The Mandibular and Hyoid Arches—From Molecular Patterning to Shaping Bone and Cartilage
Abstract
:1. Introduction
2. Anatomy and Fate of Pharyngeal Arches
3. Specification of Pharyngeal Arches by the Hox Code
4. Specification of Mandibular and Hyoid Arches by the MEIS/PBX Complex
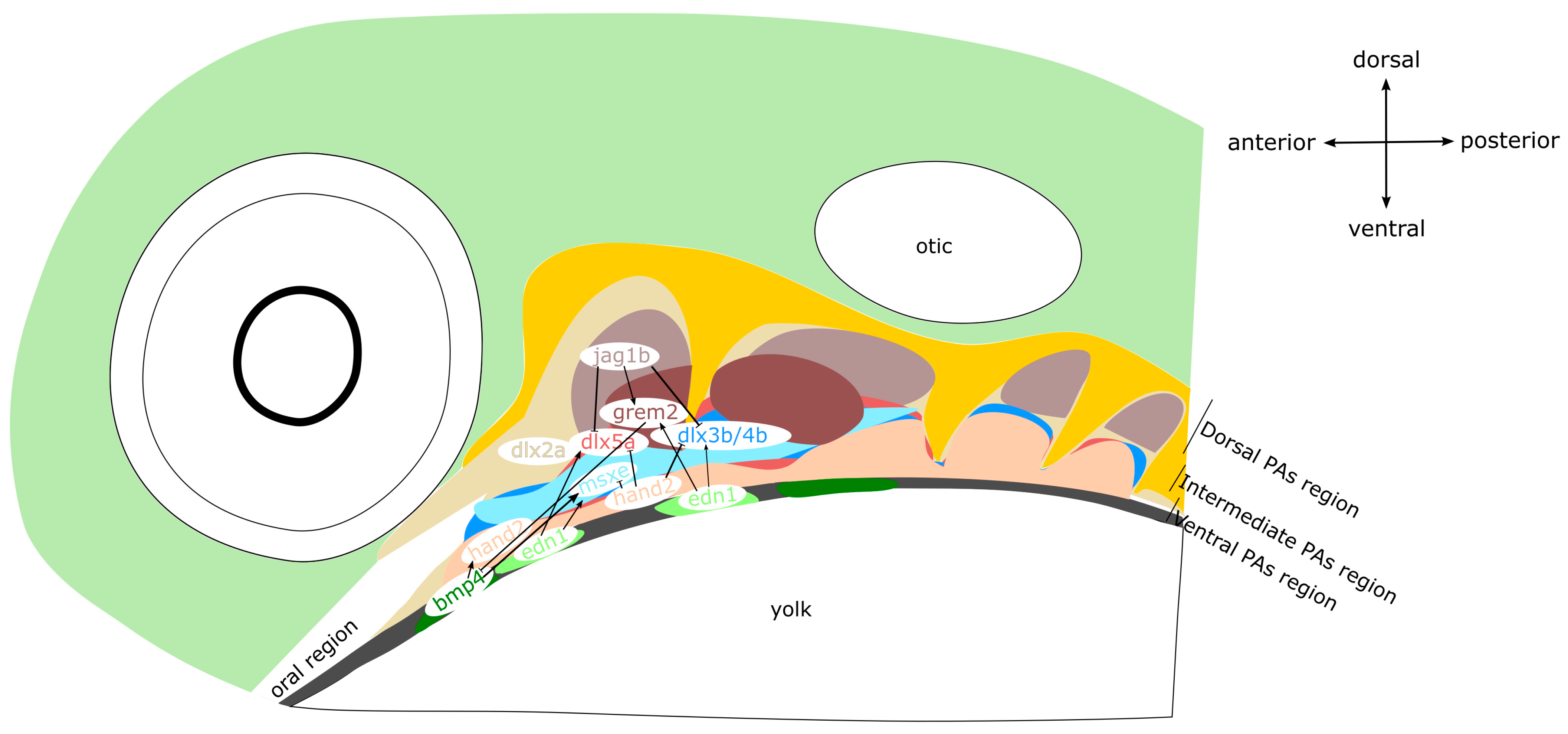
5. Endothelin–Dlx–Hand Gene Regulatory Network Controlling Anatomical Axes in Mandibular and Hyoid Arches
5.1. Mouse
5.2. Zebrafish
6. Combinatorial Action of FGF8, BMP4, and SHH Signalling Pathways during Morphogenesis of Mandibular and Hyoid Arches
6.1. Mouse
6.2. Zebrafish
7. Molecular Regulation of Osteochondrogenesis in the Mandibular and Hyoid Arches
7.1. Mouse
7.2. Zebrafish
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Hedges, S.B. A Molecular Timescale for Vertebrate Evolution. Nature 1998, 392, 917–920. [Google Scholar] [CrossRef]
- Gebuijs, I.G.E.; Raterman, S.T.; Metz, J.R.; Swanenberg, L.; Zethof, J.; Van den Bos, R.; Carels, C.E.L.; Wagener, F.A.D.T.G.; Von den Hoff, J.W. Fgf8a Mutation Affects Craniofacial Development and Skeletal Gene Expression in Zebrafish Larvae. Biol. Open 2019, 8, bio039834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Zhang, R.; Chen, H.; Du, X.; Chen, S.; Huang, J.; Liu, M.; Xu, M.; Luo, F.; Jin, M.; et al. Fgfr3 Mutation Disrupts Chondrogenesis and Bone Ossification in Zebrafish Model Mimicking CATSHL Syndrome Partially via Enhanced Wnt/β-Catenin Signaling. Theranostics 2020, 10, 7111–7130. [Google Scholar] [CrossRef]
- Schwartz, S.; Max, S.R.; Panny, S.R.; Cohen, M.M. Deletions of Proximal 15q and Non-Classical Prader-Willi Syndrome Phenotypes. Am. J. Med. Genet. 1985, 20, 255–263. [Google Scholar] [CrossRef]
- Jones, N.C.; Trainor, P.A. The Therapeutic Potential of Stem Cells in the Treatment of Craniofacial Abnormalities. Expert Opin. Biol. Ther. 2004, 4, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M.; Trainor, P.A. Relations and Interactions between Cranial Mesoderm and Neural Crest Populations. J. Anat. 2005, 207, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.S.; Charney, R.M.; García-Castro, M.I. Specification and Formation of the Neural Crest: Perspectives on Lineage Segregation. Genesis 2019, 57, e23276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauka-Spengler, T.; Bronner-Fraser, M. A Gene Regulatory Network Orchestrates Neural Crest Formation. Nat. Rev. Mol. Cell Biol. 2008, 9, 557–568. [Google Scholar] [CrossRef]
- Kontges, G.; Lumsden, A. Rhombencephalic Neural Crest Segmentation Is Preserved throughout Craniofacial Ontogeny. Development 1996, 122, 3229–3242. [Google Scholar] [CrossRef]
- D’Amico-Martel, A.; Noden, D.M. Contributions of Placodal and Neural Crest Cells to Avian Cranial Peripheral Ganglia. Am. J. Anat. 1983, 166, 445–468. [Google Scholar] [CrossRef]
- Yoshida, T.; Vivatbutsiri, P.; Morriss-Kay, G.; Saga, Y.; Iseki, S. Cell Lineage in Mammalian Craniofacial Mesenchyme. Mech. Dev. 2008, 125, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Graham, A. Deconstructing the Pharyngeal Metamere. J. Exp. Zool. B Mol. Dev. Evol. 2008, 310, 336–344. [Google Scholar] [CrossRef]
- McKinney, M.C.; McLennan, R.; Giniunaite, R.; Baker, R.E.; Maini, P.K.; Othmer, H.G.; Kulesa, P.M. Visualizing Mesoderm and Neural Crest Cell Dynamics during Chick Head Morphogenesis. Dev. Biol. 2020, 461, 184–196. [Google Scholar] [CrossRef]
- Gavalas, A.; Trainor, P.; Ariza-McNaughton, L.; Krumlauf, R. Synergy between Hoxa1 and Hoxb1: The Relationship between Arch Patterning and the Generation of Cranial Neural Crest. Development 2001, 128, 3017–3027. [Google Scholar] [CrossRef]
- Veitch, E.; Begbie, J.; Schilling, T.F.; Smith, M.M.; Graham, A. Pharyngeal Arch Patterning in the Absence of Neural Crest. Curr. Biol. 1999, 9, 1481–1484. [Google Scholar] [CrossRef] [Green Version]
- Graham, A.; Poopalasundaram, S.; Shone, V.; Kiecker, C. A Reappraisal and Revision of the Numbering of the Pharyngeal Arches. J. Anat. 2019, 235, 1019–1023. [Google Scholar] [CrossRef]
- Passos-Bueno, M.R.; Ornelas, C.C.; Fanganiello, R.D. Syndromes of the First and Second Pharyngeal Arches: A Review. Am. J. Med. Genet. Part A 2009, 149A, 1853–1859. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, J.F.; Yamamoto, M.; Abe, S.; Katori, Y.; Murakami, G. Development of the Human Incus with Special Reference to the Detachment From the Chondrocranium to Be Transferred into the Middle Ear. Anat. Rec. 2018, 301, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Woronowicz, K.C.; Schneider, R.A. Molecular and Cellular Mechanisms Underlying the Evolution of Form and Function in the Amniote Jaw. EvoDevo 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaupp, E. Über Die Ala Temporalis Des Säugerschädels Und Die Regio Orbtailis Einiger Anderer Wirbeltierschädel. Anat. Hefte 1902, 19, 155–230. [Google Scholar] [CrossRef]
- Frisdal, A.; Trainor, P.A. Development and Evolution of the Pharyngeal Apparatus. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 403–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, S.N.; Weinmann, J.P.; Schour, I. Role of Meckel’s Cartilage in the Development and Growth of the Rat Mandible. J. Dent. Res. 1953, 32, 398–410. [Google Scholar] [CrossRef]
- Ito, Y.; Bringas, P.; Mogharei, A.; Zhao, J.; Deng, C.; Chai, Y. Receptor-Regulated and Inhibitory Smads Are Critical in Regulating Transforming Growth Factorβ–Mediated Meckel’s Cartilage Development. Dev. Dyn. 2002, 224, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Shimo, T.; Kanyama, M.; Wu, C.; Sugito, H.; Billings, P.C.; Abrams, W.R.; Rosenbloom, J.; Iwamoto, M.; Pacifici, M.; Koyama, E. Expression and Roles of Connective Tissue Growth Factor in Meckel’s Cartilage Development. Dev. Dyn. 2004, 231, 136–147. [Google Scholar] [CrossRef]
- Svandova, E.; Anthwal, N.; Tucker, A.S.; Matalova, E. Diverse Fate of an Enigmatic Structure: 200 Years of Meckel’s Cartilage. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, J.F.; Mérida-Velasco, J.R.; Mérida-Velasco, J.A.; Sánchez-Montesinos, I.; Espín-Ferra, J.; Jiménez-Collado, J. Development of Meckel’s Cartilage in the Symphyseal Region in Man. Anat. Rec. 1997, 249, 249–254. [Google Scholar] [CrossRef]
- Eames, B.F.; Sharpe, P.T.; Helms, J.A. Hierarchy Revealed in the Specification of Three Skeletal Fates by Sox9 and Runx2. Dev. Biol. 2004, 274, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Harada, Y.; Ishizeki, K. Evidence for Transformation of Chondrocytes and Site-Specific Resorption during the Degradation of Meckel’s Cartilage. Anat. Embryol. 1998, 197, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Ishizeki, K. Imaging Analysis of Osteogenic Transformation of Meckel’s Chondrocytes from Green Fluorescent Protein-Transgenic Mice during Intrasplenic Transplantation. Acta Histochem. 2012, 114, 608–619. [Google Scholar] [CrossRef]
- Ishizeki, K.; Takigawa, M.; Nawa, T.; Suzuki, F. Mouse Meckel’s cartilage chondrocytes evoke bone-like matrix and further transform into osteocyte-like cells in culture. Anat. Rec. 1996, 245, 25–35. [Google Scholar] [CrossRef]
- Ishizeki, K.; Saito, H.; Shinagawa, T.; Fujiwara, N.; Nawa, T. Histochemical and Immunohistochemical Analysis of the Mechanism of Calcification of Meckel’s Cartilage during Mandible Development in Rodents. J. Anat. 1999, 194 Pt 2, 265–277. [Google Scholar] [CrossRef]
