Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges
Abstract
1. Introduction
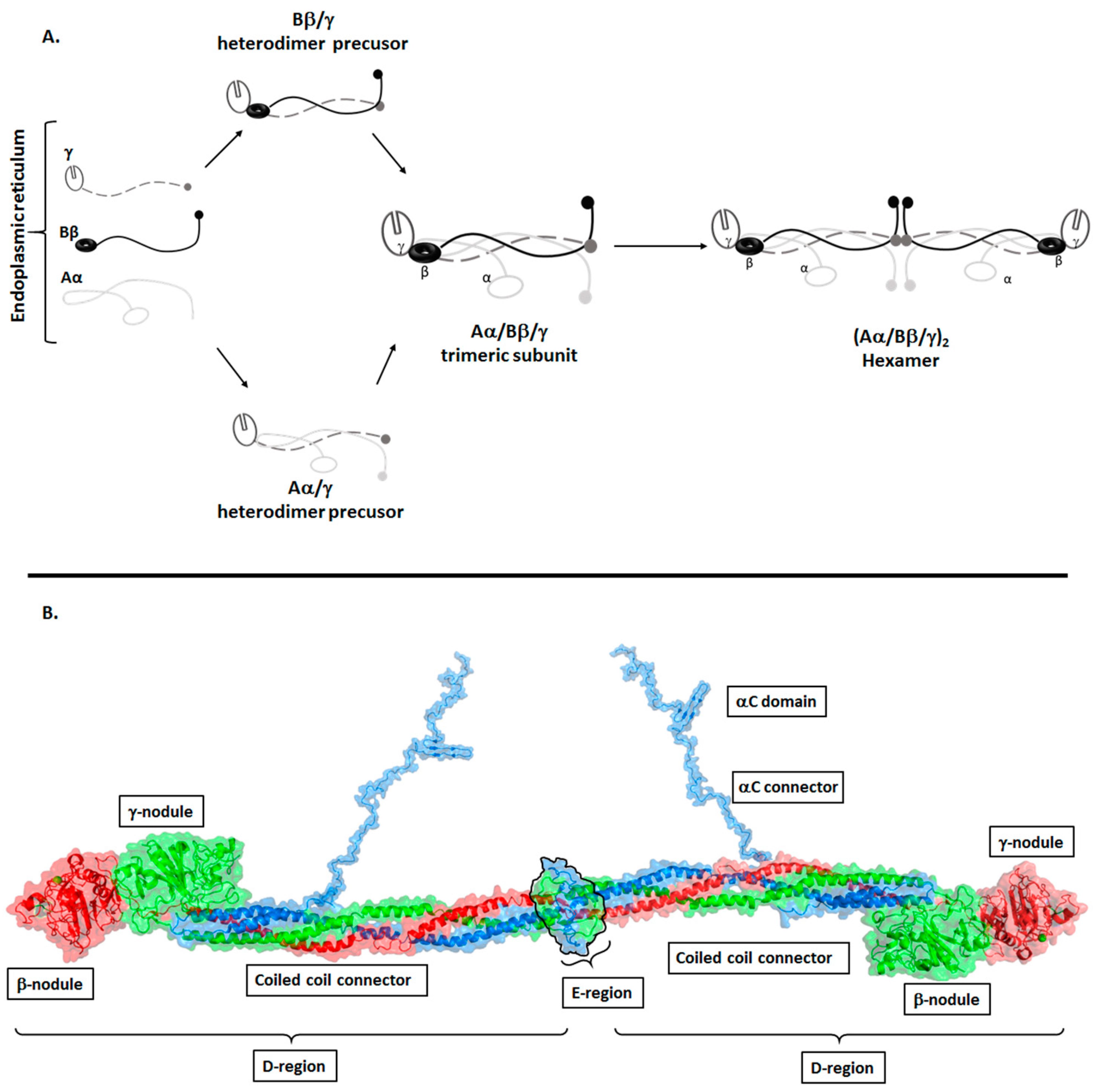
Structure of Fibrinogen
2. The Biological Role of Fibrinogen (Conversion of Fibrinogen to Fibrin)
2.1. Clot Formation
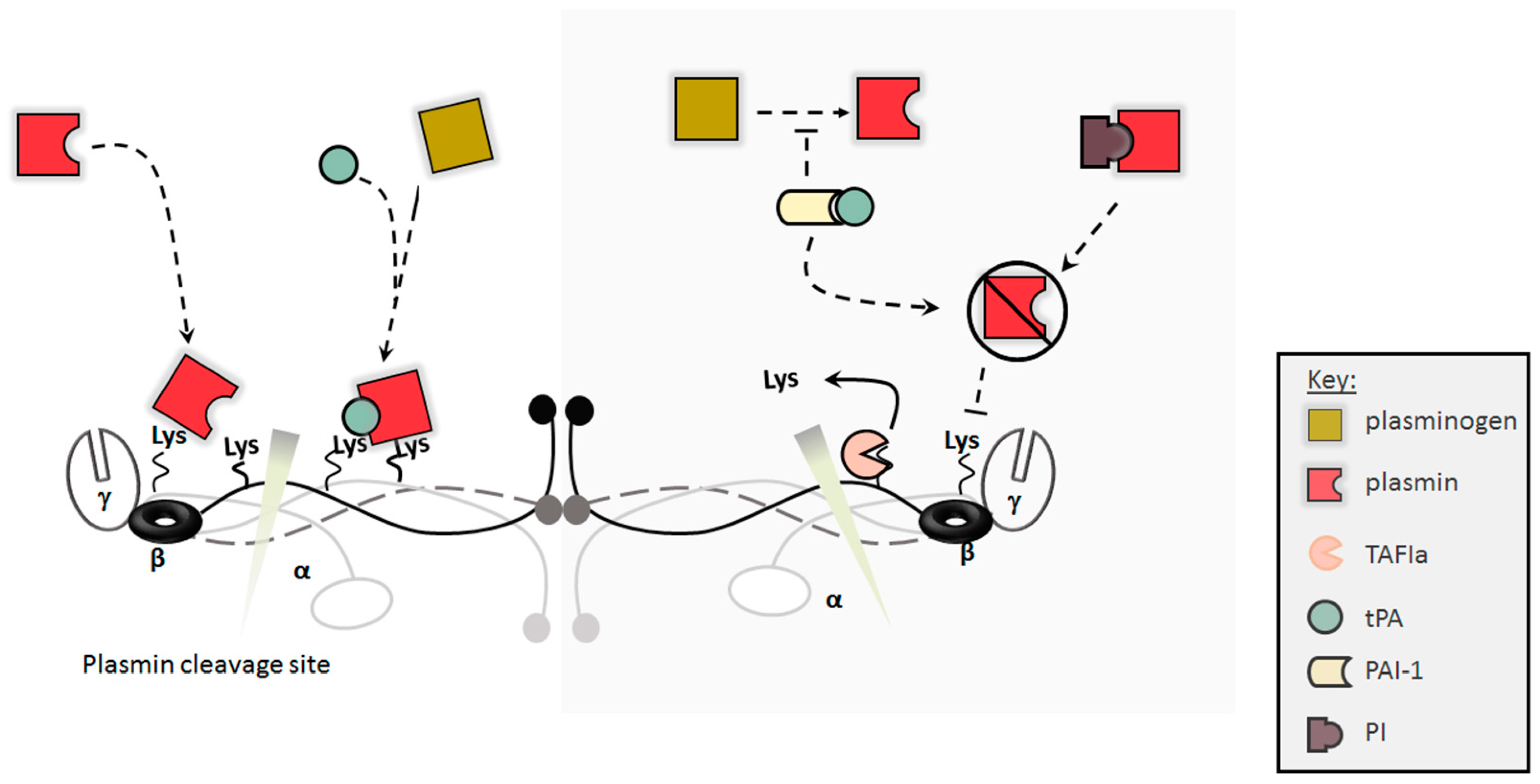
2.2. Clot Dissolution/Lysis
3. The Impact of Fibrin(ogen) Modifications and Plasma Levels on Fibrin Clots
3.1. Changes in Fibrinogen Concentrations
3.2. Post-Translational Modifications
3.3. Genetic Polymorphism and Splicing
4. Implications of Changes in Fibrin Clot Characteristics in Disease States
4.1. Fibrin(ogen) in Bleeding Disorders
4.2. Fibrin(ogen) in Thrombosis
5. Pharmacological Therapies Targeting Fibrinogen and the Fibrin Network
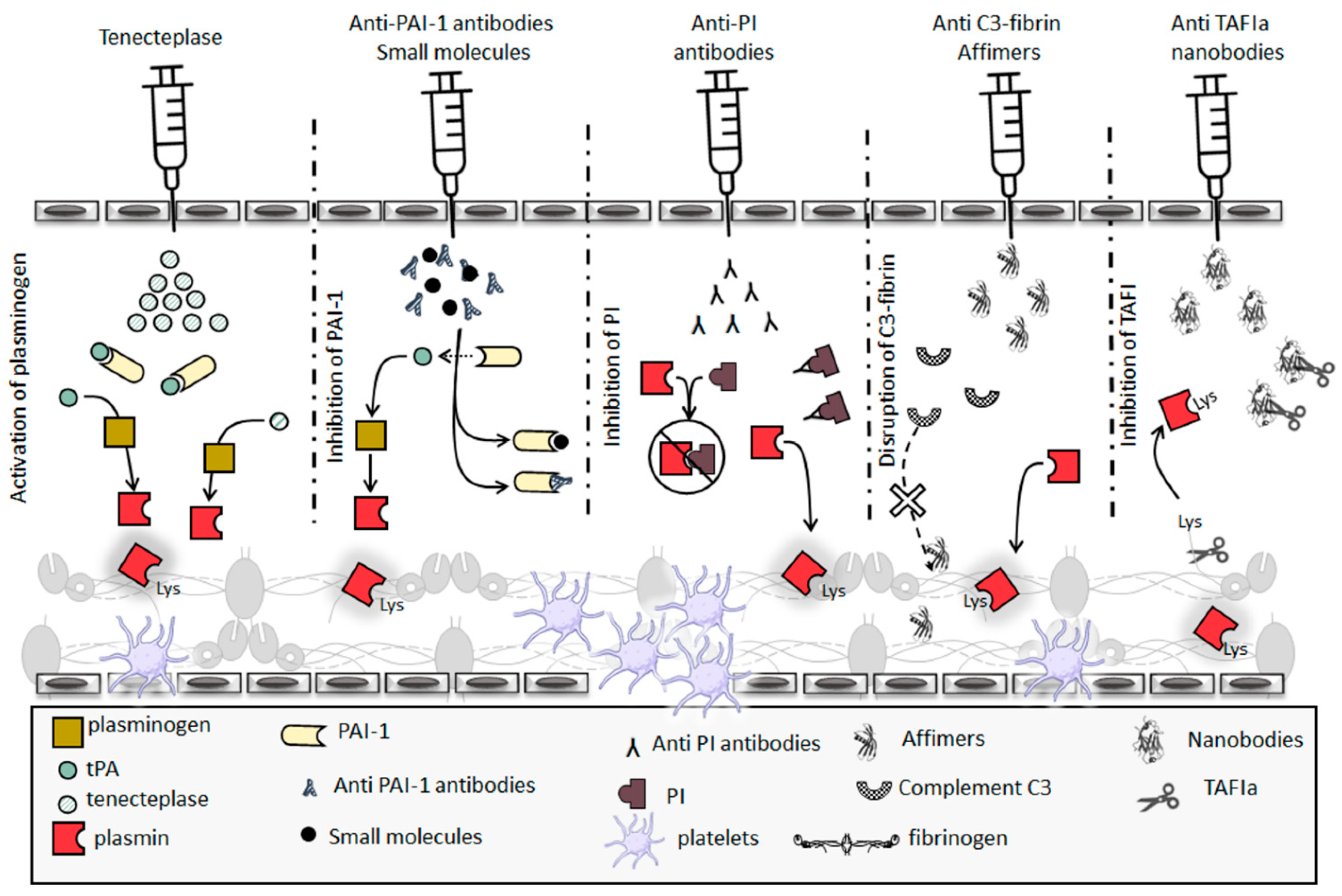
5.1. Thrombolytic Therapeutics
5.2. Hypofibrinolysis Therapeutics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, E13–E21. [Google Scholar] [CrossRef]
- Redman, C.M.; Xia, H. Fibrinogen biosynthesis—Assembly, intracellular degradation, and association with lipid synthesis and secretion. Ann. N. Y. Acad. Sci. 2001, 936, 480–495. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin formation, structure and properties. In Fibrous Proteins: Structures and Mechanisms; (Subcellular Biochemistry); Springer: Berlin, Germany, 2017; Volume 82, pp. 405–456. [Google Scholar]
- De Moerloose, P.; Boehlen, F.; Neerman-Arbez, M. Fibrinogen and the Risk of Thrombosis. Semin. Thromb. Hemost. 2010, 36, 7–17. [Google Scholar] [CrossRef]
- Weisel, J.W. Structure of fibrin: Impact on clot stability. J. Thromb. Haemost. 2007, 5, 116–124. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Hemostats, Sealants, and Adhesives: A Practical Guide for the Surgeon. Am. Surg. 2012, 78, 1305–1321. [Google Scholar] [CrossRef]
- Fish, R.J.; Neerman-Arbez, M. Fibrinogen gene regulation. Thromb. Haemost. 2012, 108, 419–426. [Google Scholar] [CrossRef]
- Fish, R.J.; Neerman-Arbez, M. A novel regulatory element between the human FGA and FGG genes. Thromb. Haemost. 2012, 108, 427–434. [Google Scholar] [CrossRef]
- Jaimes, C.E.; Fish, R.J.; Neerman-Arbez, M. Local chromatin interactions contribute to expression of the fibrinogen gene cluster. J. Thromb. Haemost. 2018, 16, 2070–2082. [Google Scholar] [CrossRef]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef]
- Tennent, G.A.; Brennan, S.O.; Stangou, A.J.; O’Grady, J.; Hawkins, P.N.; Pepys, M.B. Human plasma fibrinogen is synthesized in the liver. Blood 2007, 109, 1971–1974. [Google Scholar] [CrossRef]
- Yu, S.; Sher, B.; Kudryk, B.; Redman, C.M. Fibrinogen precursors—Order of assembly of fibrinogen chains. J. Biol. Chem. 1984, 259, 574–581. [Google Scholar] [CrossRef]
- Burton, R.A.; Tsurupa, G.; Hantgan, R.R.; Tjandra, N.; Medved, L. NMR solution structure, stability, and interaction of the recombinant bovine fibrinogen alpha C-domain fragment. Biochemistry 2007, 46, 8550–8560. [Google Scholar] [CrossRef]
- Doolittle, R.F. X-ray crystallographic studies on fibrinogen and fibrin. J. Thromb. Haemost. 2003, 1, 1559–1565. [Google Scholar] [CrossRef]
- Kollman, J.M.; Pandi, L.; Sawaya, M.R.; Riley, M.; Doolittle, R.F. Crystal Structure of Human Fibrinogen. Biochemistry 2009, 48, 3877–3886. [Google Scholar] [CrossRef]
- Kostelansky, M.S.; Betts, L.; Gorkun, O.V.; Lord, S.T. 2.8 angstrom crystal structures of recombinant fibrinogen fragment D with and without two peptide ligands: GHRP binding to the “b” site disrupts its nearby calcium-binding site. Biochemistry 2002, 41, 12124–12132. [Google Scholar] [CrossRef]
- Pechik, I.; Madrazo, J.; Mosesson, M.W.; Hernandez, I.; Gilliland, G.L.; Medved, L. Crystal structure of the complex between thrombin and the central “E” region of fibrin. Proc. Natl. Acad. Sci. USA 2004, 101, 2718–2723. [Google Scholar] [CrossRef]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Simurda, T.; Brunclikova, M.; Asselta, R.; Caccia, S.; Zolkova, J.; Kolkova, Z.; Loderer, D.; Skornova, I.; Hudecek, J.; Lasabova, Z.; et al. Genetic Variants in the FGB and FGG Genes Mapping in the Beta and Gamma Nodules of the Fibrinogen Molecule in Congenital Quantitative Fibrinogen Disorders Associated with a Thrombotic Phenotype. Int. J. Mol. Sci. 2020, 21, 4616. [Google Scholar] [CrossRef]
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin (ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef]
- Pratt, K.P.; Cote, H.C.F.; Chung, D.W.; Stenkamp, R.E.; Davies, E.W. The primary fibrin polymerization pocket: Three-dimensional structure of a 30-kDa C-terminal gamma chain fragment complexed with the peptide Gly-Pro-Arg-Pro. Proc. Natl. Acad. Sci. USA 1997, 94, 7176–7181. [Google Scholar] [CrossRef]
- Lewis, S.D.; Shields, P.P.; Shafer, J.A. Characterization of the Kinetic Pathway for Liberation of Fibrinopeptides during Assembly of Fibrin. J. Biol. Chem. 1985, 260, 192–199. [Google Scholar] [CrossRef]
- Gorkun, O.V.; Veklich, Y.I.; Medved, L.V.; Henschen, A.H.; Weisel, J.W. Role of the alpha-c domains of fibrin in clot formation. Biochemistry 1994, 33, 6986–6997. [Google Scholar] [CrossRef]
- Weisel, J.W.; Medved, L. The structure and function of the alpha C domains of fibrinogen. Ann. N. Y. Acad. Sci. 2001, 936, 312–327. [Google Scholar] [CrossRef]
- Cierniewski, C.S.; Budzynski, A.Z. Involvement of the alpha-chain in fibrin clot formation—Effect of monoclonal-antibodies. Biochemistry 1992, 31, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Moen, J.L.; Veklich, Y.I.; Gorkun, O.V.; Lord, S.T.; Montalescot, G.; Weisel, J.W. The alpha C domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood 2005, 106, 3824–3830. [Google Scholar] [CrossRef]
