Betulinic Acid Ameliorates the Severity of Acute Pancreatitis via Inhibition of the NF-κB Signaling Pathway in Mice
Abstract
:1. Introduction
2. Results
2.1. Biochemical Toxicity of BA
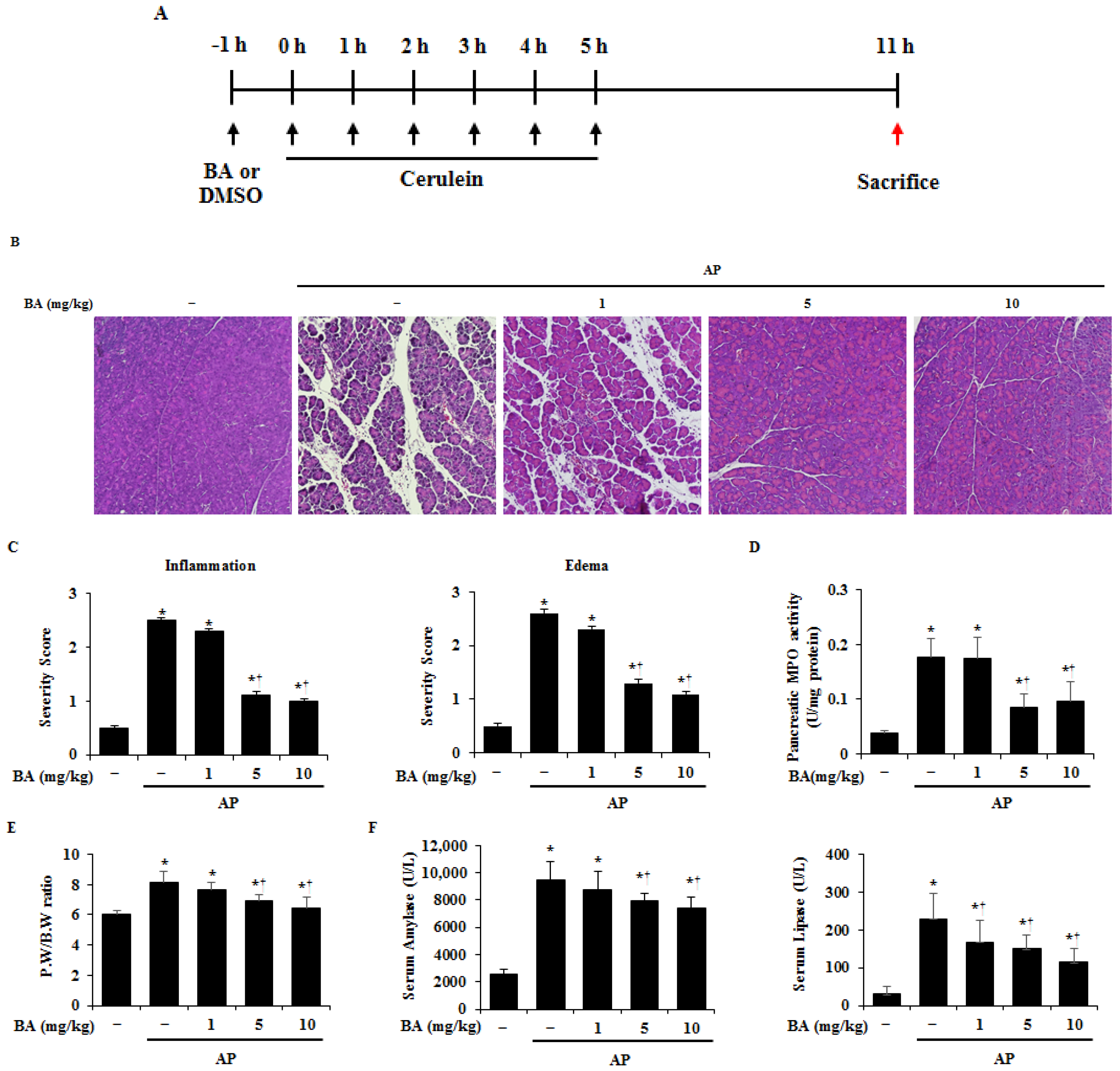
2.2. Effect of BA on Pancreatic Damage in Cerulein-Induced AP
2.3. Effect of BA on Lung Histological Damage in Cerulein-Induced AP
2.4. Effect of BA on Production of Proinflammatory Cytokines
2.5. Effect of BA on Macrophage and Neutrophil Infiltration into the Pancreas
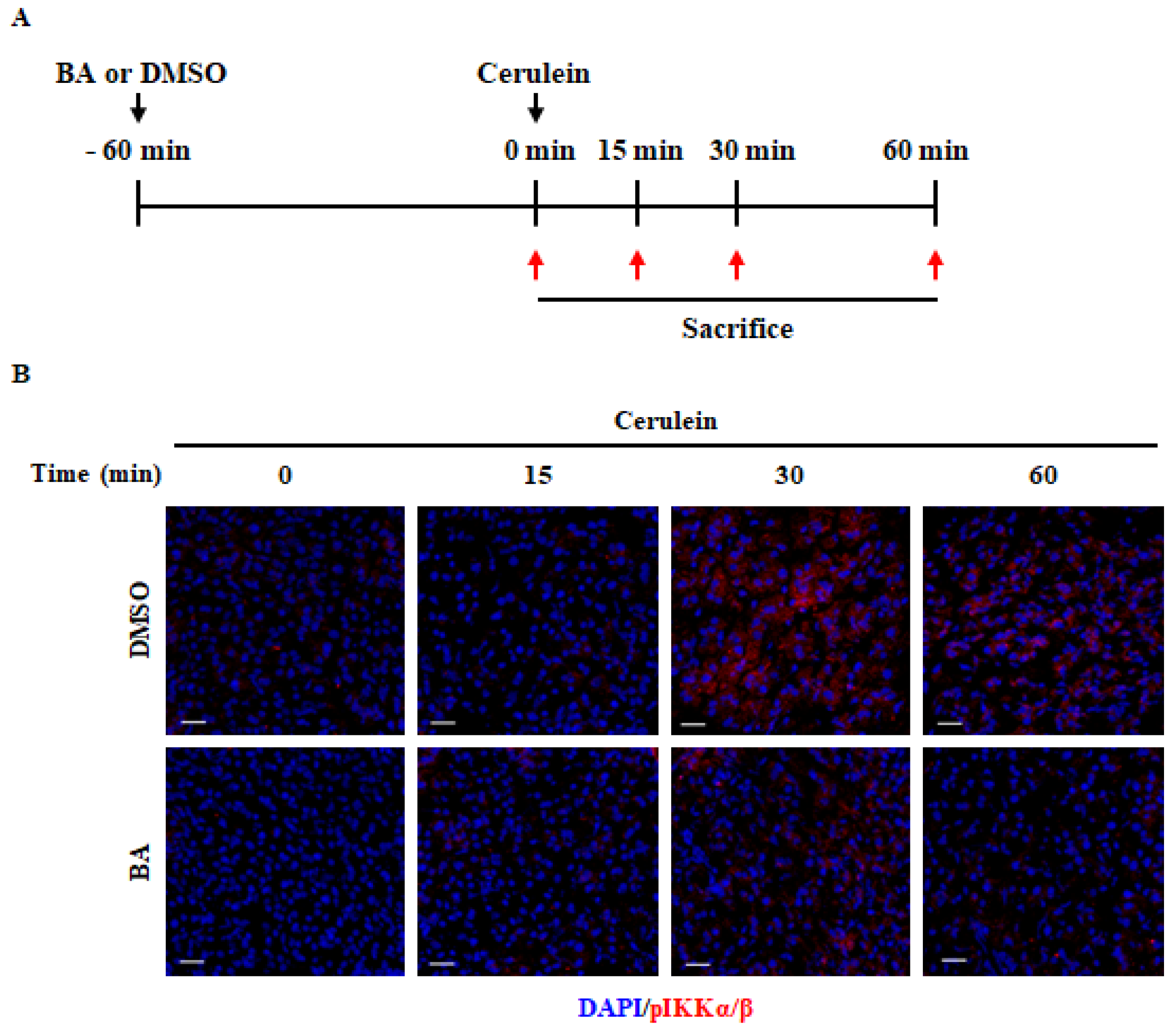
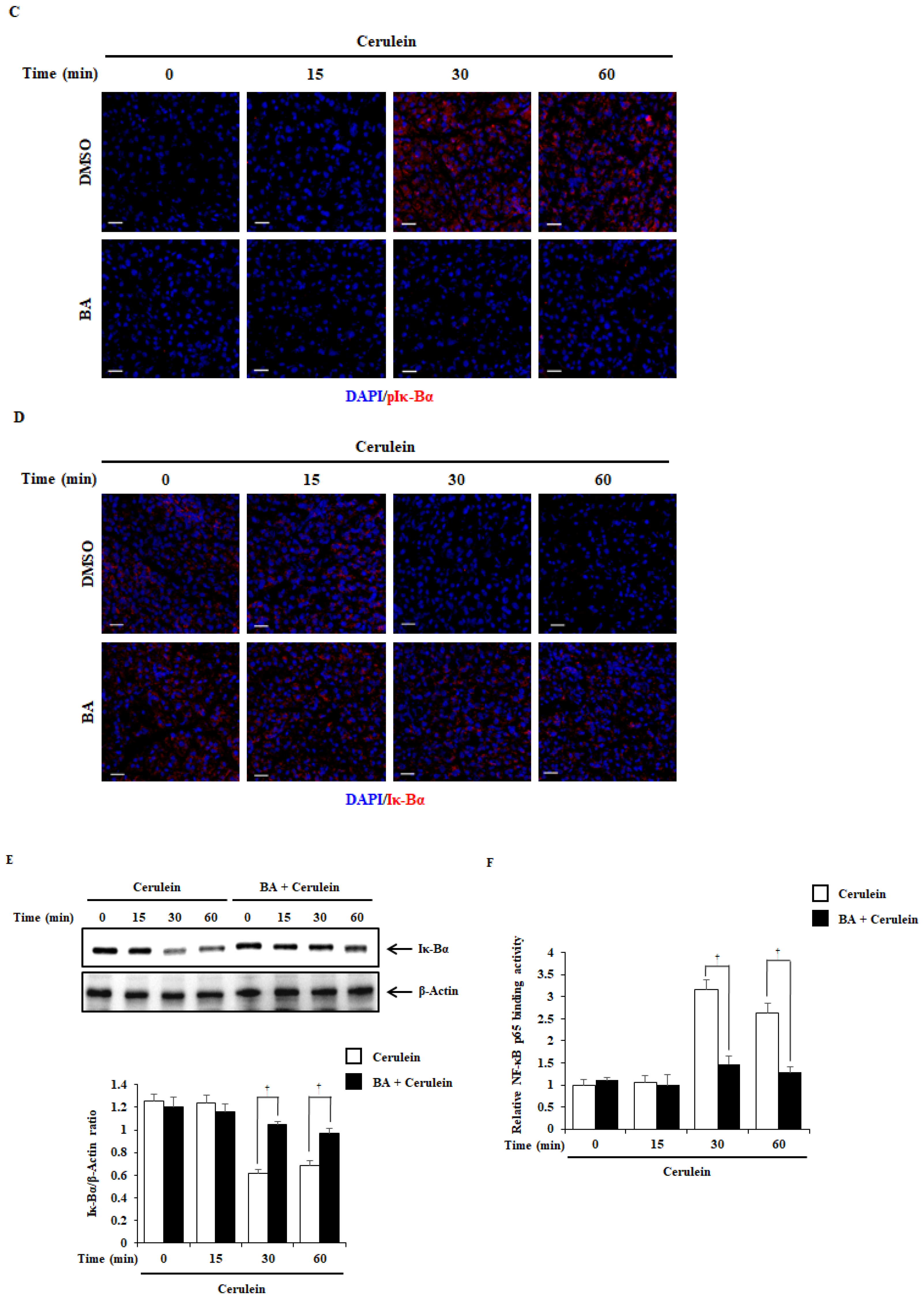
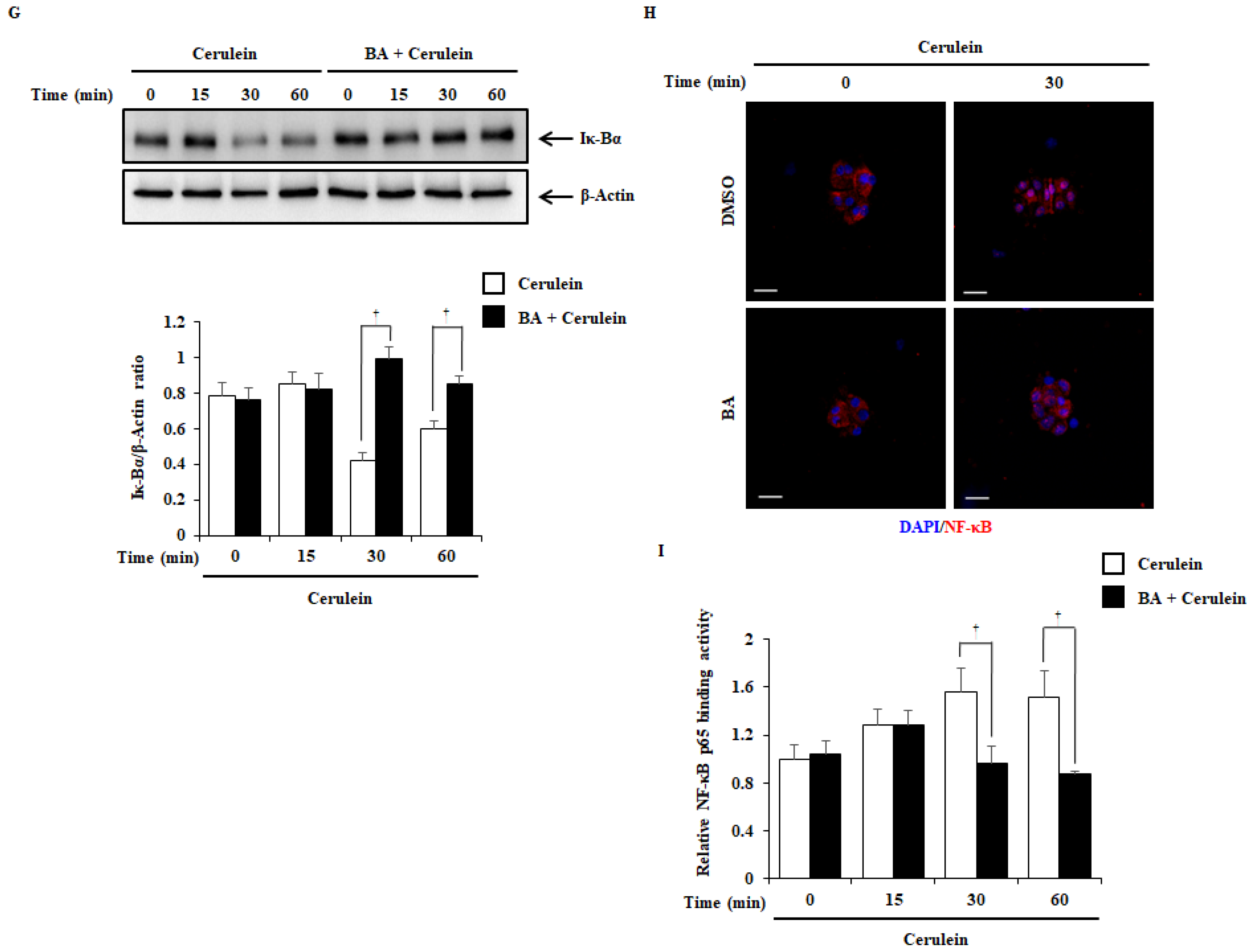
2.6. Effect of BA on NF-κB Activation
2.7. Therapeutic Effects of BA in Cerulein-Induced AP
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Models
4.3. Experimental Design
4.4. Biochemical Detections
4.5. Histological Examination
4.6. Myeloperoxidase Activity
4.7. Pancreatic Edema Analysis
4.8. Determination of Serum Amylase and Lipase Levels
4.9. Reverse Transcription-Quantitative Polymerase Chain Reaction
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Immunofluorescence
4.12. Isolation of Mouse PACs
4.13. Cell Viability Assay
4.14. Isolation of Mouse Pancreatic Cells
4.15. Flow Cytometry Analysis
4.16. Western Blot Assay
4.17. Nuclear Extraction and NF-κB Binding Activity Determination
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sendler, M.; Dummer, A.; Weiss, F.U.; Krüger, B.; Wartmann, T.; Scharffetter-Kochanek, K.; Van Rooijen, N.; Malla, S.R.; Aghdassi, A.; Halangk, W.; et al. Tumour necrosis factor alpha secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut 2013, 62, 430–439. [Google Scholar] [CrossRef]
- Talukdar, R.; Vege, S.S. Acute pancreatitis. Curr. Opin. Gastroenterol. 2015, 31, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.; Gukovskaya, A.; Reavey, P.; Gukovsky, S.; Sisk, A.; Braquet, P.; Pandol, S.; Poucell-Hatton, S. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology 1996, 111, 1081–1091. [Google Scholar] [CrossRef]
- Büchler, M.W.; Gloor, B.; Müller, C.A.; Friess, H.; Seiler, C.A.; Uhl, W. Acute necrotizing pancreatitis: Treatment strategy according to the status of infection. Ann. Surg. 2000, 232, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. The IκB kinase—A bridge between inflammation and cancer. Cell Res. 2008, 18, 334–342. [Google Scholar] [CrossRef]