- Ishizeki, K.; Kagiya, T.; Fujiwara, N.; Otsu, K.; Harada, H. Expression of Osteogenic Proteins during the Intrasplenic Transplantation of Meckel’s Chondrocytes: A Histochemical and Immunohistochemical Study. Arch. Histol. Cytol. 2009, 72, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthwal, N.; Joshi, L.; Tucker, A.S. Evolution of the Mammalian Middle Ear and Jaw: Adaptations and Novel Structures. J. Anat. 2013, 222, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Ishizeki, K.; Takahashi, N.; Nawa, T. Formation of the Sphenomandibular Ligament by Meckel’s Cartilage in the Mouse: Possible Involvement of Epidermal Growth Factor as Revealed by Studies in Vivo and in Vitro. Cell Tissue Res. 2001, 304, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Cheynet, F.; Guyot, L.; Richard, O.; Layoun, W.; Gola, R. Discomallear and Malleomandibular Ligaments: Anatomical Study and Clinical Applications. Surg. Radiol. Anat. 2003, 25, 152–157. [Google Scholar] [CrossRef]
- Amano, O.; Doi, T.; Yamada, T.; Sasaki, A.; Sakiyama, K.; Kanegae, H.; Kindaichi, K. Meckel’s Cartilage: Discovery, Embryology and Evolution: —Overview of the Specificity of Meckel’s Cartilage—. J. Oral Biosci. 2010, 52, 125–135. [Google Scholar] [CrossRef]
- Dash, S.; Trainor, P.A. The Development, Patterning and Evolution of Neural Crest Cell Differentiation into Cartilage and Bone. Bone 2020, 137, 115409. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, J.F.; Mérida-Velasco, J.R.; Verdugo-López, S.; Sánchez-Montesinos, I.; Mérida-Velasco, J.A. Morphogenesis of the Second Pharyngeal Arch Cartilage (Reichert’s Cartilage) in Human Embryos. J. Anat. 2006, 208, 179–189. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, J.F.; Verdugo-López, S.; Abe, H.; Murakami, G. The Origin of the Variations of the Hyoid Apparatus in Human. Anat. Rec. 2015, 298, 1395–1407. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, J.F.; Kim, J.H.; Verdugo-López, S.; Murakami, G.; Cho, K.H.; Asakawa, S.; Abe, S.-I. Human Fetal Hyoid Body Origin Revisited. J. Anat. 2011, 219, 143–149. [Google Scholar] [CrossRef]
- de Bakker, B.S.; de Bakker, H.M.; Soerdjbalie-Maikoe, V.; Dikkers, F.G. The Development of the Human Hyoid-Larynx Complex Revisited. Laryngoscope 2018, 128, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Poopalasundaram, S.; Richardson, J.; Scott, A.; Donovan, A.; Liu, K.; Graham, A. Diminution of Pharyngeal Segmentation and the Evolution of the Amniotes. Zool. Lett. 2019, 5, 6. [Google Scholar] [CrossRef]
- El Amm, C.A.; Denny, A. Hyoid Bone Abnormalities in Pierre Robin Patients. J. Craniofacial Surg. 2008, 19, 259–263. [Google Scholar] [CrossRef]
- Rajion, Z.A.; Townsend, G.C.; Netherway, D.J.; Anderson, P.J.; Hughes, T.; Shuaib, I.L.; Halim, A.S.; Samsudin, A.R.; McLean, N.R.; David, D.J. The Hyoid Bone in Malay Infants with Cleft Lip and Palate. Cleft Palate-Craniofacial J. 2006, 43, 532–538. [Google Scholar] [CrossRef]
- Erdinc, A.M.E.; Dincer, B.; Sabah, M.E. Evaluation of the Position of the Hyoid Bone in Relation to Vertical Facial Development. J. Clin. Pediatr. Dent. 2003, 27, 347–352. [Google Scholar] [CrossRef]
- Yoshida, K.; Yokoi, T.; Mori, S.; Achiwa, M.; Kuroiwa, Y.; Kurita, K. Abnormal Ossification of the Hyoid Bone in Cleidocranial Dysplasia Rare Case and Literature Review. Int. J. Oral Maxillofac. Surg. 2017, 46, 375–376. [Google Scholar] [CrossRef] [Green Version]
- Heliövaara, A.; Hurmerinta, K. Craniofacial Cephalometric Morphology in Children with CATCH 22 Syndrome. Orthod. Craniofacial Res. 2006, 9, 186–192. [Google Scholar] [CrossRef]
- Wells, T.R.; Gilsanz, V.; Senac, M.O.; Landing, B.H.; Vachon, L.; Takahashi, M. Ossification Centre of the Hyoid Bone in DiGeorge Syndrome and Tetralogy of Fallot. Br. J. Radiol. 1986, 59, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Milligan, B.; Harris, N.; Franz-Odendaal, T.A. Understanding Morphology: A Comparative Study on the Lower Jaw in Two Teleost Species: Lower Jaw Morphology. J. Appl. Ichthyol. 2012, 28, 346–352. [Google Scholar] [CrossRef]
- Cubbage, C.C.; Mabee, P.M. Development of the Cranium and Paired Fins in the Zebrafish Danio Rerio (Ostariophysi, Cyprinidae). J. Morphol. 1996, 229, 121–160. [Google Scholar] [CrossRef]
- Graham, A. The Development and Evolution of the Pharyngeal Arches. J. Anat. 2001, 199, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Janvier, P.; Desbiens, S.; Willett, J.A.; Arsenault, M. Lamprey-like Gills in a Gnathostome-Related Devonian Jawless Vertebrate. Nature 2006, 440, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Santagati, F.; Rijli, F.M. Cranial Neural Crest and the Building of the Vertebrate Head. Nat. Rev. Neurosci. 2003, 4, 806–818. [Google Scholar] [CrossRef]
- Trainor, P.A.; Krumlauf, R. Hox Genes, Neural Crest Cells and Branchial Arch Patterning. Curr. Opin. Cell Biol. 2001, 13, 698–705. [Google Scholar] [CrossRef]
- Pearson, J.C.; Lemons, D.; McGinnis, W. Modulating Hox Gene Functions during Animal Body Patterning. Nat. Rev. Genet. 2005, 6, 893–904. [Google Scholar] [CrossRef]
- Parker, H.J.; Pushel, I.; Krumlauf, R. Coupling the Roles of Hox Genes to Regulatory Networks Patterning Cranial Neural Crest. Dev. Biol. 2018, 444, S67–S78. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, A. Segmentation and Compartition in the Early Avian Hindbrain. Mech. Dev. 2004, 121, 1081–1088. [Google Scholar] [CrossRef]
- Couly, G.; Grapin-Botton, A.; Coltey, P.; Ruhin, B.; Le Douarin, N.M. Determination of the Identity of the Derivatives of the Cephalic Neural Crest: Incompatibility between Hox Gene Expression and Lower Jaw Development. Development 1998, 125, 3445–3459. [Google Scholar] [CrossRef]
- Jozefowicz, C.; McClintock, J.; Prince, V. The fates of zebrafish Hox gene duplicates. In Genome Evolution; Meyer, A., Van de Peer, Y., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 185–194. ISBN 978-94-010-3957-4. [Google Scholar]
- Hunt, P.; Krumlauf, R. Hox Genes Coming to a Head. Curr. Biol. 1991, 1, 304–306. [Google Scholar] [CrossRef]
- Hunt, P.; Krumlauf, R. Deciphering the Hox Code: Clues to Patterning Branchial Regions of the Head. Cell 1991, 66, 1075–1078. [Google Scholar] [CrossRef]
- Makki, N.; Capecchi, M.R. Hoxa1 Lineage Tracing Indicates a Direct Role for Hoxa1 in the Development of the Inner Ear, the Heart, and the Third Rhombomere. Dev. Biol. 2010, 341, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.; Hill, R.E. Expression of the Mouse Labial-like Homeobox-Containing Genes, Hox 2.9 and Hox 1.6, during Segmentation of the Hindbrain. Development 1991, 111, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, D.; Clarke, J.D.; Oxtoby, E.; Yan, Y.L.; Jowett, T.; Holder, N. Ectopic Expression of Hoxa-1 in the Zebrafish Alters the Fate of the Mandibular Arch Neural Crest and Phenocopies a Retinoic Acid-Induced Phenotype. Development 1996, 122, 735–746. [Google Scholar] [CrossRef]
- Gavalas, A.; Studer, M.; Lumsden, A.; Rijli, F.M.; Krumlauf, R.; Chambon, P. Hoxa1 and Hoxb1 Synergize in Patterning the Hindbrain, Cranial Nerves and Second Pharyngeal Arch. Development 1998, 125, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, B.; Kuschert, S.J.; Liu, Y.H.; Mallo, M. Hoxa-2 Restricts the Chondrogenic Domain and Inhibits Bone Formation during Development of the Branchial Area. Development 1998, 125, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Gendron-Maguire, M.; Mallo, M.; Zhang, M.; Gridley, T. Hoxa-2 Mutant Mice Exhibit Homeotic Transformation of Skeletal Elements Derived from Cranial Neural Crest. Cell 1993, 75, 1317–1331. [Google Scholar] [CrossRef]
- Rijli, F.M.; Mark, M.; Lakkaraju, S.; Dierich, A.; Dollé, P.; Chambon, P. A Homeotic Transformation Is Generated in the Rostral Branchial Region of the Head by Disruption of Hoxa-2, Which Acts as a Selector Gene. Cell 1993, 75, 1333–1349. [Google Scholar] [CrossRef]
- Grammatopoulos, G.A.; Bell, E.; Toole, L.; Lumsden, A.; Tucker, A.S. Homeotic Transformation of Branchial Arch Identity after Hoxa2 Overexpression. Development 2000, 127, 5355–5365. [Google Scholar] [CrossRef]
- Hunter, M.P.; Prince, V.E. Zebrafish Hox Paralogue Group 2 Genes Function Redundantly as Selector Genes to Pattern the Second Pharyngeal Arch. Dev. Biol. 2002, 247, 367–389. [Google Scholar] [CrossRef] [Green Version]
