From Supramolecular Hydrogels to Multifunctional Carriers for Biologically Active Substances
Abstract
:1. Introduction
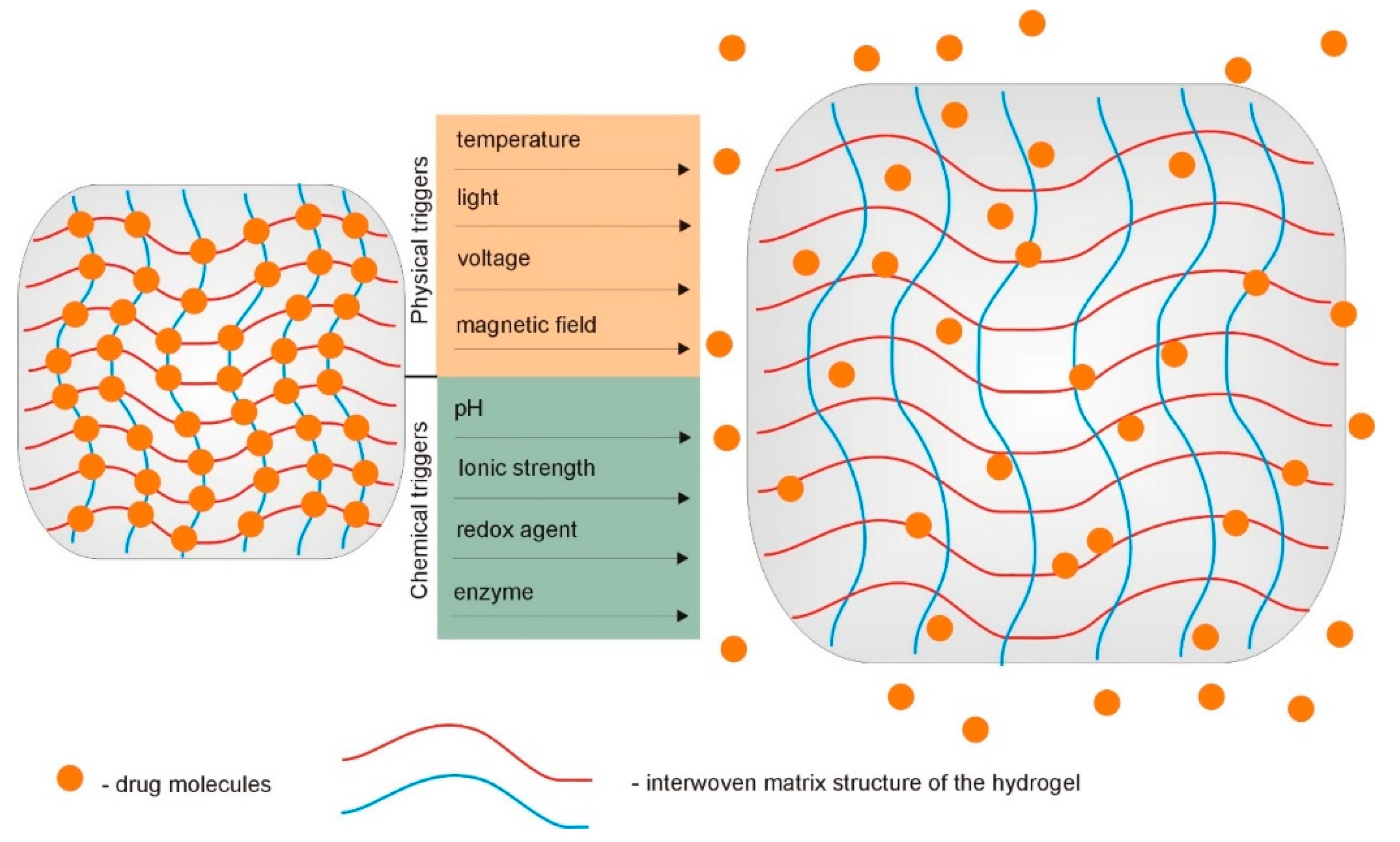
2. Interactions in Supramolecular Hydrogels
2.1. Hydrogen Bonds
2.2. Hydrophobic Interactions
2.3. Ionic Interactions
2.4. Metal–Ligand Coordination
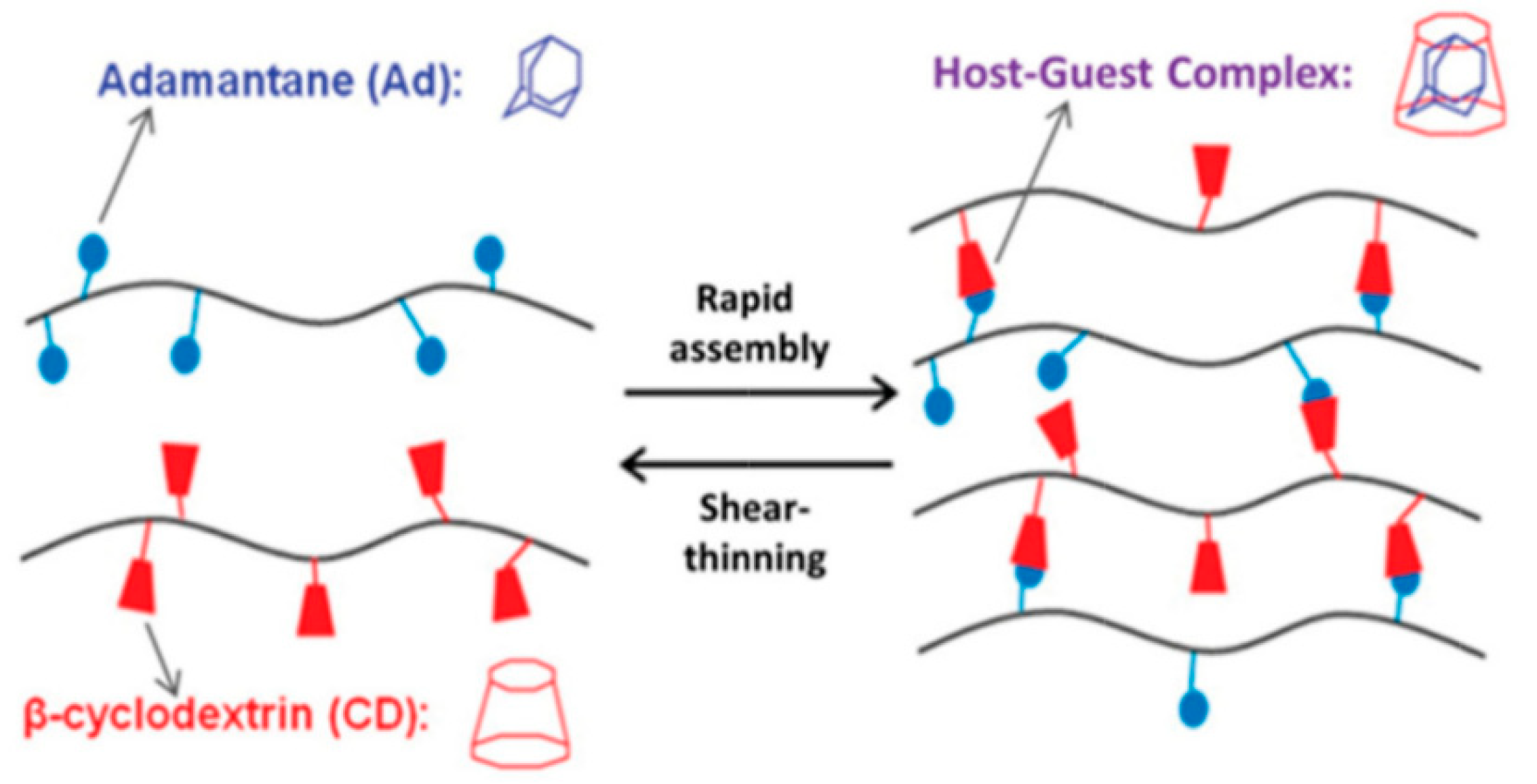
2.5. Host–Guest Interactions
3. Supramolecular Hydrogels as Carriers for Biologically Active Substances
3.1. Supramolecular Hydrogels as Drug Delivery Systems
3.1.1. Supramolecular Hydrogels for Cancer Drug Delivery
3.1.2. Supramolecular Anti-Inflammatory Hydrogels
3.2. Supramolecular Hydrogels for Antimicrobial Properties
3.2.1. Supramolecular Hydrogels with Antibacterial Activity
3.2.2. Supramolecular Hydrogels for HIV Antiretroviral Therapy
3.3. Supramolecular Hydrogels for Controlled Gene Delivery
3.4. Supramolecular Hydrogels in Tissue Engineering
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, J.; Lin, Q.; Xue, K.; Loh, X. Recent advances in supramolecular hydrogels for biomedical applications. Mater. Today Adv. 2019, 3, 100021. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. Physicochemical Properties and the Gelation Process of Supramolecular Hydrogels: A Review. Gels 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Sri, M.B.; Ashok, V.; Arkendu, C. As A Review on Hydrogels as Drug Delivery in the Pharmaceutical Field. Int. J. Pharm. Chem. Sci. 2012, 1, 642–661. [Google Scholar]
- Sandeep, C.; Harikumar, S.L.; Kanupriya, A. Hydrogels: A Smart Drug Delivery System. Int. J. Res. Pharm. Chem. 2012, 2, 603–614. [Google Scholar]
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional Hydrogels and Their Application in Drug Delivery, Biosensors, and Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Dong, R.; Pang, Y.; Su, Y.; Zhu, X. Supramolecular hydrogels: Synthesis, properties and their biomedical applications. Biomater. Sci. 2015, 3, 937–954. [Google Scholar] [CrossRef]
- Su, E.; Yurtsever, M.; Okay, O. A Self-Healing and Highly Stretchable Polyelectrolyte Hydrogel via Cooperative Hydrogen Bonding as a Superabsorbent Polymer. Macromolecules 2019, 52, 3257–3267. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Xu, B. Supramolecular Hydrogels Made of Basic Biological Building Blocks. Chem. Asian J. 2014, 9, 1446–1472. [Google Scholar] [CrossRef] [Green Version]
- Hoque, J.; Sangaj, N.; Varghese, S. Stimuli-Responsive Supramolecular Hydrogels and Their Applications in Regenerative Medicine. Macromol. Biosci. 2018, 19, e1800259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xia, Y.; Zhang, D.; Sun, X.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Hydrogen-Bonding Reinforced Injectable Hydrogels: Application As a Thermo-Triggered Drug Controlled-Release System. ACS Appl. Polym. Mater. 2020, 2, 1587–1596. [Google Scholar] [CrossRef]
- Chen, M.H.; Chung, J.J.; Mealy, J.E.; Zaman, S.; Li, E.C.; Arisi, M.F.; Atluri, P.; Burdick, J.A. Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery. Macromol. Biosci. 2019, 19, e1800248. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Yang, J.; Zheng, Q.; Wu, N.; Lv, Z.; Jiang, Z. Ultra-stretchable hydrogels with hierarchical hydrogen bonds. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T.; et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, M.; Wu, J. Hydrogels with Self-Healing Attribute. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar]
- Li, F.; Tang, J.; Geng, J.; Luo, D.; Yang, D. Polymeric DNA hydrogel: Design, synthesis and applications. Prog. Polym. Sci. 2019, 98, 101163. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Liu, H.; Wang, L. Tailoring DNA Self-assembly to Build Hydrogels. Top. Curr. Chem. 2020, 378, 32. [Google Scholar] [CrossRef]