- Traenckner, E.B.; Pahl, H.L.; Henkel, T.; Schmidt, K.; Wilk, S.; Baeuerle, P. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995, 14, 2876–2883. [Google Scholar] [CrossRef]
- Steinle, A.U.; Weidenbach, H.; Wagner, M.; Adler, G.; Schmid, R.M. NF-κB/Rel activation in cerulein pancreatitis. Gastroenterology 1999, 116, 420–430. [Google Scholar] [CrossRef]
- Aparna, J.; Jakkampudi, A.; Jangala, R.; Reddy, B.R.; Mitnala, S.; Nageshwar, R.D.; Talukdar, R. NF-kB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology 2016, 16, 477–488. [Google Scholar] [CrossRef]
- Satoh, A. Inhibition of nuclear factor-κB activation improves the survival of rats with taurocholate pancreatitis. Gut 1999, 44, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Liu, Y.; Daniluk, J.; Gaiser, S.; Chu, J.; Wang, H.; Li, Z.S.; Logsdon, C.D.; Ji, B. Activation of nuclear factor-kB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology 2013, 144, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jingbo, W.; Aimin, C.; Qi, W.; Xin, L.; Huaining, L. Betulinic acid inhibits IL-1beta-induced inflammation by activating PPAR-gamma in human osteoarthritis chondrocytes. Int. Immunopharmacol. 2015, 29, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Han, S.; Park, E.; Yim, D.; Lee, S.; Lee, C.K.; Cho, K.; Kim, K. Immunomodulatory activity of betulinic acid by producing pro-inflammatory cytokines and activation of macrophages. Arch. Pharm. Res. 2003, 26, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Xia, W.; Wu, J.; Yuan, L.; Wu, J.; Tu, D.; Fang, J.; Tan, Z. Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice. J. Vet. Sci. 2014, 15, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszen, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-alpha, TGF-beta) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic acid: A natural product with anticancer activity. Mol. Nutr. Food Res. 2009, 53, 140–146. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Z.; Xiong, F.; Chen, C.; Chao, X.; Huang, J.; Huang, H. Betulinic acid ameliorates experimental diabetic-induced renal inflammation and fibrosis via inhibiting the activation of NF-κB signaling pathway. Mol. Cell. Endocrinol. 