- Lord, S.T. Fibrinogen and fibrin: Scaffold proteins in hemostasis. Curr. Opin. Hematol. 2007, 14, 236–241. [Google Scholar] [CrossRef]
- Takagi, T.; Doolittle, R.F. Amino-acid sequence studies on factor-xiii and peptide released during its activation by thrombin. Biochemistry 1974, 13, 750–756. [Google Scholar] [CrossRef]
- Chen, R.; Doolittle, R.F. Lambda-lambda cross-linking sites in human and bovine fibrin. Biochemistry 1971, 10, 4486–4491. [Google Scholar] [CrossRef]
- Duval, C.; Allan, P.; Connell, S.D.A.; Ridger, V.C.; Philippou, H.; Ariens, R.A.S. Roles of fibrin alpha- and gamma-chain specific cross-linking by FXIIIa in fibrin structure and function. Thromb. Haemost. 2014, 111, 842–850. [Google Scholar] [CrossRef]
- Standeven, K.F.; Carter, A.M.; Grant, P.J.; Weisel, J.W.; Chernysh, I.; Masova, L.; Lord, S.T.; Ariens, R.A.S. Functional analysis of fibrin gamma-chain cross-linking by activated factor XIII: Determination of a cross-linking pattern that maximizes clot stiffness. Blood 2007, 110, 902–907. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Duval, C.; Wang, Y.M.; Hansen, C.E.; Ahn, B.; Mooberry, M.J.; Clark, M.A.; Johnsen, J.M.; Lord, S.T.; Lam, W.A.; et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin alpha-chain crosslinking. Blood 2015, 126, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Helms, C.C.; Ariens, R.A.S.; de Willige, S.U.; Standeven, K.F.; Guthold, M. Alpha-alpha Cross-Links Increase Fibrin Fiber Elasticity and Stiffness. Biophys. J. 2012, 102, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rijken, D.C.; Abdul, S.; Malfliet, J.; Leebeek, F.W.G.; de Willige, S.U. Compaction of fibrin clots reveals the antifibrinolytic effect of factor XIII. J. Thromb. Haemost. 2016, 14, 1453–1461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, J.H.; Mosher, D.F. Enhancement of thrombogenesis by plasma fibronectin cross-linked to fibrin and assembled in platelet thrombi. Blood 2006, 107, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Aoki, N. Cross-linking of alpha-2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J. Clin. Investig. 1980, 65, 290–297. [Google Scholar] [CrossRef]
- Valnickova, Z.; Enghild, J.J. Human procarboxypeptidase U, or thrombin-activable fibrinolysis inhibitor, is a substrate for transglutaminases—Evidence for transglutaminase-catalyzed cross-linking to fibrin. J. Biol. Chem. 1998, 273, 27220–27224. [Google Scholar] [CrossRef]
- Aleman, M.M.; Byrnes, J.R.; Wang, J.G.; Tran, R.; Lam, W.A.; Di Paola, J.; Mackman, N.; Degen, J.L.; Flick, M.J.; Wolberg, A.S. Factor XIII activity mediates red blood cell retention in venous thrombi. J. Clin. Investig. 2014, 124, 3590–3600. [Google Scholar] [CrossRef]
- Feng, X.D.; Clark, R.A.F.; Galanakis, D.; Tonnesen, M.G. Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: Stabilization of alpha v/beta 3 mRNA by fibrin. J. Investig. Dermatol. 1999, 113, 913–919. [Google Scholar] [CrossRef]
- Hook, P.; Litvinov, R.I.; Kim, O.V.; Xu, S.X.; Xu, Z.L.; Bennett, J.S.; Alber, M.S.; Weisel, J.W. Strong Binding of Platelet Integrin alpha IIb beta 3 to Fibrin Clots: Potential Target to Destabilize Thrombi. Sci. Rep. 2017, 7, 13001. [Google Scholar] [CrossRef]
- Muthard, R.W.; Diamond, S.L. Blood Clots Are Rapidly Assembled Hemodynamic Sensors Flow Arrest Triggers Intraluminal Thrombus Contraction. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2938–2945. [Google Scholar] [CrossRef]
- Longstaff, C.; Kolev, K. Basic mechanisms and regulation of fibrinolysis. J. Thromb. Haemost. 2015, 13, S98–S105. [Google Scholar] [CrossRef]
- Medved, L.; Nieuwenhuizen, W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb. Haemost. 2003, 89, 409–419. [Google Scholar] [PubMed]
- Thelwell, C.; Longstaff, C. The regulation by fibrinogen and fibrin of tissue plasminogen activator kinetics and inhibition by plasminogen activator inhibitor 1. J. Thromb. Haemost. 2007, 5, 804–811. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tsurupa, G.; Yakovlev, S.; McKee, P.; Medved, L. Noncovalent Interaction of alpha 2-Antiplasmin with Fibrin(ogen): Localization of alpha 2-Antiplasmin-Binding Sites. Biochemistry 2010, 49, 7643–7651. [Google Scholar] [CrossRef]
- Bosma, P.J.; Rijken, D.C.; Nieuwenhuizen, W. Binding of tissue-type plasminogen-activator to fibrinogen fragments. Eur. J. Biochem. 1988, 172, 399–404. [Google Scholar] [CrossRef]
- Yakovlev, S.; Makogonenko, E.; Kurochkina, N.; Nieuwenhuizen, W.; Ingham, K.; Medved, L. Conversion of fibrinogen to fibrin: Mechanism of exposure of tPA- and plasminogen-binding sites. Biochemistry 2000, 39, 15730–15741. [Google Scholar] [CrossRef]
- Medved, L.V.; Gorkun, O.V.; Manyakov, V.F.; Belitser, V.A. The role of fibrinogen alpha-c-domains in the fibrin assembly process. FEBS Lett. 1985, 181, 109–112. [Google Scholar] [CrossRef]
- Grailhe, P.; Nieuwenhuizen, W.; Anglescano, E. Study of tissue-type plasminogen-activator binding-sites on fibrin using distinct fragments of fibrinogen. Eur. J. Biochem. 1994, 219, 961–967. [Google Scholar] [CrossRef]
- Schielen, W.J.G.; Adams, H.; Vanleuven, K.; Voskuilen, M.; Tesser, G.I.; Nieuwenhuizen, W. The sequence gamma-(312–324) is a fibrin-specific epitope. Blood 1991, 77, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, O.; Voskuilen, M.; Nieuwenhuizen, W. Localization in the fibrinogen gamma-chain of a new site that is involved in the acceleration of the tissue-type plasminogen activator-catalyzed activation of plasminogen. Biochem. J. 1992, 283, 187–191. [Google Scholar] [CrossRef]
