The G-Protein-Coupled Membrane Estrogen Receptor Is Present in Horse Cryptorchid Testes and Mediates Downstream Pathways
Abstract
1. Introduction
2. Results
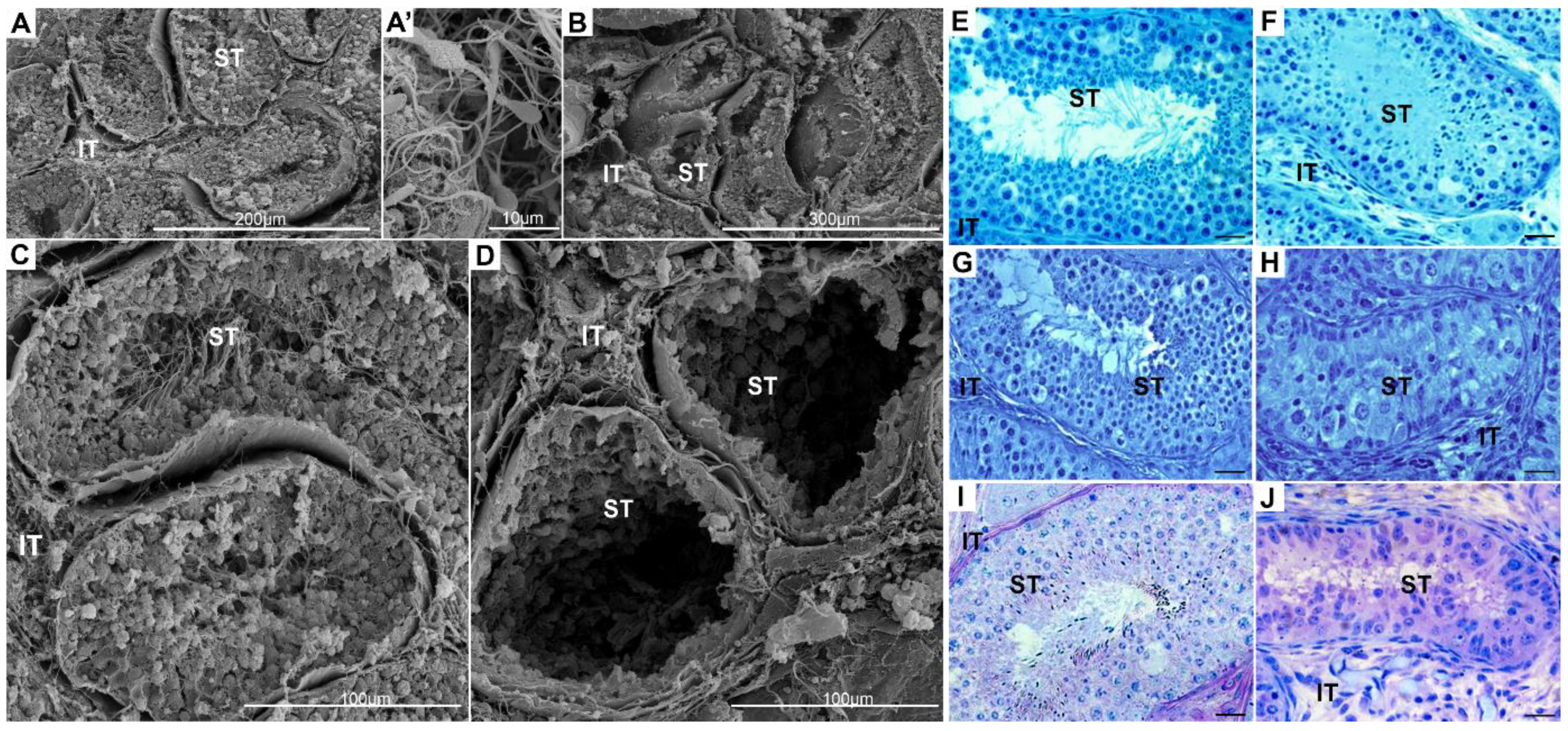
2.1. Topography and Biochemistry of Healthy and Cryptorchid Testes
2.2. GPER Protein Expression and Localization in Healthy and Cryptorchid Testes
2.3. cAMP and Ca2+ Level in Healthy and Cryptorchid Testes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Testis Topography—Scanning Electron Microscope (SEM)
4.3. Testicular Tissue Processing and Histological Staining
4.4. Immunohistochemistry
4.5. Quantitative Analysis of Immunohistochemical Signal
4.6. Western Blot
4.7. cAMP Concentration Measurement
4.8. Ca2+ Concentration Measurement
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barthold, J.S.; Robbins, A.; Wang, Y.; Pugarelli, J.; Mateson, A.; Anand-Ivell, R.; Ivell, R.; McCahan, S.M.; Akins, R.E., Jr. Cryptorchidism in the orl rat is associated with muscle patterning defects in the fetal gubernaculum and altered hormonal signaling. Biol. Reprod. 2014, 91, 41. [Google Scholar] [CrossRef]
- Nation, T.R.; Balic, A.; Southwell, B.R.; Newgreen, D.F.; Hutson, J.M. The hormonal control of testicular descent. Pediatr. Endocrinol. Rev. 2009, 7, 22–31. [Google Scholar] [PubMed]
- Toppari, J.; Virtanen, H.E.; Main, K.M.; Skakkebaek, N.E. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): Environmental connection. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Grinspon, R.P.; Gottlieb, S.; Bedecarrás, P.; Rey, R.A. Anti-Mullerian Hormone and Testicular Function in Prepubertal Boys with Cryptorchidism. Front. Endocrinol. (Lausanne) 2018, 25, 182. [Google Scholar] [CrossRef]
- Laguë, E.; Tremblay, J.J. Estradiol represses insulin-like 3 expression and promoter activity in MA-10 Leydig cells. Toxicology 2009, 258, 101–115. [Google Scholar] [CrossRef]
- Staub, C.; Rauch, M.; Ferrière, F.; Trépos, M.; Dorval-Coiffec, I.; Saunders, P.T.; Cobellis, G.; Flouriot, G.; Saligaut, C.; Jégou, B. Expression of estrogen receptor ESR1 and its 46-kDa variant in the gubernaculum testis. Biol. Reprod. 2005, 73, 703–712. [Google Scholar] [CrossRef]
- Bartlett, J.E.; Washburn, T.; Eddy, E.M.; Korach, K.S.; Temelcos, C.; Hutson, J.M. Early development of the gubernaculum and cremaster sac in estrogen receptor knockout mice. Urol. Res. 2001, 29, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ke, S.; Chen, K.H.; Li, J.H.; Ma, L.; Jiang, X.W. Diethylstilbestrol affects the expression of GPER in the gubernaculum testis. Int. J. Clin. Exp. Pathol. 2015, 8, 7217–7222. [Google Scholar]
- Han, G.; Li, F.; Yu, X.; White, R.E. GPER: A novel target for non-genomic estrogen action in the cardiovascular system. Pharmacol. Res. 2013, 71, 53–60. [Google Scholar] [CrossRef]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in vivo? Front. Endocrinol. (Lausanne) 2020, 31, 148. [Google Scholar] [CrossRef]