- Pasqualetti, M.; Ori, M.; Nardi, I.; Rijli, F.M. Ectopic Hoxa2 Induction after Neural Crest Migration Results in Homeosis of Jaw Elements in Xenopus. Development 2000, 127, 5367–5378. [Google Scholar] [CrossRef]
- Minoux, M.; Antonarakis, G.S.; Kmita, M.; Duboule, D.; Rijli, F.M. Rostral and Caudal Pharyngeal Arches Share a Common Neural Crest Ground Pattern. Development 2009, 136, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, D.; Geerts, D. MEIS Transcription Factors in Development and Disease. Development 2019, 146. [Google Scholar] [CrossRef] [Green Version]
- Longobardi, E.; Penkov, D.; Mateos, D.; Florian, G.D.; Torres, M.; Blasi, F. Biochemistry of the Tale Transcription Factors PREP, MEIS, and PBX in Vertebrates. Dev. Dyn. 2014, 243, 59–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waskiewicz, A.J.; Rikhof, H.A.; Hernandez, R.E.; Moens, C.B. Zebrafish Meis Functions to Stabilize Pbx Proteins and Regulate Hindbrain Patterning. Development 2001, 128, 4139–4151. [Google Scholar] [CrossRef]
- Choe, S.-K.; Lu, P.; Nakamura, M.; Lee, J.; Sagerström, C.G. Meis Cofactors Control HDAC and CBP Accessibility at Hox-Regulated Promoters during Zebrafish Embryogenesis. Dev. Cell 2009, 17, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Amin, S.; Donaldson, I.J.; Zannino, D.A.; Hensman, J.; Rattray, M.; Losa, M.; Spitz, F.; Ladam, F.; Sagerström, C.; Bobola, N. Hoxa2 Selectively Enhances Meis Binding to Change a Branchial Arch Ground State. Dev. Cell 2015, 32, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Machon, O.; Masek, J.; Machonova, O.; Krauss, S.; Kozmik, Z. Meis2 Is Essential for Cranial and Cardiac Neural Crest Development. BMC Dev. Biol. 2015, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Fabik, J.; Kovacova, K.; Kozmik, Z.; Machon, O. Neural Crest Cells Require Meis2 for Patterning the Mandibular Arch via the Sonic Hedgehog Pathway. Biol. Open 2020, 9. [Google Scholar] [CrossRef]
- Crowley, M.A.; Conlin, L.K.; Zackai, E.H.; Deardorff, M.A.; Thiel, B.D.; Spinner, N.B. Further Evidence for the Possible Role of MEIS2 in the Development of Cleft Palate and Cardiac Septum. Am. J. Med. Genet. A 2010, 152A, 1326–1327. [Google Scholar] [CrossRef]
- Douglas, G.; Cho, M.T.; Telegrafi, A.; Winter, S.; Carmichael, J.; Zackai, E.H.; Deardorff, M.A.; Harr, M.; Williams, L.; Psychogios, A.; et al. De Novo Missense Variants in MEIS2 Recapitulate the Microdeletion Phenotype of Cardiac and Palate Abnormalities, Developmental Delay, Intellectual Disability and Dysmorphic Features. Am. J. Med. Genet. A 2018, 176, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, F.; Ullmann, R.; Chen, W.; Schubert, M.; Adolph, S.; Hultschig, C.; Kalscheuer, V.; Ropers, H.-H.; Spaich, C.; Tzschach, A. Characterization of a 5.3 Mb Deletion in 15q14 by Comparative Genomic Hybridization Using a Whole Genome “Tiling Path” BAC Array in a Girl with Heart Defect, Cleft Palate, and Developmental Delay. Am. J. Med. Genet. A 2007, 143A, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Giliberti, A.; Currò, A.; Papa, F.T.; Frullanti, E.; Ariani, F.; Coriolani, G.; Grosso, S.; Renieri, A.; Mari, F. MEIS2 Gene Is Responsible for Intellectual Disability, Cardiac Defects and a Distinct Facial Phenotype. Eur J. Med. Genet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Berland, S.; Gradek, G.A.; Bongers, E.; de Leeuw, N.; Pfundt, R.; Fannemel, M.; Rødningen, O.; Brendehaug, A.; Haukanes, B.I.; et al. Haploinsufficiency of MEIS2 Is Associated with Orofacial Clefting and Learning Disability. Am. J. Med. Genet. Part A 2014, 164, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Verheije, R.; Kupchik, G.S.; Isidor, B.; Kroes, H.Y.; Lynch, S.A.; Hawkes, L.; Hempel, M.; Gelb, B.D.; Ghoumid, J.; D’Amours, G.; et al. Heterozygous Loss-of-Function Variants of MEIS2 Cause a Triad of Palatal Defects, Congenital Heart Defects, and Intellectual Disability. Eur. J. Hum. Genet. 2019, 27, 278–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, H.J.; De Kumar, B.; Green, S.A.; Prummel, K.D.; Hess, C.; Kaufman, C.K.; Mosimann, C.; Wiedemann, L.M.; Bronner, M.E.; Krumlauf, R. A Hox-TALE Regulatory Circuit for Neural Crest Patterning Is Conserved across Vertebrates. Nat. Commun. 2019, 10, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melvin, V.S.; Feng, W.; Hernandez-Lagunas, L.; Artinger, K.B.; Williams, T. A Morpholino-Based Screen to Identify Novel Genes Involved in Craniofacial Morphogenesis. Dev. Dyn. 2013, 242, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pöpperl, H.; Rikhof, H.; Chang, H.; Haffter, P.; Kimmel, C.B.; Moens, C.B. Lazarus Is a Novel Pbx Gene That Globally Mediates Hox Gene Function in Zebrafish. Mol. Cell 2000, 6, 255–267. [Google Scholar] [CrossRef]
- Ferretti, E.; Li, B.; Zewdu, R.; Wells, V.; Hebert, J.M.; Karner, C.; Anderson, M.J.; Williams, T.; Dixon, J.; Dixon, M.J.; et al. A Conserved Pbx-Wnt-P63-Irf6 Regulatory Module Controls Face Morphogenesis by Promoting Epithelial Apoptosis. Dev. Cell 2011, 21, 627–641. [Google Scholar] [CrossRef] [Green Version]
- Vitobello, A.; Ferretti, E.; Lampe, X.; Vilain, N.; Ducret, S.; Ori, M.; Spetz, J.-F.; Selleri, L.; Rijli, F.M. Hox and Pbx Factors Control Retinoic Acid Synthesis during Hindbrain Segmentation. Dev. Cell 2011, 20, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Selleri, L.; Depew, M.J.; Jacobs, Y.; Chanda, S.K.; Tsang, K.Y.; Cheah, K.S.E.; Rubenstein, J.L.R.; O’Gorman, S.; Cleary, M.L. Requirement for Pbx1 in Skeletal Patterning and Programming Chondrocyte Proliferation and Differentiation. Development 2001, 128, 3543–3557. [Google Scholar] [CrossRef]
- ten Berge, D.; Brouwer, A.; Korving, J.; Martin, J.F.; Meijlink, F. Prx1 and Prx2 in Skeletogenesis: Roles in the Craniofacial Region, Inner Ear and Limbs. Development 1998, 125, 3831–3842. [Google Scholar] [CrossRef]
- Czako, L.; Simko, K.; Thurzo, A.; Galis, B.; Varga, I. The Syndrome of Elongated Styloid Process, the Eagle’s Syndrome—From Anatomical, Evolutionary and Embryological Backgrounds to 3D Printing and Personalized Surgery Planning. Report of Five Cases. Medicina 2020, 56, 458. [Google Scholar] [CrossRef]
- Arai, H.; Hori, S.; Aramori, I.; Ohkubo, H.; Nakanishi, S. Cloning and Expression of a CDNA Encoding an Endothelin Receptor. Nature 1990, 348. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Yanagisawa, M.; Takuwa, Y.; Miyazaki, H.; Kimura, S.; Goto, K.; Masaki, T. Cloning of a CDNA Encoding a Non-Isopeptide-Selective Subtype of the Endothelin Receptor. Nature 1990, 348, 732–735. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, M. The Endothelin System. A New Target for Therapeutic Intervention. Circulation 1994, 89, 1320–1322. [Google Scholar] [CrossRef] [Green Version]
- Clouthier, D.E.; Williams, S.C.; Yanagisawa, H.; Wieduwilt, M.; Richardson, J.A.; Yanagisawa, M. Signaling Pathways Crucial for Craniofacial Development Revealed by Endothelin-A Receptor-Deficient Mice. Dev. Biol. 2000, 217, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuhara, S.; Kurihara, Y.; Arima, Y.; Yamada, N.; Kurihara, H. Temporal Requirement of Signaling Cascade Involving Endothelin-1/Endothelin Receptor Type A in Branchial Arch Development. Mech. Dev. 2004, 121, 1223–1233. [Google Scholar] [CrossRef]
- Thomas, T.; Kurihara, H.; Yamagishi, H.; Kurihara, Y.; Yazaki, Y.; Olson, E.N.; Srivastava, D. A Signaling Cascade Involving Endothelin-1, DHAND and Msx1 Regulates Development of Neural-Crest-Derived Branchial Arch Mesenchyme. Development 1998, 125, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, D.E.; Hosoda, K.; Richardson, J.A.; Williams, S.C.; Yanagisawa, H.; Kuwaki, T.; Kumada, M.; Hammer, R.E.; Yanagisawa, M. Cranial and Cardiac Neural Crest Defects in Endothelin-A Receptor-Deficient Mice. Development 1998, 125, 813–824. [Google Scholar] [CrossRef]