- Weisel, J.W.; Nagaswami, C.; Korsholm, B.; Petersen, L.C.; Suenson, E. Interactions of plasminogen with polymerizing fibrin and its derivatives, monitored with a photoaffinity cross-linker and electron-microscopy. J. Mol. Biol. 1994, 235, 1117–1135. [Google Scholar] [CrossRef]
- Silva, M.; Thelwell, C.; Williams, S.C.; Longstaff, C. Regulation of fibrinolysis by C-terminal lysines operates through plasminogen and plasmin but not tissue-type plasminogen activator. J. Thromb. Haemost. 2012, 10, 2354–2360. [Google Scholar] [CrossRef]
- Suenson, E.; Lutzen, O.; Thorsen, S. Initial plasmin-degradation of fibrin as the basis of a positive feedback mechanism in fibrinolysis. Eur. J. Biochem. 1984, 140, 513–522. [Google Scholar] [CrossRef]
- Hudson, N.E. Biophysical Mechanisms Mediating Fibrin Fiber Lysis. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- De Vries, J.J.; Snoek, C.J.M.; Rijken, D.C.; de Maat, M.P.M. Effects of Post-Translational Modifications of Fibrinogen on Clot Formation, Clot Structure, and Fibrinolysis A Systematic Review. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Bridge, K.I.; Philippou, H.; Ariens, R.A.S. Clot properties and cardiovascular disease. Thromb. Haemost. 2014, 112, 901–908. [Google Scholar] [CrossRef]
- Cronje, H.T.; Nienaber-Rousseau, C.; Zandberg, L.; de Lange, Z.; Green, F.R.; Pieters, M. Fibrinogen and clot-related phenotypes determined by fibrinogen polymorphisms: Independent and IL-6-interactive associations. PLoS ONE 2017, 12, e0187712. [Google Scholar] [CrossRef]
- De Maat, M.P.M.; Verschuur, M. Fibrinogen heterogeneity: Inherited and noninherited. Curr. Opin. Hematol. 2005, 12, 377–383. [Google Scholar] [CrossRef]
- De Vries, P.S.; Chasman, D.I.; Sabater-Lleal, M.; Chen, M.H.; Huffman, J.E.; Steri, M.; Tang, W.H.; Teumer, A.; Marioni, R.E.; Grossmann, V.; et al. A meta-analysis of 120 246 individuals identifies 18 new loci for fibrinogen concentration. Hum. Mol. Genet. 2016, 25, 358–370. [Google Scholar] [CrossRef]
- Hoffman, M. Alterations of Fibrinogen Structure in Human Disease. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Ariens, R.A.S.; Grant, P.J. Genetic and environmental determinants of fibrin structure and function—Relevance to clinical disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1558–1566. [Google Scholar] [CrossRef]
- Standeven, K.F.; de Willige, S.U.; Carter, A.M.; Grant, P.J. Heritability of Clot Formation. Semin. Thromb. Hemost. 2009, 35, 458–467. [Google Scholar] [CrossRef]
- Machlus, K.R.; Cardenas, J.C.; Church, F.C.; Wolberg, A.S. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 2011, 117, 4953–4963. [Google Scholar] [CrossRef]
- Blomback, B.; Carlsson, K.; Hessel, B.; Liljeborg, A.; Procyk, R.; Aslund, N. Native fibrin gel networks observed by 3d microscopy, permeation and turbidity. Biochim. Biophys. Acta 1989, 997, 96–110. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Monroe, D.M.; Roberts, H.R.; Hoffman, M. Elevated prothrombin results in clots with an altered fiber structure: A possible mechanism of the increased thrombotic risk. Blood 2003, 101, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Nagaswami, C.; Farrell, D.H.; Montalescot, G.; Weisel, J.W. Influence of γ’ fibrinogen splice variant on fibrin physical properties and fibrinolysis rate. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 382–386. [Google Scholar] [CrossRef]
- Cooper, A.V.; Standeven, K.F.; Ariens, R.A.S. Fibrinogen gamma-chain splice variant γ’ alters fibrin formation and structure. Blood 2003, 102, 535–540. [Google Scholar] [CrossRef]
- Falls, L.A.; Farrell, D.H. Resistance of gamma A/gamma’ fibrin clots to fibrinolysis. J. Biol. Chem. 1997, 272, 14251–14256. [Google Scholar] [CrossRef]
- Applegate, D.; Haraga, L.; Hertzberg, K.M.; Steben, L.S.; Zhang, J.Z.; Redman, C.M.; Grieninger, G. The alpha C-E domains of human fibrinogen (420) contain calcium binding sites but lack polymerization pockets. Blood 1998, 92, 3669–3674. [Google Scholar] [CrossRef]
- Ajjan, R.; Lim, B.C.B.; Standeven, K.F.; Harrand, R.; Dolling, S.; Phoenix, F.; Greaves, R.; Abou-Saleh, R.H.; Connell, S.; Smith, D.A.I.; et al. Common variation in the C-terminal region of the fibrinogen beta-chain: Effects on fibrin structure, fibrinolysis and clot rigidity. Blood 2008, 111, 643–650. [Google Scholar] [CrossRef]
- Lados-Krupa, A.; Konieczynska, M.; Chmiel, A.; Undas, A. Increased Oxidation as an Additional Mechanism Underlying Reduced Clot Permeability and Impaired Fibrinolysis in Type 2 Diabetes. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef]
- Shacter, E.; Williams, J.A.; Levine, R.L. Oxidative modification of fibrinogen inhibits thrombin-catalyzed clot formation. Free Radic. Biol. Med. 1995, 18, 815–821. [Google Scholar] [CrossRef]
- Upchurch, G.R.; Ramdev, N.; Walsh, M.T.; Loscalzo, J. Prothrombotic consequences of the oxidation of fibrinogen and their inhibition by aspirin. J. Thromb. Thrombolysis 1998, 5, 9–14. [Google Scholar] [CrossRef]
- Weigandt, K.M.; White, N.; Chung, D.; Ellingson, E.; Wang, Y.; Fu, X.Y.; Pozzo, D.C. Fibrin Clot Structure and Mechanics Associated with Specific Oxidation of Methionine Residues in Fibrinogen. Biophys. J. 2012, 103, 2399–2407. [Google Scholar] [CrossRef]
