Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials
Abstract
:1. Introduction
2. Antimicrobial Properties of QACs and ILs
2.1. Commercial QACs
2.2. The Latest Scientific Discoveries in the QAC Field
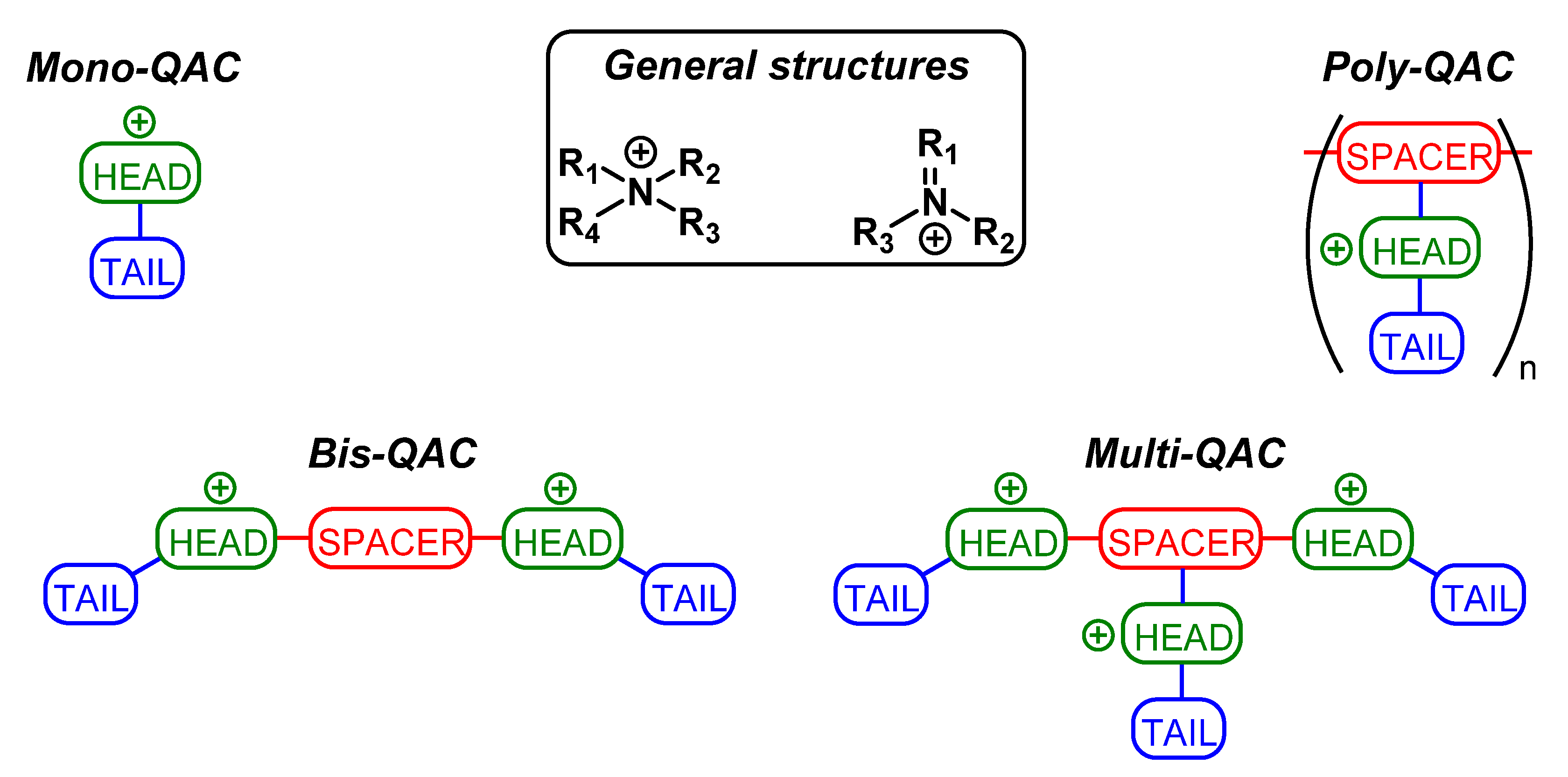
2.2.1. Single-Charged QACs (Mono-QACs)
2.2.2. Common Ionic Liquids and Ionic Liquids with Active Pharmaceutical Ingredients (API-ILs)
2.2.3. Double-Charged QACs (Bis-QACs)
2.2.4. Dicationic Ionic Liquids
2.2.5. Multiple-Charged QACs (Multi-QACs)
2.2.6. Poly-Charged QACs (Poly-QACs)
2.2.7. Polyionic Liquids
2.2.8. QAC-Containing Bactericidal Coatings
2.2.9. Ionic Liquid-Containing Bactericidal Coatings
3. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Paulson, D.S. Topical Antimicrobials. In New Biocides Development; American Chemical Society: Washington, DC, USA, 2007; Volume 967, pp. 124–150. [Google Scholar]
- Zheng, G.; Filippelli, G.M.; Salamova, A. Increased Indoor Exposure to Commonly Used Disinfectants during the COVID-19 Pandemic. Environ. Sci. Technol. Lett. 2020, 7, 760–765. [Google Scholar] [CrossRef]
- Schrank, C.L.; Minbiole, K.P.C.; Wuest, W.M. Are Quaternary Ammonium Compounds, the Workhorse Disinfectants, Effective against Severe Acute Respiratory Syndrome-Coronavirus-2? ACS Infect. Dis. 2020, 6, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, W.A. The Bactericidal Properties of The Quaternary Salts of Hexamethylenetetramine: I. The Problem of The Chemotherapy of Experimental Bacterial Infections. J. Exp. Med. 1916, 23, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, W.A.; Heidelberger, M.; Amoss, H.L. The Bactericidal Properties of The Quaternary Salts of Hexamethylenetetramine: II. The Relation Between Constitution and Bactericidal Action in the Substituted Benzylhexamethylenetetraminium. Salts. J. Exp. Med. 1916, 23, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, W.A.; Heidelberger, M.; Bull, C.G. The Bactericidal Properties of The Quaternary Salts of Hexamethylenetetramine: III. The Relation Between Constitution And Bactericidal Action in the Quaternary Salts Obtained From Halogenacetyl Compounds. J. Exp. Med. 1916, 23, 577–599. [Google Scholar] [CrossRef]
- Domagk, G. A new class of disinfectants. Dtsch. Med. Wochenschr 1935, 61, 829–832. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Directive, E.C. 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market. OJEC 1998, 123, 1–63. [Google Scholar]
- Biocides Market Size, Share & Trends Analysis Report by Product (Halogen Compounds, Quaternary Ammonium Compounds), By Application (Paints & Coatings, Water Treatment), By Region, And Segment Forecasts, 2020–2027. Available online: www.grandviewresearch.com/industry-analysis/biocides-industry (accessed on 11 January 2021).
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Simões, M.; Pereira, A.R.; Simões, L.C.; Cagide, F.; Borges, F. Biofilm control by ionic liquids. Drug Discov. Today 2021, 26, 1340–1346. [Google Scholar] [CrossRef]
- Reregistration Eligibility Decision for Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC). In EPA 739-R-06–009; National Service Center for Enviromental Publications (NSCEP): Washington, DC, USA, 17 November 2006.
- Rahn, O.; Eseltine, W.P.V. Quaternary Ammonium Compounds. Annu. Rev. Microbiol. 1947, 1, 173–192. [Google Scholar] [CrossRef]
- De Saint Jean, M.; Brignole, F.; Bringuier, A.F.; Bauchet, A.; Feldmann, G.; Baudouin, C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 619–630. [Google Scholar]
- Percival, S.L.; Finnegan, S.; Donelli, G.; Vuotto, C.; Rimmer, S.; Lipsky, B.A. Antiseptics for treating infected wounds: Efficacy on biofilms and effect of pH. Crit. Rev. Microbiol. 2016, 42, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, B.H.; Solis-Leal, A.; Lopez, J.B.; Poole, B.D.; Robison, R.A.; Berges, B.K. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Inf. 2021, 108, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Agafonova, M.N.; Kazakova, R.R.; Lubina, A.P.; Zeldi, M.I.; Nikitina, E.V.; Balakin, K.V.; Shtyrlin, Y.G. Antibacterial activity profile of miramistin in in vitro and in vivo models. Microb. Pathog. 2020, 142, 104072. [Google Scholar] [CrossRef] [PubMed]
- Turov, V.V.; Barvinchenko, V.N.; Lipkovska, N.A.; Fedyanina, T.V. Supramolecular Structures in Nanosilica/Miramistin Hydrated Composite in a Hydrophobic Medium. J. Appl. Spectrosc. 2015, 82, 175–181. [Google Scholar] [CrossRef]
- Grishin, M.N. [Use of antiseptic myramistin in the multimodality treatment of nonspecific suppurative pleuropulmonary diseases]. Probl. Tuberk. 1998, 1, 40–41. [Google Scholar]
- Vertelov, G.K.; Krutyakov, Y.A.; Efremenkova, O.V.; Olenin, A.Y.; Lisichkin, G.V. A versatile synthesis of highly bactericidal Myramistin® stabilized silver nanoparticles. Nanotechnology 2008, 19, 355707. [Google Scholar] [CrossRef]
- Quisno, R.; Foter, M.J. Cetyl Pyridinium Chloride: I. Germicidal Properties. J. Bacteriol. 1946, 52, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Bailey, D.M.; DeGrazia, C.G.; Hoff, S.J.; Schulenberg, P.L.; O’Connor, J.R.; Paris, D.A.; Slee, A.M. Bispyridinamines: A new class of topical antimicrobial agents as inhibitors of dental plaque. J. Med. Chem. 1984, 27, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Hübner, N.O.; Siebert, J.; Kramer, A. Octenidine Dihydrochloride, a Modern Antiseptic for Skin, Mucous Membranes and Wounds. Ski. Pharm. Phys. 2010, 23, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.; Braun, M.; Siebert, J.; Kietzmann, M. The percutaneous permeation of a combination of 0.1% octenidine dihydrochloride and 2% 2-phenoxyethanol (octenisept®) through skin of different species in vitro. BMC Vet. Res. 2011, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Cherian, B.; Gehlot, P.M.; Manjunath, M.K. Comparison of the Antimicrobial Efficacy of Octenidine Dihydrochloride and Chlorhexidine with and Without Passive Ultrasonic Irrigation—An Invitro Study. J. Clin. Diagn. Res. 2016, 10, ZC71–ZC77. [Google Scholar] [CrossRef] [PubMed]
- Dettenkofer, M.; Wilson, C.; Gratwohl, A.; Schmoor, C.; Bertz, H.; Frei, R.; Heim, D.; Luft, D.; Schulz, S.; Widmer, A.F. Skin disinfection with octenidine dihydrochloride for central venous catheter site care: A double-blind, randomized, controlled trial. Clin. Microbiol. Infect. 2010, 16, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadaway, L. Polyhexamethylene Biguanide Dressing—Another Promising Tool to Reduce Catheter-related Bloodstream Infection. JAVA 2010, 15, 203–205. [Google Scholar] [CrossRef]
- Roberts, W.R.; Addy, M. Comparison of the in vivo and in vitro antibacterial properties of antiseptic mouthrinses containing chlorhexidine, alexidine, cetyl pyridinium chloride and hexetidine. J. Clin. Periodontol. 1981, 8, 295–310. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Hope, C.K.; Wilson, M. Analysis of the Effects of Chlorhexidine on Oral Biofilm Vitality and Structure Based on Viability Profiling and an Indicator of Membrane Integrity. Antimicrob. Agents Chemother. 2004, 48, 1461–1468. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.; Stickler, D.J. Chlorhexidine resistance and the lipids of Providencia stuartii. Microbios 1979, 24, 141–150. [Google Scholar]
- Moore, K.; Gray, D. Using PHMB antimicrobial to prevent wound infection. Wounds UK 2007, 3, 96–102. [Google Scholar]
- Allen, M.J.; White, G.F.; Morby, A.P. The response of Escherichia coli to exposure to the biocide polyhexamethylene biguanide. Microbiology 2006, 152, 989–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Vereshchagin, A.N. Classical and interdisciplinary approaches to the design of organic and hybrid molecular systems. Russ. Chem. Bull. 2017, 66, 1765–1796. [Google Scholar] [CrossRef]
- Brown, A.C.; Fraser, T.R. On the Connection between Chemical Constitution and Physiological Action; with special reference to the Physiological Action of the Salts of the Ammonium Bases derived from Strychnia, Brucia, Thebaia, Codeia, Morphia, and Nicotia. J. Anat. Physiol. 1868, 2, 224–242. [Google Scholar] [PubMed]
- Roy, K.; Kar, S.; Das, R.N. A Primer on QSAR/QSPR Modeling; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary Ammonium Salts and Their Antimicrobial Potential: Targets or Nonspecific Interactions? Chem. Med. Chem. 2012, 7, 22–31. [Google Scholar] [CrossRef]
- Thorsteinsson, T.; Loftsson, T.; Masson, M. Soft Antibacterial Agents. Curr. Med. Chem. 2003, 10, 1129–1136. [Google Scholar] [CrossRef]
- Zubris, D.L.; Minbiole, K.P.C.; Wuest, W.M. Polymeric Quaternary Ammonium Compounds: Versatile Antimicrobial Materials. Curr. Top. Med. Chem. 2017, 17, 305–318. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [Green Version]
- Sowmiah, S.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Afonso, C.A.M. Pyridinium salts: From synthesis to reactivity and applications. Org. Chem. Front. 2018, 5, 453–493. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsson, T.; Másson, M.; Kristinsson, K.G.; Hjálmarsdóttir, M.A.; Hilmarsson, H.; Loftsson, T. Soft Antimicrobial Agents: Synthesis and Activity of Labile Environmentally Friendly Long Chain Quaternary Ammonium Compounds. J. Med. Chem. 2003, 46, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Mikláš, R.; Miklášová, N.; Bukovský, M.; Devínsky, F. Synthesis and antimicrobial properties of camphorsulfonic acid derived imidazolium salts. Acta Fac. Pharm. Univ. Comen. 2014, 61, 42–48. [Google Scholar] [CrossRef]
- Mikláš, R.; Miklášová, N.; Bukovský, M.; Horváth, B.; Kubincová, J.; Devínsky, F. Synthesis, surface and antimicrobial properties of some quaternary ammonium homochiral camphor sulfonamides. Eur. J. Pharm. Sci. 2014, 65, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Burki, S.; El-Haj, B.M.; Shafiullah; Parveen, S.; Nadeem, H.Ş.; Nadeem, S.; Shah, M.R. Synthesis and characterization of pyridine-based organic salts: Their antibacterial, antibiofilm and wound healing activities. Bioorg. Chem. 2020, 100, 103937. [Google Scholar] [CrossRef]
- Li, L.; Pu, T.; Zhanel, G.; Zhao, N.; Ens, W.; Liu, S. New Biocide with Both N-Chloramine and Quaternary Ammonium Moieties Exerts Enhanced Bactericidal Activity. Adv. Health. Mater. 2012, 1, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Li, L.; Logsetty, S.; Ghanbar, S.; Guo, M.; Ens, W.; Liu, S. Enhanced antibacterial activity of new “composite” biocides with both N-chloramine and quaternary ammonium moieties. Rsc Adv. 2015, 5, 93877–93887. [Google Scholar] [CrossRef]
- Ghanbar, S.; Kazemian, M.R.; Liu, S. New Generation of N-Chloramine/QAC Composite Biocides: Efficient Antimicrobial Agents To Target Antibiotic-Resistant Bacteria in the Presence of Organic Load. ACS Omega 2018, 3, 9699–9709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhao, Y.; Zhou, H.; Ning, A.; Zhang, F.; Zhao, Z. Synthesis of pyridinium N-chloramines for antibacterial applications. Tetrahedron Lett. 2017, 58, 321–325. [Google Scholar] [CrossRef]
- Liu, W.-S.; Wang, C.-H.; Sun, J.-F.; Hou, G.-G.; Wang, Y.-P.; Qu, R.-J. Synthesis, Characterization and Antibacterial Properties of Dihydroxy Quaternary Ammonium Salts with Long Chain Alkyl Bromides. Chem. Biol. Drug Des. 2015, 85, 91–97. [Google Scholar] [CrossRef]
- Xie, X.; Cong, W.; Zhao, F.; Li, H.; Xin, W.; Hou, G.; Wang, C. Synthesis, physiochemical property and antimicrobial activity of novel quaternary ammonium salts. J. Enzym. Inhib. Med. Chem. 2018, 33, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanov, A.V.; Zaripova, I.F.; Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Bukharov, S.V.; Voronina, J.K.; Vandyukov, A.E.; Mironov, V.F. Synthesis and Biological Evaluation of New Isatin-Based QACs with High Antimicrobial Potency. Chem. Sel. 2019, 4, 6162–6166. [Google Scholar] [CrossRef]
- Rusew, R.; Kurteva, V.; Shivachev, B. Novel Quaternary Ammonium Derivatives of 4-Pyrrolidino Pyridine: Synthesis, Structural, Thermal, and Antibacterial Studies. Crystals 2020, 10, 339. [Google Scholar] [CrossRef]
- Salajkova, S.; Benkova, M.; Marek, J.; Sleha, R.; Prchal, L.; Malinak, D.; Dolezal, R.; Sepčić, K.; Gunde-Cimerman, N.; Kuca, K.; et al. Wide-Antimicrobial Spectrum of Picolinium Salts. Molecules 2020, 25, 2254. [Google Scholar] [CrossRef] [PubMed]
- Shtyrlin, N.V.; Sapozhnikov, S.V.; Koshkin, S.A.; Iksanova, A.G.; Sabirov, A.H.; Kayumov, A.R.; Nureeva, A.A.; Zeldi, M.I.; Shtyrlin, Y.G. Synthesis and Antibacterial Activity of Novel Quaternary Ammonium Pyridoxine Derivatives. Med. Chem. 2015, 11, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikov, S.V.; Shtyrlin, N.V.; Kayumov, A.R.; Zamaldinova, A.E.; Iksanova, A.G.; Nikitina, E.V.; Krylova, E.S.; Grishaev, D.Y.; Balakin, K.V.; Shtyrlin, Y.G. New quaternary ammonium pyridoxine derivatives: Synthesis and antibacterial activity. Med. Chem. Res. 2017, 26, 3188–3202. [Google Scholar] [CrossRef]
- Kayumov, A.R.; Nureeva, A.A.; Trizna, E.Y.; Gazizova, G.R.; Bogachev, M.I.; Shtyrlin, N.V.; Pugachev, M.V.; Sapozhnikov, S.V.; Shtyrlin, Y.G. New Derivatives of Pyridoxine Exhibit High Antibacterial Activity against Biofilm-Embedded Staphylococcus Cells. Biomed Res. Int. 2015, 2015, 890968. [Google Scholar] [CrossRef] [PubMed]
