Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Fillers
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.2. UV–Vis Spectroscopy
2.1.3. Thermogravimetric Analysis
2.1.4. Wetting and Contact Angle
2.1.5. The Morphology of Fillers
2.2. Characterization of Composites
2.2.1. Rheological Properties of Rubber Mixtures
2.2.2. FTIR Analysis of Composites
2.2.3. Thermal Stability of Biocomposites (TGA)
2.2.4. Morphology of Composites (SEM)
2.2.5. Barrier Properties
2.2.6. Cross-Linking Density before and after Aging Processes
2.2.7. Mechanical Properties before and after Aging Processes
2.2.8. Colour Change
3. Materials and Methods
3.1. Materials
3.2. Preparation of Rubber Mixtures
3.3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Anderson, D.P.; Chang, P.R.; Hua, Y. Bamboo fiber and its reinforced composites: Structure and properties. Cellulose 2012, 19, 1449–1480. [Google Scholar] [CrossRef]
- Le Duigou, A.; Castro, M.; Bevan, R.; Martin, N. 3D printing of wood fibre biocomposites: From mechanical to actuation functionality. Mater. Des. 2016, 96, 106–114. [Google Scholar] [CrossRef]
- Yıldızhan, Ş.; Çalık, A.; Özcanlı, M.; Serin, H. Bio-composite materials: A short review of recent trends, mechanical and chemical properties, and applications. Eur. Mech. Sci. 2018, 2, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Progress Report on Natural Fiber Reinforced Composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- Drzal, M.L.T.; Mohanty, A.; Misra, K. Bio-Composite Materials As Alternatives To Petroleum-Based Composites for Automotive Applications. Magnesium 2001, 40, 1–3. [Google Scholar]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Dong, Y.; Ghataura, A.; Takagi, H.; Haroosh, H.J.; Nakagaito, A.N.; Lau, K.-T. Polylactic acid (PLA) biocomposites reinforced with coir fibres: Evaluation of mechanical performance and multifunctional properties. Compos. Part A Appl. Sci. Manuf. 2014, 63, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Balla, V.; Kate, K.H.; Satyavolu, J.; Singh, P.; Tadimeti, J.G.D. Additive manufacturing of natural fiber reinforced polymer composites: Processing and prospects. Compos. Part B Eng. 2019, 174, 106956. [Google Scholar] [CrossRef]
- Van Beilen, J.B.; Poirier, Y. Production of renewable polymers from crop plants. Plant J. 2008, 54, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Egwaikhide, P.A.; Akporhonor, E.E.; Okieimen, F.E. Effect of coconut fibre filler on the cure characteristics physicomechanical and swelling properties of natural rubber vulcanisates. Int. J. Phys. Sci. 2007, 2, 39–46. [Google Scholar]
- Masłowski, M.; Miedzianowska, J.; Strąkowska, A.; Strzelec, K.; Szynkowska, M.I. The use of rye, oat and triticale straw as fillers of natural rubber composites. Polym. Bull. 2018, 75, 4607–4626. [Google Scholar] [CrossRef] [Green Version]
