nasulewicz-Goldeman, A., Boguszewska-Czubara, A., Drab, M., Sycz, J., & Goszczyński, T. M. (2021). Metallacarborane Derivatives Effective against Pseudomonas aeruginosa and Yersinia enterocolitica. International Journal of Molecular Sciences, 22(13), 6762. https://doi.org/10.3390/ijms22136762