- Shtyrlin, N.V.; Sapozhnikov, S.V.; Galiullina, A.S.; Kayumov, A.R.; Bondar, O.V.; Mirchink, E.P.; Isakova, E.B.; Firsov, A.A.; Balakin, K.V.; Shtyrlin, Y.G. Synthesis and Antibacterial Activity of Quaternary Ammonium 4-Deoxypyridoxine Derivatives. Biomed Res. Int. 2016, 2016, 3864193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garipov, M.R.; Sabirova, A.E.; Pavelyev, R.S.; Shtyrlin, N.V.; Lisovskaya, S.A.; Bondar, O.V.; Laikov, A.V.; Romanova, J.G.; Bogachev, M.I.; Kayumov, A.R.; et al. Targeting pathogenic fungi, bacteria and fungal-bacterial biofilms by newly synthesized quaternary ammonium derivative of pyridoxine and terbinafine with dual action profile. Bioorg. Chem. 2020, 104, 104306. [Google Scholar] [CrossRef]
- Sapozhnikov, S.V.; Sabirova, A.E.; Shtyrlin, N.V.; Druk, A.Y.; Agafonova, M.N.; Chirkova, M.N.; Kazakova, R.R.; Grishaev, D.Y.; Nikishova, T.V.; Krylova, E.S.; et al. Design, synthesis, antibacterial activity and toxicity of novel quaternary ammonium compounds based on pyridoxine and fatty acids. Eur. J. Med. Chem. 2021, 211, 113100. [Google Scholar] [CrossRef] [PubMed]
- Paniak, T.J.; Jennings, M.C.; Shanahan, P.C.; Joyce, M.D.; Santiago, C.N.; Wuest, W.M.; Minbiole, K.P.C. The antimicrobial activity of mono-, bis-, tris-, and tetracationic amphiphiles derived from simple polyamine platforms. Bioorg. Med. Chem. Lett. 2014, 24, 5824–5828. [Google Scholar] [CrossRef]
- Mitchell, M.A.; Iannetta, A.A.; Jennings, M.C.; Fletcher, M.H.; Wuest, W.M.; Minbiole, K.P.C. Scaffold-Hopping of Multicationic Amphiphiles Yields Three New Classes of Antimicrobials. Chem. Bio. Chem. 2015, 16, 2299–2303. [Google Scholar] [CrossRef]
- Minbiole, K.P.C.; Jennings, M.C.; Ator, L.E.; Black, J.W.; Grenier, M.C.; LaDow, J.E.; Caran, K.L.; Seifert, K.; Wuest, W.M. From antimicrobial activity to mechanism of resistance: The multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron 2016, 72, 3559–3566. [Google Scholar] [CrossRef] [Green Version]
- Joyce, M.D.; Jennings, M.C.; Santiago, C.N.; Fletcher, M.H.; Wuest, W.M.; Minbiole, K.P.C. Natural product-derived quaternary ammonium compounds with potent antimicrobial activity. J. Antibiot. 2016, 69, 344–347. [Google Scholar] [CrossRef]
- Black, J.W.; Jennings, M.C.; Azarewicz, J.; Paniak, T.J.; Grenier, M.C.; Wuest, W.M.; Minbiole, K.P.C. TMEDA-derived biscationic amphiphiles: An economical preparation of potent antibacterial agents. Bioorg. Med. Chem. Lett. 2014, 24, 99–102. [Google Scholar] [CrossRef]
- Allen, R.A.; Jennings, M.C.; Mitchell, M.A.; Al-Khalifa, S.E.; Wuest, W.M.; Minbiole, K.P.C. Ester- and amide-containing multiQACs: Exploring multicationic soft antimicrobial agents. Bioorg. Med. Chem. Lett. 2017, 27, 2107–2112. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [Green Version]
- Egorova, K.S.; Ananikov, V.P. Toxicity of ionic liquids: Eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. Chem. Sus. Chem. 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Fundamental importance of ionic interactions in the liquid phase: A review of recent studies of ionic liquids in biomedical and pharmaceutical applications. J. Mol. Liq. 2018, 272, 271–300. [Google Scholar] [CrossRef]
- Moshikur, R.; Chowdhury, R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem. 2020, 22, 8116–8139. [Google Scholar] [CrossRef]
- Demberelnyamba, D.; Kim, K.-S.; Choi, S.; Park, S.-Y.; Lee, H.; Kim, C.-J.; Yoo, I.-D. Synthesis and antimicrobial properties of imidazolium and pyrrolidinonium salts. Bioorg. Med. Chem. Lett. 2004, 12, 853–857. [Google Scholar] [CrossRef]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antibacterial activity of ionic liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Prudêncio, C.; Vieira, M.; Van der Auweraer, S.; Ferraz, R. Recycling old antibiotics with ionic liquids. Antibiotics 2020, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Carson, L.; Chau, P.K.W.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; McCann, M.T.; Seddon, K.R. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009, 11, 492–497. [Google Scholar] [CrossRef]
- Gundolf, T.; Rauch, B.; Kalb, R.; Rossmanith, P.; Mester, P. Influence of bacterial lipopolysaccharide modifications on the efficacy of antimicrobial ionic liquids. J. Mol. Liq. 2018, 271, 220–227. [Google Scholar] [CrossRef]
- Cornellas, A.; Perez, L.; Comelles, F.; Ribosa, I.; Manresa, A.; Garcia, M.T. Self-aggregation and antimicrobial activity of imidazolium and pyridinium based ionic liquids in aqueous solution. J. Colloid Interface Sci. 2011, 355, 164–171. [Google Scholar] [CrossRef]
- Bergamo, V.Z.; Donato, R.K.; Dalla Lana, D.F.; Donato, K.J.Z.; Ortega, G.G.; Schrekker, H.S.; Fuentefria, A.M. Imidazolium salts as antifungal agents: Strong antibiofilm activity against multidrug-resistant Candida tropicalis isolates. Lett. Appl. Microbiol. 2015, 60, 66–71. [Google Scholar] [CrossRef]
- Qin, J.; Guo, J.; Xu, Q.; Zheng, Z.; Mao, H.; Yan, F. Synthesis of pyrrolidinium-type poly(ionic liquid) membranes for antibacterial applications. ACS Appl. Mater. Interfaces 2017, 9, 10504–10511. [Google Scholar] [CrossRef]
- Florio, W.; Becherini, S.; D’Andrea, F.; Lupetti, A.; Chiappe, C.; Guazzelli, L. Comparative evaluation of antimicrobial activity of different types of ionic liquids. Mater. Sci. Eng. C 2019, 104, 109907. [Google Scholar] [CrossRef] [PubMed]
- Florio, W.; Rizzato, C.; Becherini, S.; Guazzelli, L.; D’Andrea, F.; Lupetti, A. Synergistic activity between colistin and the ionic liquids 1-methyl-3-dodecylimidazolium bromide, 1-dodecyl-1-methylpyrrolidinium bromide, or 1-dodecyl-1-methylpiperidinium bromide against Gram-negative bacteria. J. Glob. Antimicrob. Resist. 2020, 21, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Siopa, F.; Figueiredo, T.; Frade, R.F.M.; Neto, I.; Meirinhos, A.; Reis, C.P.; Sobral, R.G.; Afonso, C.A.M.; Rijo, P. Choline-based ionic liquids: Improvement of antimicrobial activity. Chem. Sel. 2016, 1, 5909–5916. [Google Scholar] [CrossRef]
- De Leo, F.; Marchetta, A.; Capillo, G.; Germanà, A.; Primerano, P.; Schiavo, S.L.; Urzì, C. Surface active ionic liquids based coatings as subaerial anti-biofilms for stone built cultural heritage. Coatings 2020, 11, 26. [Google Scholar] [CrossRef]
- Hajfarajollah, H.; Mokhtarani, B.; Noghabi, K.A.; Sharifi, A.; Mirzaei, M. Antibacterial and antiadhesive properties of butyl-methylimidazolium ionic liquids toward pathogenic bacteria. Rsc Adv. 2014, 4, 42751–42757. [Google Scholar] [CrossRef]
- Anvari, S.; Hajfarajollah, H.; Mokhtarani, B.; Enayati, M.; Sharifi, A.; Mirzaei, M. Antibacterial and anti-adhesive properties of ionic liquids with various cationic and anionic heads toward pathogenic bacteria. J. Mol. Liq. 2016, 221, 685–690. [Google Scholar] [CrossRef]
- Weyhing-Zerrer, N.; Kalb, R.; Oßmer, R.; Rossmanith, P.; Mester, P. Evidence of a reverse side-chain effect of tris(pentafluoroethyl)trifluorophosphate [FAP]-based ionic liquids against pathogenic bacteria. Ecotoxicol. Environ. Saf. 2018, 148, 467–472. [Google Scholar] [CrossRef]
- Cole, M.R.; Li, M.; El-Zahab, B.; Janes, M.E.; Hayes, D.; Warner, I.M. Design, synthesis, and biological evaluation of β-lactam antibiotic-based imidazolium- and pyridinium-type ionic liquids. Chem. Biol. Drug Des. 2011, 78, 33–41. [Google Scholar] [CrossRef]
- Venkata Nancharaiah, Y.; Reddy, G.K.K.; Lalithamanasa, P.; Venugopalan, V.P. The ionic liquid 1-alkyl-3-methylimidazolium demonstrates comparable antimicrobial and antibiofilm behavior to a cationic surfactant. Biofouling 2012, 28, 1141–1149. [Google Scholar] [CrossRef]
- Hough-Troutman, W.L.; Smiglak, M.; Griffin, S.; Matthew Reichert, W.; Mirska, I.; Jodynis-Liebert, J.; Adamska, T.; Nawrot, J.; Stasiewicz, M.; Rogers, R.D.; et al. Ionic liquids with dual biological function: Sweet and anti-microbial, hydrophobic quaternary ammonium-based salts. N. J. Chem. 2009, 33, 26–33. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini surfactants: A new class of self-assembling molecules. J. Am. Chem. Soc. 1993, 115, 10083–10090. [Google Scholar] [CrossRef]
- Pavlíková-Mořická, M.; Lacko, I.; Devínsky, F.; Masárová, L.; Mlynarčík, D. Quantitative relationships between structure and antimicrobial activity of new “Soft” bisquaternary ammonium salts. Fol. Microbiol. 1994, 39, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Devínsky, F.; Kopecka-Leitmanová, A.; Šeršeň, F.; Balgavý, P. Cut-off Effect in Antimicrobial Activity and in Membrane Perturbation Efficiency of the Homologous Series of N,N-Dimethylalkylamine Oxides†. J. Pharm. Pharm. 1990, 42, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable Cationic Antibacterial Amphiphiles: Synthesis, Mechanism of Action, and Cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Buttaro, B.A.; Minbiole, K.P.C.; Wuest, W.M. Bioorganic Investigation of Multicationic Antimicrobials to Combat QAC-Resistant Staphylococcus aureus. ACS Infect. Dis. 2015, 1, 304–309. [Google Scholar] [CrossRef] [PubMed]
- LaDow, J.E.; Warnock, D.C.; Hamill, K.M.; Simmons, K.L.; Davis, R.W.; Schwantes, C.R.; Flaherty, D.C.; Willcox, J.A.L.; Wilson-Henjum, K.; Caran, K.L.; et al. Bicephalic amphiphile architecture affects antibacterial activity. Eur. J. Med. Chem. 2011, 46, 4219–4226. [Google Scholar] [CrossRef]
- Shtyrlin, N.V.; Pugachev, M.V.; Sapozhnikov, S.V.; Garipov, M.R.; Vafina, R.M.; Grishaev, D.Y.; Pavelyev, R.S.; Kazakova, R.R.; Agafonova, M.N.; Iksanova, A.G.; et al. Novel Bis-Ammonium Salts of Pyridoxine: Synthesis and Antimicrobial Properties. Molecules 2020, 25, 4341. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.E.; Fletcher, M.H.; Jennings, M.C.; Duggan, S.M.; Minbiole, K.P.C.; Wuest, W.M. Structure–Resistance Relationships: Interrogating Antiseptic Resistance in Bacteria with Multicationic Quaternary Ammonium Dyes. Chem. Med. Chem. 2016, 11, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Maeda, T.; Nagamune, H.; Kourai, H. Synthesis and Antimicrobial Characteristics of Novel Biocides, 1, 1’-(Decanedioyl) bis (4-methy1–4-alkylpiperazinium iodide) s with a Gemini Structure. Biocontrol Sci. 2004, 9, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Kontos, R.C.; Schallenhammer, S.A.; Bentley, B.S.; Morrison, K.R.; Feliciano, J.A.; Tasca, J.A.; Kaplan, A.R.; Bezpalko, M.W.; Kassel, W.S.; Wuest, W.M.; et al. An Investigation into Rigidity–Activity Relationships in BisQAC Amphiphilic Antiseptics. Chem. Med. Chem. 2019, 14, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, N.; Huang, M.; Wang, L.; Han, J.; Qian, H.; Che, F. Synthesis, physicochemical and antimicrobial properties of cardanol-derived quaternary ammonium compounds (QACs) with heterocyclic polar head. J. Mol. Liq. 2019, 294, 111669. [Google Scholar] [CrossRef]
- Schallenhammer, S.A.; Duggan, S.M.; Morrison, K.R.; Bentley, B.S.; Wuest, W.M.; Minbiole, K.P.C. Hybrid BisQACs: Potent Biscationic Quaternary Ammonium Compounds Merging the Structures of Two Commercial Antiseptics. Chem. Med. Chem. 2017, 12, 1931–1934. [Google Scholar] [CrossRef]
- Morrison, K.R.; Allen, R.A.; Minbiole, K.P.C.; Wuest, W.M. More QACs, more questions: Recent advances in structure activity relationships and hurdles in understanding resistance mechanisms. Tetrahedron Lett. 2019, 60, 150935. [Google Scholar] [CrossRef]
- Thomas, B.; Duval, R.E.; Fontanay, S.; Varbanov, M.; Boisbrun, M. Synthesis and Antibacterial Evaluation of Bis-thiazolium, Bis-imidazolium, and Bis-triazolium Derivatives. Chem. Med. Chem. 2019, 14, 1232–1237. [Google Scholar] [CrossRef]
- Shirai, A.; Sumitomo, T.; Yoshida, M.; Kaimura, T.; Nagamune, H.; Maeda, T.; Kourai, H. Synthesis and Biological Properties of Gemini Quaternary Ammonium Compounds, 5,5’-[2,2’-(alpha,omega-Polymethylnedicarbonyldioxy)diethyl]bis-(3-alkyl-4-methylthiazolium iodide) and 5,5’-[2,2’-(p-Phenylenedicarbonyldioxy)diethyl]bis(3-alkyl-4-methylthiazolium bromide). Chem. Pharm. Bull. 2006, 54, 639–645. [Google Scholar]
- Shrestha, J.P.; Baker, C.; Kawasaki, Y.; Subedi, Y.P.; Vincent de Paul, N.N.; Takemoto, J.Y.; Chang, C.-W.T. Synthesis and bioactivity investigation of quinone-based dimeric cationic triazolium amphiphiles selective against resistant fungal and bacterial pathogens. Eur. J. Med. Chem. 2017, 126, 696–704. [Google Scholar] [CrossRef]
- Grenier, M.C.; Davis, R.W.; Wilson-Henjum, K.L.; LaDow, J.E.; Black, J.W.; Caran, K.L.; Seifert, K.; Minbiole, K.P.C. The antibacterial activity of 4,4′-bipyridinium amphiphiles with conventional, bicephalic and gemini architectures. Bioorg. Med. Chem. Lett. 2012, 22, 4055–4058. [Google Scholar] [CrossRef]
- Ator, L.E.; Jennings, M.C.; McGettigan, A.R.; Paul, J.J.; Wuest, W.M.; Minbiole, K.P.C. Beyond paraquats: Dialkyl 3,3′- and 3,4′-bipyridinium amphiphiles as antibacterial agents. Bioorg. Med. Chem. Lett. 2014, 24, 3706–3709. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, A.J.; Feliciano, J.A.; Sanchez, H.A.; Allen, R.A.; Morrison, K.R.; Sommers, K.J.; Carden, R.G.; Wuest, W.M.; Minbiole, K.P.C. Further Investigations into Rigidity-Activity Relationships in BisQAC Amphiphilic Antiseptics. Chem. Med. Chem. 2020, 15, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Yamamoto, M.; Vereshchagin, A.N.; Dorofeev, A.S.; Geyvandova, T.A.; Agafonova, I.F.; Geyvandov, R.K. Dimeric Quaternary Pyridinium Salts Possessing Biocidal Activity. Patent #WO158045, 2 October 2014. [Google Scholar]