- Dunn, E.J.; Philippou, H.; Ariens, R.A.S.; Grant, P.J. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia 2006, 49, 1071–1080. [Google Scholar] [CrossRef]
- Gilman, P.B.; Keane, P.; Martinez, J. The role of the carbohydrate moiety in the biologic properties of fibrinogen. J. Biol. Chem. 1984, 259, 3248–3253. [Google Scholar] [CrossRef]
- Henschen-Edman, A.H. Fibrinogen non-inherited heterogeneity and its relationship to function in health and disease. Ann. N. Y. Acad. Sci. 2001, 936, 580–593. [Google Scholar] [CrossRef]
- Langer, B.G.; Weisel, J.W.; Dinauer, P.A.; Nagaswami, C.; Bell, W.R. Deglycosylation of fibrinogen accelerates polymerization and increases lateral aggregation of fibrin fibers. J. Biol. Chem. 1988, 263, 15056–15063. [Google Scholar] [CrossRef]
- Pieters, M.; Covic, N.; van der Westhuizen, F.H.; Nagaswami, C.; Baras, Y.; Loots, D.T.; Jerling, J.C.; Elgar, D.; Edmondson, K.S.; van Zyl, D.G.; et al. Glycaemic control improves fibrin network characteristics in type 2 diabetes—A purified fibrinogen model. Thromb. Haemost. 2008, 99, 691–700. [Google Scholar]
- Svensson, J.; Bergman, A.C.; Adamson, U.; Blomback, M.; Wallen, H.; Jorneskog, G. Acetylation and glycation of fibrinogen in vitro occur at specific lysine residues in a concentration dependent manner: A mass spectrometric and isotope labeling study. Biochem. Biophys. Res. Commun. 2012, 421, 335–342. [Google Scholar] [CrossRef]
- Hanna, L.S.; Scheraga, H.A.; Francis, C.W.; Marder, V.J. Comparison of structures of various human fibrinogens and a derivative thereof by a study of the kinetics of release of fibrinopeptides. Biochemistry 1984, 23, 4681–4687. [Google Scholar] [CrossRef] [PubMed]
- Heldin, P.; Hessel, B.; Humble, E.; Blomback, B.; Engstrom, L. Effect of phosphorylation invitro of human-fibrinogen with protein-kinase-c on thrombin-induced gelation. Thromb. Res. 1987, 47, 93–99. [Google Scholar] [CrossRef]
- Martin, S.C.; Forsberg, P.O.; Eriksson, S.D. The effects of invitro phosphorylation and dephosphorylation on the thrombin-induced gelation and plasmin degradation of fibrinogen. Thromb. Res. 1991, 61, 243–252. [Google Scholar] [CrossRef]
- Seydewitz, H.H.; Witt, I. Increased phosphorylation of human fibrinopeptide a under acute phase conditions. Thromb. Res. 1985, 40, 29–39. [Google Scholar] [CrossRef]
- Damiana, T.; Damgaard, D.; Sidelmann, J.J.; Nielsen, C.H.; de Maat, M.P.M.; Munster, A.M.B.; Palarasah, Y. Citrullination of fibrinogen by peptidylarginine deiminase 2 impairs fibrin clot structure. Clin. Chim. Acta 2020, 501, 6–11. [Google Scholar] [CrossRef]
- Nakayama-Hamada, M.; Suzuki, A.; Furukawa, H.; Yamada, R.; Yamamoto, K. Citrullinated fibrinogen inhibits thrombin-catalysed fibrin polymerization. J. Biochem. 2008, 144, 393–398. [Google Scholar] [CrossRef]
- Okumura, N.; Haneishi, A.; Terasawa, F. Citrullinated fibrinogen shows defects in FPA and FPB release and fibrin polymerization catalyzed by thrombin. Clin. Chim. Acta 2009, 401, 119–123. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Standeven, K.F.; Khanbhai, M.; Phoenix, F.; Gersh, K.C.; Weisel, J.W.; Kearney, M.T.; Ariens, R.A.S.; Grant, P.J. Effects of Aspirin on Clot Structure and Fibrinolysis Using a Novel In Vitro Cellular System. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 712–717. [Google Scholar] [CrossRef]
- Williams, S.; Fatah, K.; Hjemdahl, P.; Blomback, M. Better increase in fibrin gel porosity by low dose than intermediate dose acetylsalicylic acid. Eur. Heart J. 1998, 19, 1666–1672. [Google Scholar] [CrossRef]
- Lauricella, A.M.; Quintana, I.; Castanon, M.; Sassetti, B.; Kordich, L. Influence of homocysteine on fibrin network lysis. Blood Coagul. Fibrinolysis 2006, 17, 181–186. [Google Scholar] [CrossRef]
- Sauls, D.L.; Wolberg, A.S.; Hoffman, M. Elevated plasma homocysteine leads to alterations in fibrin clot structure and stability: Implications for the mechanism of thrombosis in hyperhomocysteinemia. J. Thromb. Haemost. 2003, 1, 300–306. [Google Scholar] [CrossRef]
- Schuett, K.; Savvaidis, A.; Maxeiner, S.; Lysaja, K.; Jankowski, V.; Schirmer, S.H.; Dimkovic, N.; Boor, P.; Kaesler, N.; Dekker, F.W.; et al. Clot Structure: A Potent Mortality Risk Factor in Patients on Hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 1622–1630. [Google Scholar] [CrossRef]
- Binder, V.; Bergum, B.; Jaisson, S.; Gillery, P.; Scavenius, C.; Spriet, E.; Nyhaug, A.K.; Roberts, H.M.; Chapple, I.L.C.; Hellvard, A.; et al. Impact of fibrinogen carbamylation on fibrin clot formation and stability. Thromb. Haemost. 2017, 117, 899–910. [Google Scholar]
- Parastatidis, I.; Thomson, L.; Burke, A.; Chernysh, I.; Nagaswami, C.; Visser, J.; Stamer, S.; Liebler, D.C.; Koliakos, G.; Heijnen, H.F.G.; et al. Fibrinogen beta-Chain Tyrosine Nitration Is a Prothrombotic Risk Factor. J. Biol. Chem. 2008, 283, 33846–33853. [Google Scholar] [CrossRef]
- Vadseth, C.; Souza, J.M.; Thomson, L.; Seagraves, A.; Nagaswami, C.; Scheiner, T.; Torbet, J.; Vilaire, G.; Bennett, J.S.; Murciano, J.C.; et al. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J. Biol. Chem. 2004, 279, 8820–8826. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Grant, P.J. Role of clotting factors and fibrin structure in predisposition to atherothrombotic disease. Exp. Rev. Cardiovasc. Ther. 2005, 3, 1047–1059. [Google Scholar] [CrossRef]
- Undas, A.; Plicner, D.; Stepien, E.; Drwila, R.; Sadowski, L. Altered fibrin clot structure in patients with advanced coronary artery disease: A role of C-reactive protein, lipoprotein(a) and homocysteine. J. Thromb. Haemost. 2007, 5, 1988–1990. [Google Scholar] [CrossRef]