- Pupo, M.; Maggiolini, M.; Musti, A.M. GPER Mediates Non-Genomic Effects of Estrogen. Methods Mol. Biol. 2016, 1366, 471–488. [Google Scholar]
- Sandner, F.; Welter, H.; Schwarzer, J.U.; Köhn, F.M.; Urbanski, H.F.; Mayerhofer, A. Expression of the oestrogen receptor GPER by testicular peritubular cells is linked to sexual maturation and male fertility. Andrology 2014, 5, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Zarzycka, M.; Gorowska-Wojtowicz, E.; Tworzydlo, W.; Klak, A.; Kozub, K.; Hejmej, A.; Bilinska, B.; Kotula-Balak, M. Are aryl hydrocarbon receptor and G-protein-coupled receptor 30 involved in the regulation of seasonal testis activity in photosensitive rodent-the bank vole (Myodes glareolus)? Theriogenology 2016, 86, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Kotula-Balak, M.; Pawlicki, P.; Milon, A.; Tworzydlo, W.; Sekula, M.; Pacwa, A.; Gorowska-Wojtowicz, E.; Bilinska, B.; Pawlicka, B.; Wiater, J.; et al. The role of G-protein-coupled membrane estrogen receptor in mouse Leydig cell function-in vivo and in vitro evaluation. Cell Tissue Res. 2018, 374, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Kotula-Balak, M.; Gorowska-Wojtowicz, E.; Milon, A.; Pawlicki, P.; Tworzydlo, W.; Plachno, B.J.; Krakowska, I.; Hejmej, A.; Wolski, J.K.; Bilinska, B. Towards understanding leydigioma: Do G protein-coupled estrogen receptor and peroxisome proliferator-activated receptor regulate lipid metabolism and steroidogenesis in Leydig cell tumors? Protoplasma 2020, 257, 1149–1163. [Google Scholar] [CrossRef]
- Krejčířová, R.; Maňasová, M.; Sommerová, V.; Langhamerová, E.; Rajmon, R.; Maňásková-Postlerová, P.G. Protein-coupled estrogen receptor (GPER) in adult boar testes, epididymis and spermatozoa during epididymal maturation. Int. J. Biol. Macromol. 2018, 116, 113–119. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; Nocito, M.C.; Avena, P.; La Padula, D.; Zavaglia, L.; Pezzi, V. Role of GPER-Mediated Signaling in Testicular Functions and Tumorigenesis. Cells 2020, 17, 2115. [Google Scholar] [CrossRef]
- Gautier, C.; Barrier-Battut, I.; Guénon, I.; Goux, D.; Delalande, C.; Bouraïma-Lelong, H. Implication of the estrogen receptors GPER, ESR1, ESR2 in post-testicular maturations of equine spermatozoa. Gen. Comp. Endocrinol. 2016, 1, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Raeside, J.I. The isolation of estrone sulfate and estradiol-17 beta sulfate from stallion testes. Can. J. Biochem. 1969, 47, 811–815. [Google Scholar] [CrossRef]
- Lemazurier, E.; Moslemi, S.; Sourdaine, P.; Desjardins, I.; Plainfosse, B.; Seralini, G.E. Free and conjugated estrogens and androgens in stallion semen. Gen. Comp. Endocrinol. 2002, 125, 272–282. [Google Scholar] [CrossRef]
- Hejmej, A.; Gorazd, M.; Kosiniak-Kamysz, K.; Wiszniewska, B.; Sadowska, J.; Bilińska, B. Expression of aromatase and oestrogen receptors in reproductive tissues of the stallion and a single cryptorchid visualised by means of immunohistochemistry. Domest. Anim. Endocrinol. 2005, 29, 534–547. [Google Scholar] [CrossRef]
