Novel Nanobiocomposites Based on Natural Polysaccharides as Universal Trophic Low-Dose Micronutrients
Abstract
1. Introduction
2. Results
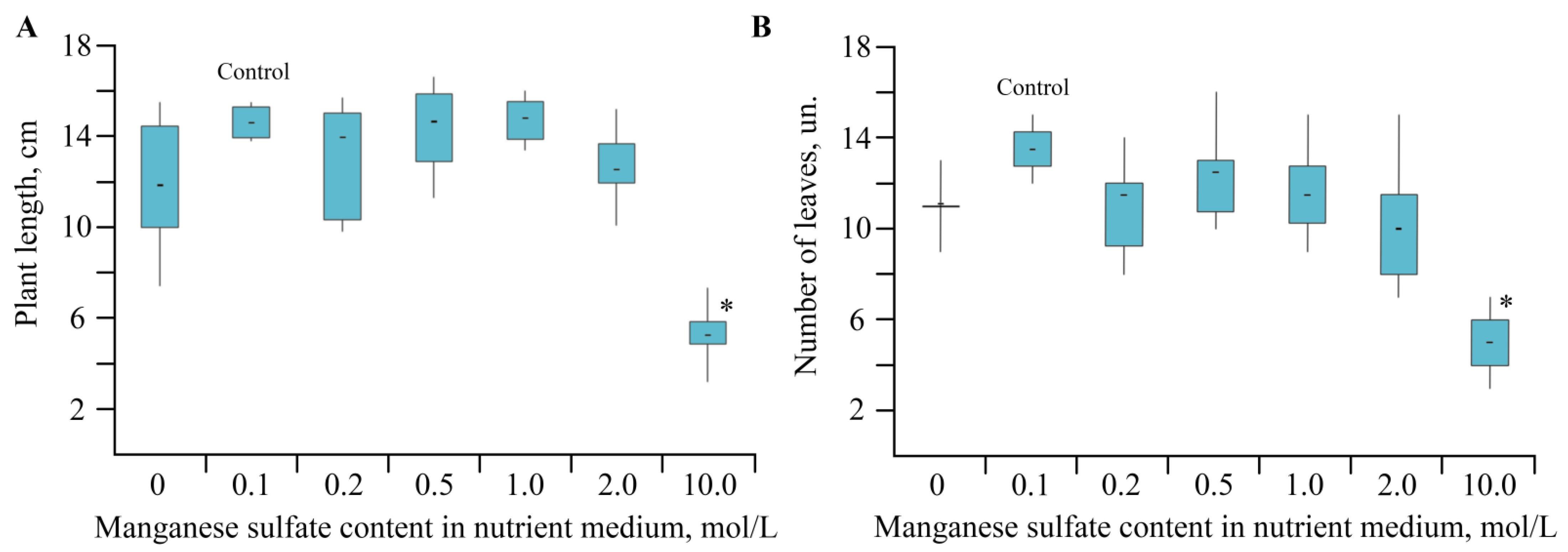
2.1. Toxic Effect of Mn and Its Accumulation during the Growing Season of Solanum tuberosum