- Miedzianowska, J.; Masłowski, M.; Strzelec, K. Thermoplastic Elastomeric Composites Filled with Lignocellulose Bioadditives. Part 1: Morphology, Processing, Thermal and Rheological Properties. Materials 2020, 13, 1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayaram, T.R. SA technical review on rubber. Int. J. Des. Manuf. Technol. 2009, 3, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Kanoth, B.P.; Thomas, T.; Joseph, J.M.; Narayanankutty, S.K. Restructuring of coir to microfibers for enhanced reinforcement in natural rubber. Polym. Compos. 2019, 40, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Modified Nanoclays/Straw Fillers as Functional Additives of Natural Rubber Biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef]
- Barana, D.; Orlandi, M.; Salanti, A.; Castellani, L.; Hanel, T.; Zoia, L. Simultaneous synthesis of cellulose nanocrystals and a lignin-silica biofiller from rice husk: Application for elastomeric compounds. Ind. Crops Prod. 2019, 141, 111822. [Google Scholar] [CrossRef]
- Mahesh, V.; Joladarashi, S.; Kulkarni, S.M. Evaluation of Tensile Strength and Slurry Erosive Behaviour of Jute Reinforced Natural Rubber Based Flexible Composite. Rev. Compos. Matér. Av. 2020, 30, 77–82. [Google Scholar] [CrossRef]
- Ekwueme, C.C.; Igwe, I.O.; Vivian, A.O. End-Use Properties of Pineapple Leaf Fibre Filled Natural Rubber. J. Miner. Mater. Charact. Eng. 2019, 07, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Rohit, K.; Dixit, S. A Review - Future Aspect of Natural Fiber Reinforced Composite. Polym. Renew. Resour. 2016, 7, 43–59. [Google Scholar] [CrossRef]
- Ansell, M.P. Wood Composites; Woodhead Publishing: Sawston, UK, 2015; pp. 1–427. [Google Scholar]
- De Lemos, A.L.; Pires, P.G.P.; De Albuquerque, M.L.; Botaro, V.R.; De Paiva, J.M.F.; Junior, N.S.D. Biocomposites reinforced with natural fibers: Thermal, morphological and mechanical characterization. Matéria 2017, 22. [Google Scholar] [CrossRef] [Green Version]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Strzelec, K. Horsetail (Equisetum Arvense) as a Functional Filler for Natural Rubber Biocomposites. Materials 2020, 13, 2526. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Crop Residues as an Alternative to Vulcanizates with Common Fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faruk, O.; Ain, M. Biofiber reinforced polymer composites for structural applications. In Developments in Fiber-Reinforced Polymer (FRP) Composites for Civil Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 18–53. [Google Scholar]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Silanized cereal straw as a novel, functional filler of natural rubber biocomposites. Cellulose 2018, 26, 1025–1040. [Google Scholar] [CrossRef] [Green Version]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Properties of Chemically Modified (Selected Silanes) Lignocellulosic Filler and its Application in Natural Rubber Biocomposites. Materials 2020, 13, 4163. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Cereals Straw Modified with Acetic and Maleic Anhydride: Preparation and Properties. J. Polym. Environ. 2018, 26, 4141–4157. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-C.; Ge, X.; Cho, U.R. Mechanical performance, water absorption behavior and biodegradability of poly(methyl methacrylate)-modified starch/SBR biocomposites. Macromol. Res. 2013, 21, 793–800. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.A.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Common Nettle (Urtica dioica L.) as an Active Filler of Natural Rubber Biocomposites. Materials 2021, 14, 1616. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural rubber biocomposites containing corn, barley and wheat straw. Polym. Test. 2017, 63, 84–91. [Google Scholar] [CrossRef]