2016, 434, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bi, Y.; Mo, C.; Zeng, T.; Huang, S.; Gao, L.; Sun, X.; Lv, Z. Betulinic acid attenuates liver fibrosis by inducing autophagy via the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. J. Nat. Med. 2019, 73, 179–189. [Google Scholar] [CrossRef]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.; Bettio, L.; Oliveira, Á.; Pazini, F.L.; Dalmarco, J.B.; Simionatto, E.L.; et al. Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 2013, 136, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Poch, B.; Gansauge, F.; Rau, B.; Wittel, U.; Gansauge, S.; Nussler, A.K.; Schoenberg, M.; Beger, H.G. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: Mediators of local destruction and activators of inflammation. FEBS Lett. 1999, 461, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Sui, J.; Fan, R.; Qu, W.; Dong, X.; Sun, D. Emodin protects against acute pancreatitis-associated lung injury by inhibiting NLPR3 inflammasome activation via Nrf2/HO-1 signaling. Drug Des. Dev. Ther. 2020, 14, 1971–1982. [Google Scholar] [CrossRef]
- Huber, W.; Algülm, H.; Lahmerm, T.; Mayr, U.; Lehmann, M.; Schmid, R.M.; Faltlhauser, A. Pancreatitis cytosorbents (CytoSorb) inflammatory cytokine removal: A prospective study (PACIFIC). Medicine 2019, 98, e13044. [Google Scholar] [CrossRef] [PubMed]
- Ethridge, R.T.; Chung, D.H.; Slogoff, M.; Ehlers, R.A.; Hellmich, M.R.; Rajaraman, S.; Saito, H.; Uchida, T.; Evers, B. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology 2002, 123, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Song, A.M.; Bhagat, L.; Singh, V.P.; Van Acker, G.G.D.; Steer, M.L.; Saluja, A.K. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G1166–G1174. [Google Scholar] [CrossRef] [Green Version]
- Polito, F.; Bitto, A.; Irrera, N.; Squadrito, F.; Fazzari, C.; Minutoli, L.; Altavilla, D. Flavocoxid, a dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, reduces pancreatic damage in an experimental model of acute pancreatitis. Br. J. Pharmacol. 2010, 161, 1002–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.-W.; Jung, W.-S.; Piao, T.-G.; Hong, S.-H.; Yun, K.-J.; Park, R.-K.; Shin, M.-K.; Song, H.-J.; Park, S.-J. Selective cyclooxygenase-2 inhibitor ameliorates cholecystokinin-octapeptide-induced acute pancreatitis in rats. World J. Gastroenterol. 2007, 13, 2298–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Ji, B.; Han, B.; Ernst, S.A.; Simeone, D.; Logsdon, C.D. NF-κB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 2002, 122, 448–457. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, J.-W.; Kim, D.-G.; Zhou, Z.Q.; Shin, Y.K.; Seo, J.H.; Song, H.-J.; Choi, B.-M.; Bae, G.-S.; Park, S.-J. Protective effects of Coenzyme Q10 against acute pancreatitis. Int. Immunopharmacol. 2020, 88, 106900. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Wong, F.L.; Cao, Y.; Lau, H.Y.; Huang, J.; Puneet, P.; Chevali, L. Pathophysiology of acute pancreatitis. Pancreatology 2005, 5, 132–144. [Google Scholar] [CrossRef]
- Gukovsky, I.; Gukovskaya, A.S.; Blinman, T.A.; Zaninovic, V.; Pandol, S.J. Early NF-κB activation is associated with hormone-induced pancreatitis. Am. J. Physiol. 1998, 275, G1402–G1414. [Google Scholar] [CrossRef]
- Spanier, B.W.; Dijkgraaf, M.G.; Bruno, M.J. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: An update. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Lowenfels, A.B. Trends in the epidemiology of the first attack of acute pancreatitis: A systematic review. Pancreas 2006, 33, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Farthing, M.; Roberts, S.E.; Samuel, D.G.; Williams, J.G.; Thorne, K.; Morrison-Rees, S.; John, A.; Akbari, A.; Williams, J.C. Survey of digestive health across Europe: Final report. Part 1: The burden of gastrointestinal diseases and the organisation and delivery of gastroenterology services across Europe. United Eur. Gastroenterol. J. 2014, 2, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.E.; Morrison-Rees, S.; John, A.; Williams, J.G.; Brown, T.H.; Samuel, D.G. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017, 17, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bildziukevich, U.; Özdemir, Z.; Wimmer, Z. Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives. Molecules 2019, 24, 3546. [Google Scholar] [CrossRef] [Green Version]
- Saneja, A.; Arora, D.; Kumar, R.; Dubey, R.D.; Panda, A.K.; Gupta, P.N. Therapeutic applications of betulinic acid nanoformulations. Ann. N. Y. Acad. Sci. 2018, 1421, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Z.; Zhao, J.; Zhu, L.; Huang, L.; Ma, Y.; Ma, C.; Luo, C.; Zhu, Z.; Yuan, Z.; Wu, J.; et al. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed. Pharmacother. 2019, 118, 109347. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Lu, C.; Chen, H.; Deng, J.; Yan, Y.; Xu, Y.