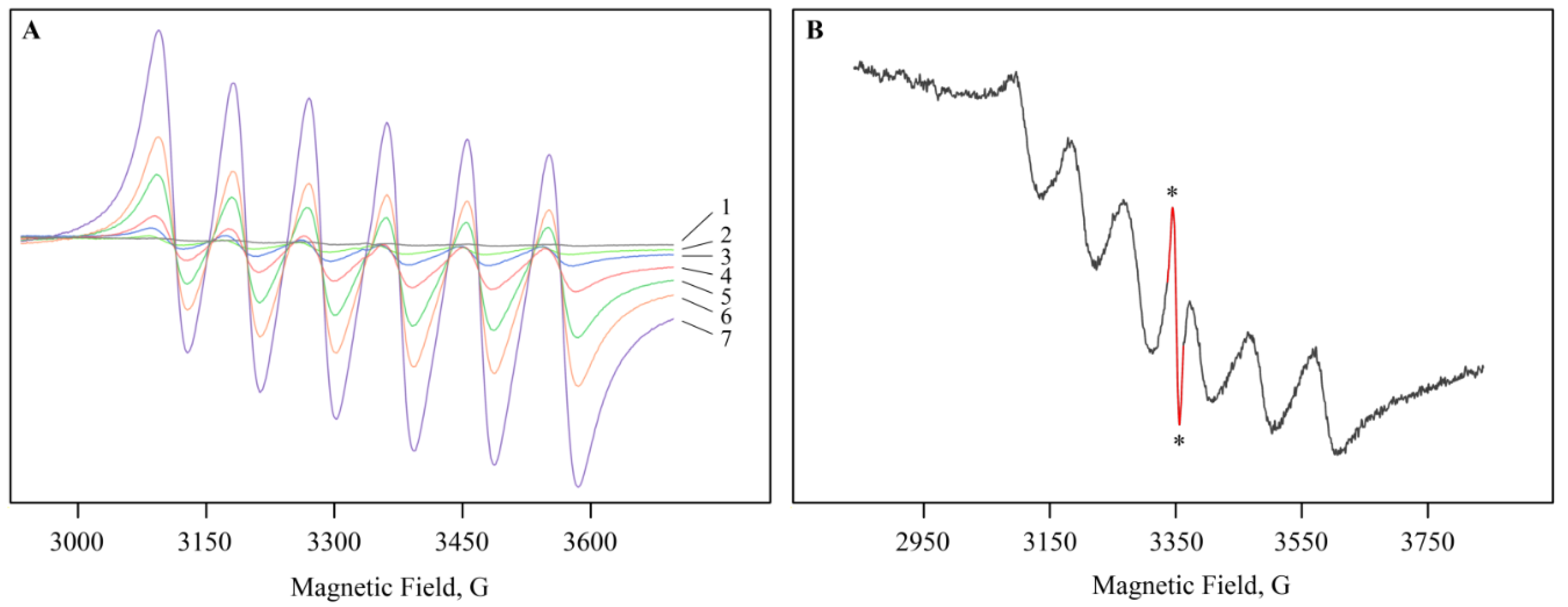
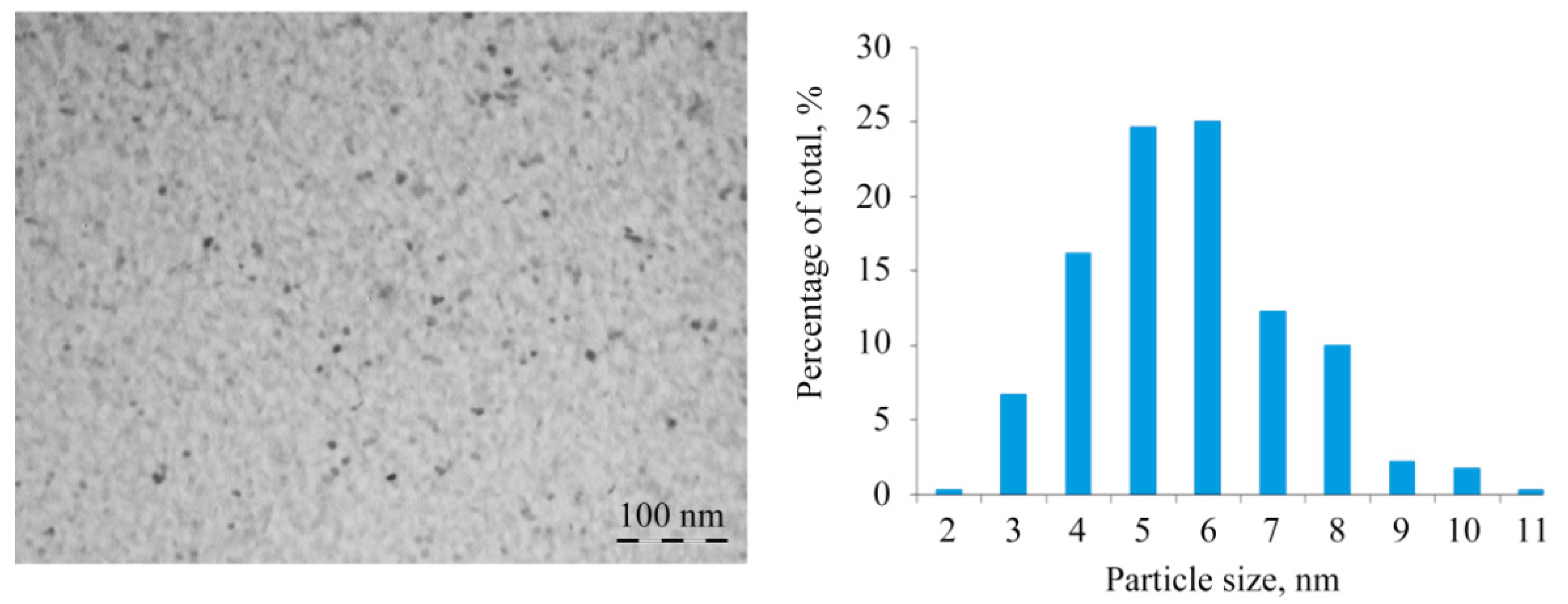
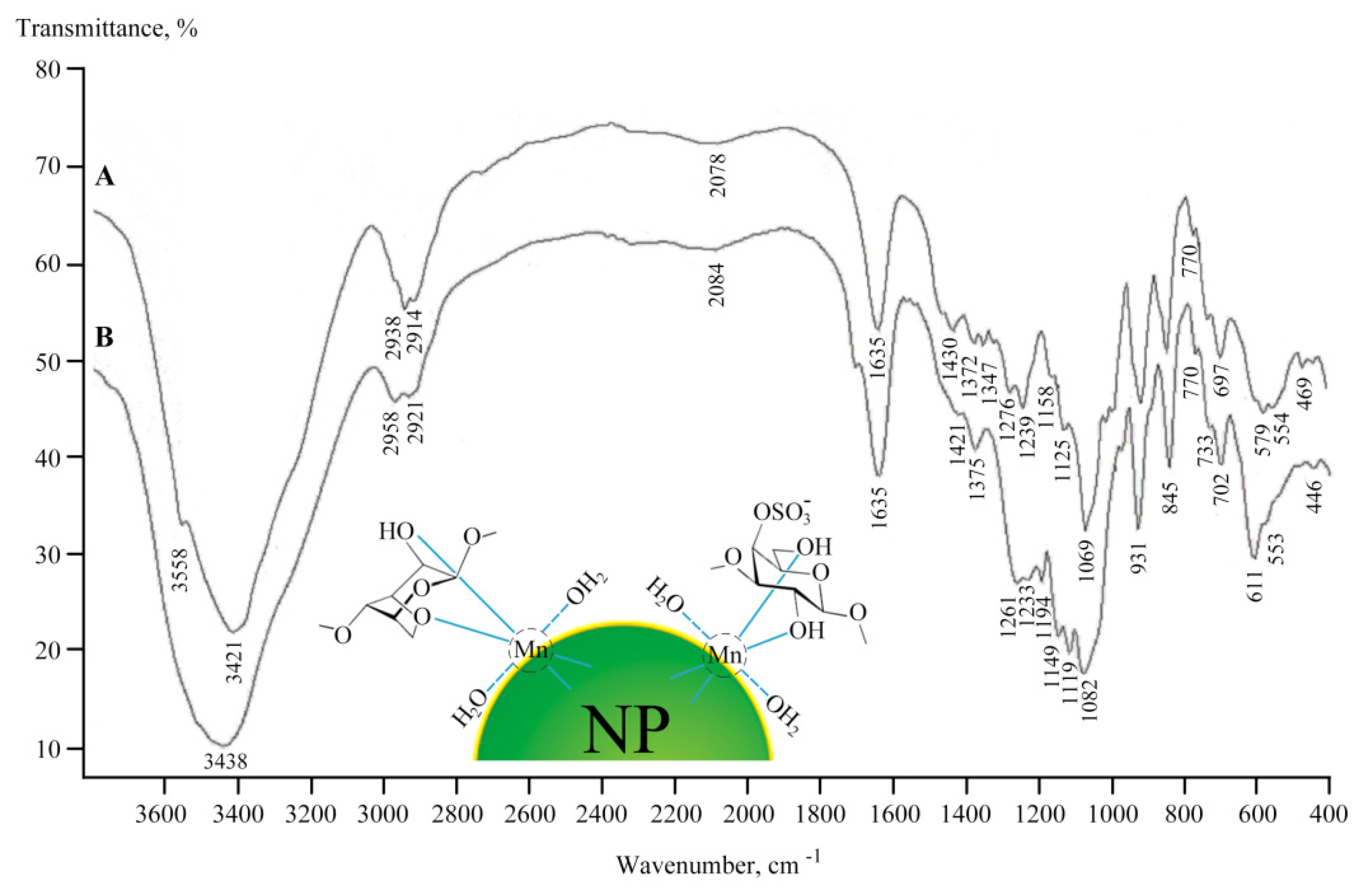