- Ernst, E.; Resch, K.L. Fibrinogen as a cardiovascular risk factor—A metaanalysis and review of the literature. Ann. Int. Med. 1993, 118, 956–963. [Google Scholar] [CrossRef]
- Kannel, W.B.; Wolf, P.A.; Castelli, W.P.; Dagostino, R.B. Fibrinogen and risk of cardiovascular-disease—The framingham-study. JAMA 1987, 258, 1183–1186. [Google Scholar] [CrossRef]
- Van Holten, T.C.; Waanders, L.F.; de Groot, P.G.; Vissers, J.; Hoefer, I.E.; Pasterkamp, G.; Prins, M.W.J.; Roest, M. Circulating Biomarkers for Predicting Cardiovascular Disease Risk; a Systematic Review and Comprehensive Overview of Meta-Analyses. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Scrutton, M.C.; Rossmurphy, S.B.; Bennett, G.M.; Stirling, Y.; Meade, T.W. Changes in clot deformability—A possible explanation for the epidemiologic association between plasma-fibrinogen concentration and myocardial-infarction. Blood Coagul. Fibrinolysis 1994, 5, 719–723. [Google Scholar] [CrossRef]
- Chung, D.W.; Davie, E.W. Gamma-chain and γ’-chain of human-fibrinogen are produced by alternative messenger-rna processing. Biochemistry 1984, 23, 4232–4236. [Google Scholar] [CrossRef] [PubMed]
- Wolfensteintodel, C.; Mosesson, M.W. Human-plasma fibrinogen heterogeneity—Evidence for an extended carboxyl-terminal sequence in a normal gamma-chain variant (γ’). Proc. Natl. Acad. Sci. USA 1980, 77, 5069–5073. [Google Scholar] [CrossRef]
- Lovely, R.S.; Moaddel, M.; Farrell, D.H. Fibrinogen γ’ chain binds thrombin exosite II. J. Thromb. Haemost. 2003, 1, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Siebenlist, K.R.; Meh, D.A.; Mosesson, M.W. Plasma factor XIII binds specifically to fibrinogen molecules containing γ’ chains. Biochemistry 1996, 35, 10448–10453. [Google Scholar] [CrossRef]
- Farrell, D.H. γ’ Fibrinogen as a novel marker of thrombotic disease. Clin. Chem. Lab. Med. 2012, 50, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Omarova, F.; de Willige, S.U.; Simioni, P.; Ariens, R.A.S.; Bertina, R.M.; Rosing, J.; Castoldi, E. Fibrinogen γ’ increases the sensitivity to activated protein C in normal and factor V Leiden plasma. Blood 2014, 124, 1531–1538. [Google Scholar] [CrossRef][Green Version]
- Behague, I.; Poirier, O.; Nicaud, V.; Evans, A.; Arveiler, D.; Luc, G.; Cambou, J.P.; Scarabin, P.Y.; Bara, L.; Green, F.; et al. Beta fibrinogen gene polymorphisms are associated with plasma fibrinogen and coronary artery disease in patients with myocardial infarction—The ECTIM study. Circulation 1996, 93, 440–449. [Google Scholar] [CrossRef]
- Carter, A.M.; Catto, A.J.; Bamford, J.M.; Grant, P.J. Gender-specific associations of the fibrinogen B beta 448 polymorphism, fibrinogen levels, and acute cerebrovascular disease. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 589–594. [Google Scholar] [CrossRef]
- De Moerloose, P.; Casini, A.; Neerman-Arbez, M. Congenital Fibrinogen Disorders: An Update. Semin. Thromb. Hemost. 2013, 39, 585–595. [Google Scholar] [PubMed]
- Simurda, T.; Snahnicanova, Z.; Loderer, D.; Sokol, J.; Stasko, J.; Lasabova, Z.; Kubisz, P. Fibrinogen Martin: A Novel Mutation in FGB (Gln180Stop) Causing Congenital Afibrinogenemia. Semin. Thromb. Hemost. 2016, 42, 455–457. [Google Scholar] [PubMed]
- Simurda, T.; Caccia, S.; Asselta, R.; Zolkova, J.; Stasko, J.; Skornova, I.; Snahnicanova, Z.; Loderer, D.; Lasabova, Z.; Kubisz, P. Congenital hypofibrinogenemia associated with a novel heterozygous nonsense mutation in the globular C-terminal domain of the γ-chain (p.Glu275Stop). J. Thromb. Thrombolysis 2020, 50, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Neerman-Arbez, M.; Ariens, R.A.; De Moerloose, P. Dysfibrinogenemia: From molecular anomalies to clinical manifestations and management. J. Thromb. Haemost. 2015, 13, 909–919. [Google Scholar] [CrossRef]
- Casini, A.; Duval, C.; Pan, X.; Tintillier, V.; Biron-Andreani, C.; Ariens, R.A.S. Fibrin clot structure in patients with congenital dysfibrinogenaemia. Thromb. Res. 2016, 137, 189–195. [Google Scholar] [CrossRef]
- Brummel-Ziedins, K.E.; Branda, R.F.; Butenas, S.; Mann, K.G. Discordant fibrin formation in hemophilia. J. Thromb. Haemost. 2009, 7, 825–832. [Google Scholar] [CrossRef]
- Broze, G.J.; Higuchi, D.A. Coagulation-dependent inhibition of fibrinolysis: Role of carboxypeptidase-U and the premature lysis of clots from hemophilic plasma. Blood 1996, 88, 3815–3823. [Google Scholar] [CrossRef]
- Foley, J.H.; Nesheim, M.E.; Rivard, G.E.; Brummel-Ziedins, K.E. Thrombin activatable fibrinolysis inhibitor activation and bleeding in haemophilia A. Haemophilia 2012, 18, e316–e322. [Google Scholar] [CrossRef] [PubMed]
- Antovic, A.; Mikovic, D.; Elezovic, I.; Zabczyk, M.; Hutenby, K.; Antovic, J.P. Improvement of fibrin clot structure after factor VIII injection in haemophilia A patients treated on demand. Thromb. Haemost. 2014, 111, 656–661. [Google Scholar]
- Wolberg, A.S.; Allen, G.A.; Monroe, D.M.; Hedner, U.; Roberts, H.R.; Hoffman, M. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. Br. J. Haematol. 2005, 131, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Zucker, M.; Seligsohn, U.; Salomon, O.; Wolberg, A.S. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J. Thromb. Haemost. 2014, 12, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Peyvandi, F.; Naderi, M.; Shapiro, A. Factor XIII deficiency diagnosis: Challenges and tools. Int. J. Lab. Hematol. 2018, 40, 3–11. [Google Scholar] [CrossRef]
