Regeneration of Bone Defects in a Rabbit Femoral Osteonecrosis Model Using 3D-Printed Poly (Epsilon-Caprolactone)/Nanoparticulate Willemite Composite Scaffolds
Abstract
:1. Introduction
2. Results
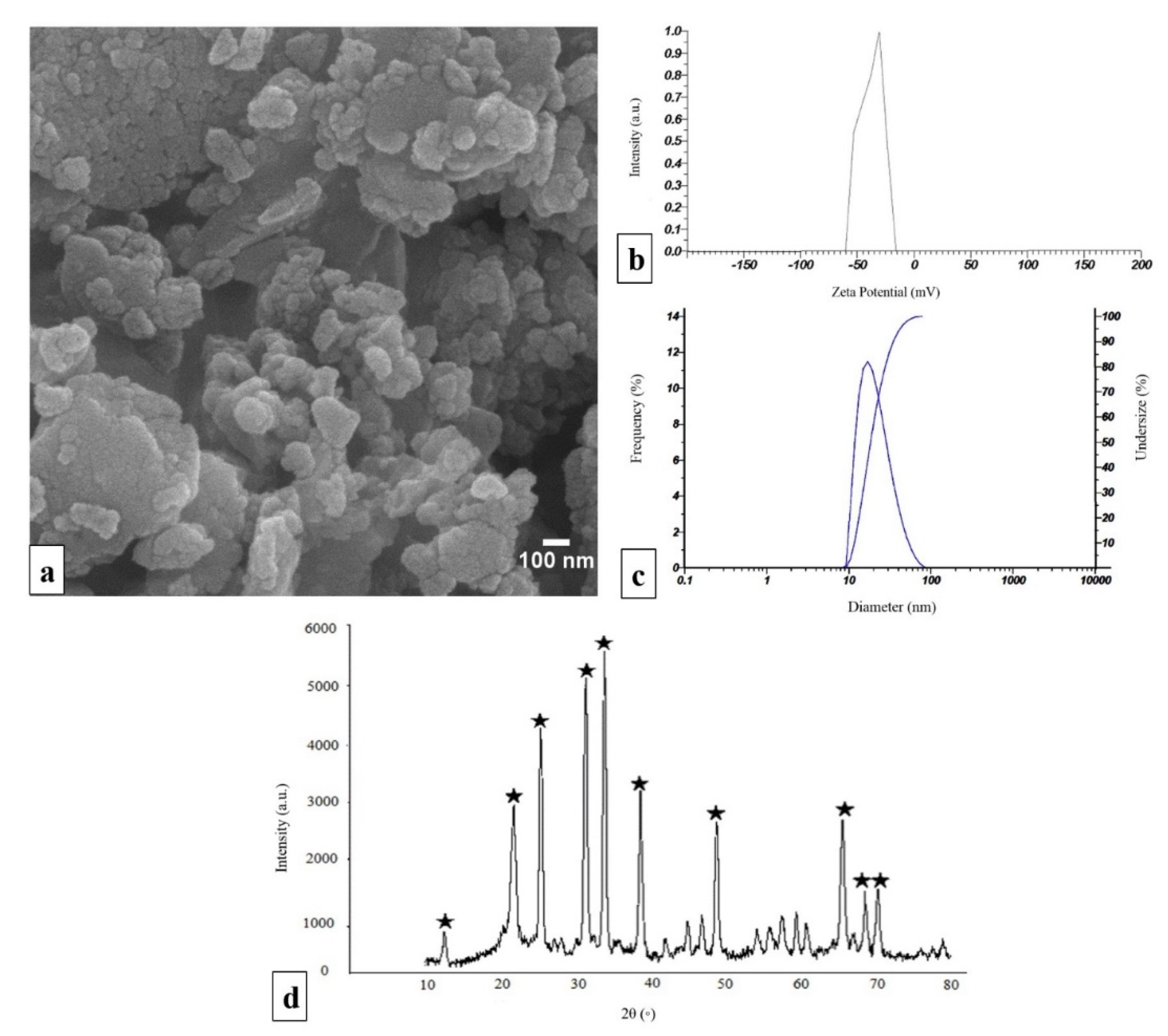
2.1. Characterization of npW
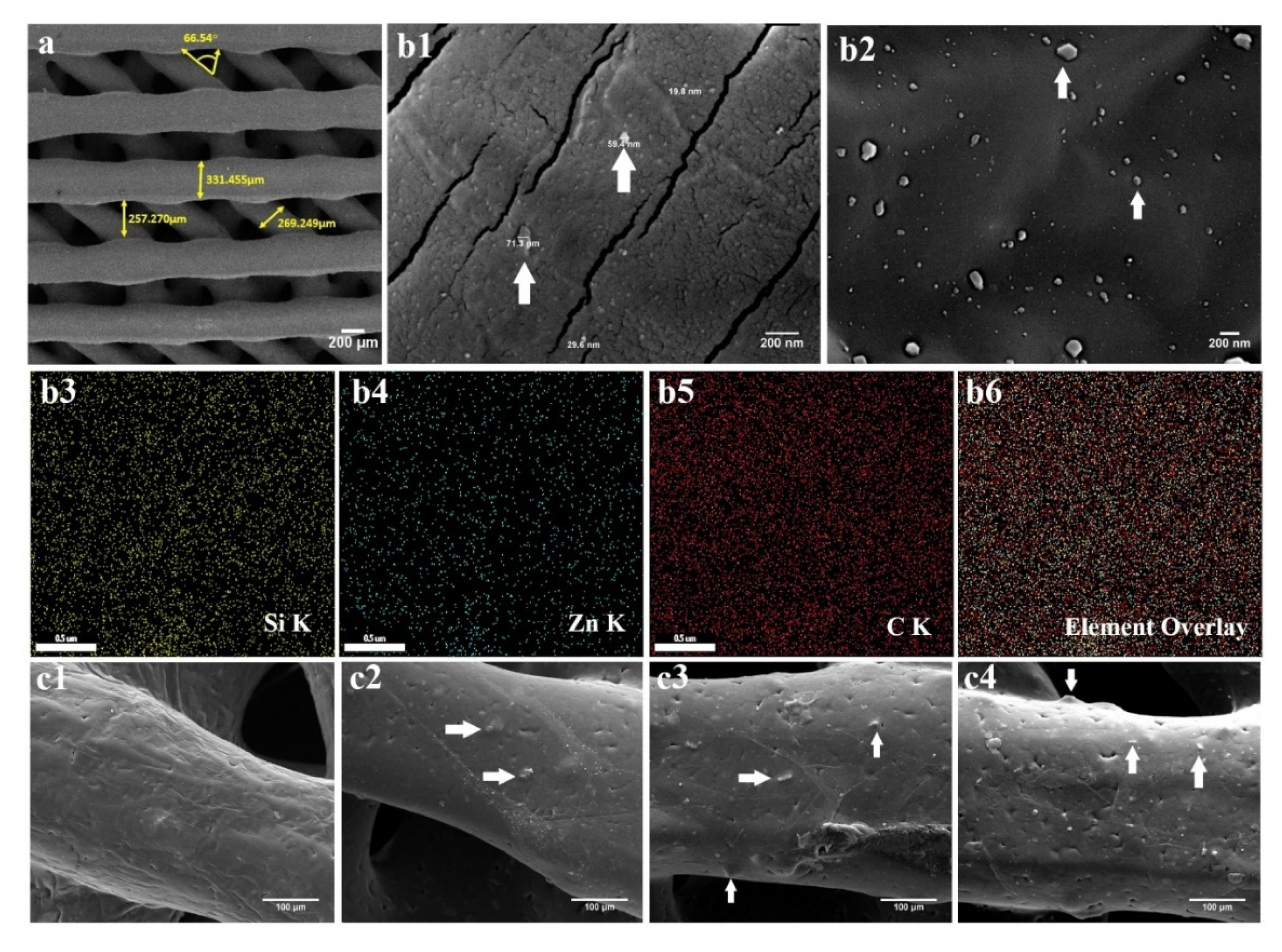
2.2. PCL/npW Composite Scaffolds Characterization
2.3. Characterization of PCL/npW Composite Scaffolds
2.3.1. Wettability and Degradation Rate
2.3.2. Chemical Analysis of the PCL/npW Composite Scaffold
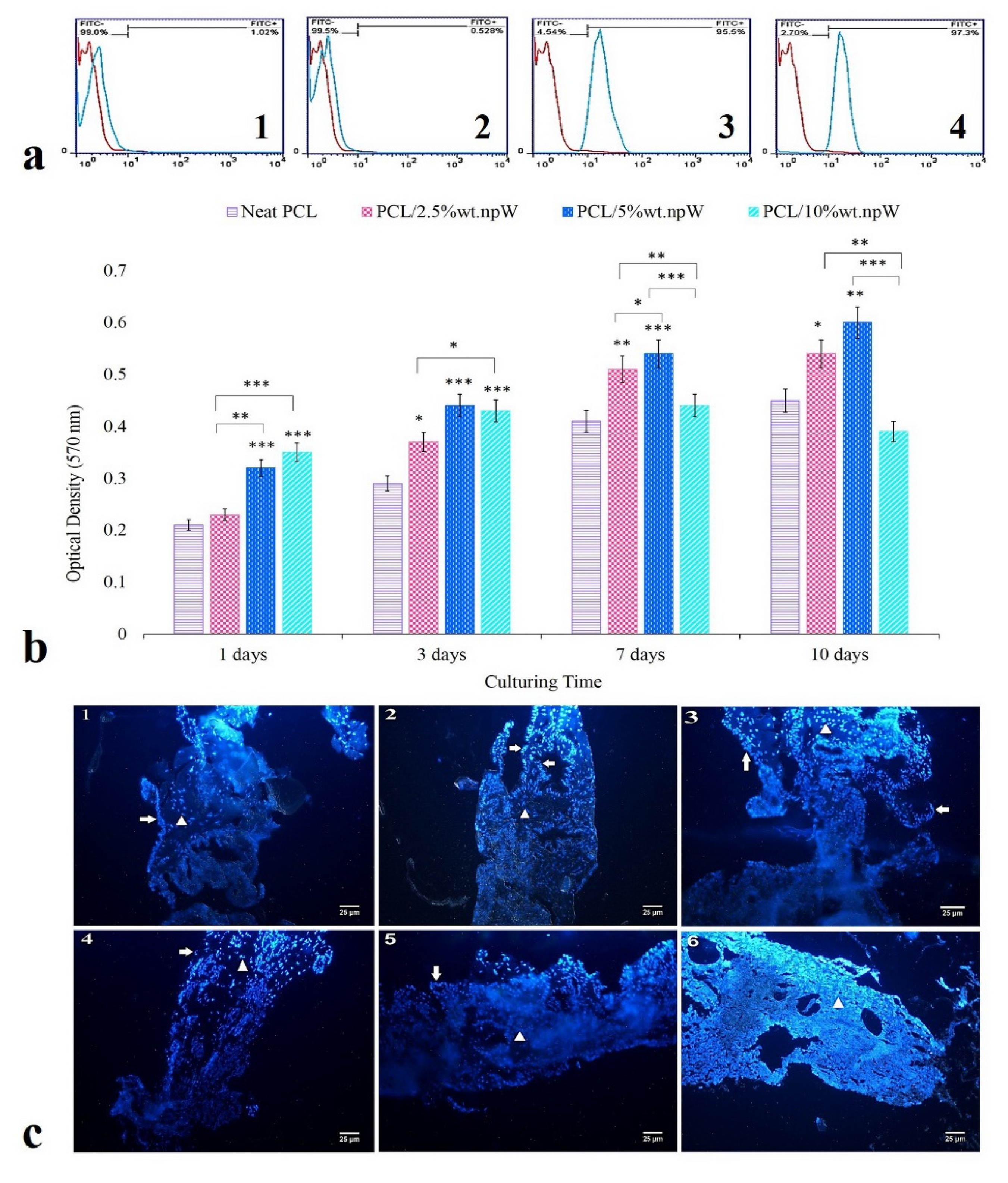
2.4. In Vitro Cytocompatibility
2.5. Osteogenic Differentiation
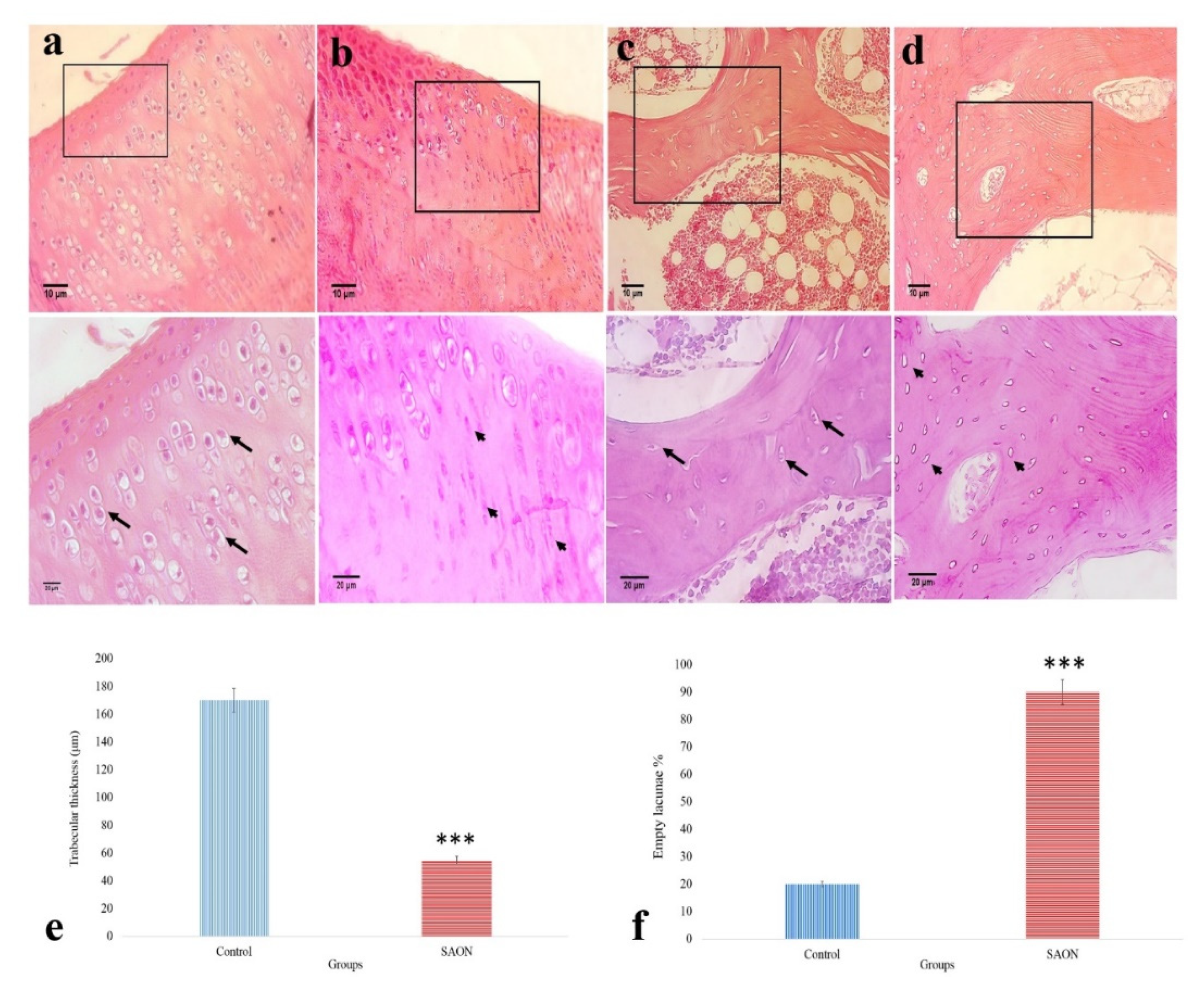
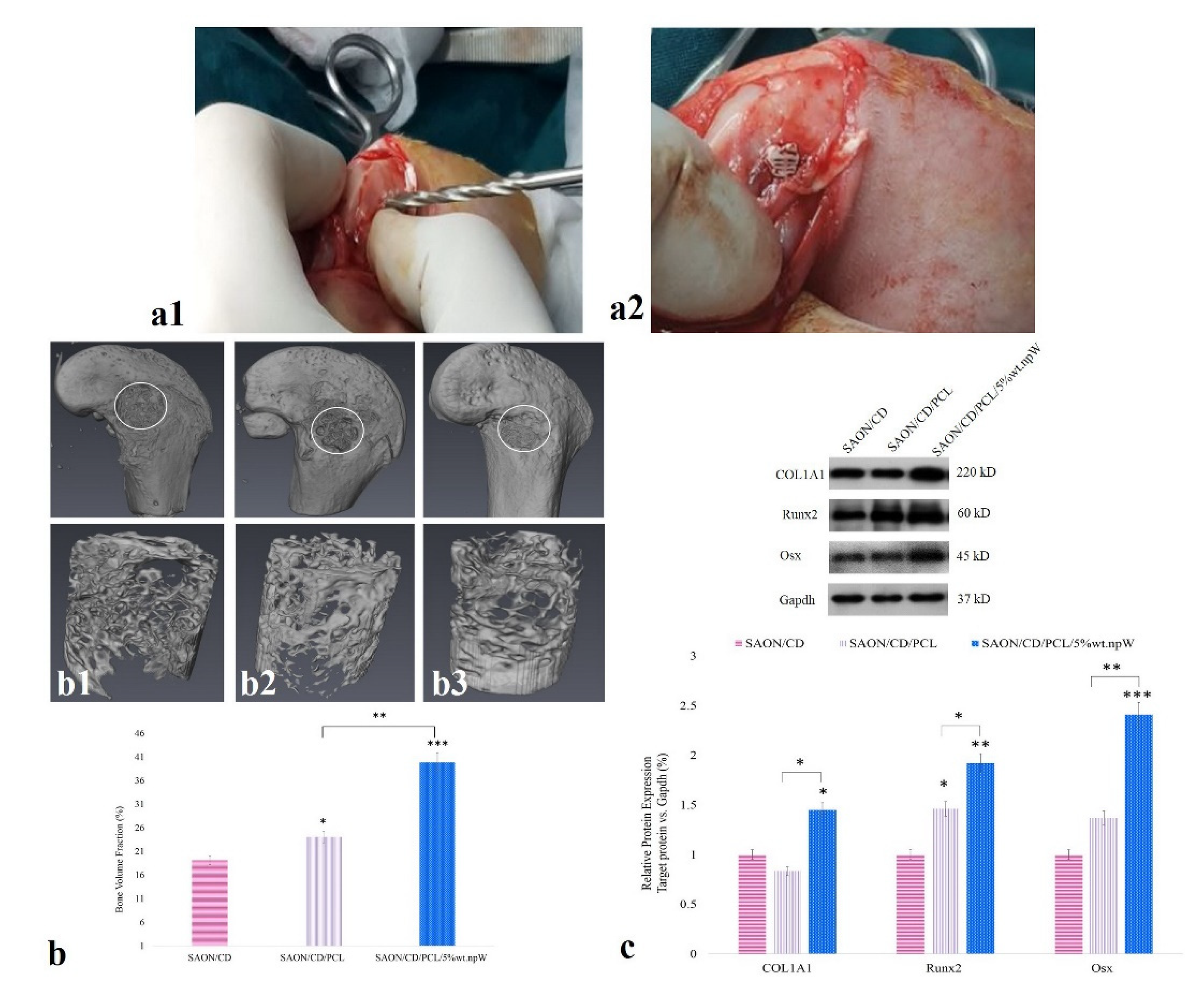
2.6. The Effect of PCL/npW Scaffold on the Reconstruction of SAON Lesion in the Rabbit Femur
2.7. PCL/npW Scaffold Effect on Col1A1, Runx2, and Osx
3. Discussion
4. Material and Methods
4.1. Materials and Experimental Reagents
4.2. Preparation of npW
4.3. Manufacturing of the 3D Interconnected Porous PCL/npW Composite Scaffolds
4.4. Characterization of PCL/npW Composites
The Water Uptake and Wettability
4.5. Ion Release and Degradation Rate
Brunauer Emmett-Teller (BET) and FTIR Analysis
4.6. Evaluation of the Mineralization Capacity (SEM-EDX)
4.7. In Vitro Evaluation
Extraction and Culture of rBMSCs
4.8. Investigating the Cytocompatibility of Scaffolds
4.9. Evaluation of Cell Distribution in Scaffolds Using SEM
4.10. Evaluation of Osteogenic Differentiation Effect of PCL/npW Scaffolds
4.11. Evaluation of Alkaline Phosphatase Activity
4.12. Calcium Content Assay
4.13. In Vivo Evaluation
Animals
4.14. Induction of Osteonecrosis of the Femur in Animals
- Group I: SAON animal undergoing core decompression (CD) surgery (SAON/CD, n = 5).
- Group II: SAON animal undergoing CD surgery and implantation of neat PCL scaffold for eight weeks (SAON/CD/neat PCL, n = 5).
- Group III: SAON animal undergoing CD surgery and implantation of PCL/5% wt. npW for eight weeks (SAON/CD/PCL/5% wt. npW, n = 5).
4.15. Surgical Procedure
4.16. Micro CT Analysis