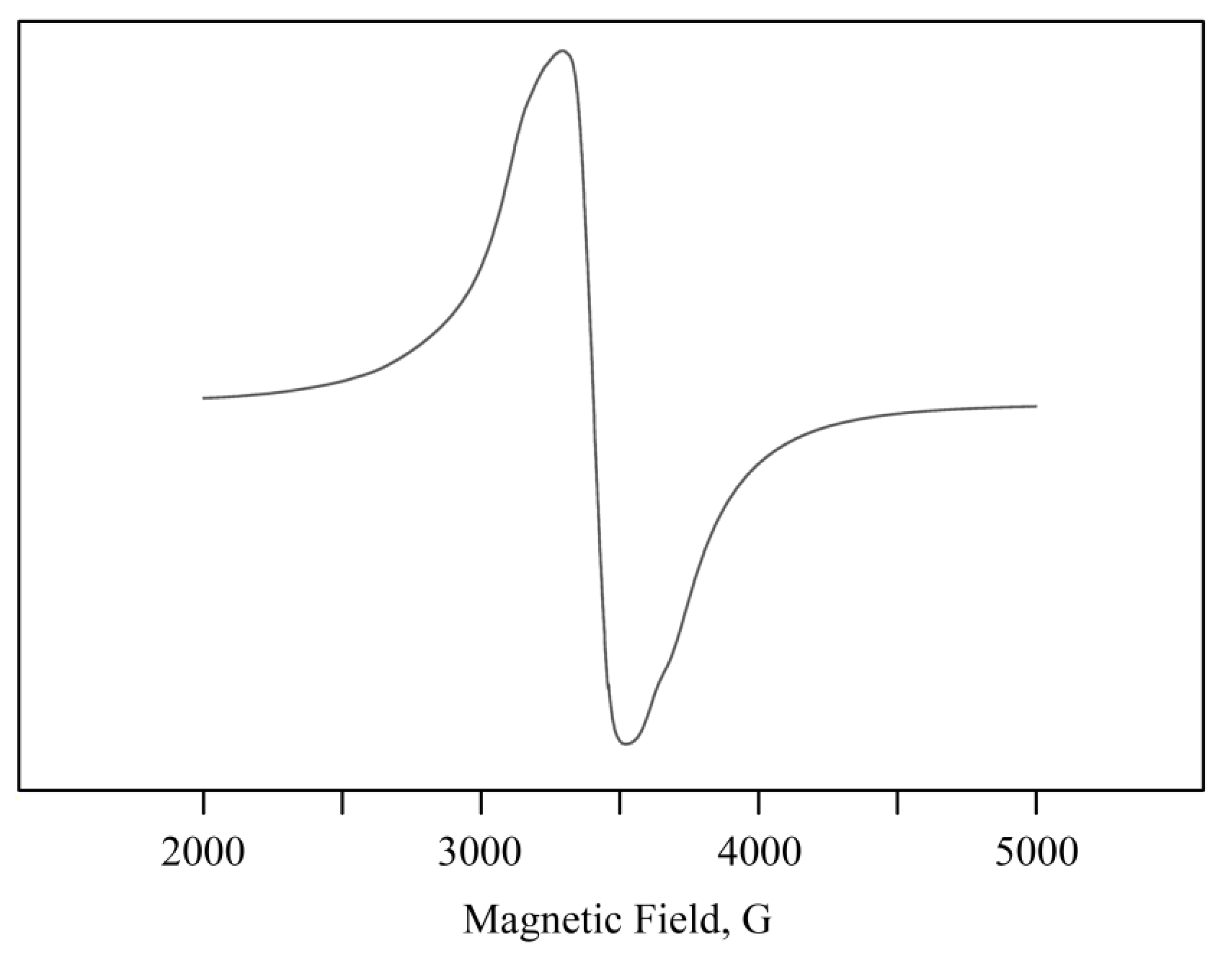
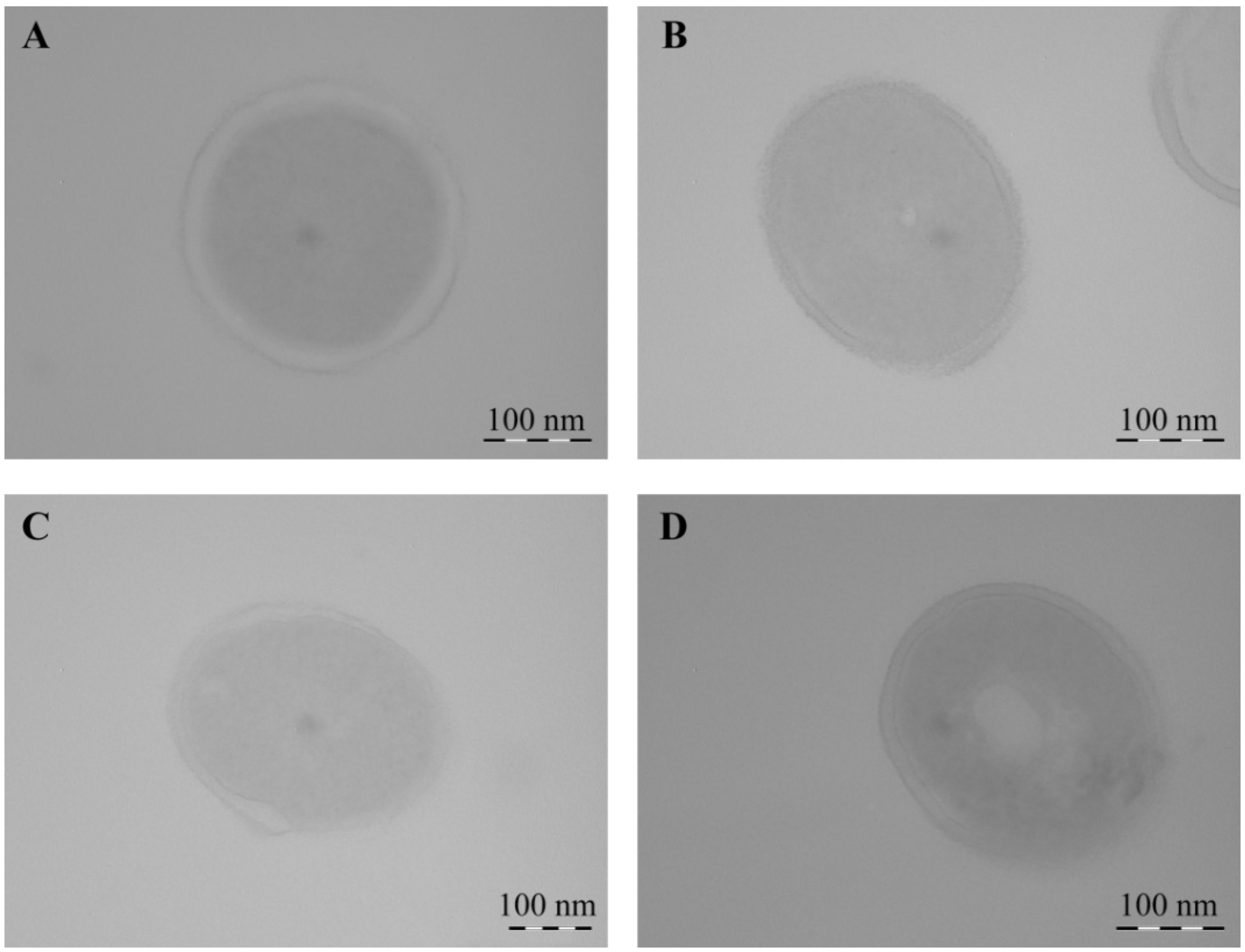
2.2. Structural Features of Mn-Containing NCs
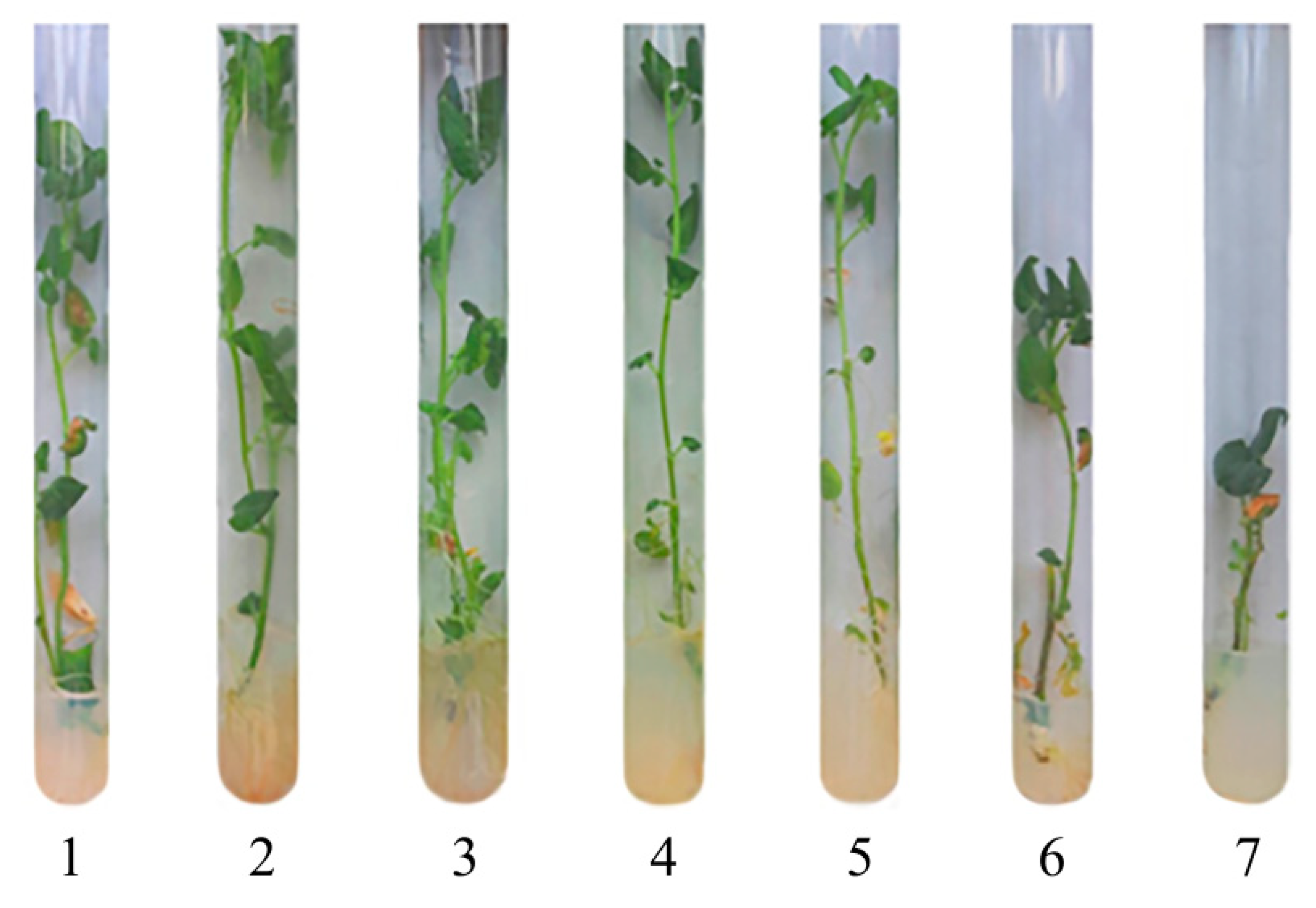