- Howes, J.M.; Richardson, V.R.; Smith, K.A.; Schroeder, V.; Somani, R.; Shore, A.; Hess, K.; Ajjan, R.; Pease, R.J.; Keen, J.N.; et al. Complement C3 is a novel plasma dot component with anti-fibrinolytic properties. Diabetes Vasc. Dis. Res. 2012, 9, 216–225. [Google Scholar] [CrossRef]
- Richardson, V.R.; Schroeder, V.; Grant, P.J.; Standeven, K.F.; Carter, A.M. Complement C3 is a substrate for activated factor XIII that is cross-linked to fibrin during clot formation. Br. J. Haematol. 2013, 160, 116–119. [Google Scholar] [CrossRef]
- Ritchie, H.; Lawrie, L.C.; Crombie, P.W.; Mosesson, M.W.; Booth, N.A. Cross-linking of plasminogen activator inhibitor 2 and alpha(2)-antiplasmin to fibrin(ogen). J. Biol. Chem. 2000, 275, 24915–24920. [Google Scholar] [CrossRef]
- Lorand, L. Factor XIII: Structure, activation, and interactions with fibrinogen and fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 291–311. [Google Scholar] [CrossRef]
- Cieslik, J.; Mrozinska, S.; Broniatowska, E.; Undas, A. Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: A cohort study. Blood 2018, 131, 797–807. [Google Scholar] [CrossRef]
- Collet, J.P.; Allali, Y.; Lesty, C.; Tanguy, M.L.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Fatah, K.; Silveira, A.; Tornvall, P.; Karpe, F.; Blomback, M.; Hamsten, A. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb. Haemost. 1996, 76, 535–540. [Google Scholar] [CrossRef]
- Leander, K.; Blomback, M.; Wallen, H.; He, S. Impaired fibrinolytic capacity and increased fibrin formation associate with myocardial infarction. Thromb. Haemost. 2012, 107, 1092–1099. [Google Scholar] [CrossRef]
- Undas, A.; Kolarz, M.; Kopec, G.; Tracz, W. Altered fibrin clot properties in patients on long-term haemodialysis: Relation to cardiovascular mortality. Nephrol. Dial. Transplant. 2008, 23, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Farmer-Boatwright, M.K.; Roubey, R.A.S. Venous Thrombosis in the Antiphospholipid Syndrome. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Pechlivani, N.; Ajjan, R.A. Thrombosis and Vascular Inflammation in Diabetes: Mechanisms and Potential Therapeutic Targets. Front. Cardiovasc. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Furie, B.C.; Furie, B. Cancer-associated thrombosis. Crit. Rev. Oncol. Hematol. 2007, 62, 126–136. [Google Scholar] [CrossRef]
- Kearney, K.; Tomlinson, D.; Smith, K.; Ajjan, R. Hypofibrinolysis in diabetes: A therapeutic target for the reduction of cardiovascular risk. Cardiovasc. Diabetol. 2017, 16, 34. [Google Scholar] [CrossRef]
- He, S.; Blomback, M.; Yoo, G.; Sinha, R.; Henschen-Edman, A.H. Modified clotting properties of fibrinogen in the presence of acetylsalicylic acid in a purified system. Ann. N. Y. Acad. Sci. 2001, 936, 531–535. [Google Scholar] [CrossRef]
- Standeven, K.F.; Ariens, R.A.S.; Whitaker, P.; Ashcroft, A.E.; Weisel, J.W.; Grant, P.J. The effect of dimethylbiguanide on thrombin activity, FXIII activation, fibrin polymerization, and fibrin clot formation. Diabetes 2002, 51, 189–197. [Google Scholar] [CrossRef]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Anticoagulant effects of statins and their clinical implications. Thromb. Haemost. 2014, 111, 392–400. [Google Scholar]
- Absar, S.; Gupta, N.; Nahar, K.; Ahsan, F. Engineering of plasminogen activators for targeting to thrombus and heightening thrombolytic efficacy. J. Thromb. Haemost. 2015, 13, 1545–1556. [Google Scholar] [CrossRef]
- Davydov, L.; Cheng, J.W.M. Tenecteplase: A review. Clin. Ther. 2001, 23, 982–997. [Google Scholar] [CrossRef]
- Keyt, B.A.; Paoni, N.F.; Refino, C.J.; Berleau, L.; Nguyen, H.; Chow, A.; Lai, J.; Pena, L.; Pater, C.; Ogez, J.; et al. Faster-acting and more potent form of tissue-plasminogen activator. Proc. Natl. Acad. Sci. USA 1994, 91, 3670–3674. [Google Scholar] [CrossRef]
- Hagemeyer, C.E.; Tomic, I.; Weirich, U.; Graeber, J.; Nordt, T.; Runge, M.S.; Bode, C.; Peter, K. Construction and characterization of a recombinant plasminogen activator composed of an anti-fibrin single-chain antibody and low-molecular-weight urokinase. J. Thromb. Haemost. 2004, 2, 797–803. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, J.; Su, Y.J.; Tang, Y.C.; Liu, J.N. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials 2009, 30, 5125–5130. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, K.; Ganguly, K.; Ding, B.S.; Atochin, D.; Zaitsev, S.; Murciano, J.C.; Huang, P.L.; Kasner, S.E.; Cines, D.B.; Muzykantov, V.R. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation 2008, 118, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.K.; Park, J.S.; Byun, Y.; Kim, C.K. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials 2009, 30, 5751–5756. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Favaloro, E.J. Novel and Emerging Therapies: Thrombus-Targeted Fibrinolysis. Semin. Thromb. Hemost. 2013, 39, 48–58. [Google Scholar]
- Wyseure, T.; Declerck, P.J. Novel or expanding current targets in fibrinolysis. Drug Dis. Today 2014, 19, 1476–1482. [Google Scholar] [CrossRef]
- Fortenberry, Y.M. Plasminogen activator inhibitor-1 inhibitors: A patent review (2006-present). Exp. Opin. Ther. Pat. 2013, 23, 801–815. [Google Scholar] [CrossRef]
- Hillmayer, K.; Vancraenenbroeck, R.; De Maeyer, M.; Compernolle, G.; Declerck, P.J.; Gils, A. Discovery of novel mechanisms and molecular targets for the inhibition of activated thrombin activatable fibrinolysis inhibitor. J. Thromb. Haemost. 2008, 6, 1892–1899. [Google Scholar] [CrossRef]