- Nef, S.; Shipman, T.; Parada, L.F. A molecular basis for estrogen-induced cryptorchidism. Dev. Biol. 2000, 224, 354–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bilińska, B.; Kotula-Balak, M.; Gancarczyk, M.; Sadowska, J.; Tabarowski, Z.; Wojtusiak, A. Androgen aromatization in cryptorchid mouse testis. Acta Histochem. 2003, 105, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Agoulnik, A.I. Cryptorchidism—An estrogen spoil? J. Clin. Endocrinol. Metab. 2005, 90, 4975–4977. [Google Scholar] [CrossRef][Green Version]
- Yoshida, R.; Fukami, M.; Sasagawa, I.; Hasegawa, T.; Kamatani, N.; Ogata, T. Association of cryptorchidism with a specific haplotype of the estrogen receptor α gene: Implication for the susceptibility to estrogenic environmental endocrine disruptors. J. Clin. Endocrinol. Metab. 2005, 90, 4716–4721. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Kojima, Y.; Kurokawa, S.; Kamisawa, H.; Kohri, K.; Hayashi, Y. Altered expression and localization of estrogen receptors alpha and beta in the testes of a cryptorchid rat model. Urology 2011, 77, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Yoo, D.Y.; Jo, Y.K.; Kim, G.A.; Chung, J.Y.; Choi, J.H.; Jang, G.; Hwang, I.K. Differential expression of estrogen receptor α and progesterone receptor in the normal and cryptorchid testis of a dog. Lab. Anim. Res. 2016, 32, 128–132. [Google Scholar] [CrossRef]
- Fénichel, P.; Chevalier, N.; Lahlou, N.; Coquillard, P.; Wagner-Mahler, K.; Pugeat, M.; Panaïa-Ferrari, P.; Brucker-Davis, F. Endocrine Disrupting Chemicals Interfere with Leydig Cell Hormone Pathways During Testicular Descent in Idiopathic Cryptorchidism. Front. Endocrinol. (Lausanne) 2019, 9, 786. [Google Scholar] [CrossRef]
- Han, H.; Dong, H.; Chen, Q.; Gao, Y.; Li, J.; Li, W.; Dang, R.; Lei, C. Transcriptomic Analysis of Testicular Gene Expression in Normal and Cryptorchid Horses. Animals 2020, 10, 102. [Google Scholar] [CrossRef]
- Eik-Nes, K.B. Secretion of testosterone by the eutopio and the cryptorchid testes in the same dog. Can. J. Physiol. Pharmacol. 1966, 144, 629–633. [Google Scholar] [CrossRef]
- Jose, M.; Vilar, J.M.; Batista, M.; Carrillo, J.M.; Rubio, M.; Sopena, J.; Álamo, D. Histological, cytogenetic and endocrine evaluation in twenty-five unilateral cryptorchid horses. J. App. Anim. Res. 2018, 46, 441–444. [Google Scholar]
- Schulz, R.W.; Menting, S.; Bogerd, J.; França, L.R.; Vilela, D.A.; Godinho, H.P. Sertoli cell proliferation in the adult testis--evidence from two fish species belonging to different orders. Biol. Reprod. 2005, 73, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.E. Testosterone concentrations in normal and cryptorchid horses. Response to human chorionic gonadotrophin. Anim. Reprod. Sci. 1989, 18, 43–50. [Google Scholar] [CrossRef]