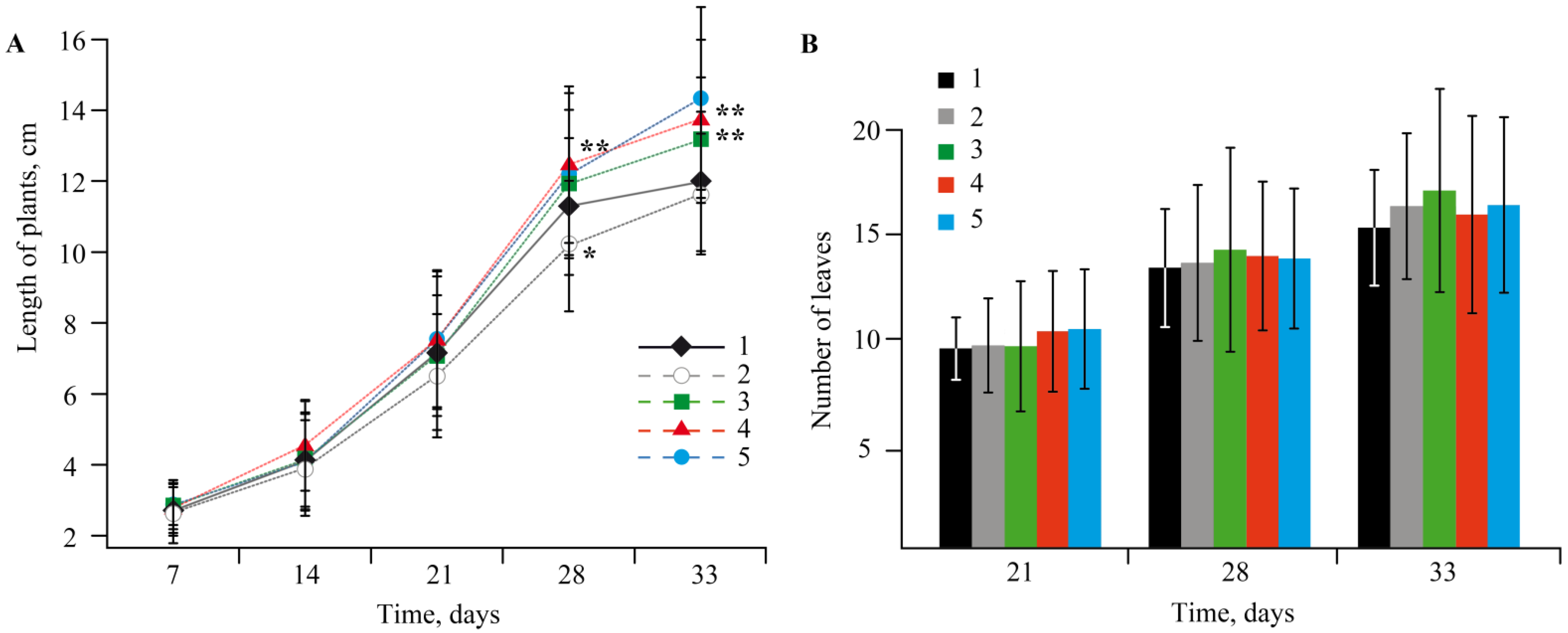
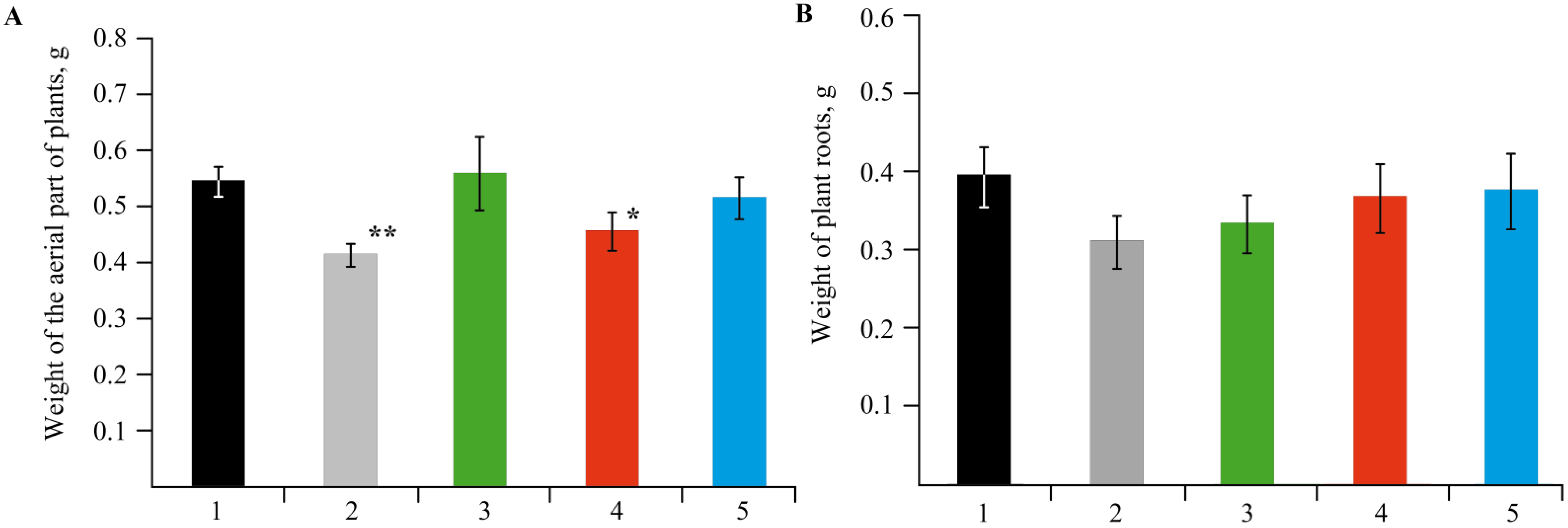
2.3. The Effect of Mn-Containing NCs