- Kurihara, Y.; Kurihara, H.; Suzuki, H.; Kodama, T.; Maemura, K.; Nagai, R.; Oda, H.; Kuwaki, T.; Cao, W.H.; Kamada, N. Elevated Blood Pressure and Craniofacial Abnormalities in Mice Deficient in Endothelin-1. Nature 1994, 368, 703–710. [Google Scholar] [CrossRef]
- Ruest, L.-B.; Xiang, X.; Lim, K.-C.; Levi, G.; Clouthier, D.E. Endothelin-A Receptor-Dependent and -Independent Signaling Pathways in Establishing Mandibular Identity. Development 2004, 131, 4413–4423. [Google Scholar] [CrossRef] [Green Version]
- Ozeki, H.; Kurihara, Y.; Tonami, K.; Watatani, S.; Kurihara, H. Endothelin-1 Regulates the Dorsoventral Branchial Arch Patterning in Mice. Mech. Dev. 2004, 121, 387–395. [Google Scholar] [CrossRef]
- Sato, T.; Kurihara, Y.; Asai, R.; Kawamura, Y.; Tonami, K.; Uchijima, Y.; Heude, E.; Ekker, M.; Levi, G.; Kurihara, H. An Endothelin-1 Switch Specifies Maxillomandibular Identity. Proc. Natl. Acad. Sci. USA 2008, 105, 18806–18811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavares, A.L.P.; Cox, T.C.; Maxson, R.M.; Ford, H.L.; Clouthier, D.E. Negative Regulation of Endothelin Signaling by SIX1 Is Required for Proper Maxillary Development. Development 2017, 144, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Narboux-Nême, N.; Gitton, Y.; de Lombares, C.; Fontaine, A.; Alfama, G.; Kitazawa, T.; Kawamura, Y.; Heude, E.; Marshall, L.; et al. Probing the Origin of Matching Functional Jaws: Roles of Dlx5/ 6 in Cranial Neural Crest Cells. Sci. Rep. 2018, 8, 14975. [Google Scholar] [CrossRef] [Green Version]
- Panganiban, G.; Rubenstein, J.L.R. Developmental Functions of the Distal-Less/Dlx Homeobox Genes. Development 2002, 129, 4371–4386. [Google Scholar] [CrossRef]
- Stock, D.W.; Ellies, D.L.; Zhao, Z.; Ekker, M.; Ruddle, F.H.; Weiss, K.M. The Evolution of the Vertebrate Dlx Gene Family. Proc. Natl. Acad. Sci. USA 1996, 93, 10858–10863. [Google Scholar] [CrossRef] [Green Version]
- Depew, M.J.; Simpson, C.A.; Morasso, M.; Rubenstein, J.L. Reassessing the Dlx Code: The Genetic Regulation of Branchial Arch Skeletal Pattern and Development. J. Anat. 2005, 207, 501–561. [Google Scholar] [CrossRef]
- Qiu, M.; Bulfone, A.; Martinez, S.; Meneses, J.J.; Shimamura, K.; Pedersen, R.A.; Rubenstein, J.L. Null Mutation of Dlx-2 Results in Abnormal Morphogenesis of Proximal First and Second Branchial Arch Derivatives and Abnormal Differentiation in the Forebrain. Genes Dev. 1995, 9, 2523–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, M.; Bulfone, A.; Ghattas, I.; Meneses, J.J.; Christensen, L.; Sharpe, P.T.; Presley, R.; Pedersen, R.A.; Rubenstein, J.L. Role of the Dlx Homeobox Genes in Proximodistal Patterning of the Branchial Arches: Mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 Alter Morphogenesis of Proximal Skeletal and Soft Tissue Structures Derived from the First and Second Arches. Dev. Biol. 1997, 185, 165–184. [Google Scholar] [CrossRef] [Green Version]
- Acampora, D.; Merlo, G.R.; Paleari, L.; Zerega, B.; Postiglione, M.P.; Mantero, S.; Bober, E.; Barbieri, O.; Simeone, A.; Levi, G. Craniofacial, Vestibular and Bone Defects in Mice Lacking the Distal-Less-Related Gene Dlx5. Development 1999, 126, 3795–3809. [Google Scholar] [CrossRef]
- Depew, M.J.; Liu, J.K.; Long, J.E.; Presley, R.; Meneses, J.J.; Pedersen, R.A.; Rubenstein, J.L. Dlx5 Regulates Regional Development of the Branchial Arches and Sensory Capsules. Development 1999, 126, 3831–3846. [Google Scholar] [CrossRef]
- Beverdam, A.; Merlo, G.R.; Paleari, L.; Mantero, S.; Genova, F.; Barbieri, O.; Janvier, P.; Levi, G. Jaw Transformation with Gain of Symmetry after Dlx5/Dlx6 Inactivation: Mirror of the Past? Genesis 2002, 34, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depew, M.J.; Lufkin, T.; Rubenstein, J.L.R. Specification of Jaw Subdivisions by Dlx Genes. Science 2002, 298, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Morasso, M.I.; Grinberg, A.; Robinson, G.; Sargent, T.D.; Mahon, K.A. Placental Failure in Mice Lacking the Homeobox Gene Dlx3. Proc. Natl. Acad. Sci. USA 1999, 96, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Li, X.; McEvilly, R.J.; Rosenfeld, M.G.; Lufkin, T.; Rubenstein, J.L.R. Dlx Genes Pattern Mammalian Jaw Primordium by Regulating Both Lower Jaw-Specific and Upper Jaw-Specific Genetic Programs. Development 2008, 135, 2905–2916. [Google Scholar] [CrossRef] [Green Version]
- Verzi, M.P.; Agarwal, P.; Brown, C.; McCulley, D.J.; Schwarz, J.J.; Black, B.L. The Transcription Factor MEF2C Is Required for Craniofacial Development. Dev. Cell 2007, 12, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Barron, F.; Woods, C.; Kuhn, K.; Bishop, J.; Howard, M.J.; Clouthier, D.E. Downregulation of Dlx5 and Dlx6 Expression by Hand2 Is Essential for Initiation of Tongue Morphogenesis. Development 2011, 138, 2249–2259. [Google Scholar] [CrossRef] [Green Version]
- Vincentz, J.W.; Casasnovas, J.J.; Barnes, R.M.; Que, J.; Clouthier, D.E.; Wang, J.; Firulli, A.B. Exclusion of Dlx5/6 Expression from the Distal-Most Mandibular Arches Enables BMP-Mediated Specification of the Distal Cap. Proc. Natl. Acad. Sci. USA 2016, 113, 7563–7568. [Google Scholar] [CrossRef] [Green Version]
- Funato, N.; Chapman, S.L.; McKee, M.D.; Funato, H.; Morris, J.A.; Shelton, J.M.; Richardson, J.A.; Yanagisawa, H. Hand2 Controls Osteoblast Differentiation in the Branchial Arch by Inhibiting DNA Binding of Runx2. Development 2009, 136, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, H.; Clouthier, D.E.; Richardson, J.A.; Charité, J.; Olson, E.N. Targeted Deletion of a Branchial Arch-Specific Enhancer Reveals a Role of DHAND in Craniofacial Development. Development 2003, 130, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.C.; Funato, N.; Chapman, S.; McKee, M.D.; Richardson, J.A.; Olson, E.N.; Yanagisawa, H. Hand Transcription Factors Cooperatively Regulate Development of the Distal Midline Mesenchyme. Dev. Biol. 2007, 310, 154–168. [Google Scholar] [CrossRef] [Green Version]
- Firulli, B.A.; Fuchs, R.K.; Vincentz, J.W.; Clouthier, D.E.; Firulli, A.B. Hand1 Phosphoregulation within the Distal Arch Neural Crest Is Essential for Craniofacial Morphogenesis. Development 2014, 141, 3050–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funato, N.; Kokubo, H.; Nakamura, M.; Yanagisawa, H.; Saga, Y. Specification of Jaw Identity by the Hand2 Transcription Factor. Sci. Rep. 2016, 6, 28405. [Google Scholar] [CrossRef]
- Nair, S.; Li, W.; Cornell, R.; Schilling, T.F. Requirements for Endothelin Type-A Receptors and Endothelin-1 Signaling in the Facial Ectoderm for the Patterning of Skeletogenic Neural Crest Cells in Zebrafish. Development 2007, 134, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ullmann, B.; Walker, M.; Miller, C.T.; Crump, J.G. Endothelin 1-Mediated Regulation of Pharyngeal Bone Development in Zebrafish. Development 2003, 130, 1339–1351. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.T.; Schilling, T.F.; Lee, K.; Parker, J.; Kimmel, C.B. Sucker Encodes a Zebrafish Endothelin-1 Required for Ventral Pharyngeal Arch Development. Development 2000, 127, 3815–3828. [Google Scholar] [CrossRef]
- Miller, C.T.; Yelon, D.; Stainier, D.Y.R.; Kimmel, C.B. Two Endothelin 1 Effectors, Hand2 and Bapx1, Pattern Ventral Pharyngeal Cartilage and the Jaw Joint. Development 2003, 130, 1353–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuniga, E.; Rippen, M.; Alexander, C.; Schilling, T.F.; Crump, J.G. Gremlin 2 Regulates Distinct Roles of BMP and Endothelin 1 Signaling in Dorsoventral Patterning of the Facial Skeleton. Development 2011, 138, 5147–5156. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.M.; Nichols, J.T.; Kimmel, C.B. Edn1 and Hand2 Interact in Early Regulation of Pharyngeal Arch Outgrowth during Zebrafish Development. PLoS ONE 2013, 8, e67522. [Google Scholar] [CrossRef] [Green Version]