- Mishra, N.; Vercauteren, E.; Develter, J.; Bammens, R.; Declerck, P.J.; Gils, A. Identification and characterisation of monoclonal antibodies that impair the activation of human thrombin activatable fibrinolysis inhibitor through different mechanisms. Thromb. Haemost. 2011, 106, 90–101. [Google Scholar] [CrossRef]
- Buelens, K.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S.; Gils, A.; Declerck, P.J. Generation and characterization of inhibitory nanobodies towards thrombin activatable fibrinolysis inhibitor. J. Thromb. Haemost. 2010, 8, 1302–1312. [Google Scholar] [CrossRef]
- Zhou, X.H.; Hendrickx, M.L.V.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S.; Declerck, P.J. Generation and in vitro characterisation of inhibitory nanobodies towards plasminogen activator inhibitor 1. Thromb. Haemost. 2016, 116, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Wyseure, T.; Rubio, M.; Denorme, F.; de Lizarrondo, S.M.; Peeters, M.; Gils, A.; De Meyer, S.F.; Vivien, D.; Declerck, P.J. Innovative thrombolytic strategy using a heterodimer diabody against TAFI and PAI-1 in mouse models of thrombosis and stroke. Blood 2015, 125, 1325–1332. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. Targeting PAI-1 in Cardiovascular Disease: Structural Insights Into PAI-1 Functionality and Inhibition. Front. Cardiovasc. Med. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.R.; Booth, N.A.; Mutch, N.J. The antifibrinolytic function of factor XIII is exclusively expressed through alpha(2)-antiplasmin cross-linking. Blood 2011, 117, 6371–6374. [Google Scholar] [CrossRef] [PubMed]
- Kumada, T.; Abiko, Y. Physiological-role of alpha-2-plasmin inhibitor in rats. Thromb. Res. 1984, 36, 153–163. [Google Scholar] [CrossRef]
- Reed, G.L.; Matsueda, G.R.; Haber, E. Inhibition of clot-bound alpha-2-antiplasmin enhances invivo thrombolysis. Circulation 1990, 82, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Tamaki, T.; Ichinose, A. Cross-linking of alpha-2-plasmin inhibitor to fibrin catalyzed by activated fibrin-stabilizing factor. J. Biol. Chem. 1982, 257, 14767–14772. [Google Scholar]
- Kimura, S.; Aoki, N. Cross-linking site in fibrinogen for alpha-2-plasmin inhibitor. J. Biol. Chem. 1986, 261, 5591–5595. [Google Scholar] [CrossRef]
- Kimura, S.; Tamaki, T.; Aoki, N. Acceleration of fibrinolysis by the n-terminal peptide of alpha-2-plasmin inhibitor. Blood 1985, 66, 157–160. [Google Scholar] [CrossRef]
- Nikolajsen, C.L.; Scavenius, C.; Enghild, J.J. Human Complement C3 Is a Substrate for Transglutaminases. A Functional Link between Non-Protease-Based Members of the Coagulation and Complement Cascades. Biochemistry 2012, 51, 4735–4742. [Google Scholar] [CrossRef]
- Hess, K.; Alzahrani, S.H.; Price, J.F.; Strachan, M.W.; Oxley, N.; King, R.; Gamlen, T.; Schroeder, V.; Baxter, P.D.; Ajjan, R.A. Hypofibrinolysis in type 2 diabetes: The role of the inflammatory pathway and complement C3. Diabetologia 2014, 57, 1737–1741. [Google Scholar] [CrossRef]
- King, R.; Tiede, C.; Simmons, K.; Fishwick, C.; Tomlinson, D.; Ajjan, R. Inhibition of complement C3 and fibrinogen interaction: A potential novel therapeutic target to reduce cardiovascular disease in diabetes. Lancet 2015, 385, S57. [Google Scholar] [CrossRef]
- Santagostino, E.; Young, G.; Carcao, M.; Mannucci, P.M.; Halimeh, S.; Austin, S. A contemporary look at FVIII inhibitor development: Still a great influence on the evolution of hemophilia therapies. Exp. Rev. Hematol. 2018, 11, 87–97. [Google Scholar] [CrossRef]
- Weyand, A.C.; Pipe, S.W. New therapies for hemophilia. Blood 2019, 133, 389–398. [Google Scholar] [CrossRef]
- Beckman, J.D.; Holle, L.A.; Wolberg, A.S. Factor XIII cotreatment with hemostatic agents in hemophilia A increases fibrin alpha-chain crosslinking. J. Thromb. Haemost. 2018, 16, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, L.O.; Lisman, T.; van den Berg, H.M.; Nieuwenhuis, H.K.; Meijers, J.C.M.; Bouma, B.N. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb. Haemost. 2001, 86, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.H.; Petersen, K.U.; Rea, C.J.; Harpell, L.; Powell, S.; Lillicrap, D.; Nesheim, M.E.; Sorensen, B. Solulin increases clot stability in whole blood from humans and dogs with hemophilia. Blood 2012, 119, 3622–3628. [Google Scholar] [CrossRef] [PubMed]
- Hoylaerts, M.; Lijnen, H.R.; Collen, D. Studies on the mechanism of the anti-fibrinolytic action of tranexamic acid. Biochim. Biophys. Acta 1981, 673, 75–85. [Google Scholar] [CrossRef]
- McCormack, P.L. Tranexamic Acid A Review of its Use in the Treatment of Hyperfibrinolysis. Drugs 2012, 72, 585–617. [Google Scholar] [CrossRef]
- Bennett, A.E.; Ingram, G.I.C.; Inglish, P.J. Antifibrinolytic treatment in hemophilia—Controlled trial of prophylaxis with tranexamic acid. Br. J. Haematol. 1973, 24, 83–88. [Google Scholar] [CrossRef]
- Holmstrom, M.; Tran, H.T.T.; Holme, P.A. Combined treatment with APCC (FEIBA (R)) and tranexamic acid in patients with haemophilia A with inhibitors and in patients with acquired haemophilia A—A two-centre experience. Haemophilia 2012, 18, 544–549. [Google Scholar] [CrossRef]
- Ghosh, K.; Shetty, S.; Jijina, F.; Mohanty, D. Role of epsilon amino caproic acid in the management of haemophilic patients with inhibitors. Haemophilia 2004, 10, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Ando, T.; Umemoto, T.; Grp, A. Seizures associated with tranexamic acid for cardiac surgery: A meta-analysis of randomized and non-randomized studies. J. Cardiovasc. Surg. 2017, 58, 633–641. [Google Scholar]