- Siddeeg, A.; Salih, Z.A.; Mukhtar, R.M.E.; Ali, A.O. Extraction and Characterization of Peppermint (Mentha piperita) Essential Oil and its Assessment as Antioxidant and Antibacterial. Gezira J. Eng. Appl. Sci. 2018, 13, 1–14. [Google Scholar]
- Rita, P.; Animesh, D.K. An updated overview on peppermint (Mentha piperita L.). Int. Res. J. Pharm. 2011, 2, 1–10. [Google Scholar]
- Zaidi, S.; Dahiya, P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha piperita. Int. Food Res. J. 2015, 22, 2040–2445. [Google Scholar]
- Sústriková, A.; Salamon, I. Essential oil of peppermint (Mentha × piperita L.) from fields in Eastern Slovakia. Hortic. Sci. 2011, 31, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Benzaid, C.; Tichati, L.; Djeribi, R.; Rouabhia, M. Evaluation of the Chemical Composition, the Antioxidant and Antimicrobial Activities ofMentha × piperitaEssential Oil against Microbial Growth and Biofilm Formation. J. Essent. Oil Bear. Plants 2019, 22, 335–346. [Google Scholar] [CrossRef]
- Verma, R.S.; Rahman, L.; Verma, R.K.; Chauhan, A.; Yadav, A.K.; Singh, A. Essential oil composition of menthol mint (Mentha arvensis) and peppermint (Mentha piperita) cultivars at different stages of plant growth from Kumaon region of Western Himalaya. Open Access J. Med. Aromat. Plants 2010, 1, 13–18. [Google Scholar]
- Trevisan, S.C.C.; Menezes, A.P.P.; Barbalho, S.M.; Guiguer, E.L. Properties of Mentha piperita: A Brief Review. World J. Pharm. Med. Res. 2017, 3, 309–313. [Google Scholar]
- Chalchat, J.-C.; Garry, R.-P.; Michet, A. Variation of the Chemical Composition of Essential Oil of Mentha piperita L. during the Growing Time. J. Essent. Oil Res. 1997, 9, 463–465. [Google Scholar] [CrossRef]
- Beigi, M.; Torki-Harchegani, M.; Pirbalouti, A.G. Quantity and chemical composition of essential oil of peppermint (Mentha × piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Iscan, G.; Kirimer, N.; Kürkcüoǧlu, M.; Baser, K.H.C.; Demirci, F. Antimicrobial Screening of Mentha piperita Essential Oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Taha, K.M.; Abozid, M.M.; Saed, H.E.E. Comparative study between anise seeds and mint leaves (chemical composition, phenolic compounds and flavonoids). Menoufia J. Agric. Biotechnol. 2019, 4, 53–60. [Google Scholar] [CrossRef]
- Mallick, B.; Sinha, S.; Roy, D. Evaluation of Antioxidative Potential of Field Grown and Tissue Culture Derived Mentha piperita L. Plants. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 382–391. [Google Scholar] [CrossRef]
- Rasooli, I.; Owlia, P.; Taghizadeh, M.; Astaneh, S.D.A.; Sharafi, S.M. Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn. Mag. 2010, 6, 147–153. [Google Scholar] [CrossRef]
- Johari, N.Z.; Ismail, I.S.; Sulaiman, M.R.; Abas, F.; Shaari, K. Acute toxicity and metabolomics analysis of hypocholesterolemic effect of Mentha piperita aqueous extract in Wistar rats. Int. J. Appl. Res. Nat. Prod. 2015, 8, 1–11. [Google Scholar]