- Talbot, J.C.; Johnson, S.L.; Kimmel, C.B. Hand2 and Dlx Genes Specify Dorsal, Intermediate and Ventral Domains within Zebrafish Pharyngeal Arches. Development 2010, 137, 2507–2517. [Google Scholar] [CrossRef] [Green Version]
- Alexander, C.; Zuniga, E.; Blitz, I.L.; Wada, N.; Le Pabic, P.; Javidan, Y.; Zhang, T.; Cho, K.W.; Crump, J.G.; Schilling, T.F. Combinatorial Roles for BMPs and Endothelin 1 in Patterning the Dorsal-Ventral Axis of the Craniofacial Skeleton. Development 2011, 138, 5135–5146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quint, E.; Zerucha, T.; Ekker, M. Differential Expression of Orthologous Dlx Genes in Zebrafish and Mice: Implications for the Evolution of the Dlx Homeobox Gene Family. J. Exp. Zool. 2000, 288, 235–241. [Google Scholar] [CrossRef]
- Trumpp, A.; Depew, M.J.; Rubenstein, J.L.R.; Bishop, J.M.; Martin, G.R. Cre-Mediated Gene Inactivation Demonstrates That FGF8 Is Required for Cell Survival and Patterning of the First Branchial Arch. Genes Dev. 1999, 13, 3136–3148. [Google Scholar] [CrossRef]
- Tucker, A.S.; Yamada, G.; Grigoriou, M.; Pachnis, V.; Sharpe, P.T. Fgf-8 Determines Rostral-Caudal Polarity in the First Branchial Arch. Development 1999, 126, 51–61. [Google Scholar] [CrossRef]
- Mina, M.; Kollar, E.J. The Induction of Odontogenesis in Non-Dental Mesenchyme Combined with Early Murine Mandibular Arch Epithelium. Arch. Oral Biol. 1987, 32, 123–127. [Google Scholar] [CrossRef]
- Lanctôt, C.; Moreau, A.; Chamberland, M.; Tremblay, M.L.; Drouin, J. Hindlimb Patterning and Mandible Development Require the Ptx1 Gene. Development 1999, 126, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.F.; Pressman, C.; Dyer, R.; Johnson, R.L.; Martin, J.F. Function of Rieger Syndrome Gene in Left-Right Asymmetry and Craniofacial Development. Nature 1999, 401, 276–278. [Google Scholar] [CrossRef]
- Liu, W.; Selever, J.; Lu, M.-F.; Martin, J.F. Genetic Dissection of Pitx2 in Craniofacial Development Uncovers New Functions in Branchial Arch Morphogenesis, Late Aspects of Tooth Morphogenesis and Cell Migration. Development 2003, 130, 6375–6385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Liu, H.; Lan, Y.; Adam, M.; Clouthier, D.E.; Potter, S.; Jiang, R. Hedgehog Signaling Patterns the Oral-Aboral Axis of the Mandibular Arch. eLife 2019, 8, e40315. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Mao, J.; Tenzen, T.; Kottmann, A.H.; McMahon, A.P. Hedgehog Signaling in the Neural Crest Cells Regulates the Patterning and Growth of Facial Primordia. Genes Dev. 2004, 18, 937–951. [Google Scholar] [CrossRef] [Green Version]
- Tucker, A.S.; Matthews, K.L.; Sharpe, P.T. Transformation of Tooth Type Induced by Inhibition of BMP Signaling. Science 1998, 282, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.; Boglev, Y.; Owens, H.; Goldie, S.J. The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival. J. Dev. Biol. 2016, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Xavier, G.M.; Seppala, M.; Barrell, W.; Birjandi, A.A.; Geoghegan, F.; Cobourne, M.T. Hedgehog Receptor Function during Craniofacial Development. Dev. Biol. 2016, 415, 198–215. [Google Scholar] [CrossRef] [Green Version]
- Billmyre, K.K.; Klingensmith, J. Sonic Hedgehog from Pharyngeal Arch 1 Epithelium Is Necessary for Early Mandibular Arch Cell Survival and Later Cartilage Condensation Differentiation. Dev. Dyn. 2015, 244, 564–576. [Google Scholar] [CrossRef]
- Millington, G.; Elliott, K.H.; Chang, Y.-T.; Chang, C.-F.; Dlugosz, A.; Brugmann, S.A. Cilia-Dependent GLI Processing in Neural Crest Cells Is Required for Tongue Development. Dev. Biol. 2017, 424, 124–137. [Google Scholar] [CrossRef]
- Yamagishi, C.; Yamagishi, H.; Maeda, J.; Tsuchihashi, T.; Ivey, K.; Hu, T.; Srivastava, D. Sonic Hedgehog Is Essential for First Pharyngeal Arch Development. Pediatr. Res. 2006, 59, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Brito, J.M.; Teillet, M.-A.; Le Douarin, N.M. Induction of Mirror-Image Supernumerary Jaws in Chicken Mandibular Mesenchyme by Sonic Hedgehog-Producing Cells. Development 2008, 135, 2311–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haworth, K.E.; Wilson, J.M.; Grevellec, A.; Cobourne, M.T.; Healy, C.; Helms, J.A.; Sharpe, P.T.; Tucker, A.S. Sonic Hedgehog in the Pharyngeal Endoderm Controls Arch Pattern via Regulation of Fgf8 in Head Ectoderm. Dev. Biol. 2007, 303, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, R.; Kawakami, H.; Taketo, M.M.; Evans, S.M.; Wada, N.; Petryk, A.; Kawakami, Y. Distinct Populations within Isl1 Lineages Contribute to Appendicular and Facial Skeletogenesis through the β-Catenin Pathway. Dev. Biol. 2014, 387, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Bu, L.; Cai, C.-L.; Zhang, X.; Evans, S. Isl1 Is Upstream of Sonic Hedgehog in a Pathway Required for Cardiac Morphogenesis. Dev. Biol. 2006, 295, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Teng, I.; Huo, R.; Rosenfeld, M.G.; Olson, L.E.; Li, X.; Li, X. Asymmetric Requirement of Surface Epithelial β-Catenin during the Upper and Lower Jaw Development. Dev. Dyn. 2012, 241, 663–674. [Google Scholar] [CrossRef]
- Li, F.; Fu, G.; Liu, Y.; Miao, X.; Li, Y.; Yang, X.; Zhang, X.; Yu, D.; Gan, L.; Qiu, M.; et al. ISLET1-Dependent β-Catenin/Hedgehog Signaling Is Required for Outgrowth of the Lower Jaw. Mol. Cell. Biol. 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crump, J.G.; Maves, L.; Lawson, N.D.; Weinstein, B.M.; Kimmel, C.B. An Essential Role for Fgfs in Endodermal Pouch Formation Influences Later Craniofacial Skeletal Patterning. Development 2004, 131, 5703–5716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperber, S.M.; Dawid, I.B. Barx1 Is Necessary for Ectomesenchyme Proliferation and Osteochondroprogenitor Condensation in the Zebrafish Pharyngeal Arches. Dev. Biol. 2008, 321, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, S. Evolution of the Structure and Function of the Vertebrate Tongue. J. Anat. 2002, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.M.; Leung, K.K.; Wheatley, S.C.; Ng, L.J.; Zhou, S.; Ling, K.W.; Sham, M.H.; Koopman, P.; Tam, P.P.; Cheah, K.S. SOX9 Directly Regulates the Type-II Collagen Gene. Nat. Genet. 1997, 16, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 Is Required for Cartilage Formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.H.; Beddington, R.S.P.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a Candidate Gene for Cleidocranial Dysplasia Syndrome, Is Essential for Osteoblast Differentiation and Bone Development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Brault, V.; Moore, R.; Kutsch, S.; Ishibashi, M.; Rowitch, D.H.; McMahon, A.P.; Sommer, L.; Boussadia, O.; Kemler, R. Inactivation of the Beta-Catenin Gene by Wnt1-Cre-Mediated Deletion Results in Dramatic Brain Malformation and Failure of Craniofacial Development. Development 2001, 128, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/Beta-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Goodnough, L.H.; DiNuoscio, G.J.; Ferguson, J.W.; Williams, T.; Lang, R.A.; Atit, R.P. Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors. PLoS Genet. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, V.; Huang, W.; Harley, V.R.; Goodfellow, P.N.; de Crombrugghe, B. SOX9 Is a Potent Activator of the Chondrocyte-Specific Enhancer of the pro Alpha1(II) Collagen Gene. Mol. Cell Biol. 1997, 17, 2336–2346. [Google Scholar] [CrossRef] [Green Version]