on the Growth and Development of Solanum tuberosum L.
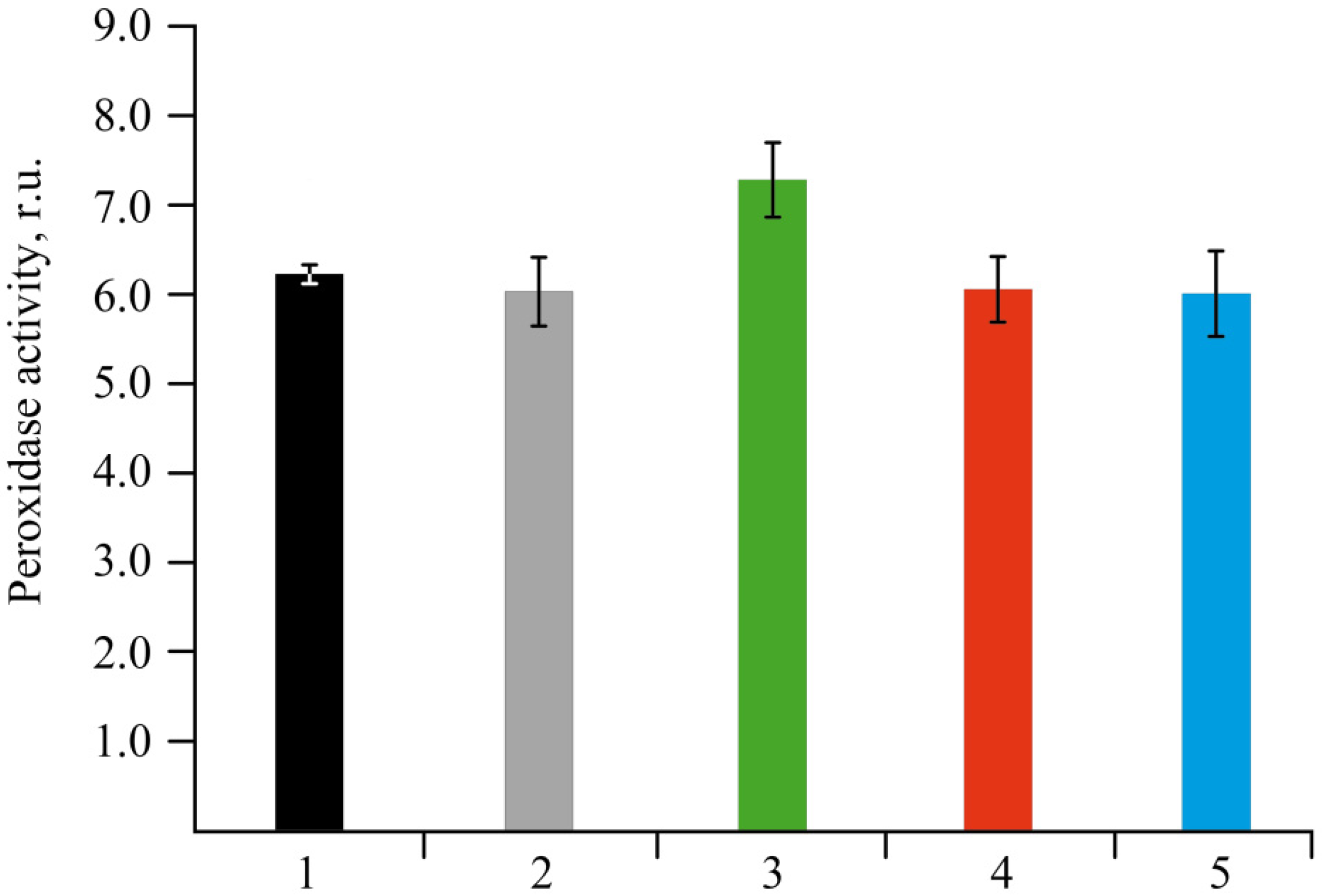
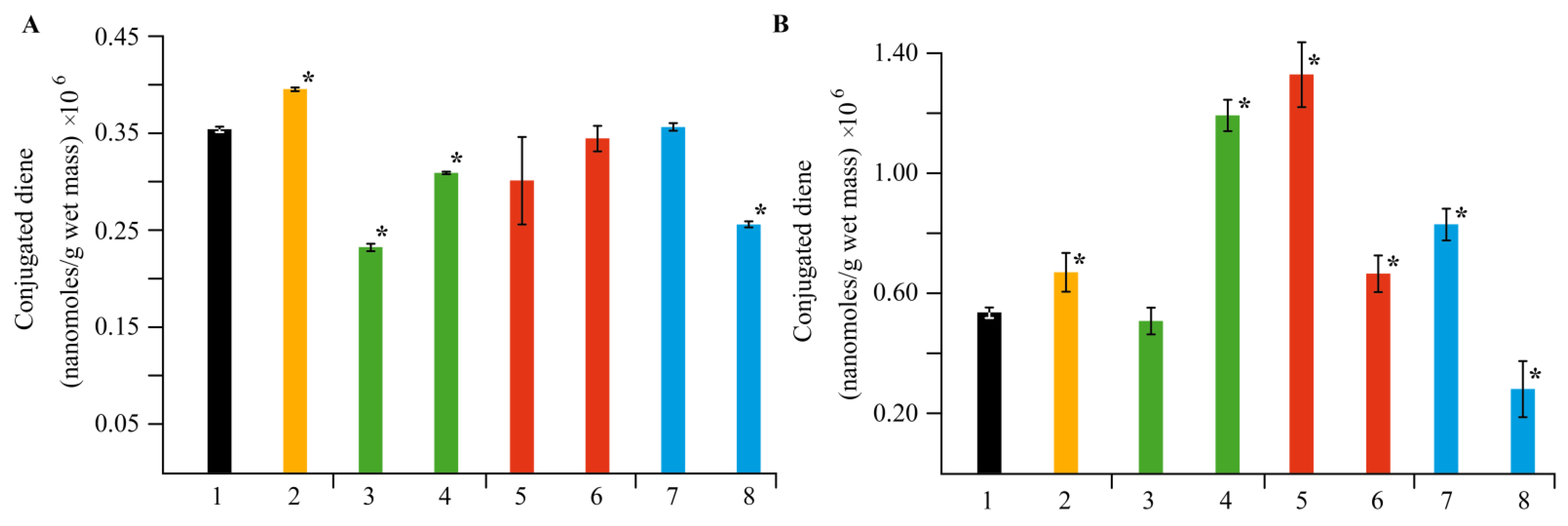