- Liu, X.; Sun, Z.-L.; Jia, A.-R.; Shi, Y.-P.; Li, R.-H.; Yang, P.-M. Extraction, Preliminary Characterization and Evaluation of in Vitro Antitumor and Antioxidant Activities of Polysaccharides from Mentha piperita. Int. J. Mol. Sci. 2014, 15, 16302–16319. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.R.; Patil, R.S.; Godghate, A.G. Mentha piperita linn: Phytochemical, antibacterial and dipterian adulticidal approach. Int. J. Pharm. Pharm. Sci. 2016, 8, 352–355. [Google Scholar]
- Mairapetyan, S.; Alexanyan, J.; Tovmasyan, A.; Daryadar, M.; Stepanian, B.; Mamikonyan, V. Productivity, biochemical indices and antioxidant activity of Peppermint (Mentha piperita L.) and Basil (Ocimum basilicum L.) in condition of hydroponics. J. Biosci. Agric. Res. 2016, 3, 191–194. [Google Scholar] [CrossRef]
- Keifer, D.; Ulbricht, C.; Abrams, T.R.; Basch, E.; Giese, N.; Giles, M.; Kirkwood, C.D.; Miranda, M.; Woods, J. Peppermint (Mentha piperita): An evidence-based systematic review by the Natural Standard Research Collaboration. J. Herb. Pharmacother. 2008, 7, 91–143. [Google Scholar] [CrossRef]
- Mahendran, G.; Rahman, L. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)—A review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Chis, M.S.; Muste, S.; Paucean, A.; Man, S.; Pop, A.; Pop, C.R.; Martis, G.S. A comprehensive review of medicinal and therapeutic uses of Mentha piperita. Hop Med. Plants 2019, 27, 38–49. [Google Scholar]
- Baghalian, K.; Abdoshah, S.; Khalighi-Sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.). Plant Physiol. Biochem. 2011, 49, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ranpariya, V.; Parmar, S.; Sheth, N.; Chandrashekhar, V. Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharm. Biol. 2011, 49, 696–701. [Google Scholar] [CrossRef]
- Sebai, H.; Jabri, M.-A.; Souli, A.; Rtibi, K.; Selmi, S.; Tebourbi, O.; El-Benna, J.; Sakly, M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J. Ethnopharmacol. 2014, 152, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R. The essential oil of Chamomilla recutita (L.) Rausch. Cultivated and wild growing in Poland. Ann. Univ. Mariae Curie-Sklodowska 2011, 24, 199–206. [Google Scholar]
- Shebbo, S.; El Joumaa, M.; Kawach, R.; Borjac, J. Hepatoprotective effect of Matricaria chamomilla aqueous extract against 1,2-Dimethylhydrazine-induced carcinogenic hepatic damage in mice. Heliyon 2020, 6, e04082. [Google Scholar] [CrossRef] [PubMed]
- Jarrahi, M.; Vafaei, A.A.; Taherian, A.A.; Miladi, H.; Rashidy-Pour, A.; Miladi-Gorji, H. Evaluation of topical Matricaria chamomilla extract activity on linear incisional wound healing in albino rats. Nat. Prod. Res. 2010, 24, 697–702. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, J.; Shankar, E. Chamomile: A herbal medicine of the past with a bright future (Review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Mahgoub, Y.A.; Shawky, E.; Darwish, F.A.; El Sebakhy, N.A.; El-Hawiet, A.M. Near-infrared spectroscopy combined with chemometrics for quality control of German chamomile (Matricaria recutita L.) and detection of its adulteration by related toxic plants. Microchem. J. 2020, 158, 105153. [Google Scholar] [CrossRef]