- Dunn, C.J.; Goa, K.L. Tranexamic acid—A review of its use in surgery and other indications. Drugs 1999, 57, 1005–1032. [Google Scholar] [CrossRef] [PubMed]
- Kolev, K.; Longstaff, C. Bleeding related to disturbed fibrinolysis. Br. J. Haematol. 2016, 175, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Groenewold, M.D.; Gribnau, A.J.; Ubbink, D.T. Topical haemostatic agents for skin wounds: A systematic review. BMC Surg. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Milne, A.A. Clinical impact of fibrin sealants. Vox Sang. 2004, 87, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Rousou, J.A. Use of Fibrin Sealants in Cardiovascular Surgery: A Systematic Review. J. Card. Surg. 2013, 28, 238–247. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xiao, L.; Guo, H.; Zhao, G.H.; Ma, J.B. The efficiency and safety of fibrin sealant for reducing blood loss in primary total hip arthroplasty: A systematic review and meta-analysis. Int. J. Surg. 2017, 37, 50–57. [Google Scholar] [CrossRef]
- Carless, P.A.; Henry, D.A. Systematic review and meta-analysis of the use of fibrin sealant to prevent seroma formation after breast cancer surgery. Br. J Surg. 2006, 93, 810–819. [Google Scholar] [CrossRef]
- Rogers, A.C.; Turley, L.P.; Cross, K.S.; McMonagle, M.P. Meta-analysis of the use of surgical sealants for suture-hole bleeding in arterial anastomoses. Br. J. Surg. 2016, 103, 1758–1767. [Google Scholar] [CrossRef]
- Wang, H.S.; Shan, L.C.; Zeng, H.; Sun, M.X.; Hua, Y.Q.; Cai, Z.D. Is fibrin sealant effective and safe in total knee arthroplasty? A meta-analysis of randomized trials. J. Orthop. Surg. Res. 2014, 9, 36. [Google Scholar] [CrossRef][Green Version]
- Somani, S.N.; Moshirfar, M.; Shmunes, K.M.; Ronquillo, Y.C. Comparison and application of commercially available fibrin sealants in ophthalmology. Ocul. Surf. 2020, 18, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.A.; Calcaterra, J.; Johanning, J.M.; Pipinos, I.I.; Cordes, C.M.; Velander, W.H. A totally recombinant human fibrin sealant. J. Surg. Res. 2014, 187, 334–342. [Google Scholar] [CrossRef]
- Chan, L.W.; Wang, X.; Wei, H.; Pozzo, L.D.; White, N.J.; Pun, S.H. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci. Transl. Med. 2015, 7, 277ra29. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.W.; Kim, C.H.; Wang, X.; Pun, S.H.; White, N.J.; Kim, T.H. PolySTAT-modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta Biomater. 2016, 31, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kearney, K.J.; Pechlivani, N.; King, R.; Tiede, C.; Phoenix, F.; Cheah, R.; Macrae, F.L.; Simmons, K.J.; Manfield, I.W.; Smith, K.A.; et al. Affimer proteins as a tool to modulate fibrinolysis, stabilize the blood clot, and reduce bleeding complications. Blood 2019, 133, 1233–1244. [Google Scholar] [CrossRef] [PubMed]


| Modifications | Associated Effects |
|---|---|
| Elevated Fibrinogen concentration | ↑ clottability, ↑ clot density, ↑ resistance to clot lysis [5,66] |
| High Thrombin concentration | ↑ fibre diameter, ↑ clot density, ↑ resistance to clot lysis [67,68] |
| Fibrinogen polymorphisms and splice variants | γ’:↑ clot stiffness, ↓ fibre thickness, ↓ clot permeability, ↑ clot density, ↑ resistance to clot lysis [69,70,71] Fibrinogen 420: ↓ fibrin degradation by plasmin, ↑ resistance to clot lysis [72] BβArg448Lys: ↓ fibre diameter, ↓ permeability, ↑ clot stiffness, ↑ resistance to fibrinolysis [73] |
| Oxidation | ↑ clottability, ↓ fibre thickness, ↓ clot stiffness, ↓ clot permeability, ↑ clot density, ↑ resistance to clot lysis [74,75,76,77] |
| Glycation | ↑ clottability, ↓ clot permeability, ↑ clot density, ↑ resistance to clot lysis [78,79,80,81,82,83] |
| Phosphorylation | ↓ fibre thickness, ↓ resistance to clot lysis [84,85,86,87] |
| Citrullination | ↓ clottability, ↓ fibre thickness, ↓ clot density, ↑ lysis [88,89,90] |
| Acetylation | ↑ fibre thickness, ↓ clot stiffness, ↑ clot permeability, ↓ clot density, ↓ resistance to clot lysis [83,91,92] |
| Homocysteinylation | ↑ clot density, ↑ resistance to clot lysis [93,94] |
| Guanidinylation | ↓ fibre thickness, ↓ clot permeability [95] |
| Carbamylation | ↓ fibre thickness, ↑ clot density, ↓ crosslinking, ↑ resistance to clot lysis [96] |
| Nitration | ↑ clot stiffness, ↑ resistance to clot lysis [97,98] |
| Aspirin | See acetylation |
| Metformin | ↓ crosslinking, ↓ resistance to clot lysis [99] |
| Elevated Lipoprotein concentrations | ↓ clot permeability, ↑ resistance to clot lysis [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaule, T.G.; Ajjan, R.A. Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges. Int. J. Mol. Sci. 2021, 22, 6916. https://doi.org/10.3390/ijms22136916
Gaule TG, Ajjan RA. Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges. International Journal of Molecular Sciences. 2021; 22(13):6916. https://doi.org/10.3390/ijms22136916
Chicago/Turabian StyleGaule, Thembaninkosi G., and Ramzi A. Ajjan. 2021. "Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges" International Journal of Molecular Sciences 22, no. 13: 6916. https://doi.org/10.3390/ijms22136916
APA StyleGaule, T. G., & Ajjan, R. A. (2021). Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges. International Journal of Molecular Sciences, 22(13), 6916. https://doi.org/10.3390/ijms22136916