4.17. Protein Preparation and Western Blot Analysis
4.18. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, P. Avascular Necrosis and Bone Infarcts of the Knee. Orthop. Nurs. 2020, 39, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Boontanapibul, K.; Amanatullah, D.F.; Huddleston, J.I.; Maloney, W.J.; Goodman, S.B. Outcomes of Cemented Total Knee Arthroplasty for Secondary Osteonecrosis of the Knee. J. Arthroplasty 2021, 36, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Boontanapibul, K.; Steere, J.T.; Amanatullah, D.F.; Huddleston, J.I.; Maloney, W.J.; Goodman, S.B. Initial Presentation and Progression of Secondary Osteonecrosis of the Knee. J. Arthroplasty 2020, 35, 2798–2806. [Google Scholar] [CrossRef]
- Zywiel, M.G.; McGrath, M.S.; Seyler, T.M.; Marker, D.R.; Bonutti, P.M.; Mont, M.A. Osteonecrosis of the Knee: A Review of Three Disorders. Orthop. Clin. N. Am. 2009, 40, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Labus, K.M.; Gadomski, B.C.; Bruyas, A.; Easley, J.; Nelson, B.; Palmer, R.H.; McGilvray, K.; Regan, D.; Puttlitz, C.M.; et al. Osteoinductive 3D printed scaffold healed 5 cm segmental bone defects in the ovine metatarsus. Sci. Rep. 2021, 11, 6704. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Theus, A.S.; Kamalakar, A.; Ning, L.; Cao, C.; Tomov, M.L.; Kaiser, J.M.; Goudy, S.; Willett, N.J.; Jang, H.W.; et al. 3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration. Polymers 2021, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, Z.; Yan, J.; Zhong, J. Synthetic Biodegradable Polymers for Bone Tissue Engineering. In Handbook of Composites from Renewable Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 355–375. [Google Scholar]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly(ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, B.; Byun, J.J.; Bártolo, P. Assessment of PCL/carbon material scaffolds for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 93, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, K.; Kwaśniak, K.; Gnilitskyi, I.; Barylyak, A.; Zinchenko, V.; Fahmi, A.; Korchynskyi, O.; Bobitski, Y. Functionalization of polycaprolactone electrospun osteoplastic scaffolds with fluorapatite and hydroxyapatite nanoparticles: Biocompatibility comparison of human versus mouse mesenchymal stem cells. Materials 2021, 14, 1333. [Google Scholar] [CrossRef]
- Bigham, A.; Salehi, A.O.M.; Rafienia, M.; Salamat, M.R.; Rahmati, S.; Raucci, M.G.; Ambrosio, L. Zn-substituted Mg2SiO4 nanoparticles-incorporated PCL-silk fibroin composite scaffold: A multifunctional platform towards bone tissue regeneration. Mater. Sci. Eng. C 2021, 127, 112242. [Google Scholar] [CrossRef] [PubMed]
- Constante, G.; Apsite, I.; Alkhamis, H.; Dulle, M.; Schwarzer, M.; Caspari, A.; Synytska, A.; Salehi, S.; Ionov, L. 4D Biofabrication Using a Combination of 3D Printing and Melt-Electrowriting of Shape-Morphing Polymers. ACS Appl. Mater. Interfaces 2021, 13, 12767–12776. [Google Scholar] [CrossRef]
- Pang, L.; Paxton, N.C.; Ren, J.; Liu, F.; Zhan, H.; Woodruff, M.A.; Bo, A.; Gu, Y. Development of mechanically enhanced polycaprolactone composites by a functionalized titanate nanofiller for melt electrowriting in 3D printing. ACS Appl. Mater. Interfaces 2020, 12, 47993–48006. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Yoshida, M.; Turner, P.R.; Ali, M.A.; Cabral, J.D. Three-Dimensional Melt-Electrowritten Polycaprolactone/Chitosan Scaffolds Enhance Mesenchymal Stem Cell Behavior. ACS Appl. Bio Mater. 2021, 4, 1319–1329. [Google Scholar] [CrossRef]
- Sawadkar, P.; Mohanakrishnan, J.; Rajasekar, P.; Rahmani, B.; Kohli, N.; Bozec, L.; García-Gareta, E. A Synergistic Relationship between Polycaprolactone and Natural Polymers Enhances the Physical Properties and Biological Activity of Scaffolds. ACS Appl. Mater. Interfaces 2020, 12, 13587–13597. [Google Scholar] [CrossRef] [PubMed]