- Ayhan, N.K. Investigation of antioxidant properties of chamomile consumed as herbal tea. J. Food Process. Preserv. 2021, 45, e15327. [Google Scholar] [CrossRef]
- Chaves, P.F.P.; Hocayen, P.D.A.S.; Dallazen, J.L.; Werner, M.F.D.P.; Iacomini, M.; Andreatini, R.; Cordeiro, L.M. Chamomile tea: Source of a glucuronoxylan with antinociceptive, sedative and anxiolytic-like effects. Int. J. Biol. Macromol. 2020, 164, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Hameed, I.H.; Mohammed, G.J.; Kamal, S.A. A Review: Uses and Pharmacological Activity of Matricaria chamomilla. Indian J. Public Health Res. Dev. 2018, 9, 200. [Google Scholar] [CrossRef]
- Gosztola, B.; Sárosi, S.; Németh, E. Variability of the Essential Oil Content and Composition of Chamomile (Matricaria recutita L.) affected by Weather Conditions. Nat. Prod. Commun. 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita. Int. J. Food Prop. 2014, 18, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Haghi, G.; Hatami, A.; Safaei, A.; Mehran, M. Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res. Pharm. Sci. 2015, 9, 31–37. [Google Scholar]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research1. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef]
- Yaeesh, S.; Jamal, Q.; Khan, A.-U.; Gilani, A.H. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother. Res. 2006, 20, 546–551. [Google Scholar] [CrossRef]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Nemeth, E. Biological Activities of Yarrow Species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151–3167. [Google Scholar] [CrossRef]
- Daniel, P.S.; Lourenço, E.L.B.; Da Cruz, R.M.S.; Gonçalves, C.H.D.S.; Das Almas, L.R.M.; Hoscheid, J.; Da Silva, C.; Jacomassi, E.; Junior, L.B.; Alberton, O. Composition and antimicrobial activity of essential oil of yarrow (Achillea millefolium L.). Aust. J. Crop. Sci. 2020, 14, 545–550. [Google Scholar] [CrossRef]
- Benedek, B.; Gjoncaj, N.; Saukel, J.; Kopp, B. Distribution of Phenolic Compounds in Middleeuropean Taxa of the Achillea millefolium L. Aggregate. Chem. Biodivers. 2007, 4, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Nadim, M.M.; Malik, A.; Bakshi, S.K. The Essential Oil Composition of Achillea millefolium L. Cultivated under Tropical Condition in India. World J. Agric. Sci. 2011, 7, 561–565. [Google Scholar]
- Judzentiene, A.; Mockute, D. Essential oil composition of two yarrow taxonomic forms. Open Life Sci. 2010, 5, 346–352. [Google Scholar] [CrossRef]
- Benedek, B.; Kopp, B. Achillea millefolium L. s.l. revisited: Recent findings confirm the traditional use. Wien. Med. Wochenschr. 2007, 157, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Moerman, D.E. Native American Ethnobotany. TAXON 1998, 47, 980. [Google Scholar] [CrossRef]
- Lakshmi, T.; Geetha, R.V.; Roy, A.; Aravind Kumar, S. Yarrow (Achillea millefolium Linn.) A herbal medicinal plant with broad therapeutic use—A review. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 136–141. [Google Scholar]
- Taheri, J.B.; Azimi, S.; Rafieian, N.; Zanjani, H.A. Herbs in dentistry. Int. Dent. J. 2011, 61, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Glasl, S.; Rothwangl-Wiltschnigg, K. Yarrow (Achillea millefolium L. s.l.): Pharmaceutical quality of commercial samples. Pharmazie 2008, 63, 23–26. [Google Scholar]