- Mori-Akiyama, Y.; Akiyama, H.; Rowitch, D.H.; de Crombrugghe, B. Sox9 Is Required for Determination of the Chondrogenic Cell Lineage in the Cranial Neural Crest. Proc. Natl. Acad. Sci. USA 2003, 100, 9360–9365. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wlodarczyk, B.J.; Niederreither, K.; Venugopalan, S.; Florez, S.; Finnell, R.H.; Amendt, B.A. Fuz Regulates Craniofacial Development through Tissue Specific Responses to Signaling Factors. PLoS ONE 2011, 6, e24608. [Google Scholar] [CrossRef] [Green Version]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.-H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation Owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Shirai, Y.; Kawabe, K.; Tosa, I.; Tsukamoto, S.; Yamada, D.; Takarada, T. Runx2 Function in Cells of Neural Crest Origin during Intramembranous Ossification. Biochem. Biophys. Res. Commun. 2019, 509, 1028–1033. [Google Scholar] [CrossRef]
- Shibata, S.; Suda, N.; Yoda, S.; Fukuoka, H.; Ohyama, K.; Yamashita, Y.; Komori, T. Runx2-Deficient Mice Lack Mandibular Condylar Cartilage and Have Deformed Meckel’s Cartilage. Anat. Embryol. 2004, 208, 273–280. [Google Scholar] [CrossRef]
- Baek, W.-Y.; Kim, Y.-J.; de Crombrugghe, B.; Kim, J.-E. Osterix Is Required for Cranial Neural Crest-Derived Craniofacial Bone Formation. Biochem. Biophys. Res. Commun. 2013, 432, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Semba, I.; Nonaka, K.; Takahashi, I.; Takahashi, K.; Dashner, R.; Shum, L.; Nuckolls, G.H.; Slavkin, H.C. Positionally-Dependent Chondrogenesis Induced by BMP4 Is Co-Regulated by Sox9 and Msx2. Dev. Dyn. 2000, 217, 401–414. [Google Scholar] [CrossRef]
- Garcia-Miñaur, S.; Mavrogiannis, L.A.; Rannan-Eliya, S.V.; Hendry, M.A.; Liston, W.A.; Porteous, M.E.M.; Wilkie, A.O.M. Parietal Foramina with Cleidocranial Dysplasia Is Caused by Mutation in MSX2. Eur. J. Hum. Genet. 2003, 11, 892–895. [Google Scholar] [CrossRef] [Green Version]
- Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Loss of Tbx1 Induces Bone Phenotypes Similar to Cleidocranial Dysplasia. Hum. Mol. Genet. 2015, 24, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Jabs, E.W.; Müller, U.; Li, X.; Ma, L.; Luo, W.; Haworth, I.S.; Klisak, I.; Sparkes, R.; Warman, M.L.; Mulliken, J.B. A Mutation in the Homeodomain of the Human MSX2 Gene in a Family Affected with Autosomal Dominant Craniosynostosis. Cell 1993, 75, 443–450. [Google Scholar] [CrossRef]
- Liu, Y.H.; Kundu, R.; Wu, L.; Luo, W.; Ignelzi, M.A.; Snead, M.L.; Maxson, R.E. Premature Suture Closure and Ectopic Cranial Bone in Mice Expressing Msx2 Transgenes in the Developing Skull. Proc. Natl. Acad. Sci. USA 1995, 92, 6137–6141. [Google Scholar] [CrossRef] [Green Version]
- Wilkie, A.O.M.; Tang, Z.; Elanko, N.; Walsh, S.; Twigg, S.R.F.; Hurst, J.A.; Wall, S.A.; Chrzanowska, K.H.; Maxson, R.E. Functional Haploinsufficiency of the Human Homeobox Gene MSX2 Causes Defects in Skull Ossification. Nat. Genet. 2000, 24, 387–390. [Google Scholar] [CrossRef]
- Jumlongras, D.; Bei, M.; Stimson, J.M.; Wang, W.-F.; DePalma, S.R.; Seidman, C.E.; Felbor, U.; Maas, R.; Seidman, J.G.; Olsen, B.R. A Nonsense Mutation in MSX1 Causes Witkop Syndrome. Am. J. Hum. Genet. 2001, 69, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Winograd, J.; Reilly, M.P.; Roe, R.; Lutz, J.; Laughner, E.; Xu, X.; Hu, L.; Asakura, T.; vander Kolk, C.; Strandberg, J.D.; et al. Perinatal Lethality and Multiple Craniofacial Malformations in MSX2 Transgenic Mice. Hum. Mol. Genet. 1997, 6, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satokata, I.; Maas, R. Msx1 Deficient Mice Exhibit Cleft Palate and Abnormalities of Craniofacial and Tooth Development. Nat. Genet. 1994, 6, 348–356. [Google Scholar] [CrossRef]
- Ishii, M.; Han, J.; Yen, H.-Y.; Sucov, H.M.; Chai, Y.; Maxson, R.E., Jr. Combined Deficiencies of Msx1 and Msx2 Cause Impaired Patterning and Survival of the Cranial Neural Crest. Development 2005, 132, 4937–4950. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Li, J.; Engleka, K.A.; Zhou, B.; Lu, M.M.; Plotkin, J.B.; Epstein, J.A. Persistent Expression of Pax3 in the Neural Crest Causes Cleft Palate and Defective Osteogenesis in Mice. J. Clin. Investig. 2008, 118, 2076–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, P.; Dietrich, S.; Mericskay, M.; Schubert, F.R.; Li, Z.; Paulin, D. A Crucial Role for Pax3 in the Development of the Hypaxial Musculature and the Long-Range Migration of Muscle Precursors. Dev. Biol. 1998, 203, 49–61. [Google Scholar] [CrossRef]
- Parry, D.A.; Logan, C.V.; Stegmann, A.P.A.; Abdelhamed, Z.A.; Calder, A.; Khan, S.; Bonthron, D.T.; Clowes, V.; Sheridan, E.; Ghali, N.; et al. SAMS, a Syndrome of Short Stature, Auditory-Canal Atresia, Mandibular Hypoplasia, and Skeletal Abnormalities Is a Unique Neurocristopathy Caused by Mutations in Goosecoid. Am. J. Hum. Genet. 2013, 93, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaunt, S.J.; Blum, M.; De Robertis, E.M. Expression of the Mouse Goosecoid Gene during Mid-Embryogenesis May Mark Mesenchymal Cell Lineages in the Developing Head, Limbs and Body Wall. Development 1993, 117, 769–778. [Google Scholar] [CrossRef]
- Rivera-Perez, J.A.; Mallo, M.; Gendron-Maguire, M.; Gridley, T.; Behringer, R.R. Goosecoid Is Not an Essential Component of the Mouse Gastrula Organizer but Is Required for Craniofacial and Rib Development. Development 1995, 121, 3005–3012. [Google Scholar] [CrossRef]
- Yamada, G.; Ueno, K.; Nakamura, S.; Hanamure, Y.; Yasui, K.; Uemura, M.; Eizuru, Y.; Mansouri, A.; Blum, M.; Sugimura, K. Nasal and Pharyngeal Abnormalities Caused by the Mouse Goosecoid Gene Mutation. Biophys. Res. Commun. 1997, 233, 161–165. [Google Scholar] [CrossRef]
- Cserjesi, P.; Lilly, B.; Bryson, L.; Wang, Y.; Sassoon, D.A.; Olson, E.N. MHox: A Mesodermally Restricted Homeodomain Protein That Binds an Essential Site in the Muscle Creatine Kinase Enhancer. Development 1992, 115, 1087–1101. [Google Scholar] [CrossRef]
- Opstelten, D.-J.E.; Vogels, R.; Robert, B.; Kalkhoven, E.; Zwartkruis, F.; de Laaf, L.; Destrée, O.H.; Deschamps, J.; Lawson, K.A.; Meijlink, F. The Mouse Homeobox Gene, S8, Is Expressed during Embryogenesis Predominantly in Mesenchyme. Mech. Dev. 1991, 34, 29–41. [Google Scholar] [CrossRef]
- Martin, J.F.; Bradley, A.; Olson, E.N. The Paired-like Homeo Box Gene MHox Is Required for Early Events of Skeletogenesis in Multiple Lineages. Genes Dev. 1995, 9, 1237–1249. [Google Scholar] [CrossRef] [Green Version]
- ten Berge, D.; Brouwer, A.; Korving, J.; Reijnen, M.J.; van Raaij, E.J.; Verbeek, F.; Gaffield, W.; Meijlink, F. Prx1 and Prx2 Are Upstream Regulators of Sonic Hedgehog and Control Cell Proliferation during Mandibular Arch Morphogenesis. Development 2001, 128, 2929–2938. [Google Scholar] [CrossRef]
- Balic, A.; Adams, D.; Mina, M. Prx1 and Prx2 Cooperatively Regulate the Morphogenesis of the Medial Region of the Mandibular Process. Dev. Dyn. 2009, 238, 2599–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çelik, T.; Simsek, P.O.; Sozen, T.; Ozyuncu, O.; Utine, G.E.; Talim, B.; Yiğit, Ş.; Boduroglu, K.; Kamnasaran, D. PRRX1 Is Mutated in an Otocephalic Newborn Infant Conceived by Consanguineous Parents. Clin. Genet. 2012, 81, 294–297. [Google Scholar] [CrossRef]
- Dasouki, M.; Andrews, B.; Parimi, P.; Kamnasaran, D. Recurrent Agnathia–Otocephaly Caused by DNA Replication Slippage in PRRX1. Am. J. Med. Genet. Part A 2013, 161, 803–808. [Google Scholar] [CrossRef]
- Donnelly, M.; Todd, E.; Wheeler, M.; Winn, V.D.; Kamnasaran, D. Prenatal Diagnosis and Identification of Heterozygous Frameshift Mutation in PRRX1 in an Infant with Agnathia-Otocephaly. Prenat. Diagn. 2012, 32, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.; Delio, M.; Morrow, B.; Samanich, J. Agnathia–Otocephaly Complex: A Case Report and Examination of the OTX2 and PRRX1 Genes. Gene 2012, 494, 124–129. [Google Scholar] [CrossRef]
- Sergi, C.; Kamnasaran, D. PRRX1 Is Mutated in a Fetus with Agnathia-Otocephaly. Clin. Genet. 2011, 79, 293–295. [Google Scholar] [CrossRef]