- Claes, A.; Ball, B.A.; Almeida, J.; Corbin, C.J.; Conley, A.J. Serum anti-Mullerian hormone concentrations in stallions: Developmental changes, seasonal variation, and differences between intact stallions, cryptorchid stallions, and geldings. Theriogenology 2013, 79, 1229–1235. [Google Scholar] [CrossRef]
- Raś, A.; Rapacz, A.; Raś-Noryńska, M.; Janowski, T.E. Clinical, hormonal and ultrasonograph approaches to diagnosing cryptorchidism in horses. Pol. J. Vet. Sci. 2010, 13, 473–477. [Google Scholar]
- Raudsepp, T. Genetics of Equine Reproductive Diseases. Vet. Clin. N. Am. Equine Pract. 2020, 36, 395–409. [Google Scholar] [CrossRef]
- Murase, H.; Ochi, A.; Tozaki, T.; Kakoi, H.; Munkhtuul, T.; Kurimoto, S.; Sato, F.; Hada, T.J. A case of equine cryptorchidism with undetectable serum anti-Müllerian hormone. Vet. Med. Sci. 2020, 18, 209–211. [Google Scholar] [CrossRef]
- Tannouz, V.G.S.; Mamprim, M.J.; Lopes, M.D.; Santos-Sousa, C.A.; Souza Junior, P.; Babinski, M.A.; Abidu-Figueiredo, M. Is the right testis more affected by cryptorchidism than the left testis? An ultrasonographic approach in dogs of different sizes and breeds. Folia Morphol. (Warszawa) 2019, 78, 847–852. [Google Scholar] [CrossRef]
- Ramisz, G.; Turek, W.; Chmurska-Gasowska, M.; Rak, A.; Pietsch-Fulbiszewska, A.; Galuszka, A.; Kotula-Balak, M.; Tarasiuk, K. Senescence and adiponectin signaling—Studies in canine testis. Ann. Anat. 2020, 20, 151606. [Google Scholar]
- Seetharam, V.; Hameed, Z.B.; Talengala, S.B.; Thomas, J. Bilateral cryptorchidism with bilateral synchronous abdominal testicular germ cell tumour. BMJ. Case Rep. 2014, 12, bcr2013203085. [Google Scholar] [CrossRef]
- Ugolini, L.W.; Cunha dos Santos, F.C.; Vicensi da Costa, G.; Ramos Oliveira, H.; Folchini, N.; Machado, T.P.; Zannela, R.; Porto Alves, L. Testicular Teratoma in a Unilateral Right-Sided Abdominal Cryptorchid Horse. Acta Sci. Vet. 2019, 47, 409. [Google Scholar] [CrossRef]
- Kaneko, S.; Takamatsu, K. Reactive Blue 2 highlights outline and inner vacuoles of human sperm and spermatid that have undergone protamination, but not somatic cells associated with histones. Int. J. Mol. Sci. 2021. submitted. [Google Scholar]
- Lustofin, K.; Niedbala, P.; Pawlicki, P.; Tuz, R.; Płachno, B.J.; Profaska-Szymik, M.; Galuszka, A.; Stolarczyk, P.; Gorowska-Wojtowicz, E.; Kotula-Balak, M. Senescent cells in rabbit, nutria and chinchilla testes. Animal Reprod. Sci. 2021, 226, 106701. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Szatkowska, M.; Blasiak, J. An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme—Role in Pathogenesis and Therapeutic Perspective. Int. J. Mol. Sci. 2018, 19, 889. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.; Korolchuk, V.I. Nutrient sensing, growth and senescence. FEBS J. 2018, 285, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Setchell, B.P.; Hink, N.T.; Voglmayr, J.K.; Scott, T.W. Amino acids in ram testicular fluid and semen and their metabolism by spermatozoa. Bioch. J. 1967, 105, 1061–1065. [Google Scholar] [CrossRef]