- Sadowska, U.; Matwijczuk, A.; Niemczynowicz, A.; Dróżdż, T.; Żabiński, A. Spectroscopic Examination and Chemometric Analysis of Essential Oils Obtained from Peppermint Herb (Mentha piperita L.) and Caraway Fruit (Carum carvi L.) Subjected to Pulsed Electric Fields. Processes 2019, 7, 466. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FTIR macro- and micro-spectroscopy coupled with chemometrics. Biotechnol. Biofuels 2018, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Schulz, H.; Ozkan, G.; Baranska, M.; Krüger, H.; Özcan, M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 2005, 39, 249–256. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Morar, M.I.; Fetea, F.; Rotar, A.M.; Nagy, M.; Semeniuc, C.A. Characterization of essential oils extracted from different aromatic plants by FTIR spectroscopy. Bull. UASVM Food Sci. Technol. 2017, 74, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef] [Green Version]
- Wyrostek, J.; Kowalski, R.; Pankiewicz, U.; Solarska, E. Estimation of the Content of Selected Active Substances in Primary and Secondary Herbal Brews by UV-VIS and GC-MS Spectroscopic Analyses. J. Anal. Methods Chem. 2020, 2020, 8891855. [Google Scholar] [CrossRef] [PubMed]
- Matěnová, M.; Horhoiu, V.L.; Dang, F.-X.; Pospíšil, P.; Alster, J.; Burda, J.V.; Balaban, T.S.; Pšenčík, J. Energy transfer in aggregates of bacteriochlorophyll c self-assembled with azulene derivatives. Phys. Chem. Chem. Phys. 2014, 16, 16755–16764. [Google Scholar] [CrossRef] [Green Version]
- Giovannetti, R.; Alibabaei, L.; Zannotti, M.; Ferraro, S.; Petetta, L. HPLC-DAD-ESI/MS Identification of Light Harvesting and Light Screening Pigments in the Lake Sediments at Edmonson Point. Sci. World J. 2013, 2013, 741906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atobe, S.; Saga, K.; Maeyama, H.; Fujiwara, K.; Okada, S.; Imou, K. Culture of the green microalga Botryococcus braunii Showa with LED irradiation eliminating violet light enhances hydrocarbon production and recovery. Biosci. Biotechnol. Biochem. 2014, 78, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
- De Faria, A.F.; De Rosso, V.V.; Mercadante, A.Z. Carotenoid Composition of Jackfruit (Artocarpus heterophyllus), Determined by HPLC-PDA-MS/MS. Plant Foods Hum. Nutr. 2009, 64, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Naughton, A. Mechanisms of thermal decomposition of natural fibre composites. Compos. Part B Eng. 2016, 88, 1–10. [Google Scholar] [CrossRef]
- Khimi, S.R.; Pickering, K.L. A new method to predict optimum cure time of rubber compound using dynamic mechanical analysis. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Marković, G.; Marinović-Cincović, M.; Valentova, H.; Ilavský, M.; Radovanović, B.; Budinski-Simendić, J. Curing Characteristics and Dynamic Mechanical Behaviour of Reinforced Acrylonitrile-Butadiene/Chlorosulfonated Polyethylene Rubber Blends. Mater. Sci. Forum 2005, 494, 475–480. [Google Scholar] [CrossRef]