- Yamamoto, M.; Takami, T.; Matsumura, R.; Dorofeev, A.; Hirata, Y.; Nagamune, H. In vitro evaluation of the biocompatibility of newly synthesized bis-quaternary ammonium compounds with spacer structures derived from pentaerythritol or hydroquinone. Biocontrol. Sci. 2016, 21, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Matsumura, R.; Hirata, Y.; Nagamune, H. A comparative study of skin irritation caused by novel bis-quaternary ammonium compounds and commonly used antiseptics by using cell culture methods. Toxicol. Vitr. 2019, 54, 75–81. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Gordeeva, A.M.; Frolov, N.A.; Proshin, P.I.; Hansford, K.A.; Egorov, M.P. Synthesis and Microbiological Properties of Novel Bis-Quaternary Ammonium Compounds Based on Biphenyl Spacer. Eur. J. Org. Chem 2019, 2019, 4123–4127. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Konyuhova, V.Y.; Hansford, K.A.; Egorov, M.P. Synthesis and microbiological properties of novel bis-quaternary ammonium compounds based on 4,4′-oxydiphenol spacer. Mendeleev Commun. 2019, 29, 523–525. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Konyuhova, V.Y.; Dorofeeva, E.O.; Hansford, K.A.; Egorov, M.P. Synthesis and biological evaluation of novel bis-quaternary ammonium compounds with p-terphenyl spacer. Mendeleev Commun. 2020, 30, 424–426. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Pakina, A.S.; Hansford, K.A.; Egorov, M.P. Synthesis and biological evaluation of novel bispyridinium salts containing naphthalene-2,7-diylbis(oxy) spacer. Mendeleev Commun. 2020, 30, 703–705. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Konyuhova, V.Y.; Kapelistaya, E.A.; Hansford, K.A.; Egorov, M.P. Investigations into the structure–activity relationship in gemini QACs based on biphenyl and oxydiphenyl linker. Rsc Adv. 2021, 11, 3429–3438. [Google Scholar] [CrossRef]
- Shirai, A.; Maeda, T.; Hara, I.; Yoshinari, A.; Nagamune, H.; Kourai, H. Antimicrobial Characteristics of Bis-quaternary Ammonium Compounds Possessing a p-Phenylene Group in Their Spacer Chains. Biocontrol Sci. 2003, 8, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Sumitomo, T.; Maeda, T.; Nagamune, H.; Kourai, H. Bacterioclastic Action of a Bis-Quaternary Ammonium Compound against Escherichia coli. Biocontrol Sci. 2004, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yabuhara, T.; Maeda, T.; Nagamune, H.; Kourai, H. Synthesis and Antimicrobial Characteristics of a Novel Biocide, 4, 4’-(1, 6-Dioxyhexamethylene) bis-(1-alkylpyridinium halide). Biocontrol. Sci. 2004, 9, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Ohkura, K.; Sukeno, A.; Nagamune, H.; Kourai, H. Bridge-linked bis-quaternary ammonium anti-microbial agents: Relationship between cytotoxicity and anti-bacterial activity of 5,5′-[2,2′-(tetramethylenedicarbonyldioxy)-diethyl]bis(3-alkyl-4-methylthiazonium iodide)s. Bioorg. Med. Chem. 2005, 13, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Kourai, H.; Yabuhara, T.; Shirai, A.; Maeda, T.; Nagamune, H. Syntheses and antimicrobial activities of a series of new bis-quaternary ammonium compounds. Eur. J. Med. Chem. 2006, 41, 437–444. [Google Scholar] [CrossRef]
- Murakami, K.; Yumoto, H.; Murakami, A.; Amoh, T.; Viducic, D.; Hirota, K.; Tabata, A.; Nagamune, H.; Kourai, H.; Matsuo, T.; et al. Evaluation of the effectiveness of the potent bis-quaternary ammonium compound, 4,4′-(α,ω-hexametylenedithio) bis (1-octylpyridinium bromide) (4DTBP-6,8) on Pseudomonas aeruginosa. J. Appl. Microbiol. 2017, 122, 893–899. [Google Scholar] [CrossRef]
- Obando, D.; Koda, Y.; Pantarat, N.; Lev, S.; Zuo, X.; Bijosono Oei, J.; Widmer, F.; Djordjevic, J.T.; Sorrell, T.C.; Jolliffe, K.A. Synthesis and Evaluation of a Series of Bis(pentylpyridinium) Compounds as Antifungal Agents. Chem. Med. Chem. 2018, 13, 1421–1436. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Qin, T.; Zhang, Y.; Li, Y.; Zhang, Y. Synthesis, surface properties and antimicrobial performance of novel gemini pyridinium surfactants. Colloids Surf. B 2019, 181, 814–821. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Egorov, M.P. Synthesis and antibacterial activity of new dimeric pyridinium chlorides based on 2,2-bis(hydroxymethyl)propane-1,3-diyl spacer. Russ. Chem. Bull. 2020, 69, 620–623. [Google Scholar] [CrossRef]
- Rezki, N.; Al-Sodies, S.A.; Ahmed, H.E.A.; Ihmaid, S.; Messali, M.; Ahmed, S.; Aouad, M.R. A novel dicationic ionic liquids encompassing pyridinium hydrazone-phenoxy conjugates as antimicrobial agents targeting diverse high resistant microbial strains. J. Mol. Liq. 2019, 284, 431–444. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Bhardwaj, P.; Rodriguez, L.C.; Palmer, K.L.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Dicationic imidazolium-based ionic liquids: A new strategy for non-toxic and antimicrobial materials. Rsc Adv. 2014, 4, 62594–62602. [Google Scholar] [CrossRef]
- Ganapathi, P.; Ganesan, K.; Vijaykanth, N.; Arunagirinathan, N. Anti-bacterial screening of water soluble carbonyl diimidazolium salts and its derivatives. J. Mol. Liq. 2016, 219, 180–185. [Google Scholar] [CrossRef]
- Ganapathi, P.; Ganesan, K. Anti-bacterial, catalytic and docking behaviours of novel di/trimeric imidazolium salts. J. Mol. Liq. 2017, 233, 452–464. [Google Scholar] [CrossRef]
- Forman, M.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P.C. Building a Better Quaternary Ammonium Compound (QAC): Branched Tetracationic Antiseptic Amphiphiles. Chem. Med. Chem. 2016, 11, 1401–1405. [Google Scholar] [CrossRef]
- Marafino, J.N.; Gallagher, T.M.; Barragan, J.; Volkers, B.L.; LaDow, J.E.; Bonifer, K.; Fitzgerald, G.; Floyd, J.L.; McKenna, K.; Minahan, N.T.; et al. Colloidal and antibacterial properties of novel triple-headed, double-tailed amphiphiles: Exploring structure–activity relationships and synergistic mixtures. Bioorg. Med. Chem. 2015, 23, 3566–3573. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, T.M.; Marafino, J.N.; Wimbish, B.K.; Volkers, B.; Fitzgerald, G.; McKenna, K.; Floyd, J.; Minahan, N.T.; Walsh, B.; Thompson, K.; et al. Hydra amphiphiles: Using three heads and one tail to influence aggregate formation and to kill pathogenic bacteria. Colloids Surf. B 2017, 157, 440–448. [Google Scholar] [CrossRef]
- Al-Khalifa, S.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P.C. The Development of Next-Generation Pyridinium-Based multiQAC Antiseptics. Chem. Med. Chem. 2017, 12, 280–283. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Minaeva, A.P.; Egorov, M.P. Synthesis and antibacterial activity of new tetrameric quaternary ammonium compounds based on pentaerythritol and 3-hydroxypyridine. Russ. Chem. Bull. 2021, 70, 545–548. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Huang, D.; Qin, A.; Tang, B.Z. CHAPTER 1 Overview of Click Polymerization. In Click Polymerization; The Royal Society of Chemistry: Croydon, UK, 2018; pp. 1–35. [Google Scholar]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Guo, J.; Qin, J.; Ren, Y.; Wang, B.; Cui, H.; Ding, Y.; Mao, H.; Yan, F. Antibacterial activity of cationic polymers: Side-chain or main-chain type? Polym. Chem. 2018, 9, 4611–4616. [Google Scholar] [CrossRef]
- Badawy, M.E.I. Structure and antimicrobial activity relationship of quaternary N-alkyl chitosan derivatives against some plant pathogens. J. Appl. Polym. Sci. 2010, 117, 960–969. [Google Scholar] [CrossRef]
- Shaban, S.M.; Aiad, I.; Moustafa, A.H.; Aljoboury, O.H. Some alginates polymeric cationic surfactants; surface study and their evaluation as biocide and corrosion inhibitors. J. Mol. Liq. 2019, 273, 164–176. [Google Scholar] [CrossRef]
- Dizman, B.; Elasri, M.O.; Mathias, L.J. Synthesis and antimicrobial activities of new water-soluble bis-quaternary ammonium methacrylate polymers. J. Appl. Polym. Sci. 2004, 94, 635–642. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Kleshcheva, N.A.; Moroz, A.F.; Didenko, L.V. Secondary and Tertiary Polydiallylammonium Salts: Novel Polymers with High Antimicrobial Activity. Biomacromolecules 2009, 10, 2976–2986. [Google Scholar] [CrossRef]
- Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.C.; Koromilas, N.D.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J.K. Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef]
- Druvari, D.; Koromilas, N.D.; Lainioti, G.C.; Bokias, G.; Vasilopoulos, G.; Vantarakis, A.; Baras, I.; Dourala, N.; Kallitsis, J.K. Polymeric Quaternary Ammonium-Containing Coatings with Potential Dual Contact-Based and Release-Based Antimicrobial Activity. ACS Appl. Mater. Interface 2016, 8, 35593–35605. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Zhao, Y.; Ren, L.; Yuan, X. Antifogging/Antibacterial Coatings Constructed by N-Hydroxyethylacrylamide and Quaternary Ammonium-Containing Copolymers. ACS Appl. Mater. Interfaces 2020, 12, 12305–12316. [Google Scholar] [CrossRef]
- Jaeger, W.; Bohrisch, J.; Laschewsky, A. Synthetic polymers with quaternary nitrogen atoms—Synthesis and structure of the most used type of cationic polyelectrolytes. Prog. Polym. Sci. 2010, 35, 511–577. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; De Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Peng, H.; Blakey, I.; Whittaker, A.K. Biocidal Polymers: A Mechanistic Overview. Polym. Rev. 2017, 57, 276–310. [Google Scholar] [CrossRef]
- Jie, Z.; Yan, X.; Zhao, L.; Worley, S.D.; Liang, J. Eco-friendly synthesis of regenerable antimicrobial polymeric resin with N-halamine and quaternary ammonium salt groups. RSC Adv. 2014, 4, 6048–6054. [Google Scholar] [CrossRef]
- Egorova, K.S.; Posvyatenko, A.V.; Larin, S.S.; Ananikov, V.P. Ionic liquids: Prospects for nucleic acid handling and delivery. Nucleic Acids Res. 2021, 49, 1201–1234. [Google Scholar] [CrossRef]
- Ran, B.; Zhang, Z.; Yin, L.; Hu, T.; Jiang, Z.; Wang, Q.; Li, Y. A facile antibacterial coating based on UV-curable acrylated imidazoliums. J. Coat. Technol. Res. 2018, 15, 345–349. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Voskian, S.; Brown, P.; Liu, A.; Lu, T.K.; Hatton, T.A.; de la Fuente-Nunez, C. Coatable and resistance-proof ionic liquid for pathogen eradication. ACS Nano 2021, 15, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xu, Q.; Guo, J.; Qin, J.; Mao, H.; Wang, B.; Yan, F. Structure–antibacterial activity relationships of imidazolium-type ionic liquid monomers, poly(ionic liquids) and poly(ionic liquid) membranes: Effect of alkyl chain length and cations. ACS Appl. Mater. Interfaces 2016, 8, 12684–12692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, B.; Guo, J.; Wang, M.; Cui, H.; Mao, H.; Wang, B.; Yan, F. Active pharmaceutical ingredient poly(ionic liquid)-based microneedles for the treatment of skin acne infection. Acta Biomater. 2020, 115, 136–147. [Google Scholar] [CrossRef]
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.; Muñoz-Bonilla, A.; Fernández-García, M. Tailoring macromolecular structure of cationic polymers towards efficient contact active antimicrobial surfaces. Polymers 2018, 10, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahiner, N.; Sagbas, S. Polymeric ionic liquid materials derived from natural source for adsorption purpose. Sep. Purif. Technol. 2018, 196, 208–216. [Google Scholar] [CrossRef]
- Ethirajan, S.K.; Sengupta, A.; Jebur, M.; Kamaz, M.; Qian, X.; Wickramasinghe, R. Single-step synthesis of novel polyionic liquids having antibacterial activity and showing π-electron mediated selectivity in separation of aromatics. ChemistrySelect 2018, 3, 4959–4968. [Google Scholar] [CrossRef]
- Claus, J.; Jastram, A.; Piktel, E.; Bucki, R.; Janmey, P.A.; Kragl, U. Polymerized ionic liquids-based hydrogels with intrinsic antibacterial activity: Modern weapons against a ntibiotic-resistant infections. J. Appl. Polym. Sci. 2020, 138, 50222. [Google Scholar] [CrossRef]
- Fang, C.; Kong, L.; Ge, Q.; Zhang, W.; Zhou, X.; Zhang, L.; Wang, X. Antibacterial activities of N-alkyl imidazolium-based poly(ionic liquid) nanoparticles. Polym. Chem. 2019, 10, 209–218. [Google Scholar] [CrossRef]
- Niesyto, K.; Neugebauer, D. Synthesis and characterization of ionic graft copolymers: Introduction and in vitro release of antibacterial drug by anion exchange. Polymers 2020, 12, 2159. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A novel high-strength poly(ionic liquid)/PVA hydrogel dressing for antibacterial applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Fang, H.; Li, D.; Xu, L.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A reusable ionic liquid-grafted antibacterial cotton gauze wound dressing. J. Mater. Sci. 2021, 56, 7598–7612. [Google Scholar] [CrossRef]
- Andresen, M.; Stenstad, P.; Møretrø, T.; Langsrud, S.; Syverud, K.; Johansson, L.-S.; Stenius, P. Nonleaching Antimicrobial Films Prepared from Surface-Modified Microfibrillated Cellulose. Biomacromolecules 2007, 8, 2149–2155. [Google Scholar] [CrossRef]
- Song, J.; Kong, H.; Jang, J. Bacterial adhesion inhibition of the quaternary ammonium functionalized silica nanoparticles. Colloids Surf. B 2011, 82, 651–656. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, K.; Li, R.; Ren, X.; Huang, T.S. Antibacterial cotton treated with N-halamine and quaternary ammonium salt. Cellulose 2013, 20, 3123–3130. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cheng, X.; Ren, X.; Huang, T.S. Self-assembled antibacterial coating by N-halamine polyelectrolytes on a cellulose substrate. J. Mater. Chem. B 2015, 3, 1446–1454. [Google Scholar] [CrossRef]