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, e1800221. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yang, H.; Zhu, H.; Jiang, L.; Yang, H. Self-healing gelatin-based shape memory hydrogels via quadruple hydrogen bonding and coordination crosslinking for controlled delivery of 5-fluorouracil. J. Biomater. Sci. Polym. Ed. 2020, 31, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Cortajarena, A.L.; Grove, T.Z. Protein-based Engineered Nanostructures. Adv. Exp. Med. Biol. 2016, 940, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, S.; Yan, T.; Fan, X.; Li, F.; Yang, X.; Ren, B.; Xu, J.; Liu, J. Injectable and fast self-healing protein hydrogels. Soft Matter 2019, 15, 7583–7589. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Xue, B.; Gao, X.; Qin, M.; Wang, W.; Chen, B.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, C.; Wang, B.; Sui, X.; Wang, L.; Yan, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Self-healing and injectable polysaccharide hydrogels with tunable mechanical properties. Cellulose 2018, 25, 559–571. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wang, L.; Dang, J.; Mi, L.; Han, J.; Mao, M.; Chen, B.; Liu, H. Reconfigurable and tunable photo-controlled hydrogel using hydrogen bonding to drive molecule self-assembly and cross-linking. J. Mater. Sci. 2020, 55, 14740–14750. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.; Liu, W.; Huang, J.; Qiu, X. Synthesis of highly conductive hydrogel with high strength and super toughness. Polymer 2020, 202, 122643. [Google Scholar] [CrossRef]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, e1800313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.G.; Nam, M.G.; Yoo, P.J.; Kim, J.-H. Hydrogen bonding-based strongly adhesive coacervate hydrogels synthesized using poly(N-vinylpyrrolidone) and tannic acid. Soft Matter 2019, 15, 785–791. [Google Scholar] [CrossRef]
- Song, G.; Zhang, L.; He, C.; Fang, D.-C.; Whitten, P.G.; Wang, H. Facile Fabrication of Tough Hydrogels Physically Cross-Linked by Strong Cooperative Hydrogen Bonding. Macromolecules 2013, 46, 7423–7435. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, H.; Yi, P.; Sun, S.; Li, Y.; Liu, X.; Li, G. Zwitterionic hydrogels formed via quadruple hydrogen-bonds with ultra-fast room-temperature self-healing ability. Mater. Lett. 2020, 269, 127665. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, N.; Sun, D.; Wang, Y.; Chen, X.; Zhu, S.; Zhang, L. A fast UV-curable PU-PAAm hydrogel with mechanical flexibility and self-adhesion for wound healing. RSC Adv. 2020, 10, 4907–4915. [Google Scholar] [CrossRef]
- Fredrick, R.; Podder, A.; Viswanathan, A.; Bhuniya, S. Synthesis and characterization of polysaccharide hydrogel based on hydrophobic interactions. J. Appl. Polym. Sci. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Maeda, T. Structures and Applications of Thermoresponsive Hydrogels and Nanocomposite-Hydrogels Based on Copolymers with Poly (Ethylene Glycol) and Poly (Lactide-Co-Glycolide) Blocks. Bioengineering 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mihajlovic, M.; Staropoli, M.; Appavou, M.-S.; Wyss, H.M.; Pyckhout-Hintzen, W.; Sijbesma, R.P. Tough Supramolecular Hydrogel Based on Strong Hydrophobic Interactions in a Multiblock Segmented Copolymer. Macromolecules 2017, 50, 3333–3346. [Google Scholar] [CrossRef]
- Deng, Y.; Hussain, I.; Kang, M.; Li, K.; Yao, F.; Liu, S.; Fu, G. Self-recoverable and mechanical-reinforced hydrogel based on hydrophobic interaction with self-healable and conductive properties. Chem. Eng. J. 2018, 353, 900–910. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Mihajlovic, M.; Dankers, P.Y.W.; Masereeuw, R.; Sijbesma, R.P. Carbon Nanotube Reinforced Supramolecular Hydrogels for Bioapplications. Macromol. Biosci. 2018, 19, e1800173. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.Y.; Lee, B.; Kang, T.W.; Noh, J.H.; Kim, M.J.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. Electrostatically Interactive Injectable Hydrogels for Drug Delivery. Tissue Eng. Regen. Med. 2018, 15, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, Y.; Yang, B.; Guo, F.; Hou, X.-J. Ionic effects on the mechanical and swelling properties of a poly(acrylic acid/acrylamide) double crosslinking hydrogel. New J. Chem. 2018, 42, 9151–9158. [Google Scholar] [CrossRef]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Inamdar, N.; Mourya, V.K. Chitosan and anionic polymers—Complex formation and applications. In Polysaccharides: Development, Properties and Applications; Tiwari, A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 333–377. [Google Scholar]