- Shibukawa, Y.; Young, B.; Wu, C.; Yamada, S.; Long, F.; Pacifici, M.; Koyama, E. Temporomandibular Joint Formation and Condyle Growth Require Indian Hedgehog Signaling. Dev. Dyn. 2007, 236, 426–434. [Google Scholar] [CrossRef]
- Sugito, H.; Shibukawa, Y.; Kinumatsu, T.; Yasuda, T.; Nagayama, M.; Yamada, S.; Minugh-Purvis, N.; Pacifici, M.; Koyama, E. Ihh Signaling Regulates Mandibular Symphysis Development and Growth. J. Dent. Res. 2011, 90, 625–631. [Google Scholar] [CrossRef]
- Yang, L.; Gu, S.; Ye, W.; Song, Y.; Chen, Y. Augmented Indian Hedgehog Signaling in Cranial Neural Crest Cells Leads to Craniofacial Abnormalities and Dysplastic Temporomandibular Joint in Mice. Cell Tissue Res. 2016, 364, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Bechtold, T.E.; Kurio, N.; Nah, H.-D.; Saunders, C.; Billings, P.C.; Koyama, E. The Roles of Indian Hedgehog Signaling in TMJ Formation. Int. J. Mol. Sci 2019, 20, 6300. [Google Scholar] [CrossRef] [Green Version]
- Bertolacini, C.D.P.; Ribeiro-Bicudo, L.A.; Petrin, A.; Richieri-Costa, A.; Murray, J.C. Clinical Findings in Patients with GLI2 Mutations--Phenotypic Variability. Clin. Genet. 2012, 81, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Y.; Mah, A.; Crohin, C.; Groff, S.; Bringas, P.; Le, T.; Santos, V.; Slavkin, H.C. Specific Transforming Growth Factor-Beta Subtypes Regulate Embryonic Mouse Meckel’s Cartilage and Tooth Development. Dev. Biol. 1994, 162, 85–103. [Google Scholar] [CrossRef]
- Zhao, H.; Oka, K.; Bringas, P.; Kaartinen, V.; Chai, Y. TGF-Beta Type I Receptor Alk5 Regulates Tooth Initiation and Mandible Patterning in a Type II Receptor-Independent Manner. Dev. Biol. 2008, 320, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Dudas, M.; Kim, J.; Li, W.-Y.; Nagy, A.; Larsson, J.; Karlsson, S.; Chai, Y.; Kaartinen, V. Epithelial and Ectomesenchymal Role of the Type I TGF-β Receptor ALK5 during Facial Morphogenesis and Palatal Fusion. Dev. Biol. 2006, 296, 298–314. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Yeo, J.Y.; Chytil, A.; Han, J.; Bringas, P.; Nakajima, A.; Shuler, C.F.; Moses, H.L.; Chai, Y. Conditional Inactivation of Tgfbr2 in Cranial Neural Crest Causes Cleft Palate and Calvaria Defects. Development 2003, 130, 5269–5280. [Google Scholar] [CrossRef] [Green Version]
- Iwata, J.; Parada, C.; Chai, Y. The Mechanism of TGF-β Signaling during Palate Development. Oral Dis. 2011, 17, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Oka, K.; Oka, S.; Hosokawa, R.; Bringas, P.; Brockhoff, H.C.; Nonaka, K.; Chai, Y. TGF-β Mediated Dlx5 Signaling Plays a Crucial Role in Osteo-Chondroprogenitor Cell Lineage Determination during Mandible Development. Dev. Biol. 2008, 321, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stottmann, R.W.; Anderson, R.M.; Klingensmith, J. The BMP Antagonists Chordin and Noggin Have Essential but Redundant Roles in Mouse Mandibular Outgrowth. Dev. Biol. 2001, 240, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zheng, Y.; Chen, D.; Chen, Y. Enhanced BMP Signaling Prevents Degeneration and Leads to Endochondral Ossification of Meckel′s Cartilage in Mice. Dev. Biol. 2013, 381, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lana-Elola, E.; Tylzanowski, P.; Takatalo, M.; Alakurtti, K.; Veistinen, L.; Mitsiadis, T.A.; Graf, D.; Rice, R.; Luyten, F.P.; Rice, D.P. Noggin Null Allele Mice Exhibit a Microform of Holoprosencephaly. Hum. Mol. Genet. 2011, 20, 4005–4015. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Hu, X.; Xiong, W.; Li, L.; Lin, L.; Shen, B.; Yang, L.; Gu, S.; Zhang, Y.; Chen, Y. Directed Bmp4 Expression in Neural Crest Cells Generates a Genetic Model for the Rare Human Bony Syngnathia Birth Defect. Dev. Biol 2014, 391, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, Z.; Chen, Y.; Zhang, Y. Conditional Deletion of Bmp2 in Cranial Neural Crest Cells Recapitulates Pierre Robin Sequence in Mice. Cell Tissue Res. 2019, 376, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Claudio, M.; Wang, J.; Bai, Y.; Klysik, E.; Selever, J.; Martin, J.F. Bmp Signaling Regulates a Dose-Dependent Transcriptional Program to Control Facial Skeletal Development. Development 2012, 139, 709–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.M.; Marker, P.C.; Peng, H.; Quintero, A.J.; Kingsley, D.M.; Huard, J. Dominant Negative Bmp5mutation Reveals Key Role of BMPs in Skeletal Response to Mechanical Stimulation. BMC Dev. Biol. 2008, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouskoura, T.; Kozlova, A.; Alexiou, M.; Blumer, S.; Zouvelou, V.; Katsaros, C.; Chiquet, M.; Mitsiadis, T.A.; Graf, D. The Etiology of Cleft Palate Formation in BMP7-Deficient Mice. PLoS ONE 2013, 8, e59463. [Google Scholar] [CrossRef] [Green Version]
- Trokovic, N.; Trokovic, R.; Mai, P.; Partanen, J. Fgfr1 Regulates Patterning of the Pharyngeal Region. Genes Dev. 2003, 17, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Trokovic, N.; Trokovic, R.; Partanen, J. Fibroblast Growth Factor Signalling and Regional Specification of the Pharyngeal Ectoderm. Int. J. Dev. Biol. 2005, 49, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Hoch, R.V.; Soriano, P. Context-Specific Requirements for Fgfr1 Signaling through Frs2 and Frs3 during Mouse Development. Development 2006, 133, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Kameda, Y.; Ito, M.; Nishimaki, T.; Gotoh, N. FRS2alpha Is Required for the Separation, Migration, and Survival of Pharyngeal-Endoderm Derived Organs Including Thyroid, Ultimobranchial Body, Parathyroid, and Thymus. Dev. Dyn. 2009, 238, 503–513. [Google Scholar] [CrossRef]
- Jackson, A.; Kasah, S.; Mansour, S.L.; Morrow, B.; Basson, M.A. Endoderm-Specific Deletion of Tbx1 Reveals an FGF-Independent Role for Tbx1 in Pharyngeal Apparatus Morphogenesis. Dev. Dyn. 2014, 243, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Chang, J.Y.F.; Yang, C.; Huang, Y.; Liu, J.; You, P.; McKeehan, W.L.; Wang, F.; Li, X. Type 1 Fibroblast Growth Factor Receptor in Cranial Neural Crest Cell-Derived Mesenchyme Is Required for Palatogenesis. J. Biol. Chem. 2013, 288, 22174–22183. [Google Scholar] [CrossRef] [Green Version]
- Motch Perrine, S.M.; Wu, M.; Stephens, N.B.; Kriti, D.; van Bakel, H.; Jabs, E.W.; Richtsmeier, J.T. Mandibular Dysmorphology Due to Abnormal Embryonic Osteogenesis in FGFR2-Related Craniosynostosis Mice. Dis. Models Mech. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Biosse Duplan, M.; Komla-Ebri, D.; Heuzé, Y.; Estibals, V.; Gaudas, E.; Kaci, N.; Benoist-Lasselin, C.; Zerah, M.; Kramer, I.; Kneissel, M.; et al. Meckel’s and Condylar Cartilages Anomalies in Achondroplasia Result in Defective Development and Growth of the Mandible. Hum. Mol. Genet. 2016, 25, 2997–3010. [Google Scholar] [CrossRef] [Green Version]
- Melnick, M.; Witcher, D.; Bringas, P.; Carlsson, P.; Jaskoll, T. Meckel’s Cartilage Differentiation Is Dependent on Hedgehog Signaling. Cells Tissues Organs 2005, 179, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Liu, C.; Song, Y.; Ye, W.; He, W.; Yuan, G.; Gu, S.; Lin, C.; Ma, L.; Zhang, Y.; et al. FGF8 Signaling Sustains Progenitor Status and Multipotency of Cranial Neural Crest-Derived Mesenchymal Cells in Vivo and in Vitro. J. Mol. Cell Biol. 2015, 7, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Issa, R.; Smyth, G.; Smoak, I.; Yamamura, K.; Meyers, E.N. Fgf8 Is Required for Pharyngeal Arch and Cardiovascular Development in the Mouse. Development 2002, 129, 4613–4625. [Google Scholar] [CrossRef]