- Asri, L.A.T.W.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; van der Mei, H.C.; Loontjens, T.J.A.; Busscher, H.J. A Shape-Adaptive, Antibacterial-Coating of Immobilized Quaternary-Ammonium Compounds Tethered on Hyperbranched Polyurea and its Mechanism of Action. Adv. Func. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Zhao, J.; Millians, W.; Tang, S.; Wu, T.; Zhu, L.; Ming, W. Self-Stratified Antimicrobial Acrylic Coatings via One-Step UV Curing. ACS Appl. Mater. Interface 2015, 7, 18467–18472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, J.; Tang, C.Y.; Wang, Z.; Ng, H.Y.; Wu, Z. Antibiofouling Polyvinylidene Fluoride Membrane Modified by Quaternary Ammonium Compound: Direct Contact-Killing versus Induced Indirect Contact-Killing. Environ. Sci. Technol. 2016, 50, 5086–5093. [Google Scholar] [CrossRef] [PubMed]
- Żywicka, A.; Fijałkowski, K.; Junka, A.F.; Grzesiak, J.; El Fray, M. Modification of Bacterial Cellulose with Quaternary Ammonium Compounds Based on Fatty Acids and Amino Acids and the Effect on Antimicrobial Activity. Biomacromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef]
- He, D.; Yu, Y.; Liu, F.; Yao, Y.; Li, P.; Chen, J.; Ning, N.; Zhang, S. Quaternary ammonium salt-based cross-linked micelle templated synthesis of highly active silver nanocomposite for synergistic anti-biofilm application. Chem. Eng. J. 2020, 382, 122976. [Google Scholar] [CrossRef]
- Alkabli, J.; El-Sayed, W.N.; Elshaarawy, R.F.M.; Khedr, A.I.M. Upgrading Oryza sativa wastes into multifunctional antimicrobial and antibiofilm nominees; Ionic Metallo-Schiff base-supported cellulosic nanofibers. Eur. Polym. J 2020, 138, 109960. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, Z.; Wang, B.; Mao, H.; Yan, F. Zinc ion coordinated poly(ionic liquid) antimicrobial membranes for wound healing. ACS Appl. Mater. Interfaces 2017, 9, 14656–14664. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jin, K.; Wong, W.; Wang, Y.; Liang, T.; He, M.; Li, H.; Lu, C.; Tang, X.; Zong, Y.; et al. Ionic liquid functionalized non-releasing antibacterial hydrogel dressing coupled with electrical stimulation for the promotion of diabetic wound healing. Chem. Eng. J. 2021, 415, 129025. [Google Scholar] [CrossRef]
- Jin, L.; Shi, Z.; Zhang, X.; Liu, X.; Li, H.; Wang, J.; Liang, F.; Zhao, W.; Zhao, C. Intelligent antibacterial surface based on ionic liquid molecular brushes for bacterial killing and release. J. Mater. Chem. B 2019, 7, 5520–5527. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Song, H.; Wang, S.; Zhao, H.; Wei, D. Polyanionic composite membranes based on bacterial cellulose and amino acid for antimicrobial application. ACS Appl. Mater. Interfaces 2020, 12, 14784–14796. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wang, Y.; Wu, S.; Li, Y.; Li, J. Durable anti-superbug polymers: Covalent bonding of ionic liquid onto the polymer chains. Biomacromolecules 2017, 18, 4364–4372. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Fasolino, I.; Pastore, S.G.; Soriente, A.; Capeletti, L.B.; Dessuy, M.B.; Giannini, C.; Schrekker, H.S.; Ambrosio, L. Antimicrobial imidazolium ionic liquids for the development of minimal invasive calcium phosphate-based bionanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 42766–42776. [Google Scholar] [CrossRef] [PubMed]
- Suner, S.S.; Sahiner, M.; Akcali, A.; Sahiner, N. Functionalization of halloysite nanotubes with polyethyleneimine and various ionic liquid forms with antimicrobial activity. J. Appl. Polym. Sci. 2019, 137, 48352. [Google Scholar] [CrossRef]
- Gindri, I.M.; Palmer, K.L.; Siddiqui, D.A.; Aghyarian, S.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Evaluation of mammalian and bacterial cell activity on titanium surface coated with dicationic imidazolium-based ionic liquids. Rsc Adv. 2016, 6, 36475–36483. [Google Scholar] [CrossRef]
- Ye, Q.; Gao, T.; Wan, F.; Yu, B.; Pei, X.; Zhou, F.; Xue, Q. Grafting poly(ionic liquid) brushes for anti-bacterial and anti-biofouling applications. J. Mater. Chem. 2012, 22, 13123–13131. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kumar, A. Ionic liquid assisted gelatin films: Green, UV shielding, antioxidant, and antibacterial food packaging materials. ACS Sustain. Chem. Eng. 2019, 7, 8631–8636. [Google Scholar] [CrossRef]
- Martini Garcia, I.; Jung Ferreira, C.; de Souza, V.S.; Castelo Branco Leitune, V.; Samuel, S.M.W.; de Souza Balbinot, G.; de Souza da Motta, A.; Visioli, F.; Damiani Scholten, J.; Mezzomo Collares, F. Ionic liquid as antibacterial agent for an experimental orthodontic adhesive. Dent. Mater. 2019, 35, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report And Recommendations; Welcome Trust: London, UK, 2016; p. 84. [Google Scholar]





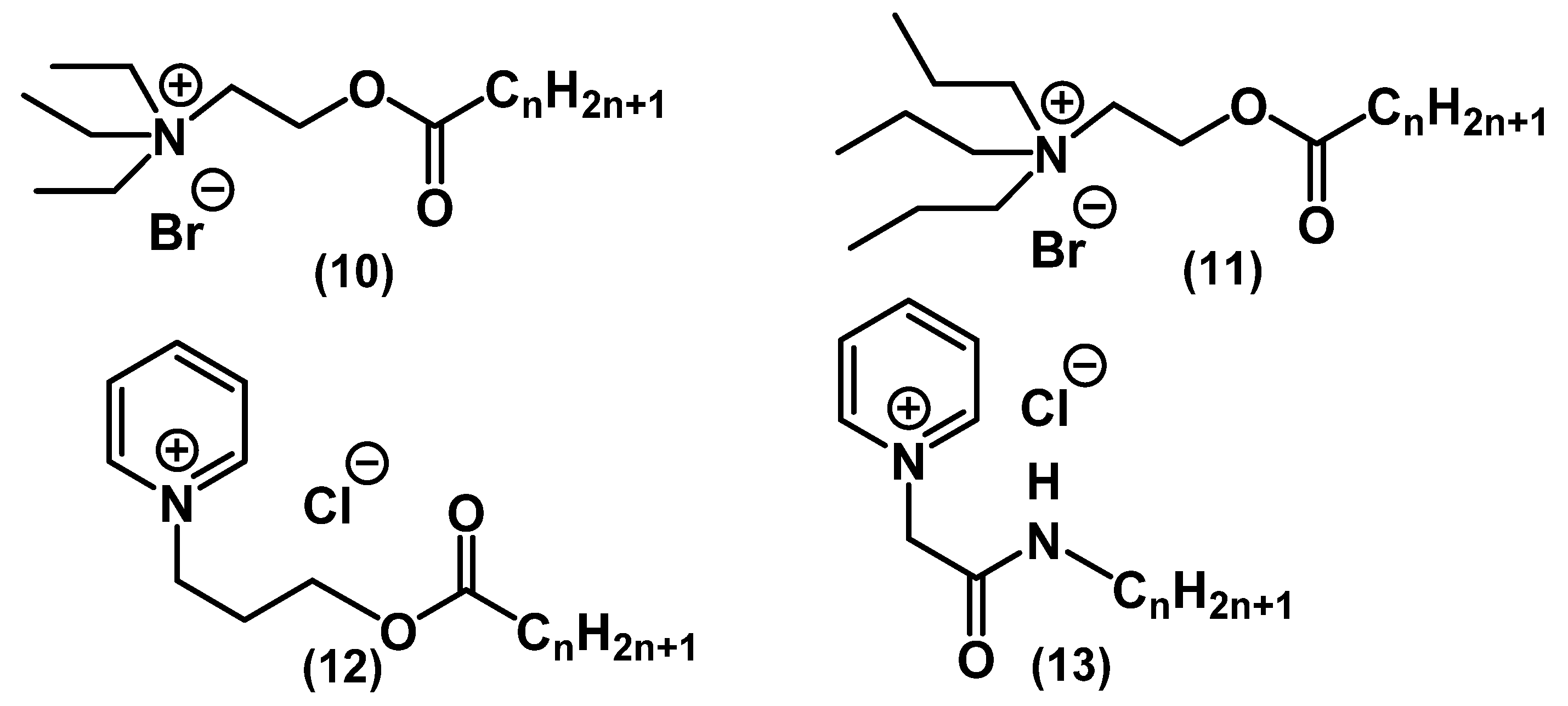




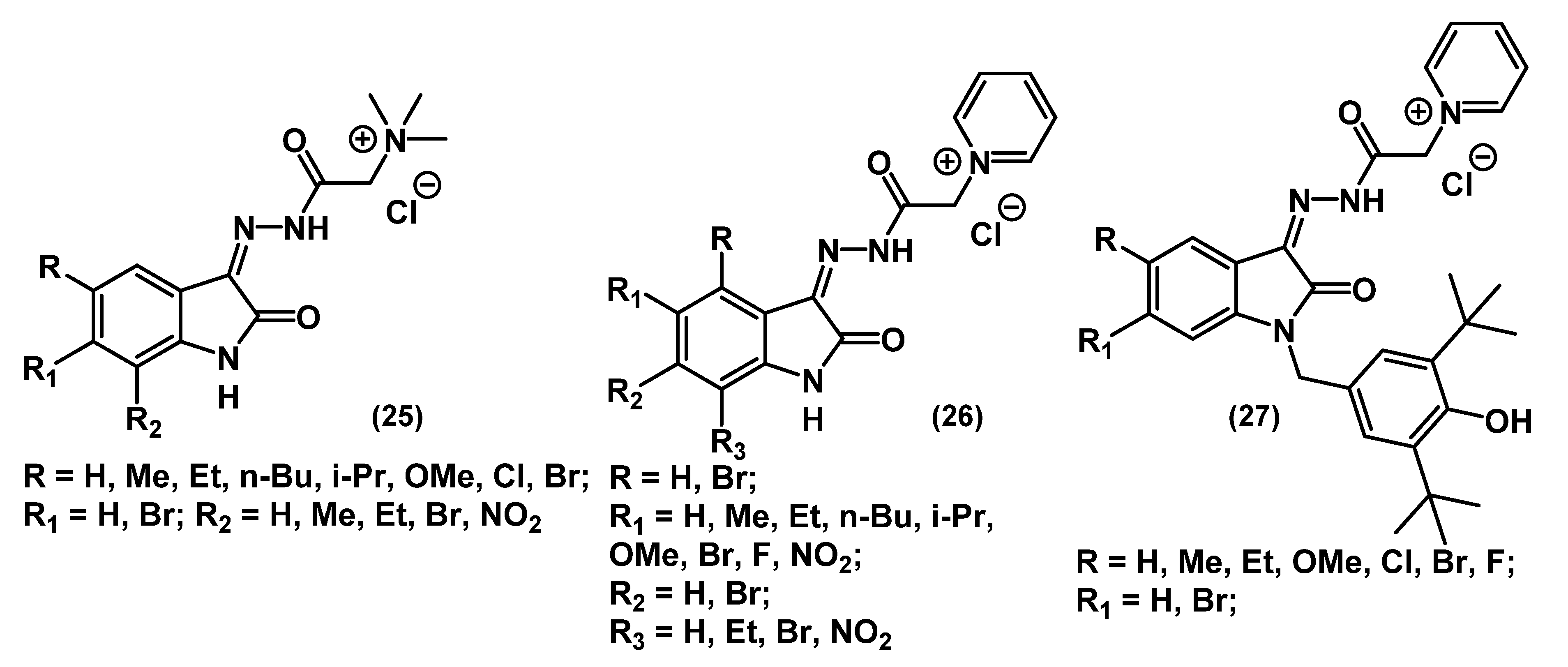


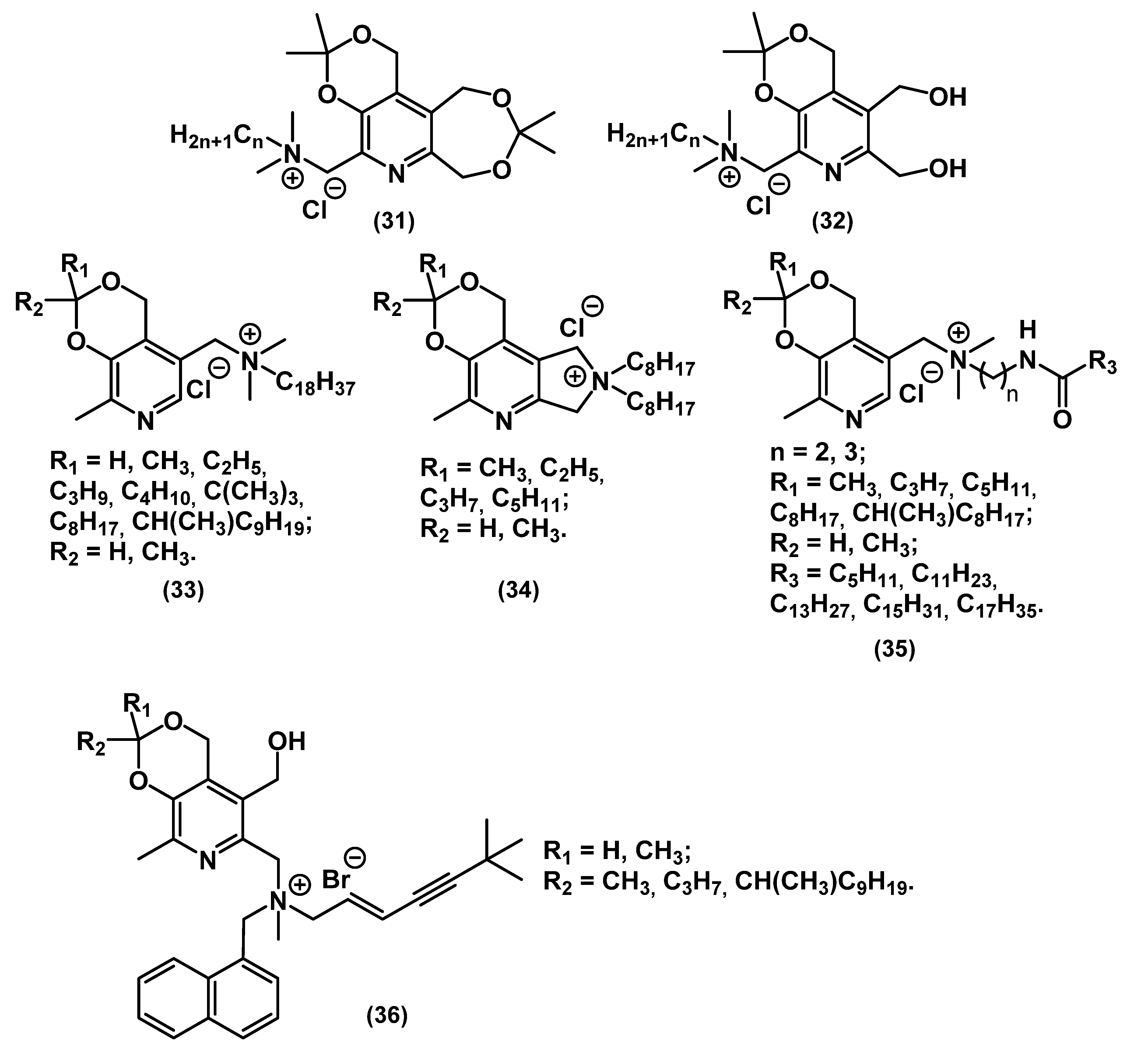
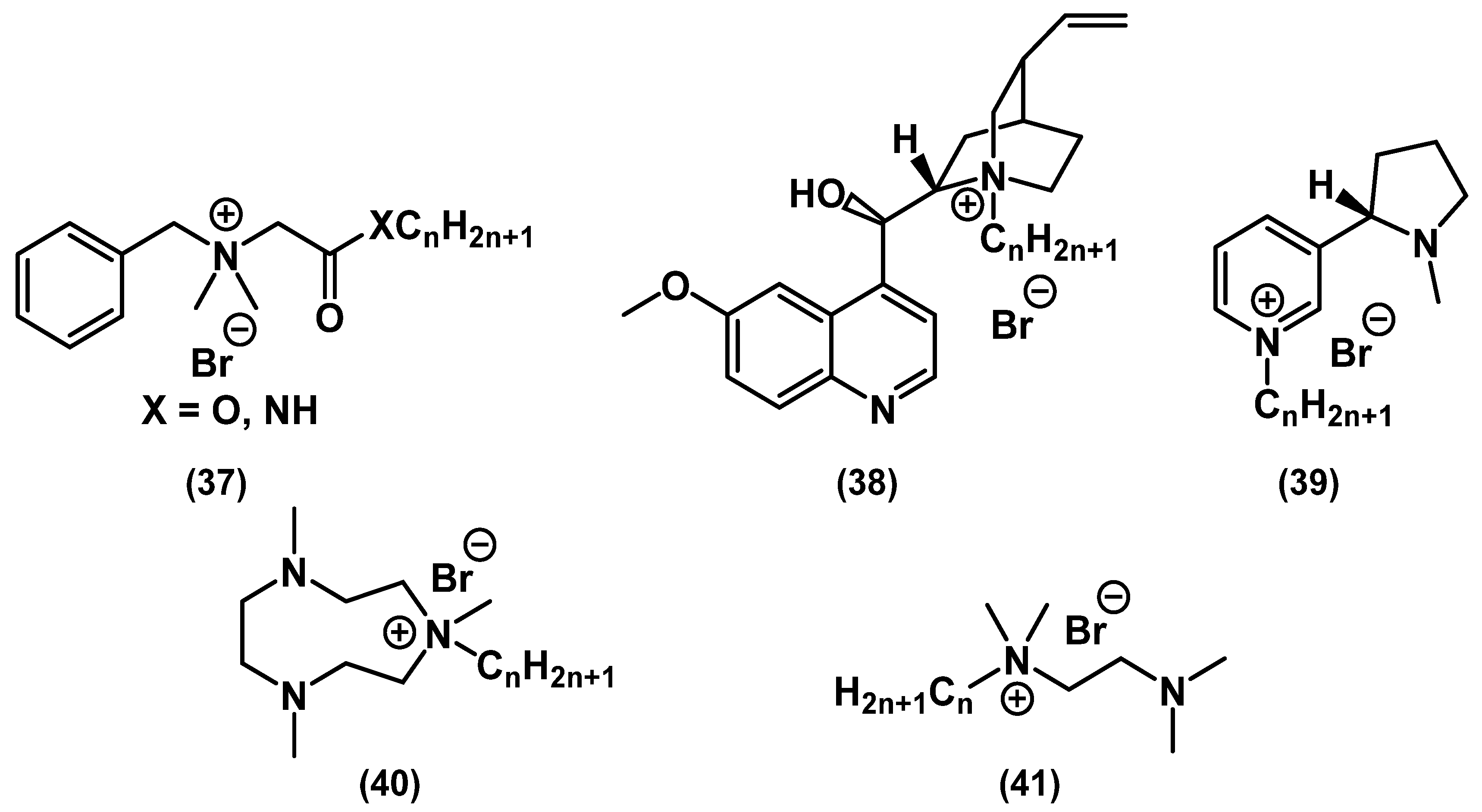
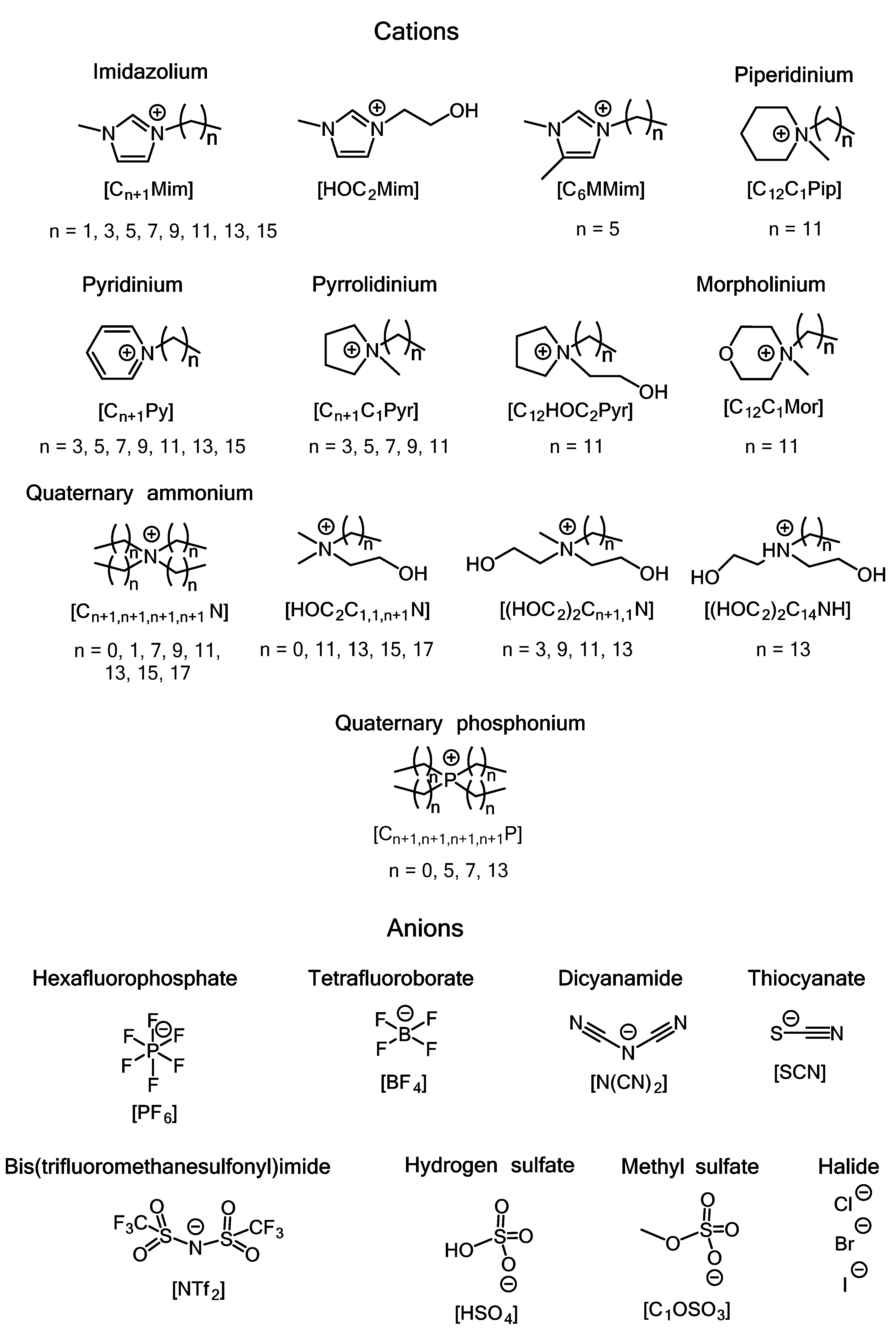

















| Series/ Compound | Strain | MIC, mg⋅L−1 | MBC, mg⋅L−1 | Method | Notes | Ref. |
|---|---|---|---|---|---|---|
| 10 | E. faecalis ATCC 29212 | 8 | 16 | Microtiter dilution | [52] | |
| S. aureus ATCC 25923 | 2 | 4 | ||||
| E. coli ATCC 25922 | 64 | 64 | ||||
| P. aeruginosa ATCC 27853 | 250 | 250 | ||||
| 11 | E. faecalis ATCC 29212 | 4 | 8 | Microtiter dilution | Active towards herpes simplex virus | [52] |
| S. aureus ATCC 25923 | 2 | 2 | ||||
| E. coli ATCC 25922 | 125 | 250 | ||||
| P. aeruginosa ATCC 27853 | 250 | 1000 | ||||
| 12 | E. faecalis ATCC 29212 | 1 | 4 | Microtiter dilution | [52] | |
| S. aureus ATCC 25923 | <0.25 | 1 | ||||
| E. coli ATCC 25922 | 250 | 250 | ||||
| P. aeruginosa ATCC 27853 | 500 | 500 | ||||
| 13 | E. faecalis ATCC 29212 | <0.25 | 8 | Microtiter dilution | [52] | |
| S. aureus ATCC 25923 | <0.25 | 4 | ||||
| E. coli ATCC 25922 | 1000 | >2000 | ||||
| P. aeruginosa ATCC 27853 | 1000 | >2000 | ||||
| 14 | S. aureus ATCC 6538 | 1.05 μM | Broth microdilution | [54] | ||
| E. coli CNCTC 377/79 | 2.2 μM | |||||
| C. albicans CCM 8186 | 1.05 μM | |||||
| 15 | S. aureus ATCC 6538 | 5.2 μM | Broth microdilution | [54] | ||
| E. coli CNCTC 377/79 | 41.2 μM | |||||
| C. albicans CCM 8186 | 164.9 μM | |||||
| 16 | S. aureus ATCC 6538 | 5.4 μM | Broth microdilution | [53] | ||
| E. coli CNCTC 377/79 | 144.1 μM | |||||
| C. albicans CCM 8186 | 5.4 μM | |||||
| 17 | S. aureus ATCC 6538 | 75% (percent of inhibition, 250 mg⋅L−1) | Broth microdilution | Active towards bacterial biofilms | [55] | |
| E. coli CNCTC 377/79 | 80% (percent of inhibition, 250 mg⋅L−1) | |||||
| 18 | MRSA 70065 | 3 min (Tk)/141 μM | [58] | |||
| E. coli ATCC 25922 | 3 min (Tk)/141 μM | |||||
| multidrug-resistant (MDR) P. aeruginosa 73104 | <1 min (Tk)/141 μM | |||||
| wild-type P. aeruginosan PA01 | 3 min (Tk)/141 μM | |||||
| 19 | methicillin-resistant S. aureus (MRSA) 70065 | 3 min (Tk (time to kill))/141 μM | [58] | |||
| E. coli ATCC 25922 | 3 min (Tk)/141 μM | |||||
| multidrug-resistant (MDR) P. aeruginosa 73104 | 5 min (Tk)/141 μM | |||||
| wild-type P. aeruginosan PA01 | 5 min (Tk)/141 μM | |||||
| 20 | S. aureus | 99% (reduction, contact time–5 min, 20 ppm) | AATCC test | [59] | ||
| E. coli | 100% (reduction, contact time–5 min, 20 ppm) | |||||
| 21 | S. aureus | 6.25 | 6.25 | Broth tube dilution | [61] | |
| a-H-tococcus | 12.5 | 12.5 | ||||
| b-H-tococcus | 1.56 | 3.125 | ||||
| E. coli | 25 | 25 | ||||
| P. aeruginosa | 25 | 25 | ||||
| P. vulgaris | 25 | 25 | ||||
| C. albicans | 6.25 | 6.25 | ||||
| C. mandshurica | 1.56 | 6.25 | ||||
| P. piricola | 3.125 | 3.125 | ||||
| A. niger | 3.125 | 6.25 | ||||
| 22 | S. aureus | 22.4 mm (IZ, 500 ppm) | Disk diffusion | [60] | ||
| B. subtilis | 17 mm (IZ, 500 ppm) | |||||
| E. coli | 24.1 mm (inhibition zone, 500 ppm) | |||||
| 23 | S. aureus | 6.25 | 6.25 | Broth tube dilution | [61] | |
| a-H-tococcus | 6.25 | 6.25 | ||||
| b-H-tococcus | 1.56 | 1.56 | ||||
| E. coli | 12.5 | 12.5 | ||||
| P. aeruginosa | 25 | 25 | ||||
| P. vulgaris | 12.5 | 12.5 | ||||
| C. albicans | 6.25 | 6.25 | ||||
| C. mandshurica | 3.125 | 3.125 | ||||
| P. piricola | 1.56 | 1.56 | ||||
| A. niger | 6.25 | 6.25 | ||||
| 24 | S. aureus | 12.5 | 25 | Broth tube dilution | [61] | |
| a-H-tococcus | 12.5 | 12.5 | ||||
| b-H-tococcus | 6.25 | 6.25 | ||||
| E. coli | 25 | 25 | ||||
| P. aeruginosa | 50 | 50 | ||||
| P. vulgaris | 25 | 25 | ||||
| C. albicans | 12.5 | 12.5 | ||||
| C. mandshurica | 12.5 | 12.5 | ||||
| P. piricola | 6.25 | 6.25 | ||||
| A. niger | 12.5 | 12.5 | ||||
| 25 | S. aureus ATCC 209p | 12.5 μM | Broth microdilution | [62] | ||
| B. cereus ATCC 8035 | 401 μM | |||||
| C. albicans 855-653 | 200 μM | |||||
| 27 | S. aureus ATCC 209p | 6.9 μM | Broth microdilution | [62] | ||
| B. cereus ATCC 8035 | 28.0 μM | |||||
| C. albicans 855-653 | 222 μM | |||||
| 29 | S. aureus | 14.3 mm (IZ, 500 ppm) | Disk diffusion | [63] | ||
| 30 | S. aureus C1947 | 0.49 μM | 1.22 μM | Broth microdilution | Active towards varicella-zoster virus | [64] |
| MRSA C1926 | 1.47 μM | 1.95 μM | ||||
| Vancomycin-reristant enterococci S2484 | 1.95 μM | 2.93 μM | ||||
| Y. bercovieri CNCTC6230 | 1.95 μM | 2.45 μM | ||||
| A. baumannii J3474 | 2.93 μM | 2.93 μM | ||||
| E. coli A1235 | 5.86 μM | 5.86 μM | ||||
| K. pneumoniae C1950 | 7.81 μM | 7.81 μM | ||||
| S. maltophilia J3552 | 5.86 μM | 5.86 μM | ||||
| Extended-spectrum β-lactamase-producing K. pneumonie C1934 | 7.81 μM | 15.63 μM | ||||
| C. parapsilosis sensu strictoEXF-8411 | 100 μM | |||||
| R. mucilaginosa EXF-8417 | 100 μM | |||||
| E. dermatitidis EXF-8470 | 30 μM | |||||
| A. melanogenum EXF-8432 | 30 μM | |||||
| B. dimerum EXF-8427 | 500 μM | |||||
| P. chrysogenum EXF-1818 | 300 μM | |||||
| A. versicolor EXF-8692 | 65 μM | |||||
| 32 | S. aureus ATCC29213 | 2 | Broth microdilution | [66] | ||
| S. epidermidis (clinical isolate) | 2 | |||||
| M. luteus (clinical isolate) | 2 | |||||
| E. coli ATCC25922 | >64 | |||||
| S. typhimurium TA100 | >64 | |||||
| P. aeruginosa ATCC27853 | >64 | |||||
| 33 | S. aureus ATCC29213 | 4 | Broth microdilution | [66] | ||
| S. epidermidis (clinical isolate) | 4 | |||||
| M. luteus (clinical isolate) | 2 | |||||
| E. coli ATCC25922 | >64 | |||||
| S. typhimurium TA100 | 4 | |||||
| P. aeruginosa ATCC27853 | >64 | |||||
| 34 | S. aureus ATCC29213 | 0.5 | Broth microdilution | [66] | ||
| S. epidermidis (clinical isolate) | 0.5 | |||||
| M. luteus (clinical isolate) | 0.5 | |||||
| E. coli ATCC25922 | 2 | |||||
| S. typhimurium TA100 | 0.5 | |||||
| P. aeruginosa ATCC27853 | >64 | |||||
| 35 | S. aureus ATCC29213 | 0.5 | Broth microdilution | Non-genotoxic and non-mutagenic | [70] | |
| S. epidermidis (clinical isolate) | 2 | |||||
| M. luteus (clinical isolate) | 1 | |||||
| E. coli ATCC25922 | 8 | |||||
| P. aeruginosa ATCC27853 | 8 | |||||
| 36 | S. aureus ATCC 29213 | 4 | 8 | Broth microdilution | Active towards bacterial, fungi and mixed biofilms | [69] |
| B. subtilis 168 | 4 | 8 | ||||
| S. epidermidis | 4 | 8 | ||||
| E. coli MG1655 | 16 | 16 | ||||
| K. pneumoniae | >64 | >64 | ||||
| P. aeruginosa ATCC 27853 | 64 | 64 | ||||
| 37 | S. aureus | 2 μM | Broth microdilution | [76] | ||
| E. faecalis | 4 μM | |||||
| E. coli | 16 μM | |||||
| P. aeruginosa | 63 μM | |||||
| MRSA 300-0114 | 2 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| 38 | S. aureus | 0.5 μM | Broth microdilution | Natural derivatives | [74] | |
| MRSA 300-0114 | 2 μM | |||||
| MRSA ATCC 33592 | 4 μM | |||||
| E. faecalis | 1 μM | |||||
| E. coli | 8 μM | |||||
| P. aeruginosa | 8 μM | |||||
| 39 | S. aureus | 1 μM | Broth microdilution | Natural derivatives | [74] | |
| MRSA 300-0114 | 4 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| E. faecalis | 1 μM | |||||
| E. coli | 4 μM | |||||
| P. aeruginosa | 63 μM | |||||
| 40 | S. aureus | 1 μM | Broth microdilution | [72] | ||
| MRSA 300-0114 | 4 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| E. faecalis | 1 μM | |||||
| E. coli | 4 μM | |||||
| P. aeruginosa | 63 μM | |||||
| 41 | S. aureus SH1000 | 1 μM | Broth microdilution | [75] | ||
| E. faecalis OG1RF | 16 μM | |||||
| E. coli MC4100 | 16 μM | |||||
| P. aeruginosa PAO1-WT | 16 μM |
| IL | Acronym | Species | MIC, μg mL−1 | MBC, μg mL−1 | Method | Notes | Ref. |
|---|---|---|---|---|---|---|---|
| 1-Ethyl-3-methylimidazolium bromide | [C2Mim][Br] | E. coli ATCC 25922 | >5000 µM | Broth microdilution | E. coli TEM CTX M9, CTX M2, and AmpC MOX2 are ampicillin-resistant strains. | [82] | |
| E. coli TEM CTX M9 | 5000 µM | ||||||
| E. coli CTX M2 | >5000 µM | ||||||
| E. coli AmpC MOX2 | >5000 µM | ||||||
| K. pneumoniae (clinical isolate) | >5000 µM | ||||||
| S. aureus ATCC 25293 | 50 µM | ||||||
| S. epidermidis (clinical isolate) | 5000 µM | ||||||
| E. faecalis (clinical isolate) | >5000 µM | ||||||
| 1-Butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide | [C4Mim][NTf2] | P. aeruginosa PTCC 1310 | 3120 | 3120 | Agar disk diffusion/agar well diffusion | Anti-adhesive activity a | [94] |
| S. aureus PTCC 1112 | 3120 | 3120 | |||||
| E. coli PTCC 1338 | <40 | 48 | |||||
| B. cereus PTCC 1015 | 3120 | 3120 | |||||
| S. typhimurium (wild type) | 390 | 390 | |||||