- Haag, S.L.; Bernards, M.T. Polyampholyte Hydrogels in Biomedical Applications. Gels 2017, 3, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Li, C.; Huang, R.; Su, R.; Qi, W.; He, Z. Amphiphilic hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 2899–2910. [Google Scholar] [CrossRef]
- Su, E.; Okay, O. Polyampholyte hydrogels formed via electrostatic and hydrophobic interactions. Eur. Polym. J. 2017, 88, 191–204. [Google Scholar] [CrossRef]
- Shi, L.; Ding, P.; Wang, Y.; Zhang, Y.; Ossipov, D.; Hilborn, J. Self-Healing Polymeric Hydrogel Formed by Metal–Ligand Coordination Assembly: Design, Fabrication, and Biomedical Applications. Macromol. Rapid Commun. 2019, 40, e1800837. [Google Scholar] [CrossRef]
- Zeng, L.; Song, M.; Gu, J.; Xu, Z.; Xue, B.; Li, Y.; Cao, Y. A Highly Stretchable, Tough, Fast Self-Healing Hydrogel Based on Peptide–Metal Ion Coordination. Biomimetics 2019, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, P.; Pageni, P.; Tang, C. Recent Advances in Metal-Containing Polymer Hydrogels. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.L.; Song, Y.; Zheng, Q. Metal-Coordination Complexes Mediated Physical Hydrogels with High Toughness, Stick–Slip Tearing Behavior, and Good Processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]
- Quan, W.-Y.; Hu, Z.; Liu, H.-Z.; Ouyang, Q.-Q.; Zhang, D.-Y.; Li, S.-D.; Yang, Z.-M. Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules 2019, 24, 2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Xue, B.; Fan, Q.; Tao, R.; Wang, C.; Wang, X.; Li, Y.; Qin, M.; Wang, W.; Chen, B.; et al. Molecular engineering of metal coordination interactions for strong, tough, and fast-recovery hydrogels. Sci. Adv. 2020, 6, eaaz9531. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Li, Z.; Xie, X.; Zhang, Z.-Y.; Bian, L. Bisphosphonate-based nanocomposite hydrogels for biomedical applications. Bioact. Mater. 2020, 5, 819–831. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Liu, S.; Yao, F.; Oderinde, O.; Fu, G. Hydroxyethyl cellulose-based self-healing hydrogels with enhanced mechanical properties via metal-ligand bond interactions. Eur. Polym. J. 2018, 100, 219–227. [Google Scholar] [CrossRef]
- Mantooth, S.M.; Munoz-Robles, B.G.; Webber, M.J. Dynamic Hydrogels from Host-Guest Supramolecular Interactions. Macromol. Biosci. 2019, 19, e1800281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, H.W.; Kocken, J.M.M.; Morgan, F.; Malheiro, A.; Zoetebier, B.; Karperien, M.; Wieringa, P.A.; Dijkstra, P.J.; Moroni, L.; Baker, M.B. Multivalency Enables Dynamic Supramolecular Host–Guest Hydrogel Formation. Biomacromolecules 2020, 21, 2208–2217. [Google Scholar] [CrossRef] [Green Version]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Biofunctional hydrogels based on host–guest interactions. Polym. J. 2020, 52, 839–859. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Liu, Y. Cyclodextrin-Based Supramolecular Hydrogel. In Handbook of Macrocyclic Supramolecular Assembly; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–26. [Google Scholar]
- Omtvedt, L.A.; Dalheim, M.Ø.; Nielsen, T.T.; Larsen, K.L.; Strand, B.L.; Aachmann, F.L. Efficient Grafting of Cyclodextrin to Alginate and Performance of the Hydrogel for Release of Model Drug. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, R.; Feng, Y.; Zhang, L.; Wang, C.; Song, J.; Jiao, T.; Zhou, J.; Peng, Q. Self-Assembled Hydrogels Based on Poly-Cyclodextrin and Poly-Azobenzene Compounds and Applications for Highly Efficient Removal of Bisphenol A and Methylene Blue. ACS Omega 2018, 3, 11663–11672. [Google Scholar] [CrossRef]
- Tarannum, N.; Kumar, D. Synthesis, characterization and applications of copolymer of β–cyclodextrin: A review. J. Polym. Res. 2020, 27, 1–30. [Google Scholar] [CrossRef]