-Y.; Liu, H.; Huang, H.; Wei, J.; et al. Betulinic acid suppresses Th17 response and ameliorates psoriasis-like murine skin inflammation. Int. Immunopharmacol. 2019, 73, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, M.C.; Pathak, N.N.; Begum, J.; Balaganur, V.; Bhat, R.A.; Ramachandra, H.D.; Ayanur, A.; Ram, M.; Singh, V.; Kumar, D.; et al. Betulinic acid attenuates lung injury by modulation of inflammatory cytokine response in experimentally-induced polymicrobial sepsis in mice. Cytokine 2015, 71, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Zhang, H.; Wang, X.; Yang, X.; Wu, S.; Wang, H.; Shen, P.; Ma, T. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Dervenis, C.; Johnson, C.D.; Bassi, C.; Bradley, E.; Imrie, C.W.; McMahon, M.J.; Modlin, I. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini consensus conference. Int. J. Pancreatol. 1999, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Dawra, R.; Sah, P.; Dudeja, V.; Rishi, L.; Talukdar, R.; Garg, P.; Saluja, A.K. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology 2011, 141, 2210–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.P.; Li, Z.J.; Zhang, J. Inflammatory mediators and microcirculatory disturbance in acute pancreatitis. HBPD Int. 2009, 8, 351–357. [Google Scholar]
- Sweiry, J.H.; Mann, G.E. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand. J. Gastroenterol. Suppl. 1996, 219, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Muhs, B.E.; Patel, S.; Yee, H.; Marcus, S.; Shamamian, P. Increased matrix metalloproteinase expression and activation following experimental acute pancreatitis. J. Surg. Res. 2001, 101, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.J.; Winter, D.C.; Redmond, H.P. Lung injury in acute pancreatitis: Mechanisms, prevention, and therapy. Curr. Opin. Crit. Care 2002, 8, 158–163. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denham, W.; Yang, J.; Fink, G.; Denham, D.; Carter, G.; Ward, K.; Norman, J. Gene targeting demonstrates additive detrimental effects of interleukin 1 and tumor necrosis factor during pancreatitis. Gastroenterology 1997, 113, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.P.; Moheyel Din, A.; Gaber, L.; Kotb, M.; Gaber, A.O. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-α polyclonal antibody. Am. J. Surg. 1994, 167, 214–218. [Google Scholar] [CrossRef]