| K. pneumonia PTCC 1290 | 3120 | 3120 | |||||
| B. subtilis PTCC 1715 | 3120 | 3120 | |||||
| 1-Octyl-3-methylimidazolium bromide | [C8Mim][Br] | M. luteus ATCC 9341 | R | Broth microdilution | R, resistant at the highest concentration tested (256 μg mL−1). | [81,87] | |
| S. epidermidis ATCC155-1 | 930 μM | ||||||
| S. aureus ATCC 25178 | R | ||||||
| S. aureus 209 KCTC1916 | 64 | ||||||
| S. aureus R209 KCTC1928 | 250 | ||||||
| E. coli ATCC 27325 | R | ||||||
| E. coli KCTC1924 | 64 | ||||||
| K. pneumonia ATCC 9721 | R | ||||||
| P. aeruginosa ATCC 9721 | R | ||||||
| C. albicans ATCC10231 | R | ||||||
| C. albicans KCTC19401 | 250 | ||||||
| B. subtilis ATCC663 | R | ||||||
| B. subtilis KCTC1914 | 500 | ||||||
| S. typhimurium KCTC1926 | 500 | ||||||
| C. regularis | 500 | ||||||
| 1-Octyl-3-methylimidazolium nitrate | [C8Mim][NO3] | S. aureus | 97 | 97 | Agar disk diffusion/agar well diffusion | Anti-adhesive activity a | [95] |
| K. pneumoniae | 780 | 780 | |||||
| S. typhimurium | 780 | 780 | |||||
| P. aeruginosa | 1560 | 1560 | |||||
| E. coli | 39 | 39 | |||||
| B. tequilensis | 19 | 19 | |||||
| B. subtilis | 19 | 19 | |||||
| 1-Decyl-3-methylimidazolium chloride | [C10Mim][Cl] | S. aureus ATCC 29213 | 40 μM (MBEC 2415 μM) | 643 μM | Broth microdilution, MBEC assay | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [81,85,86] |
| E-MRSA 15 | 40 μM (MBEC 1207 μM) | 321 μM | |||||
| MRSA (clinical strain 201) | 160 μM (MBEC 4829 μM) | 643 μM | |||||
| S. aureus 209 KCTC1916 | 16 | ||||||
| S. aureus R209 KCTC1928 | 32 | ||||||
| S. epidermidis ATCC 12228 | 40 μM | 644 μM | |||||
| S. epidermidis ATCC 35984 | 40 μM (MBEC 4829 μM) | 160 μM | |||||
| E. coli NCTC 8196 | 321 μM (MBEC 9659 μM) | 1287 μM | |||||
| E. coli KCTC1924 | 8 | ||||||
| E. coli BW25113 (wild-type) | 188.9 | ||||||
| E. coli JW3596 (ΔrfaC) | 100 | ||||||
| E. coli JW3597 (ΔrfaL) | 155 | ||||||
| E. coli JW3606 (ΔrfaG) | 67.5 | ||||||
| P. aeruginosa PA01 | >1287 μM (MBEC 2415 μM) | >1287 μM | |||||
| K. aerogenes NCTC 7427 | 643 μM (MBEC 19318 μM) | 1287 μM | |||||
| B. cenocepacia J2315 | 1287 μM (MBEC 19318 μM) | 1287 μM | |||||
| P. mirabilis NCTC 12442 | 1287 μM (MBEC 9659 μM) | 1287 μM | |||||
| C. tropicalis NCTC 7393 | 321 μM (MBEC 19318 μM) | 321 μM | |||||
| B. subtilis KCTC1914 | 125 | ||||||
| S. typhimurium KCTC1926 | 125 | ||||||
| C. albicans KCTC19401 | 250 | ||||||
| C. regularis | 250 | ||||||
| 1-Decyl-3-methylimidazolium bromide | [C10Mim][Br] | M. luteus ATCC 9341 | R | Broth microdilution | R, resistant at the highest concentration tested (256 μg mL−1). | [87] | |
| S. epidermidis ATCC155-1 | 844 μM | ||||||
| S. aureus ATCC 25178 | 106 μM | ||||||
| E. coli ATCC 27325 | R | ||||||
| K. pneumonia ATCC 9721 | R | ||||||
| P. aeruginosa ATCC 9721 | R | ||||||
| C. albicans ATCC10231 | R | ||||||
| B. subtilis ATCC6633 | 422 μM | ||||||
| 1-Dodecyl-3-methylimidazolium chloride | [C12Mim][Cl] | S. aureus ATCC 29213 | 18 μM (MBEC 272 μM) | 36 μM | Broth microdilution, MBEC assay | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [85,86] |
| E-MRSA 15 | 18 μM (MBEC 272 μM) | 73 μM | |||||
| MRSA (clinical strain 201) | 36 μM (MBEC 545 μM) | 290 μM | |||||
| S. epidermidis ATCC 12228 | 36 μM | 145 μM | |||||
| S. epidermidis ATCC 35984 | 36 μM (MBEC 272 μM) | 73 μM | |||||
| E. coli NCTC 8196 | 73 μM (MBEC 1089 μM) | 73 μM | |||||
| E. coli BW25113 (wild-type) | 47.3 | ||||||
| E. coli JW3596 (ΔrfaC) | 10.1 | ||||||
| E. coli JW3597 (ΔrfaL) | 45.4 | ||||||
| E. coli JW3606 (ΔrfaG) | 11.4 | ||||||
| P. aeruginosa PA01 | 580 μM (MBEC 1089 μM) | 1161 μM | |||||
| K. aerogenes NCTC 7427 | 73 μM (MBEC 2179 μM) | 145 μM | |||||
| B. cenocepacia J2315 | 290 μM (MBEC 2179 μM) | 580 μM | |||||
| P. mirabilis NCTC 12442 | 580 μM (MBEC 4357 μM) | 1161 μM | |||||
| C. tropicalis NCTC 7393 | 73 μM (MBEC 8714 μM) | 73 μM | |||||
| 1-Dodecyl-3-methylimidazolium bromide | [C12Mim][Br] | M. luteus ATCC 9341 | R | Broth microdilution | R, resistant at the highest concentration tested (256 μg mL−1). | [81,87,90,91] | |
| S. epidermidis ATCC155-1 | 193 μM | ||||||
| S. epidermidis ATCC 35984 | 2.5 | ||||||
| S. aureus ATCC 25178 | 97 μM | ||||||
| S. aureus ATCC 6538 | 2.5 | 40 | |||||
| S. aureus 209 KCTC1916 | 4 | ||||||
| S. aureus R209 KCTC1928 | 8 | ||||||
| E. coli ATCC 27325 | 386 μM | ||||||
| E. coli ATCC 25922 | 20 | 10 | |||||
| E. coli KCTC1924 | 8 | ||||||
| K. pneumonia ATCC 9721 | 773 μM | ||||||
| K. pneumonia ATCC BAA-1705 | 80 | ||||||
| P. aeruginosa ATCC 9721 | R | ||||||
| P. aeruginosa ATCC 27853 | 160 | 20 | |||||
| C. albicans ATCC10231 | R | ||||||
| B. subtilis ATCC6633 | 48 μM | ||||||
| B. subtilis KCTC1914 | 8 | ||||||
| S. typhimurium KCTC1926 | 32 | ||||||
| A. baumannii AB01 | 80 | ||||||
| E. faecalis ATCC 29212 | 5 | 40 | |||||
| C. albicans KCTC19401 | 32 | ||||||
| C. regularis | 16 | ||||||
| 1-Dodecyl-3-methylimidazolium iodide | [C12Mim][I] | S. aureus V329 | 0.31 μM | 5 μM | Broth microdilution | Potent anti-biofilm activity (higher against S. aureus) | [98] |
| P. aeruginosa PAO1 | 125 μM | 250 μM | |||||
| 1-Tetradecyl-3-methylimidazolim chloride | [C14Mim][Cl] | S. aureus ATCC 29213 | 16 μM (MBEC 124 μM) | 66 μM | Broth microdilution, MBEC assay | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [81,85,86] |
| E-MRSA 15 | 16 μM (MBEC 248 μM) | 66 μM | |||||
| MRSA (clinical strain 201) | 16 μM (MBEC 124 μM) | 66 μM | |||||
| S. aureus 209 KCTC1916 | 4 | ||||||
| S. aureus R209 KCTC1928 | 4 | ||||||
| S. epidermidis ATCC 12228 | 7.75 μM | 33 μM | |||||
| S. epidermidis ATCC 35984 | 7.75 μM (MBEC 124 μM) | 33 μM | |||||
| E. coli NCTC 8196 | 33 μM (MBEC 124 μM) | 33 μM | |||||
| E. coli KCTC1924 | 4 | ||||||
| E. coli BW25113 (wild-type) | 14.9 | ||||||
| E. coli JW3596 (ΔrfaC) | 2.2 | ||||||
| E. coli JW3597 (ΔrfaL) | 15.5 | ||||||
| E. coli JW3606 (ΔrfaG) | 3.3 | ||||||
| P. aeruginosa PA01 | 264 μM (MBEC 496 μM) | 264 μM | |||||
| K. aerogenes NCTC 7427 | 33 μM (MBEC 248 μM) | 66 μM | |||||
| B. cenocepacia J2315 | 132 μM (MBEC 496 μM) | 264 μM | |||||
| P. mirabilis NCTC 12442 | 264 μM (MBEC 1984 μM) | 530 μM | |||||
| C. tropicalis NCTC 7393 | 66 μM (MBEC 248 μM) | 132 μM | |||||
| B. subtilis KCTC1914 | 4 | ||||||
| S. typhimurium KCTC1926 | 8 | ||||||
| C. albicans KCTC19401 | 8 | ||||||
| C. regularis | 8 | ||||||
| 1-Tetradecyl-3-methylimidazolim bromide | [C14Mim][Br] | M. luteus ATCC 9341 | 178 μM | Broth microdilution | [81,87] | ||
| S. epidermidis ATCC155-1 | 6 μM | ||||||
| S. aureus ATCC 25178 | 45 μM | ||||||
| S. aureus 209 KCTC1916 | 4 | ||||||
| S. aureus R209 KCTC1928 | 4 | ||||||
| E. coli ATCC 27325 | 356 μM | ||||||
| E. coli KCTC1924 | 4 | ||||||
| K. pneumonia ATCC 9721 | 356 μM | ||||||
| P. aeruginosa ATCC 9721 | 356 μM | ||||||
| C. albicans ATCC10231 | 178 μM | ||||||
| B. subtilis ATCC6633 | 6 μM | ||||||
| B. subtilis KCTC1914 | 4 | ||||||
| S. typhimurium KCTC1926 | 8 | ||||||
| C. albicans KCTC19401 | 8 | ||||||
| C. regularis | 16 | ||||||
| 1-Hexadecyl-3-methylimidazolim chloride | [C16Mim][Cl] | E. coli BW25113 (wild-type) | 7.7 | Broth microdilution | The clinical isolates 72A, 72P, and 94P are resistant to fluconazole, amphotericin B, voriconazole and anidulafungin. Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86,88] | |
| E. coli JW3596 (ΔrfaC) | 3.5 | ||||||
| E. coli JW3597 (ΔrfaL) | 8.2 | ||||||
| E. coli JW3606 (ΔrfaG) | 3 | ||||||
| C. tropicalis 17A | 0.014 (MBEC 0.028) | ||||||
| C. tropicalis 57A | 0.014 (MBEC 0.056) | ||||||
| C. tropicalis 72A | 0.014 (MBEC 0.056) | ||||||
| C. tropicalis 72P | 0.014 (MBEC 0.056) | ||||||
| C. tropicalis 94P | 0.014 (MBEC 0.225) | ||||||
| C. tropicalis 102A | 0.014 (MBEC 0.056) | ||||||
| 1-Hexadecyl-3-methylimidazolim bromide | [C16Mim][Br] | S. aureus 209 KCTC1916 | 8 | Broth microdilution | [81,97] | ||
| S. aureus R209 KCTC1928 | 4 | ||||||
| S. aureus ATCC 6538 | 15 µM | ||||||
| E. coli KCTC1924 | 8 | ||||||
| E. coli O157:H7 ATCC 43895 | 10 µM | ||||||
| B. subtilis KCTC1914 | 4 | ||||||
| S. typhimurium KCTC1926 | 4 | ||||||
| E. faecium ATCC 49474 | 1 µM | ||||||
| K. pneumonia ATCC 4352 | 15 µM | ||||||
| C. albicans KCTC19401 | 8 | ||||||
| C. regularis | 8 | ||||||
| 1-Hexyl-2,3-dimethylimidazolium bromide | [C6MMim][Br] | S. aureus ATCC 6538 | 23 µM | Broth microdilution | [97] | ||
| E. coli O157:H7 ATCC 43895 | 12 µM | ||||||
| E. faecium ATCC 49474 | 9 µM | ||||||
| K. pneumonia ATCC 4352 | 15 µM | ||||||
| N-Dodecylpyridinium bromide | [C12Py][Br] | M. luteus ATCC 9341 | R | Broth microdilution | R, resistant at the highest concentration tested (256 μg mL−1). | [87] | |
| S. epidermidis ATCC155-1 | 49 μM | ||||||
| S. aureus ATCC 25178 | 195 μM | ||||||
| E. coli ATCC 27325 | 97 μM | ||||||
| K. pneumonia ATCC 9721 | 780 μM | ||||||
| P. aeruginosa ATCC 9721 | 780 μM | ||||||
| C. albicans ATCC10231 | R | ||||||
| B. subtilis ATCC6633 | 24 μM | ||||||
| N-Tetradecylpyridinium bromide | [C14Py][Br] | M. luteus ATCC 9341 | 90 μM | Broth microdilution | [87] | ||
| S. epidermidis ATCC155-1 | 6 μM | ||||||
| S. aureus ATCC 25178 | 22 μM | ||||||
| E. coli ATCC 27325 | 45 μM | ||||||
| K. pneumonia ATCC 9721 | 359 μM | ||||||
| P. aeruginosa ATCC 9721 | 359 μM | ||||||
| C. albicans ATCC10231 | 359 μM | ||||||
| B. subtilis ATCC6633 | 6 μM | ||||||
| N-Hexadecylpyridinium chloride | [C16Py][Cl] | E. coli ATCC 25922 | 500 μM | Broth microdilution | E. coli TEM CTX M9, CTX M2, and AmpC MOX2 are ampicillin-resistant strains. | [81,82] | |
| E. coli TEM CTX M9 | 500 μM | ||||||
| E. coli CTX M2 | >5000 μM | ||||||
| E. coli AmpC MOX2 | >5000 μM | ||||||
| K. pneumoniae (clinical isolate) | 2500 μM | ||||||
| S. aureus ATCC 25293 | 500 μM | ||||||
| S. aureus 209 KCTC1916 | 8 | ||||||
| S. aureus R209 KCTC1928 | 8 | ||||||
| S. epidermidis (clinical isolate) | 2500 μM | ||||||
| E. faecalis (clinical isolate) | 500 μM | ||||||
| B. subtilis KCTC1914 | 8 | ||||||
| N-Hexadecylpyridinium bromide | [C16Py][Br] | S. aureus ATCC 6538 | 15 μM | Broth microdilution | [97] | ||
| E. coli O157:H7 ATCC 43895 | 13 μM | ||||||
| E. faecium ATCC 49474 | 2 μM | ||||||
| K. pneumonia ATCC 4352 | 13 μM | ||||||
| N-Dodecyl-N-methylpyrrolidinium bromide | [C12C1Pyr][Br] | S. epidermidis ATCC 35984 | 10 | Broth microdilution | [89,90,91] | ||
| S. aureus | 15 µM | ||||||
| S. aureus ATCC 6538 | 10 | 80 | |||||
| E. coli | 20 µM | ||||||
| E. coli ATCC 25922 | 80 | 20 | |||||
| P. aeruginosa ATCC 27853 | 320 | 80 | |||||
| K. pneumonia ATCC BAA-1705 | 160 | ||||||
| A. baumannii AB01 | 80 | ||||||
| E. faecalis ATCC 29212 | 20 | 40 | |||||
| N-Dodecyl-N-hydroxyethylpyrrolidinium chloride | [C12HOC2Pyr][Cl] | E. coli KCTC1924 | 8 | Broth microdilution | [81] | ||
| S. typhimurium KCTC1926 | 16 | ||||||
| B. subtilis KCTC1914 | 4 | ||||||
| C. regularis | 8 | ||||||
| N-Dodecyl-N-methylpiperidinium bromide | [C12C1Pip][Br] | S. epidermidis ATCC 35984 | 5 | Broth microdilution | [90,91] | ||
| S. aureus ATCC 6538 | 5 | 80 | |||||
| E. coli ATCC 25922 | 40 | 20 | |||||
| P. aeruginosa ATCC 27853 | 320 | 80 | |||||
| K. pneumonia ATCC BAA-1705 | 160 | ||||||
| A. baumannii AB01 | 320 | ||||||
| E. faecalis ATCC 29212 | 10 | 40 | |||||
| N-Dodecyl-N-methylmorpholinium bromide | [C12C1Mor][Br] | S. epidermidis ATCC 35984 | 20 | Broth microdilution | [90] | ||
| S. aureus ATCC 6538 | 20 | ||||||
| E. coli ATCC 25922 | 156.2 | ||||||
| P. aeruginosa ATCC 27853 | 312.5 | ||||||
| E. faecalis ATCC 29212 | 40 | ||||||
| Dioctyldimethylammonium chloride | [C8,8,1,1N][Cl] | E. coli BW25113 (wild-type) | 104.2 | Broth microdilution | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | |
| E. coli JW3596 (ΔrfaC) | 20.8 | ||||||
| E. coli JW3597 (ΔrfaL) | 91.7 | ||||||
| E. coli JW3606 (ΔrfaG) | 22.9 | ||||||
| Trioctylmethylammonium chloride | [C8,8,8,1N][Cl] | E. coli BW25113 (wild-type) | 6.8 | Broth microdilution | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | |
| E. coli JW3596 (ΔrfaC) | 1.7 | ||||||
| E. coli JW3597 (ΔrfaL) | 6.9 | ||||||
| E. coli JW3606 (ΔrfaG) | 2.5 | ||||||
| Trimethyldecylammonium chloride | [C1,1,1,10N][Cl] | E. coli BW25113 (wild-type) | 119.4 | Broth microdilution | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | |
| E. coli JW3596 (ΔrfaC) | 83 | ||||||
| E. coli JW3597 (ΔrfaL) | 130 | ||||||
| E. coli JW3606 (ΔrfaG) | 80 | ||||||
| Trimethylhexadecylammonium chloride | [C1,1,1,16N][Cl] | E. coli BW25113 (wild-type) | 13.1 | Broth microdilution | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | |
| E. coli JW3596 (ΔrfaC) | 2.8 | ||||||
| E. coli JW3597 (ΔrfaL) | 13 | ||||||
| E. coli JW3606 (ΔrfaG) | 3.3 | ||||||
| Trimethylhexadecylammonium bromide (cetyltrimethylammonium bromide) | [C1,1,1,16N][Br] (CTAB) | S. aureus V329 | 0.31 μM | 5 μM | Broth microdilution | Potent anti-biofilm activity against S. aureus | [98] |
| P. aeruginosa PAO1 | 125 μM | 250 μM | |||||
| Dimethyldodecyl(2-hydroxyethyl)ammonium bromide | [HOC2C1,1,12N][Br] | B. subtilis ATCC 6633 | 15.62 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | 15.62 | ||||||
| K. pneumonia ATCC 9997 | N.T. | ||||||
| E. faecalis ATCC 29212 | N.T. | ||||||
| VRE ATCC 51299 | 62.5 | ||||||
| S. aureus | 31.25 | ||||||
| MRSA CIP 106760 | 62.5 | ||||||
| E. coli ATCC 25922 | 62.5 | ||||||