- Permyakova, E.S.; Kiryukhantsev-Korneev, P.V.; Gudz, K.Y.; Konopatsky, A.S.; Polčak, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Shtansky, D.V.; Manakhov, A.M. Comparison of different approaches to surface functionalization of biodegradable polycaprolactone scaffolds. Nanomaterials 2019, 9, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartnikowski, M.; Abdal-hay, A.; Bartnikowski, N.J.; Kyoung Kim, Y.; Ivanovski, S. A comprehensive study of acid and base treatment of 3D printed poly(ε-caprolactone) scaffolds to tailor surface characteristics. Appl. Surf. Sci. 2021, 555. [Google Scholar] [CrossRef]
- Adegani, F.J.; Langroudi, L.; Ardeshirylajimi, A.; Dinarvand, P.; Dodel, M.; Doostmohammadi, A.; Rahimian, A.; Zohrabi, P.; Seyedjafari, E.; Soleimani, M. Coating of electrospun poly(lactic-co-glycolic acid) nanofibers with willemite bioceramic: Improvement of bone reconstruction in rat model. Cell Biol. Int. 2014, 38, 1271–1279. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-based bioactive glasses and their applications in hard tissue regeneration: A review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef] [PubMed]
- Arango-Ospina, M.; Nawaza, Q.; Boccaccini, A.R. Silicate-based nanoceramics in regenerative medicine. In Nanostructured Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 255–273. ISBN 9780081025949. [Google Scholar]
- Amiri, B.; Ghollasi, M.; Shahrousvand, M.; Kamali, M.; Salimi, A. Osteoblast differentiation of mesenchymal stem cells on modified PES-PEG electrospun fibrous composites loaded with Zn2SiO4 bioceramic nanoparticles. Differentiation 2016, 92, 148–158. [Google Scholar] [CrossRef]
- Halabian, R.; Moridi, K.; Korani, M.; Ghollasi, M. Composite nanoscaffolds modified with bio-ceramic nanoparticles (Zn2SiO4) prompted osteogenic differentiation of human induced pluripotent stem cells. Int. J. Mol. Cell. Med. 2019, 8, 24–38. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Ramezanifard, R.; Seyedjafari, E.; Ardeshirylajimi, A.; Soleimani, M. Biomimetic scaffolds containing nanofibers coated with willemite nanoparticles for improvement of stem cell osteogenesis. Mater. Sci. Eng. C 2016, 62, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhang, L.; Wang, G.; Zhao, Z.; Peng, S.; Shuai, C. 3D Printed Zn-doped Mesoporous Silica-incorporated Poly-L-lactic Acid Scaffolds for Bone Repair. Int. J. Bioprinting 2021, 7, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J.; et al. Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Goh, C.; Shrestha, A. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci. 2021, 21, 2000365. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Hashemi, J.; Barati, G.; Enderami, S.E.; Safdari, M. Osteogenic Differentiation of Induced Pluripotent Stem Cells on Electrospun Nanofibers: A Review of Literature. Mater. Today Commun. 2020, 25, 101561. [Google Scholar] [CrossRef]
- Moses, J.C.; Dey, M.; Devi, K.B.; Roy, M.; Nandi, S.K.; Mandal, B.B. Synergistic Effects of Silicon/Zinc Doped Brushite and Silk Scaffolding in Augmenting the Osteogenic and Angiogenic Potential of Composite Biomimetic Bone Grafts. ACS Biomater. Sci. Eng. 2019, 5. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Osorio, R.; Carrasco-Carmona, Á.; Gutiérrez-Pérez, J.-L.; Gutiérrez-Corrales, A.; Serrera-Figallo, M.-A.; Lynch, C.D.; Torres-Lagares, D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers 2020, 12, 1201. [Google Scholar] [CrossRef]
- Du, R.L.; Chang, J.; Ni, S.Y.; Zhai, W.Y.; Wang, J.Y. Characterization and in vitro Bioactivity of Zinc-containing Bioactive Glass and Glass-ceramics. J. Biomater. Appl. 2006, 20, 341–360. [Google Scholar] [CrossRef]
- Kwun, I.-S.; Cho, Y.-E.; Lomeda, R.-A.R.; Shin, H.-I.; Choi, J.-Y.; Kang, Y.-H.; Beattie, J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone 2010, 46, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Yamamoto, O.; Fukuda, M.; Koyota, S.; Koizumi, Y.; Sugiyama, T. In vitro prominent bone regeneration by release zinc ion from Zn-modified implant. Biochem. Biophys. Res. Commun. 