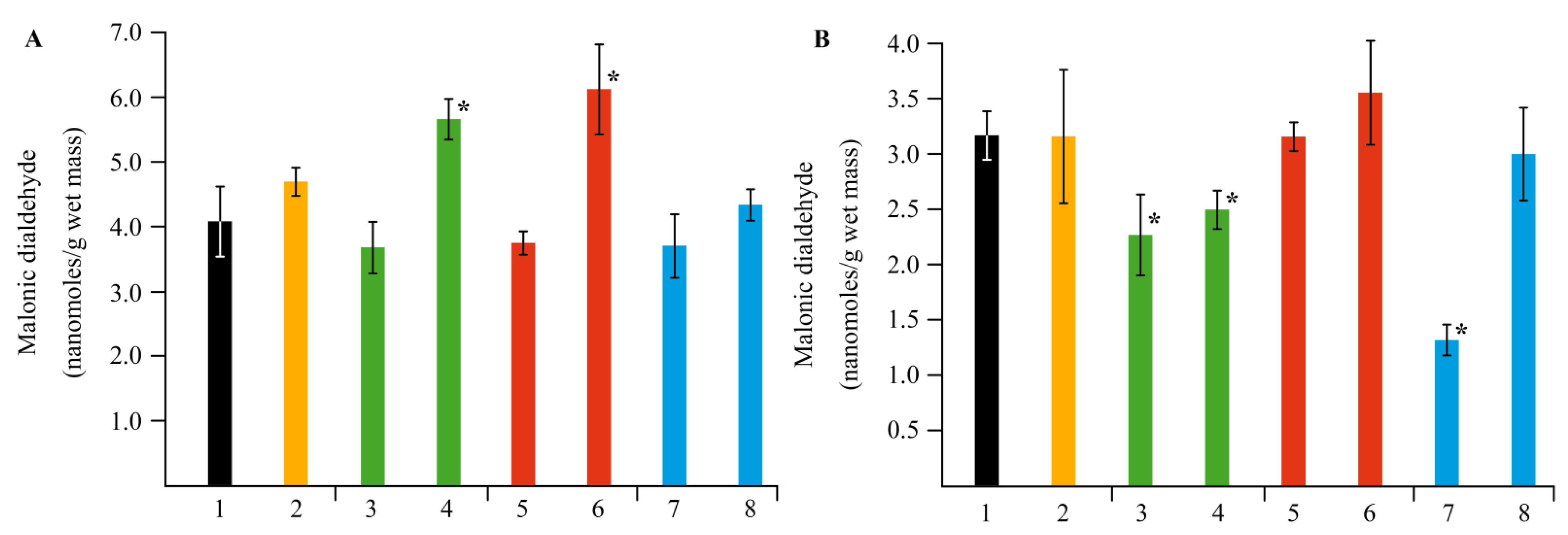
2.4. Effect of NCs on the Stress Resistance of Potato Plants
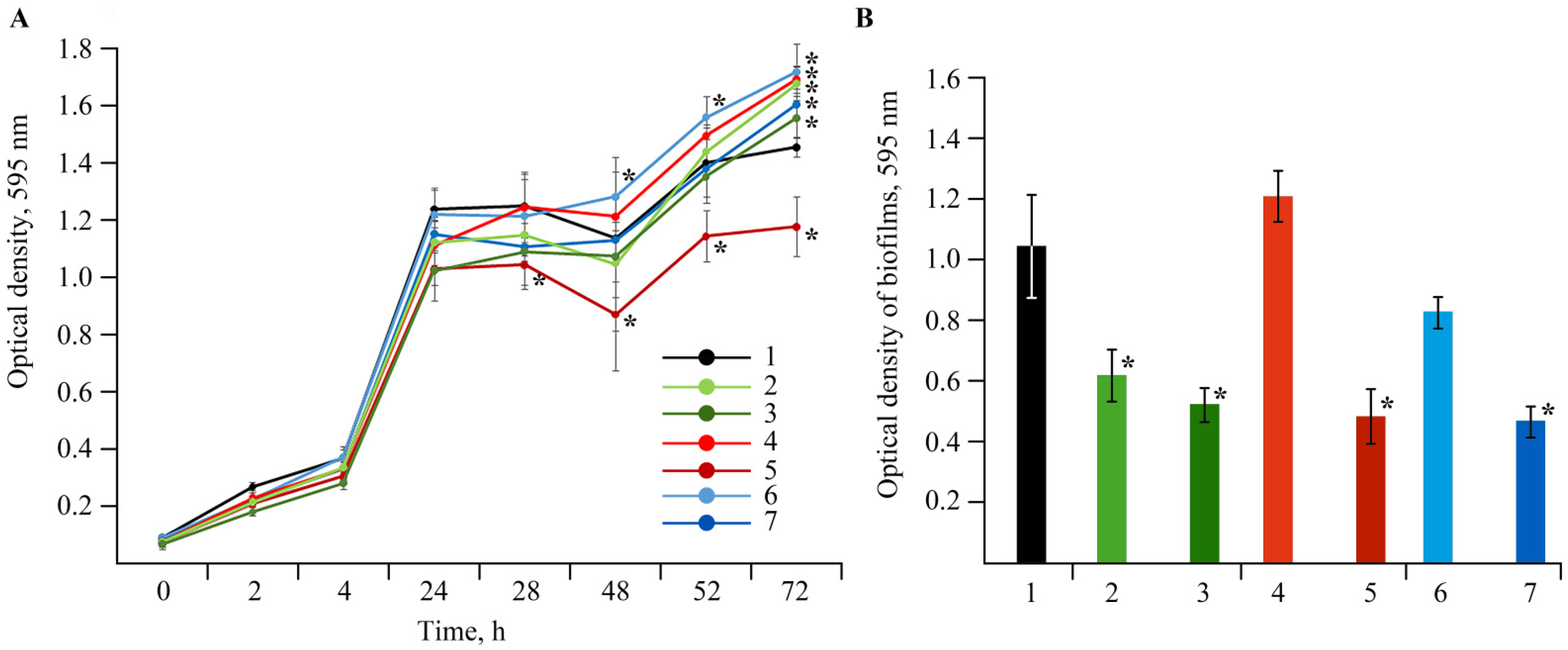