- John, D.S.; Aschenbach, J.; Krüger, B.; Sendler, M.; Weiss, F.U.; Mayerle, J.; Lerch, M.M.; Aghdassi, A.A. Deficiency of cathepsin C ameliorates severity of acute pancreatitis by reduction of neutrophil elastase activation and cleavage of E-cadherin. J. Biol. Chem. 2019, 294, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Liu, Y.; Song, Y.; Wang, Q.; Liu, Y.; Yang, S.; Li, D.; Zhang, Y.; Cheng, B. Deletion of macrophage migration inhibitory factor ameliorates inflammation in mice model severe acute pancreatitis. Biomed. Pharmacother. 2020, 125, 109919. [Google Scholar] [CrossRef] [PubMed]
- Perides, G.; Weiss, E.R.; Michae, E.S. TNF-alpha-dependent regulation of acute pancreatitis severity by Ly-6C(hi) monocytes in mice. J. Biol. Chem. 2011, 286, 13327–13335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, K.C.; Chao, K.F.; Chuang, C.C.; Liu, S.H. Blockade of interleukin 6 accelerates acinar cell apoptosis and attenuates experimental acute pancreatitis in vivo. Br. J. Surg. 2006, 93, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Frossard, J.L.; Lenglet, S.; Montecucco, F.; Steffens, S.; Galan, K.; Pelli, G.; Spahr, L.; Mach, F.; Hadengue, A. Role of CCL-2, CCR-2 and CCR-4 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. J. Clin. Pathol. 2011, 64, 387–393. [Google Scholar] [CrossRef]
- Bhatia, M.; Ramnath, R.D.; Chevali, L.; Guglielmotti, A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1259–G1265. [Google Scholar] [CrossRef] [Green Version]
- Rau, B.; Baumgart, K.; Krüger, C.M.; Schilling, M.; Beger, H.G. CC-chemokine activation in acute pancreatitis: Enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003, 29, 622–629. [Google Scholar] [CrossRef]
- Makhija, R.; Kingsnorth, A.N. Cytokine storm in acute pancreatitis. J. Hepato-Biliary-Pancreat. Surg. 2002, 9, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Minassi, A.; Rogati, F.; Cruz, C.; Prados, M.E.; Galera, N.; Jinénez, C.; Appendino, G.; Bellido, M.L.; A Calzado, M.; Caprioglio, D.; et al. Triterpenoid Hydroxamates as HIF Prolyl Hydrolase Inhibitors. J. Nat. Prod. 2018, 81, 2235–2243. [Google Scholar] [CrossRef]
- Prados, M.E.; García-Martín, A.; Unciti-Broceta, J.D.; Palomares, B.; Collado, J.A.; Minassi, A.; Calzado, M.A.; Appendino, G.; Muñoz, E. Betulinic acid hydroxamate prevents colonic inflammation and fibrosis in murine models of inflammatory bowel disease. Acta Pharmacol. Sin. 2021, 42, 1124–1138. [Google Scholar] [CrossRef]