- Zięba-Palus, J. The usefulness of infrared spectroscopy in examinations of adhesive tapes for forensic purposes. Forensic Sci. Criminol. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Gorassini, A.; Adami, G.; Calvini, P.; Giacomello, A. ATR-FTIR characterization of old pressure sensitive adhesive tapes in historic papers. J. Cult. Herit. 2016, 21, 775–785. [Google Scholar] [CrossRef]
- Farias-Aguilar, J.C.; Ramírez-Moreno, M.J.; Téllez-Jurado, L.; Balmori-Ramírez, H. Low pressure and low temperature synthesis of polyamide-6 (PA6) using Na 0 as catalyst. Mater. Lett. 2014, 136, 388–392. [Google Scholar] [CrossRef]
- Janowska, G.; Kucharska-Jastrząbek, A.; Rybiński, P. Thermal stability, flammability and fire hazard of butadiene-acrylonitrile rubber nanocomposites. J. Therm. Anal. Calorim. 2011, 103, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Lazár, M.; Rado, R.; Rychlý, J. Crosslinking of polyolefins. Polym. Phys. 1990, 95, 149–197. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Wu, X.-F.; Dzenis, Y.A. Droplet on a fiber: Geometrical shape and contact angle. Acta Mech. 2006, 185, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Maniukiewicz, W.; Zaborski, M. Aluminum-Magnesium Hydroxycarbonate/Azo Dye Hybrids as Novel Multifunctional Colorants for Elastomer Composites. Polymers 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, M.; Sowińska, A.; Kucharska, J. Organic Zinc Salts as Pro-Ecological Activators for Sulfur Vulcanization of Styrene–Butadiene Rubber. Polymers 2019, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Rybiński, P.; Maniukiewicz, W. Novel eco-friendly hybrid pigment with improved stability as a multifunctional additive for elastomer composites with reduced flammability and pH sensing properties. Dyes Pigments 2021, 186, 108965. [Google Scholar] [CrossRef]
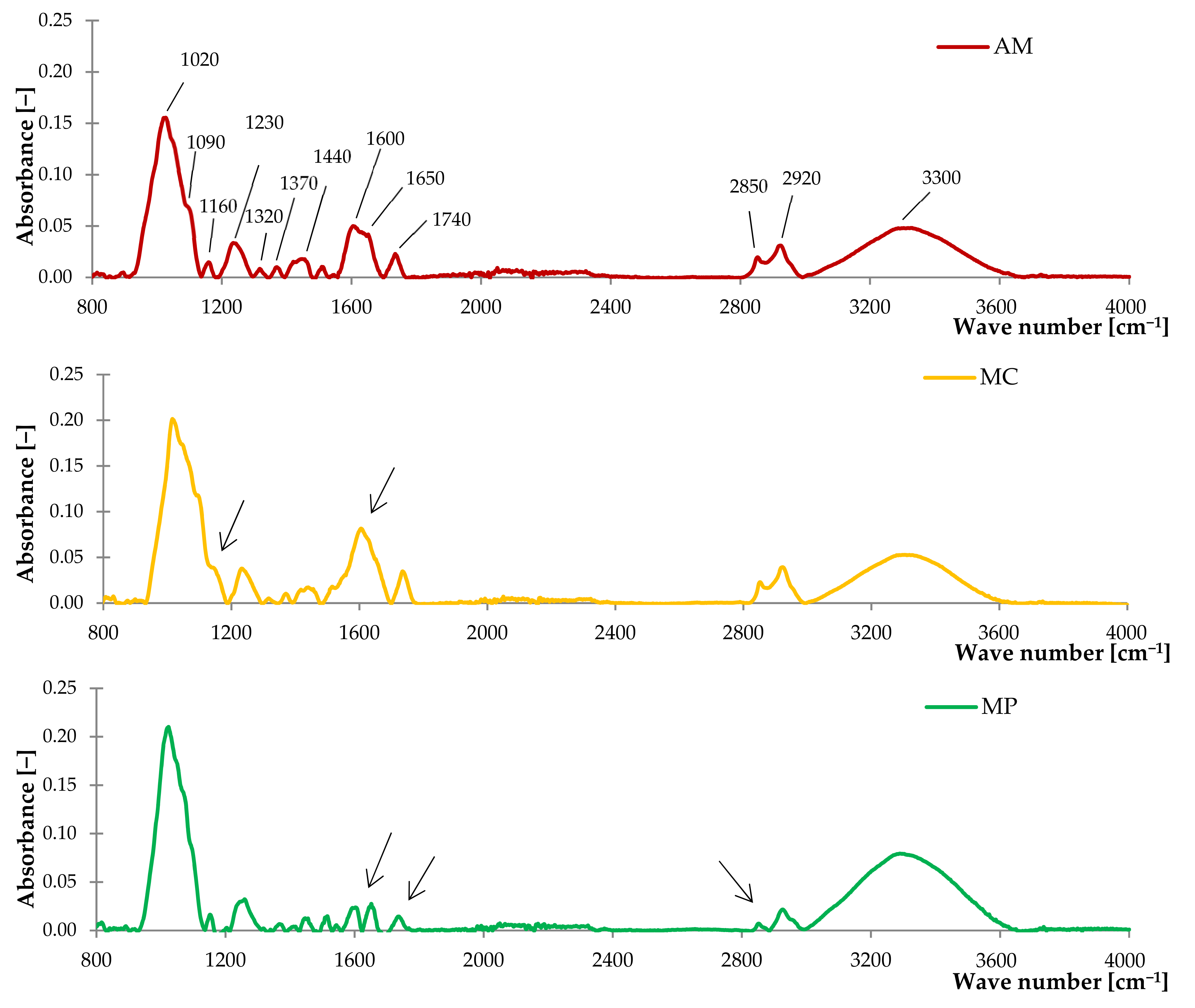















| Peak Assignments and Type of Vibration | Wavenumber [cm−1] |
|---|---|
| v (O–H) phenols and alcohols, –C=Ow (overtone) and v (=C–Hvw) | 3650–3200 |
| v (C–H) vinyl & acrylic | 3100–3010 |
| v (C–H) aliphatic and νas(–C–Hm, –CH3, –CH2) | 2970–2800 |
| v (C=O) | 1740 |
| v (C=O) | 1730–1690 |
| v (–C=Ovw) in acids | 1708 |
| v (C=C) alkenes, amide | 1680–1610 |
| vvw(–C=C–, cis-) and δ(–OH) | 1675–1648 |
| v (COOH) v (C=C) stretching | 1634–1643 1600–1620 |
| v(C=C) aryl, dvw(–CH2) and (–CH3) bending (scissoring) or vvw(–C–H) bending (rocking) | 1600–1500 |
| v(C–C) aliphatic | 1500–600 |
| sv(C=C) aromatic | 1441 |
| d(C–H) aliphatic | 1370–1340 |
| vw,m,vw(–C–H, –CH3) | 1372/1337 |
| vas,s(C–O, C–C, –C–O–C–) | 1285–1150 |
| v(C–O), (C–C) | 1020–1030 |
| vm(–C–O) or dm(–CH2–) | 1285/1244 |
| vst(–C–O) or dst(–CH2–) | 1116 |
| νm(–C–O) | 1094 |
| da(C–O–C) | 1060 |
| vm,vw(–C–O) | 1044/1023 |
| dw(–HC=CH–) | 991/923 |
| v(C=C) | 880 |
| Sample | T10 1 (°C) | T50 2 (°C) | Δm100 3 (%) | Δm320 4 (%) | Residue at 600 °C 5 (%) |
|---|---|---|---|---|---|
| MP | 203.33 | 343.75 | 4.99 | 41.63 | 31.30 |
| MC | 223.33 | 328.34 | 3.83 | 44.70 | 28.53 |
| AM | 192.50 | 322.50 | 3.18 | 48.67 | 27.93 |
| Sample Name | T10 1 (°C) | Δm380 2 (%) | Residue at 600 °C 3 (%) |
|---|---|---|---|
| Ref. Sample (NR) | 362.64 | 41.02 | 7.13 |
| NR_MP | 328.71 | 51.82 | 11.57 |
| NR_MC | 307.78 | 46.38 | 11.74 |
| NR_AM | 318.25 | 54.21 | 11.56 |