2011, 412, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Patrício, T.; Bártolo, P. Thermal Stability of PCL/PLA Blends Produced by Physical Blending Process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Wen, Z.; Liu, W.; Zhang, L.; Su, J. Double-edged effects and mechanisms of Zn2+ microenvironments on osteogenic activity of BMSCs: Osteogenic differentiation or apoptosis. RSC Adv. 2020, 10, 14915–14927. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Weitzmann, M.N. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. 2011, 355, 179–186. [Google Scholar] [CrossRef]
- Kawamura, H.; Ito, A.; Miyakawa, S.; Layrolle, P.; Ojima, K.; Ichinose, N.; Tateishi, T. Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. J. Biomed. Mater. Res. 2000, 50, 184–190. [Google Scholar] [CrossRef]
- Ramaswamy, Y.; Wu, C.; Zhou, H.; Zreiqat, H. Biological response of human bone cells to zinc-modified Ca–Si-based ceramics. Acta Biomater. 2008, 4. [Google Scholar] [CrossRef]
- Lewis, S.; Haynes, V.; Wheeler-Jones, R.; Sly, J.; Perks, R.M.; Piccirillo, L. Surface characterization of poly(methylmethacrylate) based nanocomposite thin films containing Al2O3 and TiO2 nanoparticles. Thin Solid Films 2010, 518, 2683–2687. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the wettability of polymer surfaces using nanoparticles. Prog. Org. Coat. 2014, 77. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef]
- Jallot, E.; Nedelec, J.M.; Grimault, A.S.; Chassot, E.; Grandjean-Laquerriere, A.; Laquerriere, P.; Laurent-Maquin, D. STEM and EDXS characterisation of physico-chemical reactions at the periphery of sol–gel derived Zn-substituted hydroxyapatites during interactions with biological fluids. Colloids Surf. B Biointerfaces 2005, 42, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predoi, D.; Iconaru, S.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef] [Green Version]
- Fielding, G.A.; Smoot, W.; Bose, S. Effects of SiO2, SrO, MgO, and ZnO dopants in tricalcium phosphates on osteoblastic Runx2 expression. J. Biomed. Mater. Res. Part A 2014, 102, 2417–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Wu, X.; Yao, R.; He, J.; Yao, W.; Wu, F. Effect of zinc substitution in hydroxyapatite coating on osteoblast and osteoclast differentiation under osteoblast/osteoclast co-culture. Regen. Biomater. 