- Ma, R.; Yuan, F.; Wang, S.; Liu, Y.; Fan, T.; Wang, F. Calycosin alleviates cerulein-induced acute pancreatitis by inhibiting the inflammatory response and oxidative stress via the p38 MAPK and NF-κB signal pathways in mice. Biomed. Pharmacother. 2018, 105, 599–605. [Google Scholar] [CrossRef]
- Jo, I.-J.; Bae, G.-S.; Choi, S.B.; Kim, D.-G.; Shin, J.-Y.; Seo, S.-H.; Choi, M.-O.; Kim, T.-H.; Song, H.-J.; Park, S.-J. Fisetin attenuates cerulein-induced acute pancreatitis through down regulation of JNK and NF-κB signaling pathways. Eur. J. Pharmacol. 2014, 737, 149–158. [Google Scholar] [CrossRef]
- Kim, M.-J.; Bae, G.-S.; Jo, I.-J.; Choi, S.-B.; Kim, D.-G.; Shin, J.-Y.; Lee, S.-K.; Kim, M.-J.; Shin, S.; Song, H.-J.; et al. Loganin protects against pancreatitis by inhibiting NF-κB activation. Eur. J. Pharmacol. 2015, 765, 541–550. [Google Scholar] [CrossRef]
- Altavilla, D.; Famulari, C.; Passaniti, M.; Galeano, M.; Macrì, A.; Seminara, P.; Minutoli, L.; Marini, H.R.; Calò, M.; Venuti, F.S.; et al. Attenuated cerulein-induced pancreatitis in nuclear factor-κB-deficient mice. Lab. Investig. 2003, 83, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Rabi, T.; Shukla, S.; Gupta, S. Betulinic acid suppresses constitutive and TNFα-induced NF-κB activation and induces apoptosis in human prostate carcinoma PC-3 cells. Mol. Carcinog. 2008, 47, 964–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Huang, C.; Zhu, L.; Kong, L.; Yuan, Z.; Wen, L.; Li, R.; Wu, J.; Yi, J. Betulinic acid ameliorates the T-2 toxin-triggered intestinal impairment in mice by inhibiting inflammation and mucosal barrier dysfunction through the NF-κB signaling pathway. Toxins 2020, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Shin, J.Y.; Jo, I.-J.; Kim, D.-G.; Song, H.-J.; Yoon, C.-S.; Oh, H.; Kim, Y.-C.; Bae, G.-S.; Park, S.-J. 8α-Hydroxypinoresinol isolated from Nardostachys jatamansi ameliorates cerulein-induced acute pancreatitis through inhibition of NF-κB activation. Mol. Immunol. 2019, 114, 620–628. [Google Scholar] [CrossRef]