- Terao, F.; Takahashi, I.; Mitani, H.; Haruyama, N.; Sasano, Y.; Suzuki, O.; Takano-Yamamoto, T. Fibroblast Growth Factor 10 Regulates Meckel’s Cartilage Formation during Early Mandibular Morphogenesis in Rats. Dev. Biol. 2011, 350, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Cruz, C.V.; Mattos, C.T.; Maia, J.C.; Granjeiro, J.M.; Reis, M.F.; Mucha, J.N.; Vilella, B.; Ruellas, A.C.; Luiz, R.R.; Costa, M.C.; et al. Genetic Polymorphisms Underlying the Skeletal Class III Phenotype. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Ayada, T.; Ichiyama, K.; Kohno, R.-I.; Yonemitsu, Y.; Minami, Y.; Kikuchi, A.; Maehara, Y.; Yoshimura, A. Sprouty2 and Sprouty4 Are Essential for Embryonic Morphogenesis and Regulation of FGF Signaling. Biochem. Biophys. Res. Commun. 2007, 352, 896–902. [Google Scholar] [CrossRef]
- Curtin, E.; Hickey, G.; Kamel, G.; Davidson, A.J.; Liao, E.C. Zebrafish Wnt9a Is Expressed in Pharyngeal Ectoderm and Is Required for Palate and Lower Jaw Development. Mech. Dev. 2011, 128, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kamel, G.; Hoyos, T.; Rochard, L.; Dougherty, M.; Kong, Y.; Tse, W.; Shubinets, V.; Grimaldi, M.; Liao, E.C. Requirement for Frzb and Fzd7a in Cranial Neural Crest Convergence and Extension Mechanisms during Zebrafish Palate and Jaw Morphogenesis. Dev. Biol. 2013, 381, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; He, Y.; Gui, J.-F.; Mei, J. Sox9a, Not Sox9b Is Required for Normal Cartilage Development in Zebrafish. Aquac. Fish. 2021, 6, 254–259. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Willoughby, J.; Liu, D.; Crump, J.G.; Wilson, C.; Miller, C.T.; Singer, A.; Kimmel, C.; Westerfield, M.; Postlethwait, J.H. A Pair of Sox: Distinct and Overlapping Functions of Zebrafish Sox9 Co-Orthologs in Craniofacial and Pectoral Fin Development. Development 2005, 132, 1069–1083. [Google Scholar] [CrossRef] [Green Version]
- Flores, M.V.; Tsang, V.W.K.; Hu, W.; Kalev-Zylinska, M.; Postlethwait, J.; Crosier, P.; Crosier, K.; Fisher, S. Duplicate Zebrafish Runx2 Orthologues Are Expressed in Developing Skeletal Elements. Gene Expr. Patterns 2004, 4, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.V.; Lam, E.Y.N.; Crosier, P.; Crosier, K. A Hierarchy of Runx Transcription Factors Modulate the Onset of Chondrogenesis in Craniofacial Endochondral Bones in Zebrafish. Dev. Dyn. 2006, 235, 3166–3176. [Google Scholar] [CrossRef]
- Felber, K.; Elks, P.M.; Lecca, M.; Roehl, H.H. Expression of Osterix Is Regulated by FGF and Wnt/β-Catenin Signalling during Osteoblast Differentiation. PLoS ONE 2015, 10, e0144982. [Google Scholar] [CrossRef] [Green Version]
- Dalcq, J.; Pasque, V.; Ghaye, A.; Larbuisson, A.; Motte, P.; Martial, J.A.; Muller, M. RUNX3, EGR1 and SOX9B Form a Regulatory Cascade Required to Modulate BMP-Signaling during Cranial Cartilage Development in Zebrafish. PLoS ONE 2012, 7, e50140. [Google Scholar] [CrossRef] [Green Version]
- Nakada, C.; Iida, A.; Tabata, Y.; Watanabe, S. Forkhead Transcription Factor Foxe1 Regulates Chondrogenesis in Zebrafish. J. Exp. Zool. 2009, 312B, 827–840. [Google Scholar] [CrossRef]
- Li, N.; Felber, K.; Elks, P.; Croucher, P.; Roehl, H.H. Tracking Gene Expression during Zebrafish Osteoblast Differentiation. Dev. Dyn. 2009, 238, 459–466. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Z.; Yang, J.; Huang, J.; Jiang, H. Sp7/Osterix Positively Regulates Dlx2b and Bglap to Affect Tooth Development and Bone Mineralization in Zebrafish Larvae. J. Biosci. 2019, 44, 127. [Google Scholar] [CrossRef]
- Niu, P.; Zhong, Z.; Wang, M.; Huang, G.; Xu, S.; Hou, Y.; Yan, Y.; Wang, H. Zinc Finger Transcription Factor Sp7/Osterix Acts on Bone Formation and Regulates Col10a1a Expression in Zebrafish. Sci. Bull. 2017, 62, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Eberhart, J.K.; Swartz, M.E.; Crump, J.G.; Kimmel, C.B. Early Hedgehog Signaling from Neural to Oral Epithelium Organizes Anterior Craniofacial Development. Development 2006, 133, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwend, T.; Ahlgren, S.C. Zebrafish Con/Disp1 Reveals Multiple Spatiotemporal Requirements for Hedgehog-Signaling in Craniofacial Development. BMC Dev. Biol. 2009, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felber, K.; Croucher, P.; Roehl, H.H. Hedgehog Signalling Is Required for Perichondral Osteoblast Differentiation in Zebrafish. Mech. Dev. 2011, 128, 141–152. [Google Scholar] [CrossRef]
- Hammond, C.L.; Schulte-Merker, S. Two Populations of Endochondral Osteoblasts with Differential Sensitivity to Hedgehog Signalling. Development 2009, 136, 3991–4000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huycke, T.R.; Eames, B.F.; Kimmel, C.B. Hedgehog-Dependent Proliferation Drives Modular Growth during Morphogenesis of a Dermal Bone. Development 2012, 139, 2371–2380. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Chen, B.; Zhao, Q. Hedgehog Signaling Regulates Osteoblast Differentiation in Zebrafish Larvae through Modulation of Autophagy. Biol. Open 2019, 8, bio040840. [Google Scholar] [CrossRef] [Green Version]
- Barske, L.; Askary, A.; Zuniga, E.; Balczerski, B.; Bump, P.; Nichols, J.T.; Crump, J.G. Competition between Jagged-Notch and Endothelin1 Signaling Selectively Restricts Cartilage Formation in the Zebrafish Upper Face. PLoS Genet. 2016, 12, e1005967. [Google Scholar] [CrossRef] [Green Version]
- Swartz, M.E.; Sheehan-Rooney, K.; Dixon, M.J.; Eberhart, J.K. Examination of a Palatogenic Gene Program in Zebrafish. Dev. Dyn. 2011, 240, 2204–2220. [Google Scholar] [CrossRef] [Green Version]
- Cheah, F.S.H.; Winkler, C.; Jabs, E.W.; Chong, S.S. Tgfβ3 Regulation of Chondrogenesis and Osteogenesis in Zebrafish Is Mediated through Formation and Survival of a Subpopulation of the Cranial Neural Crest. Mech. Dev. 2010, 127, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Windhausen, T.; Squifflet, S.; Renn, J.; Muller, M. BMP Signaling Regulates Bone Morphogenesis in Zebrafish through Promoting Osteoblast Function as Assessed by Their Nitric Oxide Production. Molecules 2015, 20, 7586–7601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.T.; Kwon, H.-J.; Melton, C.; Houghtaling, P.; Fritz, A.; Riley, B.B. Zebrafish MsxB, MsxC and MsxE Function Together to Refine the Neural–Nonneural Border and Regulate Cranial Placodes and Neural Crest Development. Dev. Biol. 2006, 294, 376–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Viscerocranium | Mouse | Zebrafish | ||
|---|---|---|---|---|
| Cartilaginous | Membranous | Cartilaginous | Membranous | |
| First pharyngeal arch (the mandibular) | Palatoquadrate cartilage: Alisphenoid Incus | Premaxilla Maxilla Zygomatic bone Temporal squama | Palatoquadrate cartilage: Quadrate Metapterygoids Palatines | Premaxilla Maxilla Ectopterygoid Entopterygoid |
| Meckel’s cartilage: Mandibular symphysis Lingula of mandible Sphenomandibular ligament Spine of sphenoid Anterior ligament of malleus Malleus | Dentary bone | Meckel’s cartilage: Retroarticular | Dentary Anguloarticular Coronomeckelian | |
| Second pharyngeal arch (the hyoid) | Stapes Styloid process of the temporal bone Stylohyoid ligament Lesser horns of the hyoid bone | Basihyal Ceratohyal Epihyal Hypohyal Hyomandibula Interhyal Symplectic | Urohyals Branchiostegal rays Interopercle Opercles Preopercles Subopercles | |
| Third pharyngeal arch | Greater horns of the hyoid bone | Basibranchials Ceratobranchials Epibranchials Hypobranchials Pharyngobranchials | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabik, J.; Psutkova, V.; Machon, O. The Mandibular and Hyoid Arches—From Molecular Patterning to Shaping Bone and Cartilage. Int. J. Mol. Sci. 2021, 22, 7529. https://doi.org/10.3390/ijms22147529
Fabik J, Psutkova V, Machon O. The Mandibular and Hyoid Arches—From Molecular Patterning to Shaping Bone and Cartilage. International Journal of Molecular Sciences. 2021; 22(14):7529. https://doi.org/10.3390/ijms22147529
Chicago/Turabian StyleFabik, Jaroslav, Viktorie Psutkova, and Ondrej Machon. 2021. "The Mandibular and Hyoid Arches—From Molecular Patterning to Shaping Bone and Cartilage" International Journal of Molecular Sciences 22, no. 14: 7529. https://doi.org/10.3390/ijms22147529