| P. aeruginosa ATCC 27853 | 250 | ||||||
| C. albicans ATCC 10231 | 62.5 | ||||||
| S. cerevisiae ATCC 2601 | 7.81 | ||||||
| Dimethyltetradecyl(2-hydroxyethyl)ammonium bromide | [HOC2C1,1,14N][Br] | B. subtilis ATCC 6633 | 0.98 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | 1.95 | ||||||
| K. pneumonia ATCC 9997 | 7.82 | ||||||
| E. faecalis ATCC 29212 | 1.95 | ||||||
| VRE ATCC 51299 | 1.95 | ||||||
| S. aureus | 7.81 | ||||||
| MRSA CIP 106760 | 15.62 | ||||||
| E. coli ATCC 25922 | 15.62 | ||||||
| P. aeruginosa ATCC 27853 | 125 | ||||||
| C. albicans ATCC 10231 | 31.25 | ||||||
| S. cerevisiae ATCC 2601 | 1.95 | ||||||
| Dimethylhexadecyl(2-hydroxyethyl)ammonium bromide | [HOC2C1,1,16N][Br] | B. subtilis ATCC 6633 | <0.49 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | 3.91 | ||||||
| K. pneumonia ATCC 9997 | 0.98 | ||||||
| E. faecalis ATCC 29212 | 0.98 | ||||||
| VRE ATCC 51299 | 0.98 | ||||||
| S. aureus | 1.95 | ||||||
| MRSA CIP 106760 | 3.91 | ||||||
| E. coli ATCC 25922 | 7.81 | ||||||
| P. aeruginosa ATCC 27853 | 250 | ||||||
| C. albicans ATCC 10231 | 3.91 | ||||||
| S. cerevisiae ATCC 2601 | 1.95 | ||||||
| Dimethyloctadecyl(2-hydroxyethyl)ammonium bromide | [HOC2C1,1,18N][Br] | B. subtilis ATCC 6633 | 1.95 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | 3.91 | ||||||
| K. pneumonia ATCC 9997 | 1.95 | ||||||
| E. faecalis ATCC 29212 | 1.95 | ||||||
| VRE ATCC 51299 | 0.98 | ||||||
| S. aureus | 1.95 | ||||||
| MRSA CIP 106760 | 0.98 | ||||||
| E. coli ATCC 25922 | 31.25 | ||||||
| P. aeruginosa ATCC 27853 | 125 | ||||||
| C. albicans ATCC 10231 | <0.48 | ||||||
| S. cerevisiae ATCC 2601 | <0.48 | ||||||
| Di(2-hydroxyethyl)tetradecylammonium bromide | [(HOC2)2C14NH][Br] | B. subtilis ATCC 6633 | 7.81 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | 15.62 | ||||||
| K. pneumonia ATCC 9997 | 7.81 | ||||||
| E. faecalis ATCC 29212 | 15.62 | ||||||
| VRE ATCC 51299 | 7.81 | ||||||
| S. aureus | 15.62 | ||||||
| MRSA CIP 106760 | 15.62 | ||||||
| E. coli ATCC 25922 | 31.25 | ||||||
| P. aeruginosa ATCC 27853 | N.T. | ||||||
| C. albicans ATCC 10231 | 15.62 | ||||||
| S. cerevisiae ATCC 2601 | N.T. | ||||||
| Di(2-hydroxyethyl)decylmethylammonium bromide | [(HOC2)2C10,1N][Br] | B. subtilis ATCC 6633 | 250 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | 62.5 | ||||||
| K. pneumonia ATCC 9997 | N.A. | ||||||
| E. faecalis ATCC 29212 | N.A. | ||||||
| VRE ATCC 51299 | N.A. | ||||||
| S. aureus | N.A. | ||||||
| MRSA CIP 106760 | N.A. | ||||||
| E. coli ATCC 25922 | N.A. | ||||||
| P. aeruginosa ATCC 27853 | N.A. | ||||||
| C. albicans ATCC 10231 | N.T. | ||||||
| S. cerevisiae ATCC 2601 | N.T. | ||||||
| Di(2-hydroxyethyl)dodecylmethylammonium bromide | [(HOC2)2C12,1N][Br] | B. subtilis ATCC 6633 | 31.25 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | <7.82 | ||||||
| K. pneumonia ATCC 9997 | 62.5 | ||||||
| E. faecalis ATCC 29212 | 62.25 | ||||||
| VRE ATCC 51299 | 62.5 | ||||||
| S. aureus | 31.25 | ||||||
| MRSA CIP 106760 | 62.5 | ||||||
| E. coli ATCC 25922 | 125 | ||||||
| P. aeruginosa ATCC 27853 | 250 | ||||||
| C. albicans ATCC 10231 | 250 | ||||||
| S. cerevisiae ATCC 2601 | 31.25 | ||||||
| Di(2-hydroxyethyl)tetradecylmethylammonium bromide | [(HOC2)2C14,1N][Br] | B. subtilis ATCC 6633 | 1.95 | Broth microdilution | [92] | ||
| M. smegmatis ATCC 607 | 1.95 | ||||||
| K. pneumonia ATCC 9997 | 7.82 | ||||||
| E. faecalis ATCC 29212 | N.T. | ||||||
| VRE ATCC 51299 | N.T. | ||||||
| S. aureus | 3.91 | ||||||
| MRSA CIP 106760 | 1.95 | ||||||
| E. coli ATCC 25922 | 15.62 | ||||||
| P. aeruginosa ATCC 27853 | 62.5 | ||||||
| C. albicans ATCC 10231 | 31.25 | ||||||
| S. cerevisiae ATCC 2601 | 1.95 | ||||||
| Trioctylmethylphosphonium chloride | [C8,8,8,1P][Cl] | E. coli BW25113 (wild-type) | 6.8 | Broth microdilution | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | |
| E. coli JW3596 (ΔrfaC) | 2.2 | ||||||
| E. coli JW3597 (ΔrfaL) | 5.6 | ||||||
| E. coli JW3606 (ΔrfaG) | 2.8 | ||||||
| Trihexyltetradecylphosphonium chloride | [C6,6,6,14P][Cl] | L. monocytogenes ATCC13932 | 5.7 | Broth microdilution | [96] | ||
| B. cereus ATCC 11778 | 9.77 | ||||||
| S. aureus ATCC 6538 | 8.14 | ||||||
| E. faecalis ATCC 19433 | 11.39 | ||||||
| L. sakei ATCC 15521 | 8.14 | ||||||
| L. lactis ATCC 19435 | 8.14 | ||||||
| S. typhimurium ATCC 14028 | 625 | ||||||
| E. coli ATCC 25922 | 5000 | ||||||
| C. freundii ATCC 27853 | 5000 | ||||||
| Gentamycin | S. typhimurium ATCC 14028 | 0.25 | Broth microdilution | [81] | |||
| E. coli ATCC 25922 | 0.25 | ||||||
| C. freundii ATCC 27853 | 1 | ||||||
| B. subtilis KCTC1914 | 1 | ||||||
| S. typhimurium KCTC1926 | 0.5 | ||||||
| Kanamycin | S. aureus 209 KCTC1916 | 2 | Broth microdilution | [81] | |||
| S. aureus R209 KCTC1928 | 1 | ||||||
| E. coli KCTC1924 | 16 | ||||||
| B. subtilis KCTC1914 | 2 | ||||||
| S. typhimurium KCTC1926 | 1 | ||||||
| Fuconazole | C. tropicalis 17A | 0.125 (MBEC 4) | Broth microdilution | The clinical isolates 72A, 72P, and 94P are resistant to fluconazole, amphotericin B, voriconazole and anidulafungin. | [88] | ||
| C. tropicalis 57A | 0.125 (MBEC 64) | ||||||
| C. tropicalis 72A | 128 (MBEC 8) | ||||||
| C. tropicalis 72P | 128 (MBEC 128) | ||||||
| C. tropicalis 94P | 64 (MBEC 32) | ||||||
| C. tropicalis 102A | 0.125 (MBEC 128) | ||||||
| Colistin | E. coli ATCC 25922 | 2 | Broth microdilution | [91] | |||
| P. aeruginosa ATCC 27853 | 1 | ||||||
| K. pneumonia ATCC BAA-1705 | 2 | ||||||
| A. baumannii AB01 | 4 | ||||||
| Vancomycin | B. subtilis ATCC 6633 | <0.48 | Broth microdilution | [92] | |||
| K. pneumonia ATCC 9997 | 15.62 | ||||||
| E. faecalis ATCC 29212 | 1.95 | ||||||
| VRE ATCC 51299 | 3.91 | ||||||
| S. aureus | 7.82 | ||||||
| MRSA CIP 106760 | 3.91 | ||||||
| Rifampicin | M. smegmatis ATCC 607 | <0.48 | Broth microdilution | [92] | |||
| E. coli ATCC 25922 | 0.98 | ||||||
| Norfloxacin | P. aeruginosa ATCC 27853 | <0.48 | Broth microdilution | [92] | |||
| Amphotericin B | C. albicans ATCC 10231 | <0.48 | Broth microdilution | [92] | |||
| S. cerevisiae ATCC 2601 | <0.48 |
| IL | Acronym | Species | IZ, mm | MIC μg mL−1 | MBC, μg mL−1 | Method | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1-Ethyl-3-methylimidazolium nalidixate | [C2Mim][Nal] | E. coli BW25113 (wild-type) | 11 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 20 | |||||||
| E. coli JW3597 (ΔrfaL) | 11 | |||||||
| E. coli JW3606 (ΔrfaG) | 18 | |||||||
| 1-Hexadecyl-3-methylimidazolium ampicillinate | [C16Mim][Amp] | S. aureus ATCC 6538 | 30 µM | Broth microdilution | [97] | |||
| E. coli O157:H7 ATCC 43895 | 9 µM | |||||||
| E. faecium ATCC 49474 | 13 µM | |||||||
| K. pneumonia ATCC 4352 | 15 µM | |||||||
| 1-Hexadecyl-2,3-dimethylimidazolium ampicillinate | [C16MMim][Amp] | S. aureus ATCC 6538 | 14 µM | Broth microdilution | [97] | |||
| E. coli O157:H7 ATCC 43895 | 9 µM | |||||||
| E. faecium ATCC 49474 | 0.4 µM | |||||||
| K. pneumonia ATCC 4352 | 15 µM | |||||||
| 1-Hexadecylpyridinium ampicillinate | [C16Py][Amp] | S. aureus ATCC 6538 | 8 µM | Broth microdilution | E. coli TEM CTX M9, CTX M2, and AmpC MOX2 are ampicillin-resistant strains. | [82,97] | ||
| S. aureus ATCC 25293 | 5 µM | |||||||
| S. epidermidis (clinical isolate) | 5 µM | |||||||
| E. coli O157:H7 ATCC 43895 | 6 µM | |||||||
| E. coli ATCC 25922 | 500 µM | |||||||
| E. coli TEM CTX M9 | 5 µM | |||||||
| E. coli CTX M2 | 50 µM | |||||||
| E. coli AmpC MOX2 | >5000 µM | |||||||
| E. faecium ATCC 49474 | 0.4 µM | |||||||
| E. faecalis (clinical isolate) | 5 µM | |||||||
| K. pneumonia ATCC 4352 | 9 µM | |||||||
| K. pneumoniae (clinical isolate) | 50 µM | |||||||
| N-Ethyl-N-methylpiperidinium nalidixate | [C2C1Pip][Nal] | E. coli BW25113 (wild-type) | 12.9 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 22.9 | |||||||
| E. coli JW3597 (ΔrfaL) | 12.8 | |||||||
| E. coli JW3606 (ΔrfaG) | 21 | |||||||
| Trimethylhexadecylammonium nalidixate | [C1,1,1,16N][Nal] | E. coli BW25113 (wild-type) | 12.6 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 22.7 | |||||||
| E. coli JW3597 (ΔrfaL) | 12.2 | |||||||
| E. coli JW3606 (ΔrfaG) | 20.2 | |||||||
| Dioctyldimethylammonium nalidixate | [C8,8,1,1N][Nal] | E. coli BW25113 (wild-type) | 13.3 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 23.3 | |||||||
| E. coli JW3597 (ΔrfaL) | 13.6 | |||||||
| E. coli JW3606 (ΔrfaG) | 20.3 | |||||||
| Trioctylmethylammonium nalidixate | [C8,8,8,1N][Nal] | E. coli BW25113 (wild-type) | 11.3 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 22.2 | |||||||
| E. coli JW3597 (ΔrfaL) | 11 | |||||||
| E. coli JW3606 (ΔrfaG) | 18.7 | |||||||
| Tetramethylammonium nalidixate | [C1,1,1,1N][Nal] | E. coli BW25113 (wild-type) | 13.3 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 22.9 | |||||||
| E. coli JW3597 (ΔrfaL) | 13.4 | |||||||
| E. coli JW3606 (ΔrfaG) | 20.6 | |||||||
| Tetrabutylammonium nalidixate | [C4,4,4,4N][Nal] | E. coli BW25113 (wild-type) | 13.3 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 22.7 | |||||||
| E. coli JW3597 (ΔrfaL) | 13.6 | |||||||
| E. coli JW3606 (ΔrfaG) | 21.3 | |||||||
| Didecyldimethylammonium saccharinate | [C10,10,1,1N][Sac] | S. aureus ATCC 6538 | 4 ppm | 62.5 ppm | Tube dilution | [99] | ||
| MRSA ATCC 43300 | 4 ppm | 31.2 ppm | ||||||
| E. faecium ATCC 49474 | 8 ppm | 16 ppm | ||||||
| E. coli ATCC25922 | 16 ppm | 16 ppm | ||||||
| M. luteus ATCC 9341 | 4 ppm | 31.2 ppm | ||||||
| S. epidermidis ATCC 12228 | 4 ppm | 16 ppm | ||||||
| K. pneumonia ATCC 4352 | 4 ppm | 16 ppm | ||||||
| C. albicans ATCC 10231 | 16 ppm | 16 ppm | ||||||
| R. rubra PhB | 16 ppm | 31.2 ppm | ||||||
| S. mutans PCM | 31 ppm | 62.5 ppm | ||||||
| Didecyldimethylammonium acesulfamate | [C10,10,1,1N][Ace] | S. aureus ATCC 6538 | 8 ppm | 16 ppm | Tube dilution | [99] | ||
| MRSA ATCC 43300 | 4 ppm | 31.2 ppm | ||||||
| E. faecium ATCC 49474 | 8 ppm | 31.2 ppm | ||||||
| E. coli ATCC25922 | 16 ppm | 62.5 ppm | ||||||
| M. luteus ATCC 9341 | 8 ppm | 62.5 ppm | ||||||
| S. epidermidis ATCC 12228 | 4 ppm | 31.2 ppm | ||||||
| K. pneumonia ATCC 4352 | 4 ppm | 31.2 ppm | ||||||
| C. albicans ATCC 10231 | 16 ppm | 31.2 ppm | ||||||
| R. rubra PhB | 16 ppm | 62.5 ppm | ||||||
| S. mutans PCM | 16 ppm | 125 ppm | ||||||
| Tetrabutylphosphonium nalidixate | [C4,4,4,4P][Nal] | E. coli BW25113 (wild-type) | 13.3 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | ||
| E. coli JW3596 (ΔrfaC) | 22.6 | |||||||
| E. coli JW3597 (ΔrfaL) | 12.9 | |||||||
| E. coli JW3606 (ΔrfaG) | 20.4 | |||||||
| Trihexyltetradecylphosphonium ampicillinate | [C6,6,6,14P][Amp] | E. coli ATCC 25922 | 2500 µM | Broth microdilution | E. coli TEM CTX M9, CTX M2, and AmpC MOX2 are ampicillin-resistant strains. | [82] | ||
| E. coli TEM CTX M9 | 500 µM | |||||||
| E. coli CTX M2 | 500 µM | |||||||
| E. coli AmpC MOX2 | >5000 µM | |||||||
| K. pneumoniae (clinical isolate) | 5000 µM | |||||||
| S. aureus ATCC 25293 | 50 µM | |||||||
| S. epidermidis (clinical isolate) | 50 µM | |||||||
| E. faecalis (clinical isolate) | 50 µM | |||||||
| Benzalkonium saccharinate | [BA][Sac] | S. aureus ATCC 6538 | 4 ppm | 31.2 ppm | Tube dilution | [99] | ||
| MRSA ATCC 43300 | 4 ppm | 31.2 ppm | ||||||
| E. faecium ATCC 49474 | 8 ppm | 16 ppm | ||||||
| E. coli ATCC25922 | 16 ppm | 62.5 ppm | ||||||
| M. luteus ATCC 9341 | 8 ppm | 62.5 ppm | ||||||
| S. epidermidis ATCC 12228 | 4 ppm | 31.2 ppm | ||||||
| K. pneumonia ATCC 4352 | 4 ppm | 62.5 ppm | ||||||
| C. albicans ATCC 10231 | 16 ppm | 31.2 ppm | ||||||
| R. rubra PhB | 16 ppm | 62.5 ppm | ||||||
| S. mutans PCM | 0.1 ppm | 0.5 ppm | ||||||
| Benzalkonium acesulfamate | [BA][Ace] | S. aureus ATCC 6538 | 4 ppm | 31.2 ppm | Tube dilution | [99] | ||
| MRSA ATCC 43300 | 4 ppm | 31.2 ppm | ||||||
| E. faecium ATCC 49474 | 8 ppm | 31.2 ppm | ||||||
| E. coli ATCC25922 | 31 ppm | 125 ppm | ||||||
| M. luteus ATCC 9341 | 8 ppm | 62.5 ppm | ||||||
| S. epidermidis ATCC 12228 | 4 ppm | 62.5 ppm | ||||||
| K. pneumonia ATCC 4352 | 8 ppm | 31.2 ppm | ||||||
| C. albicans ATCC 10231 | 16 ppm | 31.2 ppm | ||||||
| R. rubra PhB | 16 ppm | 62.5 ppm | ||||||
| S. mutans PCM | 1 ppm | 16 ppm | ||||||
| Nalidixic acid | E. coli BW25113 (wild-type) | 11 | Disk diffusion test, 10 µg per disk | Deletions ΔrfaC, ΔrfaL, and ΔrfaG affect the cell surface hydrophobicity and membrane permeability. | [86] | |||
| E. coli JW3596 (ΔrfaC) | 20 | |||||||
| E. coli JW3597 (ΔrfaL) | 11 | |||||||
| E. coli JW3606 (ΔrfaG) | 18 | |||||||
| Ampicillin sodium salt | S. aureus ATCC 6538 | 27 µM | Broth microdilution | E. coli TEM CTX M9, CTX M2, and AmpC MOX2 are ampicillin-resistant strains. | [82,97] | |||
| S. aureus ATCC 25293 | 5 µM | |||||||
| S. epidermidis (clinical isolate) | 50 µM | |||||||
| E. coli O157:H7 ATCC 43895 | 12 µM | |||||||
| E. coli ATCC 25922 | 50 µM | |||||||
| E. coli TEM CTX M9 | >5000 µM | |||||||
| E. coli CTX M2 | >5000 µM | |||||||
| E. coli AmpC MOX2 | >5000 µM | |||||||
| E. faecium ATCC 49474 | 17 µM | |||||||
| E. faecalis (clinical isolate) | 50 µM | |||||||
| K. pneumonia ATCC 4352 | 20 µM | |||||||
| K. pneumoniae (clinical isolate) | 2500 µM | |||||||
| Benzalkonium chloride | S. aureus ATCC 6538 | 2 ppm | 62.5 ppm | Tube dilution, broth microdilution | [81,99] | |||