| Sample | Filler Content (phr) | νe × 105 (mol/cm3) | ||
|---|---|---|---|---|
| Ref | UV | Therm | ||
| Ref. sample (NR) | 0 | 1.71 ± 0.01 | 1.90 ± 0.01 | 1.81 ± 0.02 |
| NR_MP | 10 | 1.76 ± 0.01 | 2.12 ± 0.02 | 2.03 ± 0.02 |
| 20 | 1.82 ± 0.03 | 2.29 ± 0.04 | 2.07 ± 0.02 | |
| 30 | 1.84 ± 0.03 | 2.63 ± 0.03 | 2.50 ± 0.04 | |
| NR_MC | 10 | 1.84 ± 0.02 | 2.19 ± 0.03 | 2.40 ± 0.05 |
| 20 | 1.94 ± 0.03 | 1.94 ± 0.05 | 2.21 ± 0.04 | |
| 30 | 2.01 ± 0.04 | 2.09 ± 0.04 | 2.26 ± 0.03 | |
| NR_AM | 10 | 2.07 ± 0.01 | 2.34 ± 0.02 | 2.43 ± 0.03 |
| 20 | 2.09 ± 0.03 | 3.21 ± 0.04 | 2.46 ± 0.03 | |
| 30 | 2.36 ± 0.03 | 3.55 ± 0.03 | 2.56 ± 0.04 | |
| Sample | Filler Content (phr) | Eb (%) | EbUV (%) | EbTherm (%) |
|---|---|---|---|---|
| Ref. Sample (NR) | 0 | 656.04 ± 3.48 | 736.90 ± 9.56 | 593.53 ± 6.46 |
| NR_MP | 10 | 740.99 ± 2.12 | 798.99 ± 3.49 | 707.03 ± 5.25 |
| 20 | 752.50 ± 2.21 | 775.71 ± 6.13 | 687.38 ± 5.68 | |
| 30 | 754.34 ± 3.25 | 743.81 ± 1.59 | 675.81 ± 5.91 | |
| NR_MC | 10 | 716.54 ± 4.94 | 751.65 ± 9.45 | 655.90 ± 7.34 |
| 20 | 735.85 ± 6.71 | 730.04 ± 4.44 | 655.14 ± 10.29 | |
| 30 | 640.91 ± 9.11 | 682.60 ±11.07 | 637.35 ±12.34 | |
| NR_AM | 10 | 671.99 ± 1.49 | 739.40 ± 4.47 | 598.76 ± 1.52 |
| 20 | 712.27 ± 3.33 | 737.79 ± 3.05 | 668.72 ± 0.38 | |
| 30 | 683.16 ± 2.54 | 705.77 ± 7.61 | 647.15 ± 2.34 |
| Fraction [mm] | Sieve Sizes [mm] | MP 1 | MC 2 | AM 3 | |||
|---|---|---|---|---|---|---|---|
| (g) | (%) | (g) | (%) | (g) | (%) | ||
| 1.000–2.000 | 1.000 | 1.30 | 2.60 | 0.29 | 0.57 | 0.71 | 1.41 |
| 0.500–1.000 | 0.500 | 9.54 | 19.07 | 10.57 | 21.13 | 11.68 | 23.35 |
| 0.250–0.500 | 0.250 | 28.32 | 56.64 | 30.06 | 60.12 | 29.22 | 58.43 |
| 0.125–0.250 | 0.125 | 10.11 | 20.21 | 8.69 | 17.38 | 7.81 | 15.61 |
| 0.065–0.125 | 0.065 | 0.74 | 1.48 | 0.40 | 0.80 | 0.60 | 1.20 |
| Σ | 50.00 | 100.00 | 50.00 | 100.00 | 50.00 | 100.00 | |
| Sample Name | Filler | NR | Stearin | ZnO | MBT | Sulfur |
|---|---|---|---|---|---|---|
| (phr 1) | ||||||
| Reference Sample (NR) | 0 | 100 | 1 | 5 | 2 | 2 |
| NR_MP10 | 10 | 100 | 1 | 5 | 2 | 2 |
| NR_MP20 | 20 | 100 | 1 | 5 | 2 | 2 |
| NR_MP30 | 30 | 100 | 1 | 5 | 2 | 2 |
| NR_MC10 | 10 | 100 | 1 | 5 | 2 | 2 |
| NR_MC20 | 20 | 100 | 1 | 5 | 2 | 2 |
| NR_MC30 | 30 | 100 | 1 | 5 | 2 | 2 |
| NR_AM10 | 10 | 100 | 1 | 5 | 2 | 2 |
| NR_AM20 | 20 | 100 | 1 | 5 | 2 | 2 |
| NR_AM30 | 30 | 100 | 1 | 5 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. https://doi.org/10.3390/ijms22147530
Masłowski M, Aleksieiev A, Miedzianowska J, Strzelec K. Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites. International Journal of Molecular Sciences. 2021; 22(14):7530. https://doi.org/10.3390/ijms22147530
Chicago/Turabian StyleMasłowski, Marcin, Andrii Aleksieiev, Justyna Miedzianowska, and Krzysztof Strzelec. 2021. "Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites" International Journal of Molecular Sciences 22, no. 14: 7530. https://doi.org/10.3390/ijms22147530
APA StyleMasłowski, M., Aleksieiev, A., Miedzianowska, J., & Strzelec, K. (2021). Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites. International Journal of Molecular Sciences, 22(14), 7530. https://doi.org/10.3390/ijms22147530