- Das, D.; Assaf, K.I.; Nau, W.M. Applications of Cucurbiturils in Medicinal Chemistry and Chemical Biology. Front. Chem. 2019, 7, 619. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Thakur, V.K.; Arotiba, O.A. History, Classification, Properties and Application of Hydrogels: An Overview. In Hydrogels; Springer: Berlin/Heidelberg, Germany, 2018; pp. 29–50. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Tripathi, P.; Kumar, A.; Jain, P.K.; Patel, J.R. Carbomer gel bearing methotrexate loaded lipid nanocontainers shows improved topical delivery intended for effective management of psoriasis. Int. J. Biol. Macromol. 2018, 120, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Carafa, M.; Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Di Meo, C.; Matricardi, P.; Alhaique, F.; Coviello, T. A New Vesicle-loaded Hydrogel System Suitable for Topical Applications: Preparation and Characterization. J. Pharm. Pharm. Sci. 2011, 14, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuramalingam, K.; Choi, S.I.; Hyun, C.; Kim, Y.M.; Cho, M. β-Glucan-Based Wet Dressing for Cutaneous Wound Healing. Adv. Wound Care 2019, 8, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.; Li, G.; Huang, J.; Zhang, J.; Lu, M.; Lu, W.; Huan, J.; Huang, Q. Development of chitosan-collagen hydrogel incorporated with lysostaphin (CCHL) burn dressing with anti-methicillin-resistant Staphylococcus aureus and promotion wound healing properties. Drug Deliv. 2010, 18, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Shixuan, C.; Zhang, M.; Chen, Y.; Wang, X.; Zhang, L.; Tian, Z.; Yan, Y.; Li, Q.; Zhong, W.; et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci. Rep. 2016, 5, 18104. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, R.K.; Maitra, U. Supramolecular gels ‘in action’. J. Mater. Chem. 2009, 19, 6649–6687. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saboktakin, M.R.; Tabatabaei, R.M. Supramolecular hydrogels as drug delivery systems. Int. J. Biol. Macromol. 2015, 75, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Knipe, J.M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, S.A.; Brüßler, J.; Alawak, M.; El-Sayed, M.M.H.; Bakowsky, U.; Shoeib, T. Chemotherapy Based on Supramolecular Chemistry: A Promising Strategy in Cancer Therapy. Pharmaceutics 2019, 11, 292. [Google Scholar] [CrossRef] [Green Version]
- Mayr, J.; Saldías, C.; Díaz, D.D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K. The genetics of hereditary colon cancer. Genes Dev. 2007, 21, 2525–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.-Y.; Tian, Y.; Liu, Z.-J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Nair, P.R. Delivering Combination Chemotherapies and Targeting Oncogenic Pathways via Polymeric Drug Delivery Systems. Polymers 2019, 11, 630. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.-P.; Wang, W.; Wei, C.-D. Bortezomib inhibits cell proliferation in prostate cancer. Exp. Ther. Med. 2015, 10, 1219–1223. [Google Scholar] [CrossRef] [Green Version]
- Alyafee, Y.A.; Al-Aamery, M.; Bawazeer, S.; Almutairi, M.S.; Alghamdi, B.; Alomran, N.; Sheereen, A.; Daghestani, M.; Massadeh, S. Preparation of anastrozole loaded PEG-PLA nanoparticles: Evaluation of apoptotic response of breast cancer cell lines. Int. J. Nanomed. 2017, 13, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Zhang, Y.; Li, D.; Fu, S.; Tang, M.; Wan, L.; Chen, K.; Liu, Z.; Xue, L.; Peng, A.; et al. Discovery and synthesis of novel magnolol derivatives with potent anticancer activity in non-small cell lung cancer. Eur. J. Med. Chem. 2018, 156, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheu, M.-T.; Jhan, H.-J.; Su, C.-Y.; Chen, L.-C.; Chang, C.-E.; Liu, D.-Z.; Ho, H.-O. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointerfaces 2016, 143, 260–270. [Google Scholar] [CrossRef]
- Fong, Y.T.; Chen, C.-H.; Chen, J.-P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, W.; Yu, J.; Song, X.-Y.; Chen, J.; Shi, Y.-P. Tunable Temperature-Responsive Supramolecular Hydrogels Formed by Prodrugs As a Codelivery System. ACS Appl. Mater. Interfaces 2014, 6, 10623–10630. [Google Scholar] [CrossRef] [PubMed]