| MRSA ATCC 43300 | 2 ppm | 31.2 ppm | ||||||
| S. aureus 209 KCTC1916 | 8 | |||||||
| S. aureus R209 KCTC1928 | 8 | |||||||
| E. faecium ATCC 49474 | 4 ppm | 31.2 ppm | ||||||
| E. coli ATCC25922 | 8 ppm | 62.5 ppm | ||||||
| M. luteus ATCC 9341 | 4 ppm | 31.2 ppm | ||||||
| S. epidermidis ATCC 12228 | 2 ppm | 16 ppm | ||||||
| K. pneumonia ATCC 4352 | 4 ppm | 31.2 ppm | ||||||
| B. subtilis KCTC1914 | 8 | |||||||
| C. albicans ATCC 10231 | 8 ppm | 16 ppm | ||||||
| R. rubra PhB | 8 ppm | 31.2 ppm | ||||||
| S. mutans PCM | 2 ppm | 16 ppm | ||||||
| Didecyldimethylammonium chloride | S. aureus ATCC 6538 | 2 ppm | 31.2 ppm | Tube dilution | [99] | |||
| MRSA ATCC 43300 | 2 ppm | 31.2 ppm | ||||||
| E. faecium ATCC 49474 | 4 ppm | 31.2 ppm | ||||||
| E. coli ATCC25922 | 8 ppm | 31.2 ppm | ||||||
| M. luteus ATCC 9341 | 2 ppm | 31.2 ppm | ||||||
| S. epidermidis ATCC 12228 | 2 ppm | 31.2 ppm | ||||||
| K. pneumonia ATCC 4352 | 4 ppm | 16 ppm | ||||||
| C. albicans ATCC 10231 | 8 ppm | 16 ppm | ||||||
| R. rubra PhB | 4 ppm | 31.2 ppm | ||||||
| S. mutans PCM | 2 ppm | 16 ppm |
| Series/ Compound | Strain | MIC, mg⋅L−1 | MBC, mg⋅L−1 | Method | Notes | Ref. |
|---|---|---|---|---|---|---|
| 42 | S. aureus SH1000 | 1 μM | Broth microdilution | [75] | ||
| E. faecalis OG1RF | 1 μM | |||||
| E. coli MC4100 | 2 μM | |||||
| P. aeruginosa PAO1-WT | 4 μM | |||||
| 43 | S. aureus SH1000 | 1 μM | Broth microdilution | [71] | ||
| E. faecalis OG1RF | 1 μM | |||||
| E. coli MC4100 | 2 μM | |||||
| P. aeruginosa PAO1-WT | 4 μM | |||||
| 44 | S. aureus SH1000я | 1 μM | Broth microdilution | [71] | ||
| E. faecalis OG1RF | 1 μM | |||||
| E. coli MC4100 | 1 μM | |||||
| P. aeruginosa PAO1-WT | 4 μM | |||||
| 46 | S. aureus Mau 29/58 | 0.4 μM | Suspension micromethod | [101] | ||
| E. coli 377/79 | 3.1 μM | |||||
| C. albicans 45/54 | 1.5 μM | |||||
| 47 | S. aureus | 13 μM | Broth microdilution | [103] | ||
| E. coli | 10 μM | |||||
| 48 | S. aureus SH1000 | 2 | 2 | Broth microdilution | [105] | |
| E. faecalis OG1RF | 18 | 18 | ||||
| E. coli MC4100 | 18 | 18 | ||||
| P. aeruginosa PAO1-WT | 37 | 37 | ||||
| 49 | S. aureus SH1000 | 10 | 10 | Broth microdilution | [105] | |
| E. faecalis OG1RF | 18 | 18 | ||||
| E. coli MC4100 | 37 | 37 | ||||
| P. aeruginosa PAO1-WT | 149 | 149 | ||||
| 50 | S. aureus SH1000 | 10 | 10 | Broth microdilution | [105] | |
| E. faecalis OG1RF | 30 | 30 | ||||
| E. coli MC4100 | 74 | 74 | ||||
| P. aeruginosa PAO1-WT | 297 | 297 | ||||
| 51 | S. aureus SH1000 | 4 | 4 | Broth microdilution | [105] | |
| E. faecalis OG1RF | 18 | 18 | ||||
| E. coli MC4100 | 37 | 37 | ||||
| P. aeruginosa PAO1-WT | 74 | 74 | ||||
| 52 | S. aureus SH1000 | 4 | 4 | Broth microdilution | [105] | |
| E. faecalis OG1RF | 10 | 10 | ||||
| E. coli MC4100 | 18 | 18 | ||||
| P. aeruginosa PAO1-WT | 74 | 74 | ||||
| 53 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [107] | ||
| MRSA 300-0114 | 1 μM | |||||
| MRSA ATCC 33592 | 0.25 μM | |||||
| E. faecalis OG1RF | 0.25 μM | |||||
| E. coli MC4100 | 1 μM | |||||
| P. aeruginosa PAO1-WT | 2 μM | |||||
| 54 | S. aureus ATCC 29213 | 0.5 | Broth microdilution | Tested in vivo with proved efficiency | [106] | |
| S. epidermidis (clinical) | 2 | |||||
| B. subtilis 168 | 1 | |||||
| E. coli ATCC 25922 | 0.5 | |||||
| K. pneumoniae 1813 | 4 | |||||
| P. aeruginosa ATCC 27853 | 0.5 | |||||
| T. rubrum 1336 (clinical) | 32 | |||||
| A. niger F-1119 | 16 | |||||
| C. albicans NCTC- 885-653 | 16 | |||||
| F. oxysporum KM-19 (clinical) | 32 | |||||
| 55 | S. aureus ATCC 29213 | 4 | Broth microdilution | [65] | ||
| 57 | P. aeruginosa ATCC 27583 | 6.3 μM | Broth microdilution | [108] | ||
| P. aeruginosa ATCC 10145 | 5.2 μM | |||||
| P. aeruginosa ATCC 3080 | 1.6 μM | |||||
| K. pneumoniae ATCC 4352 | 0.4 μM | |||||
| K. pneumoniae ATCC 13883 | 0.8 μM | |||||
| P. vulgaris ATCC 13315 | 0.4 μM | |||||
| P. mirabilis NBRC 3849 | 6.3 μM | |||||
| E. coli K12 W3110 | 0.8 μM | |||||
| E. coli IFO 3301 | 0.2 μM | |||||
| E. coli IFO 3972 | 1.3 μM | |||||
| B. subtilis IFO 3134 | 0.8 μM | |||||
| B. subtilis ATCC 6633 | 0.8 μM | |||||
| B. cereus IFO 3001 | 0.4 μM | |||||
| B. megaterium IFO 3003 | 0.3 μM | |||||
| S. aureus ATCC 25923 | 0.3 μM | |||||
| S. aureus IFO 12732 | 0.4 μM | |||||
| A. niger IFO 6341 | 8 μM | |||||
| A. niger IFO 6342 | 4 μM | |||||
| A. niger IFO 4414 | 4 μM | |||||
| C. globosum IFO 6347 | 8 μM | |||||
| R. oryzae IFO 31005 | 2 μM | |||||
| P. citrinum IFO 6352 | 8 μM | |||||
| A. pullulans IFO 6353 | 16 μM | |||||
| C. cladosporioides IFO 6348 | 4 μM | |||||
| G. virens IFO 6355 | 8 μM | |||||
| 58 | S. aureus SH1000 | 1 μM | Broth microdilution | [109] | ||
| MRSA 300-0114 | 1 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| E. faecalis OG1RF | 8 μM | |||||
| E. coli MC4100 | 8 μM | |||||
| P. aeruginosa PAO1-WT | 8 μM | |||||
| 59 | S. aureus SH1000 | 0.25 μM | Broth microdilution | [109] | ||
| MRSA 300-0114 | 2 μM | |||||
| MRSA ATCC 33592 | 0.5 μM | |||||
| E. faecalis OG1RF | 4 μM | |||||
| E. coli MC4100 | 2 μM | |||||
| P. aeruginosa PAO1-WT | 8 μM | |||||
| 60 | S. aureus ATCC 25923 | 64 | 128 | Broth microdilution | Surfactant | [110] |
| B. subtilis ATCC 6633 | 16 | 32 | ||||
| E. coli ATCC 25922 | 16 | 64 | ||||
| 61 | S. aureus SH1000 | 1 μM | Broth microdilution | Natural derivatives | [74] | |
| MRSA 300-0114 | 4 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| E. faecalis OG1RF | 2 μM | |||||
| E. coli MC4100 | 4 μM | |||||
| P. aeruginosa PAO1-WT | 32 μM | |||||
| 62 | S. aureus SH1000 | 1 μM | Broth microdilution | Natural derivatives | [74] | |
| MRSA 300-0114 | 1 μM | |||||
| MRSA ATCC 33592 | 1 μM | |||||
| E. faecalis OG1RF | 2 μM | |||||
| E. coli MC4100 | 2 μM | |||||
| P. aeruginosa PAO1-WT | 8 μM | |||||
| 63 | S. aureus SH1000 | 2 μM | Broth microdilution | [111] | ||
| MRSA 300-0114 | 1 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| E. faecalis OG1RF | 4 μM | |||||
| E. coli MC4100 | 1 μM | |||||
| P. aeruginosa PAO1-WT | 4 μM | |||||
| 64 | S. aureus SH1000 | 2 μM | Broth microdilution | [111] | ||
| MRSA 300-0114 | 2 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| E. faecalis OG1RF | 4 μM | |||||
| E. coli MC4100 | 2 μM | |||||
| P. aeruginosa PAO1-WT | 4 μM | |||||
| 65 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [112] | ||
| MRSA 300-0114 | 0.5 μM | |||||
| E. coli MC4100 | 1 μM | |||||
| P. aeruginosa PAO1-WT | 2 μM | |||||
| 66 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [72] | ||
| MRSA 300-0114 | 0.5 μM | |||||
| MRSA ATCC 33592 | 0.5 μM | |||||
| 67 | S. aureus ATCC 29213 | 16 | Broth microdilution | [113] | ||
| E. faecalis ATCC 29212 | 64 | |||||
| E. coli ATCC 25922 | 128 | |||||
| P. aeruginosa ATCC 27853 | 256 | |||||
| 68 | S. aureus ATCC 29213 | 0.25 | Broth microdilution | [113] | ||
| MRSA (mecA) | 0.5 | |||||
| E. faecalis ATCC 29212 | 0.5 | |||||
| Vancomycin-resistant E. faecalis (vanA) | 0.5 | |||||
| E. coli ATCC 25922 | 0.5 | |||||
| Extended-spectrum b-lactamase-producing E. coli | 1 | |||||
| P. aeruginosa ATCC 27853 | 4 | |||||
| P. aeruginosa resistant, efflux pump | 8 | |||||
| 69 | S. aureus ATCC 29213 | 0.5 | Broth microdilution | [113] | ||
| MRSA (mecA) | 0.5 | |||||
| E. faecalis ATCC 29212 | 0.5 | |||||
| Vancomycin-resistant E. faecalis (vanA) | 0.5 | |||||
| E. coli ATCC 25922 | 0.5 | |||||
| Extended-spectrum b-lactamase-producing E. coli | 1 | |||||
| P. aeruginosa ATCC 27853 | 2 | |||||
| P. aeruginosa resistant, efflux pump | 2 | |||||
| 70 | P. aeruginosa ATCC 27853 | 17 μM | Broth microdilution | [114] | ||
| K. pneumoniae ATCC 4352 | 2.1 μM | |||||
| P. mirabilis NBRC 3849 | 3.1 μM | |||||
| E. coli IFO 12713 | 1.6 μM | |||||
| S. marcescens ATCC 13880 | 3.1 μM | |||||
| M. luteus IFO 12708 | 0.65 μM | |||||
| B. subtilis ATCC 6633 | 0.91 μM | |||||
| B. cereus IFO 3001 | 1.6 μM | |||||
| S. aureus IFO 12732 | 0.23 μM | |||||
| MRSA COL 1 | 1.6 μM | |||||
| 71 | P. aeruginosa ATCC 27853 | 13 μM | Broth microdilution | [114] | ||
| K. pneumoniae ATCC 4352 | 1.6 μM | |||||
| P. mirabilis NBRC 3849 | 5.2 μM | |||||
| E. coli IFO 12713 | 1.6 μM | |||||
| S. marcescens ATCC 13880 | 6.3 μM | |||||
| M. luteus IFO 12708 | 0.78 μM | |||||
| B. subtilis ATCC 6633 | 1.0 μM | |||||
| B. cereus IFO 3001 | 1.3 μM | |||||
| S. aureus IFO 12732 | 0.33 μM | |||||
| MRSA COL 1 | 1.3 μM | |||||
| 72 | S. aureus ATCC 25923 | 4 | Broth microdilution | [115] | ||
| MRSA ATCC 33591 | 4 | |||||
| E. faecalis ATCC 1299 | 1 | |||||
| E. coli ATCC 25922 | 2 | |||||
| P. aeruginosa ATCC 27853 | 4 | |||||
| K. pneumoniae ATCC 13883 | 16 | |||||
| A. flavus | 15.63 | |||||
| C. albicans 64124 | 3.91 | |||||
| C. albicans MYA2876 | 3.91 | |||||
| C. neoformans | 3.9 | |||||
| R. pilimanae | 2.0 | |||||
| 73 | S. aureus SH1000 | 2 μM | Broth microdilution | [117] | ||
| E. faecalis OG1RF | 2 μM | |||||
| E. coli MC4100 | 2 μM | |||||
| P. aeruginosa PAO1-WT | 16 μM | |||||
| 74 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [117] | ||
| E. faecalis OG1RF | 0.5 μM | |||||
| E. coli MC4100 | 0.5 μM | |||||
| P. aeruginosa PAO1-WT | 1 μM | |||||
| 75 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [117] | ||
| E. faecalis OG1RF | 1 μM | |||||
| E. coli MC4100 | 1 μM | |||||
| P. aeruginosa PAO1-WT | 2 μM | |||||
| 76 | S. aureus SH1000 | 1 μM | Broth microdilution | [118] | ||
| MRSA 300-0114 | 1 μM | |||||
| MRSA ATCC 33592 | 1 μM | |||||
| E. faecalis OG1RF | 4 μM | |||||
| E. coli MC4100 | 1 μM | |||||
| P. aeruginosa PAO1-WT | 4 μM | |||||
| 77 | S. aureus SH1000 | 1 μM | Broth microdilution | [118] | ||
| MRSA 300-0114 | 0.5 μM | |||||
| MRSA ATCC 33592 | 2 μM | |||||
| E. faecalis OG1RF | 2 μM | |||||
| E. coli MC4100 | 1 μM | |||||
| P. aeruginosa PAO1-WT | 2 μM | |||||
| 78 | S. aureus SH1000 | 16 μM | Broth microdilution | [118] | ||
| MRSA 300-0114 | 32 μM | |||||
| MRSA ATCC 33592 | 16 μM | |||||
| E. faecalis OG1RF | 63 μM | |||||
| E. coli MC4100 | 32 μM | |||||
| P. aeruginosa PAO1-WT | 63 μM | |||||
| 79 | MRSA ATCC 43300 | 0.25 | Broth microdilution | [119] | ||
| E. coli ATCC 25922 | 4 | |||||
| K. pneumoniae ATCC 700603 | 16 | |||||
| A. baumannii ATCC 19606 | 4 | |||||
| P. aeruginosa ATCC 27853 | 8 | |||||
| C. albicans ATCC 90028 | 0.25 | |||||
| C. neoformans ATCC 208821 | 0.25 | |||||
| 80 | MRSA ATCC 43300 | 0.25 | Broth microdilution | [122,126] | ||
| E. coli ATCC 25922 | 1 | |||||
| K. pneumoniae ATCC 700603 | 8 | |||||
| A. baumannii ATCC 19606 | 2 | |||||
| P. aeruginosa ATCC 27853 | 4 | |||||
| C. albicans ATCC 90028 | 0.25 | |||||
| C. neoformans ATCC 208821 | 0.25 | |||||
| 81 | MRSA ATCC 43300 | 0.25 | Broth microdilution | [123,126] | ||
| E. coli ATCC 25922 | 0.25 | |||||
| K. pneumoniae ATCC 700603 | 0.25 | |||||
| A. baumannii ATCC 19606 | 0.25 | |||||
| P. aeruginosa ATCC 27853 | 0.25 | |||||
| C. albicans ATCC 90028 | 0.25 | |||||
| C. neoformans ATCC 208821 | 4 | |||||
| 82 | MRSA ATCC 43300 | 0.25 | Broth microdilution | [124] | ||
| E. coli ATCC 25922 | 0.25 | |||||
| K. pneumoniae ATCC 700603 | 16 | |||||
| A. baumannii ATCC 19606 | 0.25 | |||||
| P. aeruginosa ATCC 27853 | 0.25 | |||||
| C. albicans ATCC 90028 | 0.25 | |||||
| C. neoformans ATCC 208821 | 0.25 | |||||
| 83 | MRSA ATCC 43300 | 0.25 | Broth microdilution | [125] | ||
| E. coli ATCC 25922 | 0.25 | |||||
| K. pneumoniae ATCC 700603 | 0.25 | |||||
| A. baumannii ATCC 19606 | 8 | |||||
| P. aeruginosa ATCC 27853 | 0.25 | |||||
| C. albicans ATCC 90028 | 0.25 | |||||
| C. neoformans ATCC 208821 | 0.25 | |||||
| 84 | P. aeruginosa ATCC 27583 | 6.3 μM | Broth microdilution | [127] | ||
| K. pneumoniae ATCC 13883 | 3.1 μM | |||||
| P. mirabilis IFO 3849 | 6.3 μM | |||||
| E. coli K12 W3110 | 3.1 μM | |||||
| M. luteus IFO 12708 | 0.78 μM | |||||
| B. cereus IFO 3001 | 3.1 μM | |||||
| S. aureus IFO 12732 | 0.39 μM | |||||
| MRSA IID 1677 | 3.1 μM | |||||
| P. funiculosam IFO 6345 | 1.6 μM | |||||
| C. globosum IFO 6347 | 3.1 μM | |||||
| A. pullulans IFO 6353 | 6.3 μM | |||||
| R. stolonifera IFO 4781 | 25 μM | |||||
| A. terreus IFO 6346 | 25 μM | |||||
| A. niger IFO 6342 | 12.5 μM | |||||
| 85 | E. coli | 2.7 | Broth microdilution | [134] | ||
| 86 | P. aeruginosa ATCC 27583 | 13 μM | Broth microdilution | [127] | ||
| K. pneumoniae ATCC 13883 | 1.6 μM | |||||
| P. mirabilis IFO 3849 | 13 μM | |||||
| E. coli K12 W3110 | 6.3 μM | |||||
| M. luteus IFO 12708 | 0.39 μM | |||||
| B. cereus IFO 3001 | 1.6 μM | |||||
| S. aureus IFO 12732 | 0.39 μM | |||||
| MRSA IID 1677 | 6.3 μM | |||||
| P. funiculosam IFO 6345 | 1.6 μM | |||||
| C. globosum IFO 6347 | 0.78 μM | |||||
| A. pullulans IFO 6353 | 6.3 μM | |||||
| R. stolonifera IFO 4781 | 25 μM | |||||
| A. terreus IFO 6346 | 12.5 μM | |||||
| A. niger IFO 6342 | 6.3 μM | |||||
| 87 | P. aeruginosa ATCC 27583 | 25 μM | Broth microdilution | [132] | ||
| K. pneumoniae ATCC 13883 | 1.6 μM | |||||
| P. mirabilis IFO 3849 | 13 μM | |||||
| E. coli K12 W3110 | 6.3 μM | |||||
| M. luteus IFO 12708 | 0.78 μM | |||||
| B. cereus IFO 3001 | 3.1 μM | |||||
| S. aureus IFO 12732 | 0.39 μM | |||||
| MRSA IID 1677 | 6.3 μM | |||||
| P. funiculosum IFO 6345 | 0.78 μM | |||||
| C. globosum IFO 6347 | 0.78 μM | |||||
| A. pullulans IFO 6353 | 3.1 μM | |||||
| R. stolonifera IFO 4781 | 6.3 μM | |||||