- Hutson, J.M.; Hasthorpe, S.; Heyns, C.F. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr. Rev. 1997, 18, 259–280. [Google Scholar] [PubMed]
- Arya, M.; Vanha-Perttula, T. Comparison of lectin-staining pattern in testis and epididymis of gerbil, guinea pig, mouse, and nutria. Am. J. Anat. 1986, 1754, 449–469. [Google Scholar] [CrossRef]
- Su, L.; Mruk, D.D.; Lui, W.Y.; Lee, W.M.; Cheng, C.Y. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK). Proc. Natl. Acad. Sci. USA 2011, 108, 19623–19628. [Google Scholar] [CrossRef]
- Wang, X.; Li, S. Protein mislocalization: Mechanisms, functions and clinical applications in cancer. Biochim. Biophys Acta 2014, 1846, 13–25. [Google Scholar] [CrossRef]
- Hejmej, A.; Bilińska, B. The effects of cryptorchidism on the regulation of steroidogenesis and gap junctional communication in equine testes. Endokrynol. Pol. 2008, 59, 112–128. [Google Scholar]
- Gizejewski, Z. Effect of season on characteristics of red deer /Cervus elaphus L./semen collected using modified artificial vagina. Reprod. Biol. 2004, 4, 51–66. [Google Scholar] [PubMed]
- Kotula-Balak, M.; Lenartowicz, M.; Kowal, M.; Styrna, J.; Bilińska, B. Testicular morphology and expression of aromatase in testes of mice with the mosaic mutation (Atp7a mo-ms). Theriogenology 2007, 67, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M. Environmental/lifestyle effects on spermatogenesis. Phil. Trans. Royal. Soc. Lond. Biol. Sci. 2010, 365, 1697–1712. [Google Scholar] [CrossRef]
- Hejmej, A.; Kotula-Balak, M.; Chojnacka, K.; Kuras, P.; Lydka-Zarzycka, M.; Bilinska, B. Photoperiod-Dependent Effects of 4-tert-Octylphenol on Adherens and Gap Junction Proteins in Bank Vole Seminiferous Tubules. Int. J. Endocrinol. 2013, 2013, 134589. [Google Scholar] [CrossRef] [PubMed]
- Carreau, S.; Hess, R.A. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1517–1535. [Google Scholar] [CrossRef]
- Gill, W.B.; Schumacher, G.F.; Bibbo, M.; Straus, F.H., 2nd; Schoenberg, H.W. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J. Urol. 1979, 122, 36–39. [Google Scholar] [CrossRef]
- Hess, R.A. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 2003, 9, 52. [Google Scholar] [CrossRef]
- Claus, R.; Dimmick, M.A.; Gimenez, T.; Hudson, L.W. Estrogens and prostaglandin F2a in the semen and blood plasma of stallions. Theriogenology 1992, 38, 687–693. [Google Scholar] [CrossRef]
- Aldahhan, R.A.; Stanton, P.G. Heat stress response of somatic cells in the testis. Mol. Cell Endocrinol. 2021, 1, 111216. [Google Scholar] [CrossRef]