- Pesoa, J.I.; Rico, M.J.; Rozados, V.R.; Scharovsky, O.G.; Luna, J.A.; Mengatto, L.N. Paclitaxel delivery system based on poly(lactide-co-glycolide) microparticles and chitosan thermo-sensitive gel for mammary adenocarcinoma treatment. J. Pharm. Pharmacol. 2018, 70, 1494–1502. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Guo, J.; Li, L.; Xi, X.; Yu, Y. Biocompatible supramolecular pseudorotaxane hydrogels for controllable release of doxorubicin in ovarian cancer SKOV-3 cells. RSC Adv. 2020, 10, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, G.; Wang, C.; Li, C.; Yao, P. Shear-responsive injectable supramolecular hydrogel releasing doxorubicin loaded micelles with pH-sensitivity for local tumor chemotherapy. Int. J. Pharm. 2017, 530, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vaishagya, K.; Verma, R.K.; Shukla, R. Temperature/pH-Triggered PNIPAM-Based Smart Nanogel System Loaded With Anastrozole Delivery for Application in Cancer Chemotherapy. AAPS PharmSciTech 2019, 20, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Z.; Aslan, H.; Zhang, C.; Yu, M. NIR-responsive reversible phase transition of supramolecular hydrogels for tumor treatment. J. Mater. Chem. B 2020, 8, 6429–6437. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Park, J.Y.; Oh, Y.-K. A Microbial Siderophore-Inspired Self-Gelling Hydrogel for Noninvasive Anticancer Phototherapy. Cancer Res. 2019, 79, 6178–6189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, W.; Zhao, X.-B.; Jiang, K.; Kang, Y.; Chen, J.; Li, B.-J.; Shi, Y.-P. A three-dimensional graphene oxide supramolecular hydrogel for infrared light-responsive cascade release of two anticancer drugs. Chem. Commun. 2016, 52, 14384–14387. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Wei, Y.; Zhao, L. Injectable and Self-Healing Thermosensitive Magnetic Hydrogel for Asynchronous Control Release of Doxorubicin and Docetaxel to Treat Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673. [Google Scholar] [CrossRef]
- Wu, H.; Song, L.; Chen, L.; Zhang, W.; Chen, Y.; Zang, F.; Chen, H.; Ma, M.; Gu, N.; Zhang, Y. Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents post-operative recurrence in a breast cancer model. Acta Biomater. 2018, 74, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Song, S.-C. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 2016, 106, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Zhu, L.; Xue, B.; Zhu, X.; Yan, D. Supramolecular nanoscale drug-delivery system with ordered structure. Natl. Sci. Rev. 2019, 6, 1128–1137. [Google Scholar] [CrossRef]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef]
- Truong, W.T.; Su, Y.; Gloria, D.; Braet, F.; Thordarson, P. Dissolution and degradation of Fmoc-diphenylalanine self-assembled gels results in necrosis at high concentrations in vitro. Biomater. Sci. 2015, 3, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Basu, K.; Baral, A.; Basak, S.; Dehsorkhi, A.; Nanda, J.; Bhunia, D.; Ghosh, S.; Castelletto, V.; Hamley, I.W.; Banerjee, A. Peptide based hydrogels for cancer drug release: Modulation of stiffness, drug release and proteolytic stability of hydrogels by incorporating d-amino acid residue(s). Chem. Commun. 2016, 52, 5045–5048. [Google Scholar] [CrossRef]
- Pu, G.; Ren, C.; Li, N.; Wang, L.; Sun, J. A supramolecular hydrogel for the delivery of bortezomib. RSC Adv. 2014, 4, 50145–50147. [Google Scholar] [CrossRef]
- Ren, C.; Chu, L.; Huang, F.; Yang, L.; Fan, H.; Liu, J.; Yang, C. A novel H2O2 responsive supramolecular hydrogel for controllable drug release. RSC Adv. 2017, 7, 1313–1317. [Google Scholar] [CrossRef] [Green Version]
- Cinar, G.; Ozdemir, A.; Hamsici, S.; Gunay, G.; Dana, A.; Tekinay, A.B.; Guler, M.O. Local delivery of doxorubicin through supramolecular peptide amphiphile nanofiber gels. Biomater. Sci. 2016, 5, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, J.; Cai, Y.; Zhan, J.; Gao, J.; Song, M.; Shi, Y.; Yang, Z. A Glycyrrhetinic Acid-Modified Curcumin Supramolecular Hydrogel for liver tumor targeting therapy. Sci. Rep. 2017, 7, 44210. [Google Scholar] [CrossRef] [PubMed]