| A. terreus IFO 6346 | 1.6 μM | |||||
| A. niger IFO 6342 | 6.3 μM | |||||
| 88 | P. aeruginosa ATCC 27583 | 6.3 μM | Broth microdilution | [129] | ||
| P. aeruginosa ATCC 10145 | 8.3 μM | |||||
| K. pneumoniae ATCC 4352 | 1.0 μM | |||||
| P. rettgeri NIH 96 | 2.1 μM | |||||
| P. mirabilis IFO 3849 | 25 μM | |||||
| E. coli IFO 12713 | 1.8 μM | |||||
| S. enteritidis IFO 3313 | 1.3 μM | |||||
| B. subtilis IFO 3134 | 0.57 μM | |||||
| B. subtilis ATCC 6633 | 1.0 μM | |||||
| B. cereus IFO 3001 | 3.1 μM | |||||
| S. aureus IFO 12732 | 0.46 μM | |||||
| MRSA IID 1677 | 1.1 μM | |||||
| M. luteus IFO 12708 | 0.26 μM | |||||
| A. niger IFO 6342 | 25 μM | |||||
| A. niger TSY 0013 | 13 μM | |||||
| A. pullulans IFO 6353 | 3.1 μM | |||||
| P. citrinum IFO 6345 | 25 μM | |||||
| P. funiculosum IFO 6345 | 8.3 μM | |||||
| R. oryzae IFO 31005 | 13 μM | |||||
| T. viride IFO 30498 | 25 μM | |||||
| C. albicans IFO 1061 | 29 μM | |||||
| 89 | C. neoformans ATCC 90112 | 1.3 μM | Broth microdilution | [133] | ||
| C. albicans ATCC 10231 | 1.3 μM | |||||
| A. fumigatus ATCC 204305 | 88 μM | |||||
| 90 | E. coli ATCC 25922 | 8 | 18 | Broth microdilution | [120] | |
| P. aeruginosa ATCC 6538 | 32 | 8.3 | ||||
| S. aureus ATCC 278530 | 2.3 | 8.3 | ||||
| A. baumannii JCM 6841 | 11 | |||||
| B. cepacia JCM 5964 | 19 | |||||
| E. hirae ATCC 10541 | 5.3 | |||||
| E. faecalis ATCC 29212 | 6.7 | |||||
| MRSA ATCC 700698 | 11 | |||||
| S. epidermidis ATCC 12228 | 5.3 | |||||
| C. albicans ATCC 10231 | 13 | |||||
| 91 | E. coli ATCC 25922 | 1.7 | 15 | Broth microdilution | [120] | |
| P. aeruginosa ATCC 6538 | 21 | 8.3 | ||||
| S. aureus ATCC 278530 | 1.7 | 33 | ||||
| A. baumannii JCM 6841 | 16 | |||||
| B. cepacia JCM 5964 | 64 | |||||
| E. hirae ATCC 10541 | 16 | |||||
| E. faecalis ATCC 29212 | 19 | |||||
| MRSA ATCC 700698 | 8 | |||||
| S. epidermidis ATCC 12228 | 9.3 | |||||
| C. albicans ATCC 10231 | 27 | |||||
| 92 | MRSA ATCC 25923 | 2 ppm | Broth microdilution | [135] | ||
| E. coli ATCC 25922 | 4 pmm | |||||
| P. aeruginosa ATCC 27853 | 16 ppm |
| IL | Acronym | Species | IZ, mm | MIC, μg mL−1 | MBC, μg mL−1 | Method | Ref. |
|---|---|---|---|---|---|---|---|
| 2-Methyl-3-(4-(2-methyl-5-nitro-1H-imidazolium bromide)butyl-5-nitro-1H-imidazolium bromide | ([NO2C1Im]-C4-[NO2C1Im])[Br]2 | S. aureus | 16 | 0.25 | 0.25 | Disk diffusion (100 µg per well); broth microdilution | [139] |
| E. coli | 15 | 0.25 | 0.25 | ||||
| K. pneumoniae | 16 | 0.255 | 0.255 | ||||
| P. aeruginosa | 14 | 0.255 | 0.255 | ||||
| P. vulgaris | 15 | 0.27 | 0.27 | ||||
| 2-Methyl-3-(4-(2-methyl-5-nitro-1H-imidazolium tetrafluoroborate)butyl-5-nitro-1H-imidazolium tetrafluoroborate | ([NO2C1Im]-C4-[NO2C1Im])[BF4]2 | S. aureus | 15 | 0.27 | 0.27 | Disk diffusion (100 µg per well); broth microdilution | [139] |
| E. coli | 16 | 0.27 | 0.27 | ||||
| K. pneumoniae | 12 | 0.27 | 0.27 | ||||
| P. aeruginosa | 12 | 0.27 | 0.27 | ||||
| P. vulgaris | 14 | 0.27 | 0.27 | ||||
| 2-Methyl-3-(4-(2-methyl-5-nitro-1H-imidazolium hexafluorophosphate)butyl-5-nitro-1H-imidazolium hexafluorophosphate | ([NO2C1Im]-C4-[NO2C1Im])[PF6]2 | S. aureus | 16.5 | 0.255 | 0.255 | Disk diffusion (100 µg per well); broth microdilution | [139] |
| E. coli | 16 | 0.255 | 0.255 | ||||
| K. pneumoniae | 15.5 | 0.255 | 0.255 | ||||
| P. aeruginosa | 15 | 0.27 | 0.27 | ||||
| P. vulgaris | 16 | 0.27 | 0.27 | ||||
| 2-Methyl-3-(4-(2-methyl-5-nitro-1H-imidazolium trifluoromethanesulfonate)butyl-5-nitro-1H-imidazolium trifluoromethanesulfonate | ([NO2C1Im]-C4-[NO2C1Im])[TfO]2 | S. aureus | 16 | 0.27 | 0.27 | Disk diffusion (100 µg per well); broth microdilution | [139] |
| E. coli | 14 | 0.255 | 0.255 | ||||
| K. pneumoniae | 14 | 0.27 | 0.27 | ||||
| P. aeruginosa | 13 | 0.27 | 0.27 | ||||
| P. vulgaris | 15 | 0.27 | 0.27 | ||||
| Erythromycin | S. aureus | 24 | 0.23 | 0.23 | Disk diffusion (30 µg per well); broth microdilution | [139] | |
| E. coli | 27 | 0.23 | 0.23 | ||||
| K. pneumoniae | 26 | 0.23 | 0.23 | ||||
| P. aeruginosa | 25 | 0.23 | 0.23 | ||||
| P. vulgaris | 32 | 0.23 | 0.23 | ||||
| Nalidixic acid | S. aureus | 22 | 0.23 | 0.23 | Disk diffusion (30 µg per well); broth microdilution | [139] | |
| E. coli | 22 | 0.23 | 0.23 | ||||
| K. pneumoniae | 27 | 0.23 | 0.23 | ||||
| P. aeruginosa | 21 | 0.23 | 0.23 | ||||
| P. vulgaris | 24 | 0.23 | 0.23 | ||||
| Amikacin | S. aureus | 19 | 0.23 | 0.23 | Disk diffusion (30 µg per well); broth microdilution | [139] | |
| E. coli | 20 | 0.23 | 0.23 | ||||
| K. pneumoniae | 19 | 0.23 | 0.23 | ||||
| P. aeruginosa | 17 | 0.23 | 0.23 | ||||
| P. vulgaris | 17 | 0.23 | 0.23 |
| Series/ Compound | Strain | MIC, mg⋅L−1 | Method | Notes | Ref. |
|---|---|---|---|---|---|
| 93 | S. aureus SH1000 | 1 μM | Broth microdilution | [71] | |
| E. faecalis OG1RF | 1 μM | ||||
| E. coli MC4100 | 1 μM | ||||
| P. aeruginosa PAO1-WT | 2 μM | ||||
| 94 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [71] | |
| E. faecalis OG1RF | 1 μM | ||||
| E. coli MC4100 | 1 μM | ||||
| P. aeruginosa PAO1-WT | 4 μM | ||||
| 95 | S. aureus SH1000 | 1 μM | Broth microdilution | [112] | |
| MRSA 300-0114 | 0.5 μM | ||||
| MRSA ATCC 33592 | 1 μM | ||||
| 96 | S. aureus SH1000 | 1 μM | Broth microdilution | [72] | |
| MRSA 300-0114 | 1 μM | ||||
| E. coli MC4100 | 2 μM | ||||
| P. aeruginosa PAO1-WT | 4 μM | ||||
| 96 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [140] | |
| MRSA 300-0114 | 0.5 μM | ||||
| MRSA ATCC 33592 | 0.5 μM | ||||
| E. faecalis OG1RF | 1 μM | ||||
| E. coli MC4100 | 0.5 μM | ||||
| P. aeruginosa PAO1-WT | 0.5 μM | ||||
| 98 | S. aureus SH1000 | 1 μM | Broth microdilution | [107] | |
| MRSA 300-0114 | 0.5 μM | ||||
| MRSA ATCC 33592 | 0.5 μM | ||||
| E. faecalis OG1RF | 1 μM | ||||
| E. coli MC4100 | 0.5 μM | ||||
| P. aeruginosa PAO1-WT | 4 μM | ||||
| 99 | B. cereus | 2 μM | Broth microdilution | [141] | |
| E. faecalis ATCC 29212 | 2 μM | ||||
| S. agalactiae J48 | 2 μM | ||||
| S. aureus ATCC 29213 | 2 μM | ||||
| E. coli ATCC 25922 | 4 μM | ||||
| P. aeruginosa ATCC 27853 | 16 μM | ||||
| 100 | S. aureus SH1000 | 0.5 μM | Broth microdilution | [143] | |
| E. faecalis OG1RF | 1 μM | ||||
| E. coli MC4100 | 1 μM | ||||
| P. aeruginosa PAO1-WT | 2 μM | ||||
| MRSA 300-0114 | 0.5 μM | ||||
| MRSA ATCC 33592 | 0.5 μM | ||||
| 101 | MRSA ATCC 25923 | 4 | Broth microdilution | The first tetra-pyridinic salts | [144] |
| E. coli ATCC 25922 | 4 | ||||
| P. aeruginosa ATCC 27853 | 32 |
| Series/ Compound | Strain | MIC, mg⋅L−1 | MBC, mg⋅L−1 | Method | Notes | Ref. |
|---|---|---|---|---|---|---|
| 102 | E. coli ATCC 25922 | 1.56 | Broth microdilution | [148] | ||
| S. aureus ATCC 25923 | 1.56 | |||||
| 103 | E. coli ATCC 8099 | 0.78 | Broth microdilution | [149] | ||
| S. aureus ATCC 6538 | 0.91 | |||||
| 104 | E. coli ATCC 8099 | 0.13 | Broth microdilution | [149] | ||
| S. aureus ATCC 6538 | 0.28 | |||||
| 105 | E. coli ATCC 25922 | 7 | Broth tube dilution | [153] | ||
| S. aureus ATCC 6538 P | 7 | |||||
| C. albicans ATCC 865-653 | 3.5 | |||||
| P. aeruginosa ATCC 9027 | 31 | |||||
| P. mirabilis 47 | 31 | |||||
| K. pneumoniae ATCC 13883 | 62 | |||||
| 106 | E. coli | 62.5 | 62.5 | Broth dilution | [152] | |
| S. aureus | 62.5 | 62.5 | ||||
| 107 | E. coli | 22 mm/mg (IZ) | Disk diffusion | Possesses anticorrosion activity | [151] | |
| S. aureus | 20 mm/mg (IZ) | |||||
| C. albicans | 13 mm/mg (IZ) | |||||
| P. aeruginosa | 24 mm/mg (IZ) | |||||
| A. niger | 12 mm/mg (IZ) | |||||
| 108 | B. cinerea | 106 | Radial growth technique | Efficient against fungal spores | [150] | |
| F. oxysporum | 720 | |||||
| P. debaryanum | 164 | |||||
| 109 | S. aureus | 5.3 (log reduction, 24 h contact) | Plate count | Prevent biofouling | [155] | |
| P. aeruginosa | 5.4 (log reduction, 24 h contact) | |||||
| 110 | S. aureus | 1.7 (log reduction, 24 h contact) | Plate count | [155] | ||
| P. aeruginosa | 1.9 (log reduction, 24 h contact) | |||||
| 111 | S. aureus | 6 (log reduction, 24 h contact) | Plate count | [154] | ||
| E. coli | 6 (log reduction, 24 h contact) | |||||
| P. aeruginosa | 4.5 (log reduction, 24 h contact) | |||||
| 112 | S. aureus | 128 | Plate count | [156] | ||
| E. coli | 256 | |||||
| 113 | S. aureus ATCC 6538P | 7.26 (log reduction, 1 min contact) | Plate count | [160] | ||
| E. coli ATCC 1122 | 8.26 (log reduction, 1 min contact) |
| Series/ Compound | IL | Species | MIC, μM | MBC, μM | Method | Notes | Ref. |
|---|---|---|---|---|---|---|---|
| 103 | Poly-(vinylbenzyl dimethylhexylammonium chloride) | S. aureus ATCC 6538 | 910 | Broth microdilution | Side-chain polymer | [149] | |
| E. coli ATCC 8099 | 780 | ||||||
| 104 | Poly-((N,N-dimethyl-N-(4-((trimethylammonio)methyl)benzyl)hexan-1-aminium) dibromide) | S. aureus ATCC 6538 | 280 | Broth microdilution | Main-chain polymer | [149] | |
| E. coli ATCC 8099 | 130 | ||||||
| 114 | 3-(2-(Methacryloyloxy)ethyl)-1-hexylimidazolium bromide-based polymer | E. coli ATCC 25922 | 3.62 | Shake flask test | Antibacterial coating | [162] | |
| 115 | 3-(2-(Methacryloyloxy)ethyl)-1-octylimidazolium bromide-based polymer | E. coli ATCC 25922 | 1.67 | Shake flask test | Antibacterial coating | [162] | |
| 116 | 3-(2-(Methacryloyloxy)ethyl)-1-dodecylimidazolium bromide-based polymer | E. coli ATCC 25922 | <0.46 | Shake flask test | Antibacterial coating | [162] | |
| 117 | Poly(1-ethyl-3-vinylimidazolium bromide) | S. aureus ATCC 6538 | 110345 | Broth microdilution | [164] | ||
| E. coli ATCC 8099 | 110345 | ||||||
| 118 | Poly(1-butyl-3-vinylimidazolium bromide) | S. aureus ATCC 6538 | 2961 | Broth microdilution | [164] | ||
| E. coli ATCC 8099 | 5922 | ||||||
| 119 | Poly(1-octyl-3-vinylimidazolium bromide) | S. aureus ATCC 6538 | 1491 (3.71 for NPs) | Broth microdilution | [164,170] | ||
| E. coli ATCC 8099 | 1192 (1.85 for NPs) | ||||||
| 120 | Poly(1-decyl-3-vinylimidazolium bromide) | S. aureus ATCC 6538 | 3.57 | Broth microdilution | NPs | [170] | |
| E. coli ATCC 8099 | 1.84 | ||||||
| 121 | Poly(1-dodecyl-3-vinylimidazolium bromide) | S. aureus ATCC 6538 | 61 (2.52 for NPs) | Broth microdilution | [164,170] | ||
| E. coli ATCC 8099 | 122 (1.19 for NPs) | ||||||
| 122 | Poly(1-hexadecyl-3-vinylimidazolium bromide) | S. aureus ATCC 6538 | 3.15 | Broth microdilution | NPs | [170] | |
| E. coli ATCC 8099 | 2.72 | ||||||
| 123 | Poly(1-ethyl-3-(1-vinylimidazolium-3-hexyl)imidazolium bromide) | S. aureus ATCC 6538 | 33180 | Broth microdilution | [164] | ||
| E. coli ATCC 8099 | 33180 | ||||||
| 124 | Poly(1-butyl-3-(1-vinylimidazolium-3-hexyl)imidazolium bromide) | S. aureus ATCC 6538 | 918 | Broth microdilution | [164] | ||
| E. coli ATCC 8099 | 1853 | ||||||
| 125 | Poly(1-octyl-3-(1-vinylimidazolium-3-hexyl)imidazolium bromide) | S. aureus ATCC 6538 | 81 | Broth microdilution | [164] | ||
| E. coli ATCC 8099 | 41 | ||||||
| 126 | Poly(1-dodecyl-3-(1-vinylimidazolium-3-hexyl)imidazolium bromide) | S. aureus ATCC 6538 | 9 | Broth microdilution | [164] | ||
| E. coli ATCC 8099 | 18 | ||||||
| 127 | Poly-(N-Butyl-N-methylpyrrolidinonium bromide) | S. aureus | 549 | Broth microdilution | [89] | ||
| E. coli | 2196 | ||||||
| 128 | Poly-(N-Hexyl-N-methylpyrrolidinonium bromide) | S. aureus | 236 | Broth microdilution | [89] | ||
| E. coli | 548 | ||||||
| 129 | Poly-(N-Octyl-N-methylpyrrolidinonium bromide) | S. aureus | 147 | Broth microdilution | [89] | ||
| E. coli | 424 | ||||||
| 130 | Poly-(N-Decyl-N-methylpyrrolidinonium bromide) | S. aureus | 112 | Broth microdilution | [89] | ||
| E. coli | 224 | ||||||
| 131 | Poly-(N-Dodecyl-N-methylpyrrolidinonium bromide) | S. aureus | 61 | Broth microdilution | [89] | ||
| E. coli | 90 | ||||||
| 132 | Poly-(1-vinylbenzyl-3-hexylimidazolium chloride) | S. aureus ATCC 6538 | 900 | Broth microdilution | Side-chain polymer | [149] | |
| E. coli ATCC 8099 | 770 | ||||||
| 133 | Poly-(1-vinylbenzyl-4-hexyl-1,4-diazoniabicyclo[2 .2.2]octane-1,4-diium chloride bromide) | S. aureus ATCC 6538 | 1280 | Broth microdilution | Side-chain polymer | [149] | |
| E. coli ATCC 8099 | 1160 | ||||||
| 134 | Poly-(1-hexyl-3-methylimidazolium bromide) | S. aureus ATCC 6538 | 230 | Broth microdilution | Main-chain polymer | [149] | |
| E. coli ATCC 8099 | 110 | ||||||
| 135 | Poly-(1-hexyl-4-methyl-1,4-diazoniabicyclo[2.2.2]octane-1,4-diium dibromide) | S. aureus ATCC 6538 | 560 | Broth microdilution | Main-chain polymer | [149] | |
| E. coli ATCC 8099 | 510 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. https://doi.org/10.3390/ijms22136793
Vereshchagin AN, Frolov NA, Egorova KS, Seitkalieva MM, Ananikov VP. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. International Journal of Molecular Sciences. 2021; 22(13):6793. https://doi.org/10.3390/ijms22136793
Chicago/Turabian StyleVereshchagin, Anatoly N., Nikita A. Frolov, Ksenia S. Egorova, Marina M. Seitkalieva, and Valentine P. Ananikov. 2021. "Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials" International Journal of Molecular Sciences 22, no. 13: 6793. https://doi.org/10.3390/ijms22136793
APA StyleVereshchagin, A. N., Frolov, N. A., Egorova, K. S., Seitkalieva, M. M., & Ananikov, V. P. (2021). Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. International Journal of Molecular Sciences, 22(13), 6793. https://doi.org/10.3390/ijms22136793