2019, 6, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Zhang, J.; Wang, Z.; Lu, J.; Chang, J.; Liu, J.; Meng, G.; Dong, X. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed. Mater. 2011, 6, 015007. [Google Scholar] [CrossRef]
- Knychala, J.; Bouropoulos, N.; Catt, C.J.; Katsamenis, O.L.; Please, C.P.; Sengers, B.G. Pore Geometry Regulates Early Stage Human Bone Marrow Cell Tissue Formation and Organisation. Ann. Biomed. Eng. 2013, 41, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, S.; Sakata-Sogawa, K.; Hasegawa, A.; Suzuki, T.; Kabu, K.; Sato, E.; Kurosaki, T.; Yamashita, S.; Tokunaga, M.; Nishida, K.; et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007, 177, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Zreiqat, H.; Valenzuela, S.M.; Nissan, B.B.; Roest, R.; Knabe, C.; Radlanski, R.J.; Renz, H.; Evans, P.J. The effect of surface chemistry modification of titanium alloy on signalling pathways in human osteoblasts. Biomaterials 2005, 26, 7579–7586. [Google Scholar] [CrossRef]
- Cerovic, A.; Miletic, I.; Sobajic, S.; Blagojevic, D.; Radusinovic, M.; El-Sohemy, A. Effects of zinc on the mineralization of bone nodules from human osteoblast-like cells. Biol. Trace Elem. Res. 2007, 116, 61–71. [Google Scholar] [CrossRef] [PubMed]
- De Araujo Bastos Santana, L.; Oliveira Junior, P.H.; Damia, C.; dos Santos Tavares, D.; dos Santos, E.A. Bioactivity in SBF versus trace element effects: The isolated role of Mg2+ and Zn2+ in osteoblast behavior. Mater. Sci. Eng. C 2021, 118, 111320. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Young-Eun, C.; In-Sook, K. Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts. J. Nutr. Health 2018, 51, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.J.; Jeong, J.B.; Cho, Y.E.; Kwun, I.S. Zinc modulation of osterix in MC3T3-E1 cells. J. Nutr. Health 2020, 53, 347–355. [Google Scholar] [CrossRef]