- Limón, D.; Amirthalingam, E.; Rodrigues, M.; Halbaut, L.; Andrade, B.; Garduño-Ramírez, M.L.; Amabilino, D.B.; Perez-Garcia, L.; Calpena, A.C. Novel nanostructured supramolecular hydrogels for the topical delivery of anionic drugs. Eur. J. Pharm. Biopharm. 2015, 96, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Vemula, P.K.; Li, A.J.; John, G. Enzyme Catalysis: Tool to Make and Break Amygdalin Hydrogelators from Renewable Resources: A Delivery Model for Hydrophobic Drugs. J. Am. Chem. Soc. 2006, 128, 8932–8938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Song, Z.; Wen, Y.; Xu, H.; Zhu, L.; Feng, R. Transdermal delivery of curcumin-loaded supramolecular hydrogels for dermatitis treatment. J. Mater. Sci. Mater. Med. 2019, 30, 11. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Wall, S. Prevention of antibiotic resistance—An epidemiological scoping review to identify research categories and knowledge gaps. Glob. Health Action 2019, 12, 1756191. [Google Scholar] [CrossRef]
- Hu, B.; Owh, C.; Chee, P.L.; Leow, W.R.; Liu, X.; Wu, Y.-L.; Guo, P.; Loh, X.J.; Chen, X. Supramolecular hydrogels for antimicrobial therapy. Chem. Soc. Rev. 2018, 47, 6917–6929. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Luo, X.-L.; Mo, L.-D.; Su, G.-S.; Huang, J.-P.; Wu, J.-Y.; Su, H.-Z.; Huang, W.-H.; Luo, S.-D.; Ni, Z.-Y. Incidence and types of HIV-1 drug resistance mutation among patients failing first-line antiretroviral therapy. J. Pharmacol. Sci. 2019, 139, 275–279. [Google Scholar] [CrossRef] [PubMed]
- More, M.P.; Chitalkar, R.V.; Bhadane, M.; Dhole, S.D.; Patil, A.G.; Patil, P.; Deshmukh, P.K. Development of graphene-drug nanoparticle based supramolecular self assembled pH sensitive hydrogel as potential carrier for targeting MDR tuberculosis. Mater. Technol. 2018, 34, 324–335. [Google Scholar] [CrossRef]
- Vigier-Carrière, C.; Boulmedais, F.; Schaaf, P.; Jierry, L. Surface-Assisted Self-Assembly Strategies Leading to Supramolecular Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, L.; Yuan, S.; Sun, J.; Li, Z. pH-Responsive Peptide Supramolecular Hydrogels with Antibacterial Activity. Langmuir 2017, 33, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Vegners, R.; Shestakova, I.; Kalvinsh, I.; Ezzell, R.M.; Janmey, P.A. Use of a gel-forming dipeptide derivative as a carrier for antigen presentation. J. Pept. Sci. 1995, 1, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Adler-Abramovich, L.; Zigerson, S.; Mironi-Harpaz, I.; Seliktar, D.; Gazit, E. Self-Assembled Fmoc-Peptides as a Platform for the Formation of Nanostructures and Hydrogels. Biomacromolecules 2009, 10, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An injectable dipeptide–fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J. Mater. Chem. B 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Zhang, Y.-W.; Qin, X.-T.; Liu, L.-P.; Wahid, F.; Zhong, C.; Jia, S.-R. Structure-Dependent Antibacterial Activity of Amino Acid-Based Supramolecular Hydrogels. Colloids Surf. B Biointerfaces 2020, 193, 111099. [Google Scholar] [CrossRef]
- Snigdha, K.; Singh, B.K.; Mehta, A.S.; Tewari, R.; Dutta, P. Self-assembling N -(9-Fluorenylmethoxycarbonyl)- l -Phenylalanine hydrogel as novel drug carrier. Int. J. Biol. Macromol. 2016, 93, 1639–1646. [Google Scholar] [CrossRef]
- Singh, V.; Snigdha, K.; Singh, C.; Sinha, N.; Thakur, A.K. Understanding the self-assembly of Fmoc–phenylalanine to hydrogel formation. Soft Matter 2015, 11, 5353–5364. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Chen, J.; Wei, Z.; Song, C.; Huang, R. N-(9-Fluorenylmethoxycarbonyl)-L-Phenylalanine/nano-hydroxyapatite hybrid supramolecular hydrogels as drug delivery vehicles with antibacterial property and cytocompatibility. J. Mater. Sci. Mater. Med. 2020, 31, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. Self-Delivery Multifunctional Anti-HIV Hydrogels for Sustained Release. Adv. Health Mater. 2013, 2, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Ylä-Herttuala, S. Gene Therapy Used in Cancer Treatment. Biomedicines 2014, 2, 149–162. [Google Scholar] [CrossRef]
- Altaner, C. Gene therapy for cancer (present status). Neoplasma 1995, 42, 209–213. [Google Scholar] [PubMed]