- Muldoon, T.G. Regulation of Steroid Hormone Receptor Activity. Endocr. Rev. Fall. 1980, 1, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Milon, A.; Pawlicki, P.; Rak, A.; Mlyczynska, E.; Płachno, B.J.; Tworzydlo, W.; Gorowska-Wojtowicz, E.; Bilinska, B.; Kotula-Balak, M. Telocytes are localized to testis of the bank vole (Myodes glareolus) and are affected by lighting conditions and G-coupled membrane estrogen receptor (GPER) signaling. Gen. Comp. Endocrinol. 2019, 15, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, P.; Hejmej, A.; Milon, A.; Lustofin, K.; Płachno, B.J.; Tworzydlo, W.; Gorowska-Wojtowicz, E.; Pawlicka, B.; Kotula-Balak, M.; Bilinska, B. Telocytes in the mouse testicular interstitium: Implications of G-protein-coupled estrogen receptor (GPER) and estrogen-related receptor (ERR) in the regulation of mouse testicular interstitial cells. Protoplasma 2019, 256, 393–408. [Google Scholar] [CrossRef]
- Lucas, T.F.; Siu, E.R.; Esteves, C.A.; Monteiro, H.P.; Oliveira, C.A.; Porto, C.S.; Lazari, M.F. 17beta-estradiolinduces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol. Reprod. 2008, 78, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.C.; Chen, Z.J.; Liu, H.Y.; Zhang, K.S.; Liu, H.; Huang, H.B.; Zhang, G.; Wong, C.K.; Giesy, J.P.; Du, J.; et al. Involvement of activating ERK1/2 through G protein coupled receptor 30 and estrogen receptor alpha/beta in low doses of bisphenol A promoting growth of Sertoli TM4 cells. Toxicol. Lett. 2014, 226, 81–89. [Google Scholar] [CrossRef]
- Vaucher, L.; Funaro, M.G.; Mehta, A.; Mielnik, A.; Bolyakov, A.; Prossnitz, E.R.; Schlegel, P.N.; Paduch, D.A. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PLoS ONE 2014, 15, e92425. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 16, 715–726. [Google Scholar] [CrossRef]
- Hawkins, M.B.; Skipper, J.K.; Crews, D.; Thomas, P. Cloning of three estrogen receptor mRNAs from Atlantic croaker. In Proceedings of the Xth International Congress on Hormonal Steroids, Quebec City, QC, Canada, 17–21 June 1998. Abstract 6. [Google Scholar]
- Prossnitz, E.R.; Arterburn, J.B. International Union of Basic and Clinical Pharmacology. XCVII. G Protein–Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol. Rev. 2015, 67, 505–540. [Google Scholar] [CrossRef]
- Duliban, M.; Gorowska-Wojtowicz, E.; Tworzydlo, W.; Rak, A.; Brzoskwinia, M.; Krakowska, I.; Wolski, J.K.; Kotula-Balak, M.; Płachno, B.J.; Bilinska, B. Interstitial Leydig Cell Tumorigenesis-Leptin and Adiponectin Signaling in Relation to Aromatase. Int. J. Mol. Sci. 2020, 21, 3649. [Google Scholar] [CrossRef]
- Gaudet, H.M.; Cheng, S.B.; Christensen, E.M.; Filardo, E.J. The G-protein coupled estrogen receptor, GPER: The inside and inside-out story. Mol. Cell Endocrinol. 2015, 15, 207–219. [Google Scholar] [CrossRef]
- Sharma, G.; Mauvais-Jarvis, F.; Prossnitz, E.R. Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. J. Steroid Biochem. Mol. Biol. 2018, 176, 31–37. [Google Scholar] [CrossRef]
- Mital, P.; Hinton, B.T.; Dufour, J.M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 2011, 84, 851–858. [Google Scholar] [CrossRef]
- Rune, G.M.; Mayr, J.; Neugebauer, H.; Anders, C.; Sauer, H. Pattern of Sertoli cell degeneration in cryptorchid prepubertal testes. Int. J. Androl. 1992, 15, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M. The role of the gubernaculum in the descent and undescent of the testis. Ther. Adv. Urol. 2009, 1, 115–121. [Google Scholar] [CrossRef]