- Kannan, S.; Ghosh, J.; Dhara, S.K. Osteogenic differentiation potential of porcine bone marrow mesenchymal stem cell subpopulations selected in different basal media. Biol. Open 2020, 9, bio053280. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Xie, X.H.; Wang, X.L.; He, Y.X.; Liu, Z.; Sheng, H.; Zhang, G.; Qin, L. Promotion of bone repair by implantation of cryopreserved bone marrowderived mononuclear cells in a rabbit model of steroid-associated osteonecrosis. Arthritis Rheum. 2012, 64, 1562–1571. [Google Scholar] [CrossRef] [Green Version]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimzadeh Bardeei, L.; Seyedjafari, E.; Hossein, G.; Nabiuni, M.; Majles Ara, M.H.; Salber, J. Regeneration of Bone Defects in a Rabbit Femoral Osteonecrosis Model Using 3D-Printed Poly (Epsilon-Caprolactone)/Nanoparticulate Willemite Composite Scaffolds. Int. J. Mol. Sci. 2021, 22, 10332. https://doi.org/10.3390/ijms221910332
Karimzadeh Bardeei L, Seyedjafari E, Hossein G, Nabiuni M, Majles Ara MH, Salber J. Regeneration of Bone Defects in a Rabbit Femoral Osteonecrosis Model Using 3D-Printed Poly (Epsilon-Caprolactone)/Nanoparticulate Willemite Composite Scaffolds. International Journal of Molecular Sciences. 2021; 22(19):10332. https://doi.org/10.3390/ijms221910332
Chicago/Turabian StyleKarimzadeh Bardeei, Latifeh, Ehsan Seyedjafari, Ghamartaj Hossein, Mohammad Nabiuni, Mohammad Hosein Majles Ara, and Jochen Salber. 2021. "Regeneration of Bone Defects in a Rabbit Femoral Osteonecrosis Model Using 3D-Printed Poly (Epsilon-Caprolactone)/Nanoparticulate Willemite Composite Scaffolds" International Journal of Molecular Sciences 22, no. 19: 10332. https://doi.org/10.3390/ijms221910332
APA StyleKarimzadeh Bardeei, L., Seyedjafari, E., Hossein, G., Nabiuni, M., Majles Ara, M. H., & Salber, J. (2021). Regeneration of Bone Defects in a Rabbit Femoral Osteonecrosis Model Using 3D-Printed Poly (Epsilon-Caprolactone)/Nanoparticulate Willemite Composite Scaffolds. International Journal of Molecular Sciences, 22(19), 10332. https://doi.org/10.3390/ijms221910332