- Liu, G.; Yuan, Q.-J.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Cucchiarini, M. Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery. Polymers 2019, 11, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motoyama, K.; Hayashida, K.; Higashi, T.; Arima, H. Polypseudorotaxanes of pegylated α-cyclodextrin/polyamidoamine dendrimer conjugate with cyclodextrins as a sustained release system for DNA. Bioorganic Med. Chem. 2012, 20, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, H.-B.; Chen, D.-H.; Zhang, L.-M. Novel supramolecular gelation route to in situ entrapment and sustained delivery of plasmid DNA. J. Colloid Interface Sci. 2011, 364, 566–573. [Google Scholar] [CrossRef]
- Li, Z.; Yin, H.; Zhang, Z.; Liu, K.L.; Li, J. Supramolecular Anchoring of DNA Polyplexes in Cyclodextrin-Based Polypseudorotaxane Hydrogels for Sustained Gene Delivery. Biomacromolecules 2012, 13, 3162–3172. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, Y.; Hu, Q.; Guo, Z.; Liu, T.; Xu, J.; Wu, J.-P.; Kirk, T.B.; Ma, D.; Xue, W. Injectable supramolecular hydrogel formed from α-cyclodextrin and PEGylated arginine-functionalized poly(l-lysine) dendron for sustained MMP-9 shRNA plasmid delivery. Acta Biomater. 2017, 49, 456–471. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Loh, X.J.; Chen, K.; Li, Z.; Wu, Y.-L. Targeted and Sustained Corelease of Chemotherapeutics and Gene by Injectable Supramolecular Hydrogel for Drug-Resistant Cancer Therapy. Macromol. Rapid Commun. 2019, 40, e1800117. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Cheng, Y.; Li, D.; Gong, Y.; Liu, J.; Tian, H.; Chen, X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014, 35, 8723–8734. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, J.J.; Hondt, M.D. Tissue engineering and surgery: From translational studies to human trials. Innov. Surg. Sci. 2017, 2, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Castells-Sala, C.; Alemany-Ribes, M.; Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Aloy-Reverté, C.; Caballero-Camino, J.; Márquez-Gil, A.; Semino, C.E. Current applications of tissue engineering in biomedicine. J. Biochips Tiss. Chips 2013, S2. [Google Scholar] [CrossRef]
- Seiffert, S. Supramolecular Polymer Networks and Gels. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2015; Volume 268, p. 288. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, H.J.; Jeong, B. Injectable Polypeptide Thermogel as a Tissue Engineering System for Hepatogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 11568–11576. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Rodell, C.B.; Chen, M.H.; Morrow, M.G.; Anseth, K.S.; Burdick, J.A. Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels. Bioconjug. Chem. 2018, 29, 905–913. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Y.-R.; Song, Y.-F.; Ye, J.; Yang, M.; Xu, B.-B.; Zhang, J.-Y.; Wang, X.; Yu, J.-K. Advances in the Application of Supramolecular Hydrogels for Stem Cell Delivery and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 847. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Park, S.; Jin, X.; Ma, P.X. Rapid Self-Integrating, Injectable Hydrogel for Tissue Complex Regeneration. Adv. Health Mater. 2015, 4, 1491–1495. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, K.; Lin, S.; Xu, Z.; Sun, Y.; Shi, P.; Li, G.; Bian, L. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 2016, 101, 217–228. [Google Scholar] [CrossRef]
- Xu, J.; Feng, Q.; Lin, S.; Yuan, W.; Li, R.; Li, J.; Wei, K.; Chen, X.; Zhang, K.; Yang, Y.; et al. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials 2019, 210, 51–61. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.-A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef]


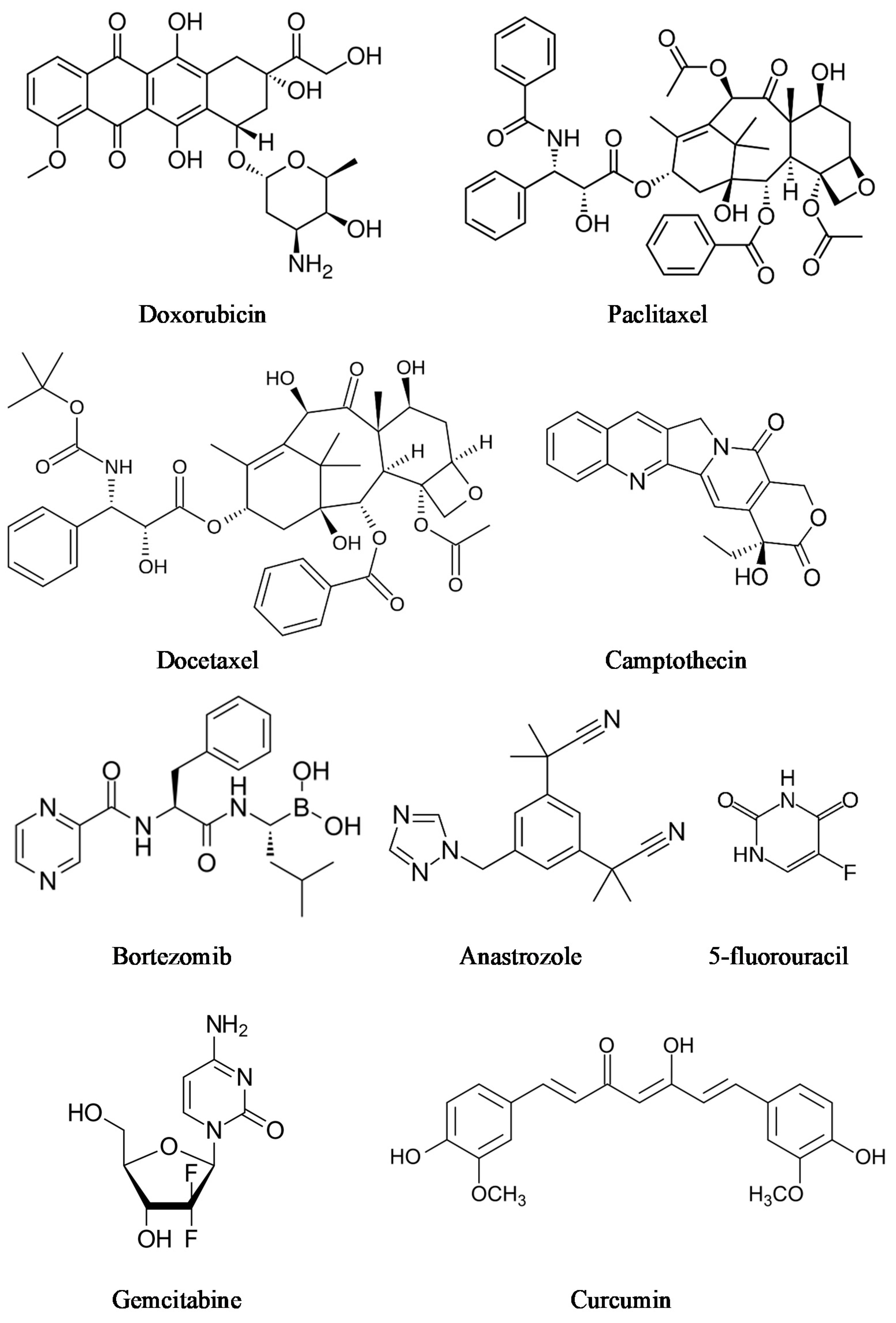
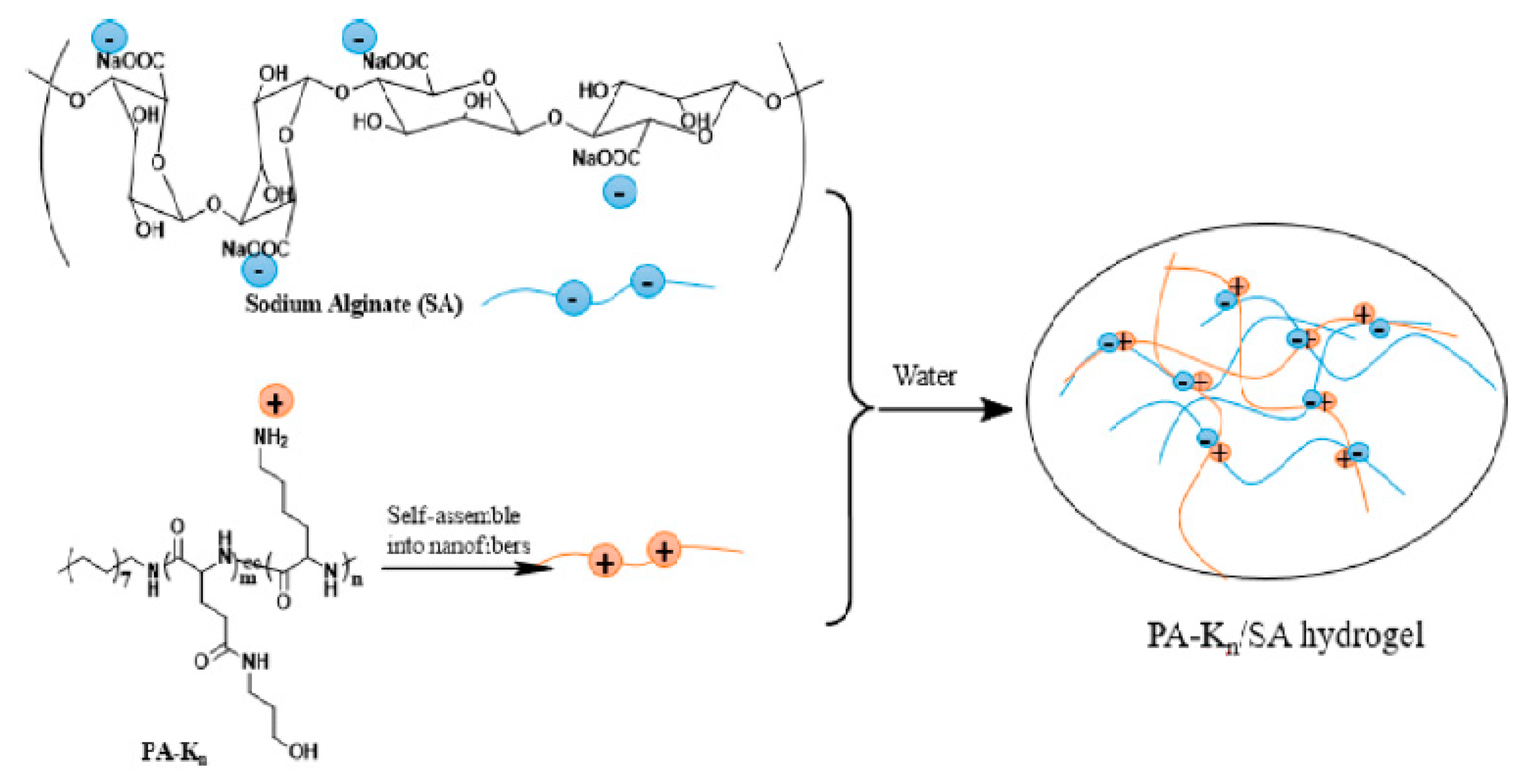
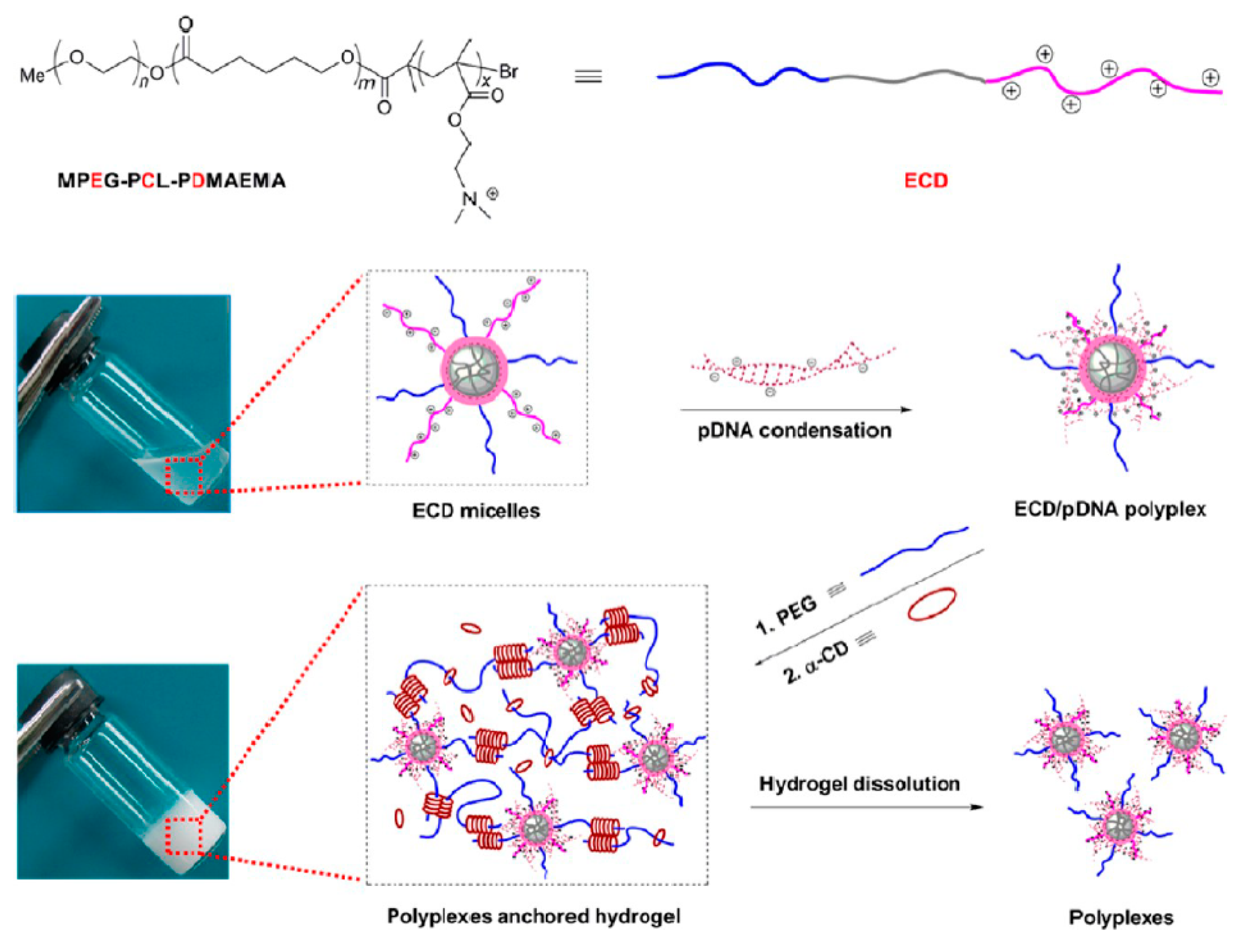
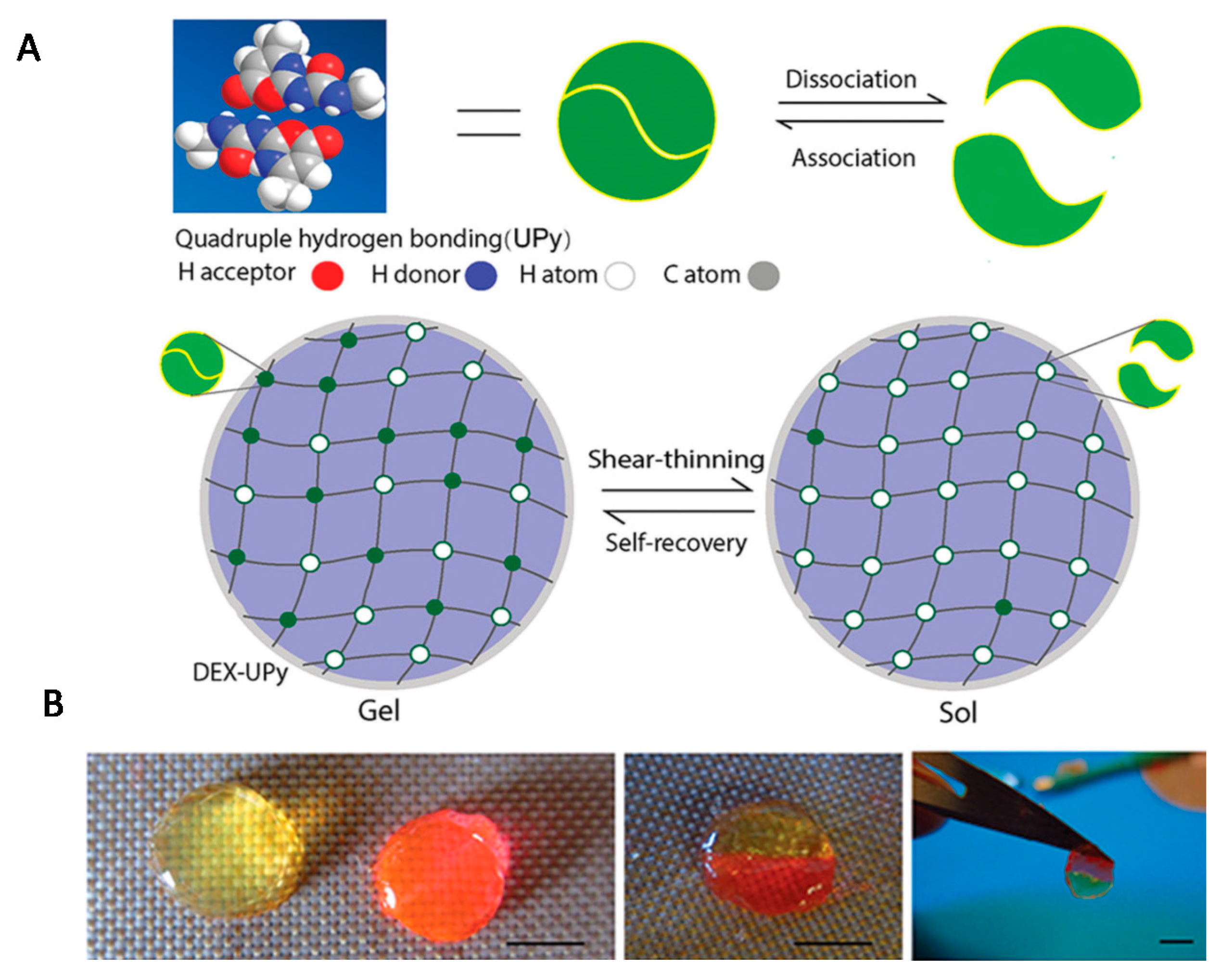
| Interaction | Strength | Description | Example | References |
|---|---|---|---|---|
| Hydrogen bond | weak (mostly about 20 kJ/mol) | interaction between hydrogen atom (e.g., -OH, -NH2) and electronegative atom (e.g., N, O, F) | proteins, nucleic acids, polysaccharides, PVA, PVP, PAAm, UPy | [10,11,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] |