- Xiao, P.-J.; Hu, L.; Li, J.; Lin, W.; Chen, X.; Xu, P. NSSR1 is regulated in testes development and cryptorchidism and promotes the exon 5-included splicing of CREB transcripts. Mol. Endocrinol. 2002, 16, 184–199. [Google Scholar] [CrossRef]
- Manna, P.R.; Dyson, M.T.; Eubank, D.W.; Clark, B.J.; Lalli, E.; Sassone-Corsi, P.; Zeleznik, A.J.; Stocco, D.M. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol. Endocrinol. 2002, 16, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.N.; Gorelick, D.A. Crosstalk between nuclear and G protein-coupled estrogen receptors. Gen. Comp. Endocrinol. 2018, 261, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Tischner, M. Zaburzenia płodności (Reproduction Disturbances). Weterynaryjne i Hodowlane Aspekty Rozrodu koni. Ogier. (Veterinary and Breeding Aspects of Horse Reproduction. Equine); Wydanie 1; Wydawnictwo Uniwersytetu Rolniczego w Krakowie: Krakow, Poland, 2010; Volume 1, pp. 180–205. ISBN 978-83-930023-0-6. [Google Scholar]
- Kotula-Balak, M.; Duliban, M.; Pawlicki, P.; Tuz, R.; Bilinska, B.; Płachno, B.J.; Arent, Z.J.; Krakowska, I.; Tarasiuk, K. The meaning of non-classical estrogen receptors and peroxisome proliferator-activated receptor for boar Leydig cell of immature testis. Acta Histochem. 2020, 122, 151526. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowski, M.; Pardyak, L.; Pawlicki, P.; Galuszka, A.; Profaska-Szymik, M.; Plachno, B.J.; Kantor, S.; Duliban, M.; Kotula-Balak, M. The G-Protein-Coupled Membrane Estrogen Receptor Is Present in Horse Cryptorchid Testes and Mediates Downstream Pathways. Int. J. Mol. Sci. 2021, 22, 7131. https://doi.org/10.3390/ijms22137131
Witkowski M, Pardyak L, Pawlicki P, Galuszka A, Profaska-Szymik M, Plachno BJ, Kantor S, Duliban M, Kotula-Balak M. The G-Protein-Coupled Membrane Estrogen Receptor Is Present in Horse Cryptorchid Testes and Mediates Downstream Pathways. International Journal of Molecular Sciences. 2021; 22(13):7131. https://doi.org/10.3390/ijms22137131
Chicago/Turabian StyleWitkowski, Maciej, Laura Pardyak, Piotr Pawlicki, Anna Galuszka, Magdalena Profaska-Szymik, Bartosz J. Plachno, Samuel Kantor, Michal Duliban, and Malgorzata Kotula-Balak. 2021. "The G-Protein-Coupled Membrane Estrogen Receptor Is Present in Horse Cryptorchid Testes and Mediates Downstream Pathways" International Journal of Molecular Sciences 22, no. 13: 7131. https://doi.org/10.3390/ijms22137131
APA StyleWitkowski, M., Pardyak, L., Pawlicki, P., Galuszka, A., Profaska-Szymik, M., Plachno, B. J., Kantor, S., Duliban, M., & Kotula-Balak, M. (2021). The G-Protein-Coupled Membrane Estrogen Receptor Is Present in Horse Cryptorchid Testes and Mediates Downstream Pathways. International Journal of Molecular Sciences, 22(13), 7131. https://doi.org/10.3390/ijms22137131