- Medhora, M.; Gao, F.; Glisch, C.; Narayanan, J.; Sharma, A.; Harmann, L.M.; Lawlor, M.W.; Snyder, L.A.; Fish, B.L.; Down, J.D.; et al. Whole-thorax irradiation induces hypoxic respiratory failure, pleural effusions and cardiac remodeling. J. Radiat. Res. 2015, 56, 248–260. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Fish, B.L.; Szabo, A.; Doctrow, S.R.; Kma, L.; Molthen, R.C.; Moulder, J.E.; Jacobs, E.R.; Medhora, M. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat. Res. 2012, 178, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Dawra, R.; Sharif, R.; Phillips, P.; Dudeja, V.; Dhaulakhandi, D.; Saluja, A.K. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1009–G1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gout, J.; Pommier, R.M.; Vincent, D.F.; Kaniewski, B.; Martel, S.; Valcourt, U.; Bartholin, L. Isolation and culture of mouse primary pancreatic acinar cells. J. Vis. Exp. 2013, 78, 50514. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Group | ALP (IU/L) | ALT (IU/L) | AST (IU/L) | BUN (mg/dL) | CREA (mg/dL) |
|---|---|---|---|---|---|
| DMSO | 241.50 ± 38.41 | 29.50 ± 4.95 | 159.33 ± 29.91 | 21.25 ± 0.96 | 0.15 ± 0.03 |
| BA 0.1 mg/kg | 242.60 ± 19.32 | 25.50 ± 3.54 | 155.67 ± 9.29 | 19.60 ± 1.34 | 0.14 ± 0.01 |
| BA 1 mg/kg | 236.20 ± 32.91 | 29.20 ± 5.58 | 157.00 ± 13.93 | 20.25 ± 0.50 | 0.15 ± 0.02 |
| BA 5 mg/kg | 240.00 ± 20.77 | 29.17 ± 7.36 | 161.20 ± 29.63 | 19.33 ± 4.16 | 0.16 ± 0.02 |
| BA 10 mg/kg | 234.50 ± 17.25 | 28.33 ± 0.58 | 156.00 ± 30.17 | 17.50 ± 1.00 | 0.13 ± 0.01 |
| BA 20 mg/kg | 237.33 ± 25.48 | 53.50 ± 7.78 * | 156.20 ± 22.38 | 21.00 ± 4.64 | 0.16 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Choi, J.-W.; Shin, J.Y.; Kim, D.-U.; Kweon, B.; Oh, H.; Kim, Y.-C.; Song, H.-J.; Bae, G.-S.; Park, S.-J. Betulinic Acid Ameliorates the Severity of Acute Pancreatitis via Inhibition of the NF-κB Signaling Pathway in Mice. Int. J. Mol. Sci. 2021, 22, 6871. https://doi.org/10.3390/ijms22136871
Zhou Z, Choi J-W, Shin JY, Kim D-U, Kweon B, Oh H, Kim Y-C, Song H-J, Bae G-S, Park S-J. Betulinic Acid Ameliorates the Severity of Acute Pancreatitis via Inhibition of the NF-κB Signaling Pathway in Mice. International Journal of Molecular Sciences. 2021; 22(13):6871. https://doi.org/10.3390/ijms22136871
Chicago/Turabian StyleZhou, Ziqi, Ji-Won Choi, Joon Yeon Shin, Dong-Uk Kim, Bitna Kweon, Hyuncheol Oh, Youn-Chul Kim, Ho-Joon Song, Gi-Sang Bae, and Sung-Joo Park. 2021. "Betulinic Acid Ameliorates the Severity of Acute Pancreatitis via Inhibition of the NF-κB Signaling Pathway in Mice" International Journal of Molecular Sciences 22, no. 13: 6871. https://doi.org/10.3390/ijms22136871