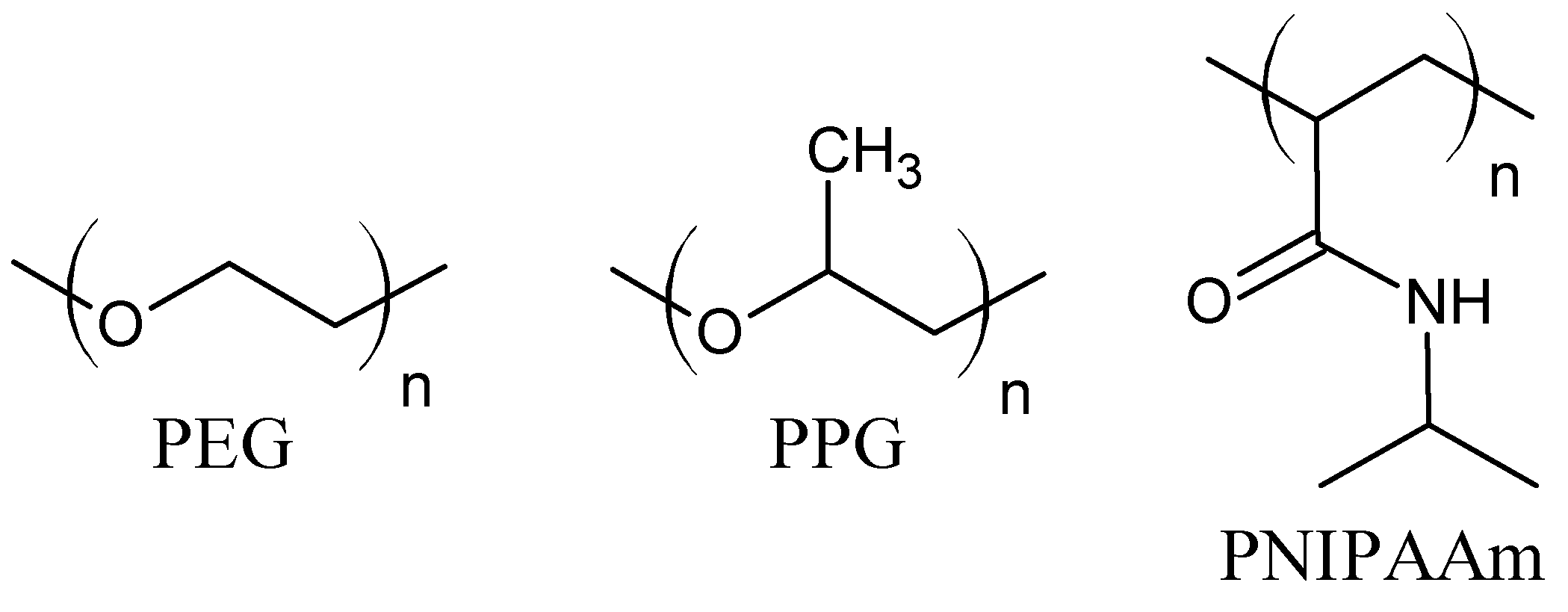
| Hydrophobic interactions | medium–strong (stronger than hydrogen bonds and Van der Waals) | interaction between nonpolar moieties of amphiphilic molecules | proteins, PNIPAAm, PEO-PPO-PEO (Pluronic), copolymers PEG-PLA, PEG-PLGA, PEG-DFA, CMC-NIPAAm | [11,34,35,36,37,38,39,40,41,42] |
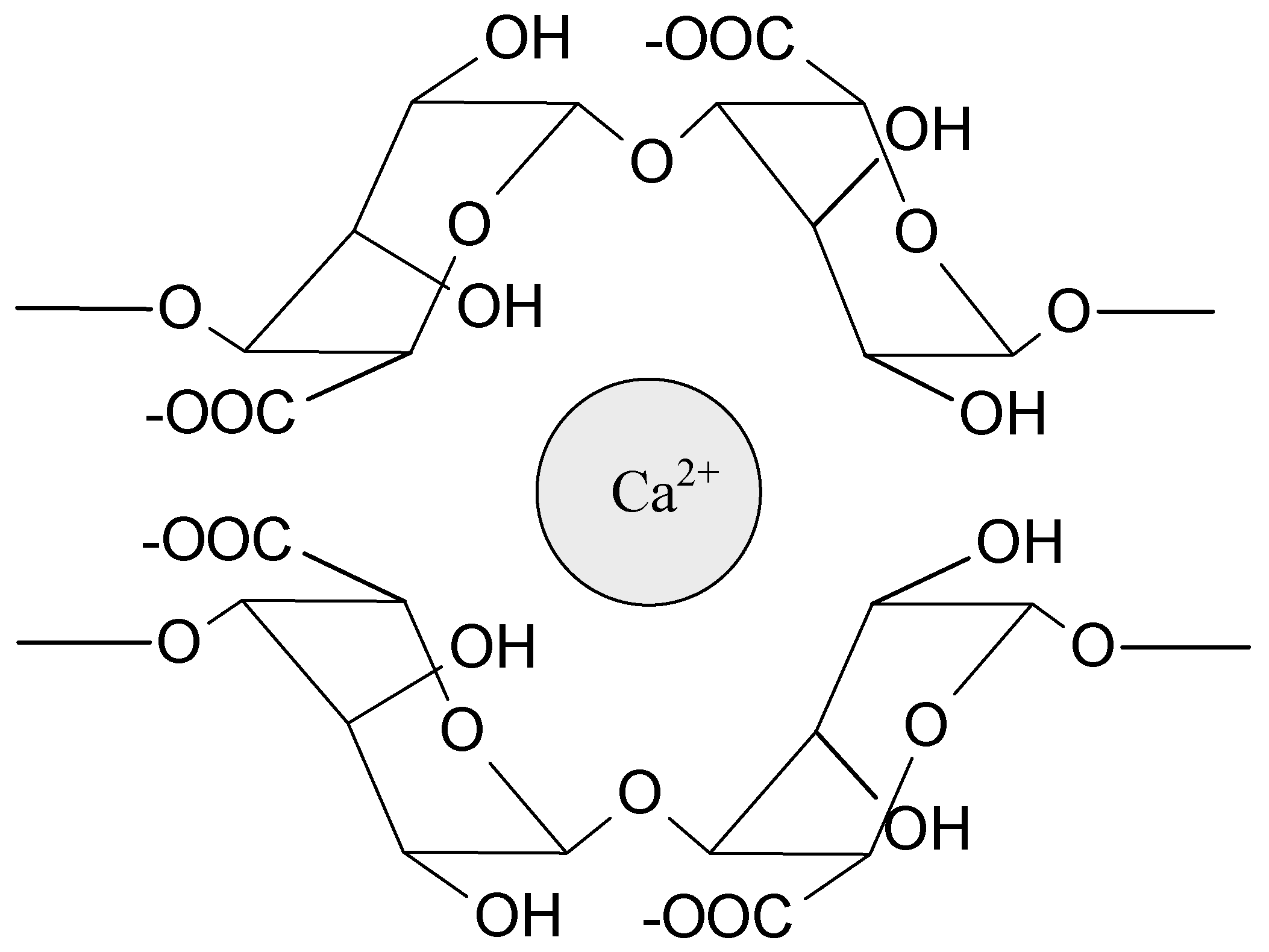
| Ionic interactions | relatively strong | based on electrostatic attraction of oppositely charged ions or dipoles | sodium alginate and Ca2+; chitosan and phosphate salts/carboxylate salts/polysaccharides; sodium alginate and chitosan | [1,36,43,44,45,46,47,48,49] |
| Metal–ligand coordination | strong (comparable to the strength of a covalent bond) | interaction between central metal atom or ion and electron donor group(s) (ligands) | ferric ions and catechol ligands; zinc ions and histidine ligands; calcium ions and bisphophonates | [50,51,52,53,54,55,56,57] |
| Host–guest interaction | wide range of strength | complex hydrogen bonds, Van der Waals, hydrophobic, electrostatic interactions, coordination bonds | cyclodextrins, cucrbiturils, crown ethers, calixarenes, pillarenes | [11,13,36,58,59,60,61,62,63,64,65] |
| Gelation Trigger | Hydrogel | Therapeutic Agent/Drug | Cell Line (In Vitro) | Cancer (In Vivo) | References |
|---|---|---|---|---|---|
| Temperature | HA/PF127 | Doxorubicin/ Docetaxel | CT-26 | Bowel cancer | [92] |
| Temperature | GO-FA/HA-CS-g-PNIPAAm | Doxorubicin | MCF-7 | Breast cancer | [93] |
| Temperature | PEG/α-CD | Camptothecin/5-fluorouracil | - | - | [94] |
| Temperature | PLGA/CS | Paclitaxel | M234-p | Mammary tumor | [95] |
| pH | α-CD/β-CD/PF127 | Doxorubicin | SKOV-3 | - | [96] |
| pH | CS-DA/OP | Doxorubicin | HCT116 | - | [97] |
| pH | CS/PNIPAAm-co-IA | Doxorubicin | MCF-7 | Breast cancer | [98] |
| pH | GC-PF127 | H22 | Breast cancer | [99] | |
| Temperature-pH | PNIPAAm | Anastrozole | MCF-7 | - | [100] |
| Light | Laponite/α-CD | Doxorubicin Near infrared | HepG2 | Liver cancer | [101] |
| Light | HA/GA/iron ions | Near infrared | KB, 4T1/A375 | Breast cancer | [102] |
| Light | GO/PEG/α-CD | Camptothecin/5-fluorouracil Near infrared | A549 | Ascites sarcoma | [103] |
| Magnetic field | Iron oxide magnetic nanoparticles/CS/DF-PEG-DF | Doxorubicin/ Docetaxel | MDA-MB-231 | Breast cancer | [104] |
| Magnetic field | PEGylated iron oxide nanoparticles/α-CD | Paclitaxel/ Doxorubicin | - | Breast cancer | [105] |
| Temperature-magnetic field | Magnetic iron oxide nanoparticles/PPZ | Magnetic heat | U87-MG | Glioblastoma | [106] |
| Hydrogel | Vector | Drug | In Vitro | In Vivo | References |
|---|---|---|---|---|---|
| PEG-α-CD/CD PPRX | pDNA | - | Colon-26 | Male Balb/C mice | [140] |
| PF68-PLL/α-CD | pDNA | - | mouse fibroblast cells 3T3 | - | [141] |
| MPEG-PCL-PDMAEMA/α-CD | pDNA | - | COS-7 | - | [142] |
| MPEG-PLLD-Arg/α-CD | pMMP-9 | - | HNE-1 | Nude mice bearing HNE-1 tumors | [143] |
| MPEG-PCL-PEI-FA/α-CD | pDNA-Nur77 | Paclitaxel | HEK293 H460 | Male Balb/C nude mice, tumor model | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopinska-Wisniewska, J.; De la Flor, S.; Kozlowska, J. From Supramolecular Hydrogels to Multifunctional Carriers for Biologically Active Substances. Int. J. Mol. Sci. 2021, 22, 7402. https://doi.org/10.3390/ijms22147402
Skopinska-Wisniewska J, De la Flor S, Kozlowska J. From Supramolecular Hydrogels to Multifunctional Carriers for Biologically Active Substances. International Journal of Molecular Sciences. 2021; 22(14):7402. https://doi.org/10.3390/ijms22147402
Chicago/Turabian StyleSkopinska-Wisniewska, Joanna, Silvia De la Flor, and Justyna Kozlowska. 2021. "From Supramolecular Hydrogels to Multifunctional Carriers for Biologically Active Substances" International Journal of Molecular Sciences 22, no. 14: 7402. https://doi.org/10.3390/ijms22147402
APA StyleSkopinska-Wisniewska, J., De la Flor, S., & Kozlowska, J. (2021). From Supramolecular Hydrogels to Multifunctional Carriers for Biologically Active Substances. International Journal of Molecular Sciences, 22(14), 7402. https://doi.org/10.3390/ijms22147402