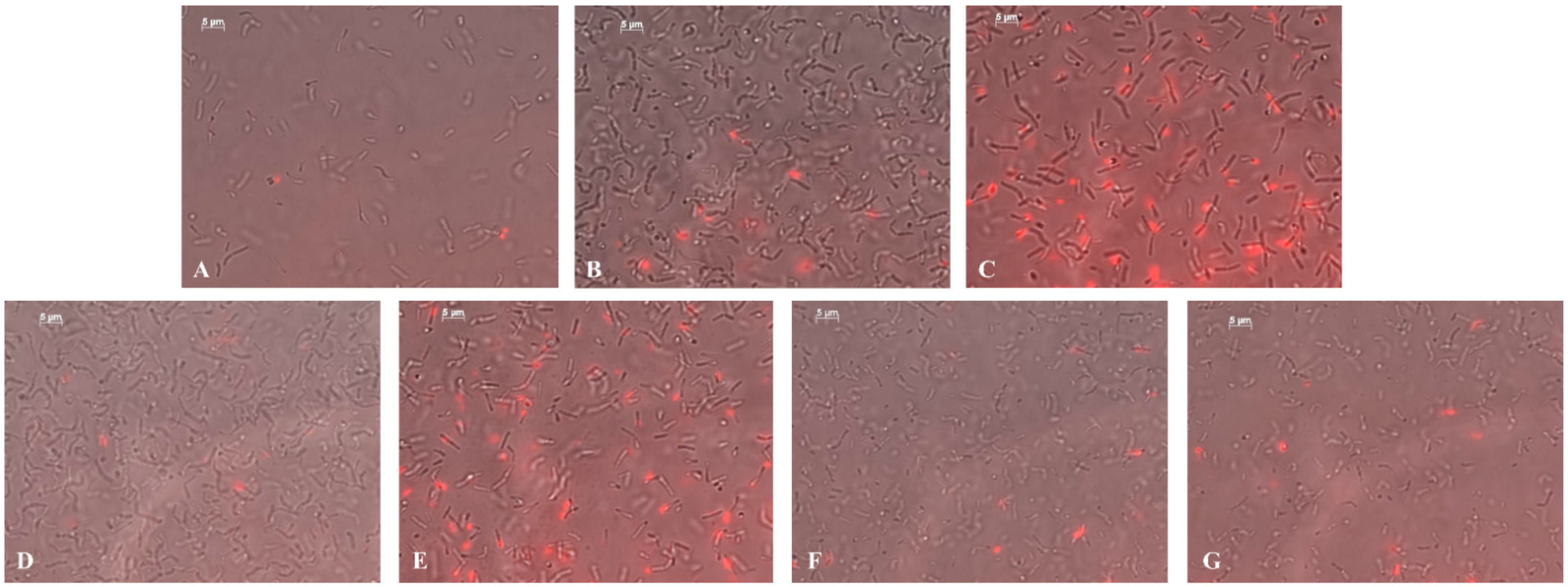
2.5. Antibacterial Activity of the NCs against the Clavibacter sepedonicus Phytopathogen
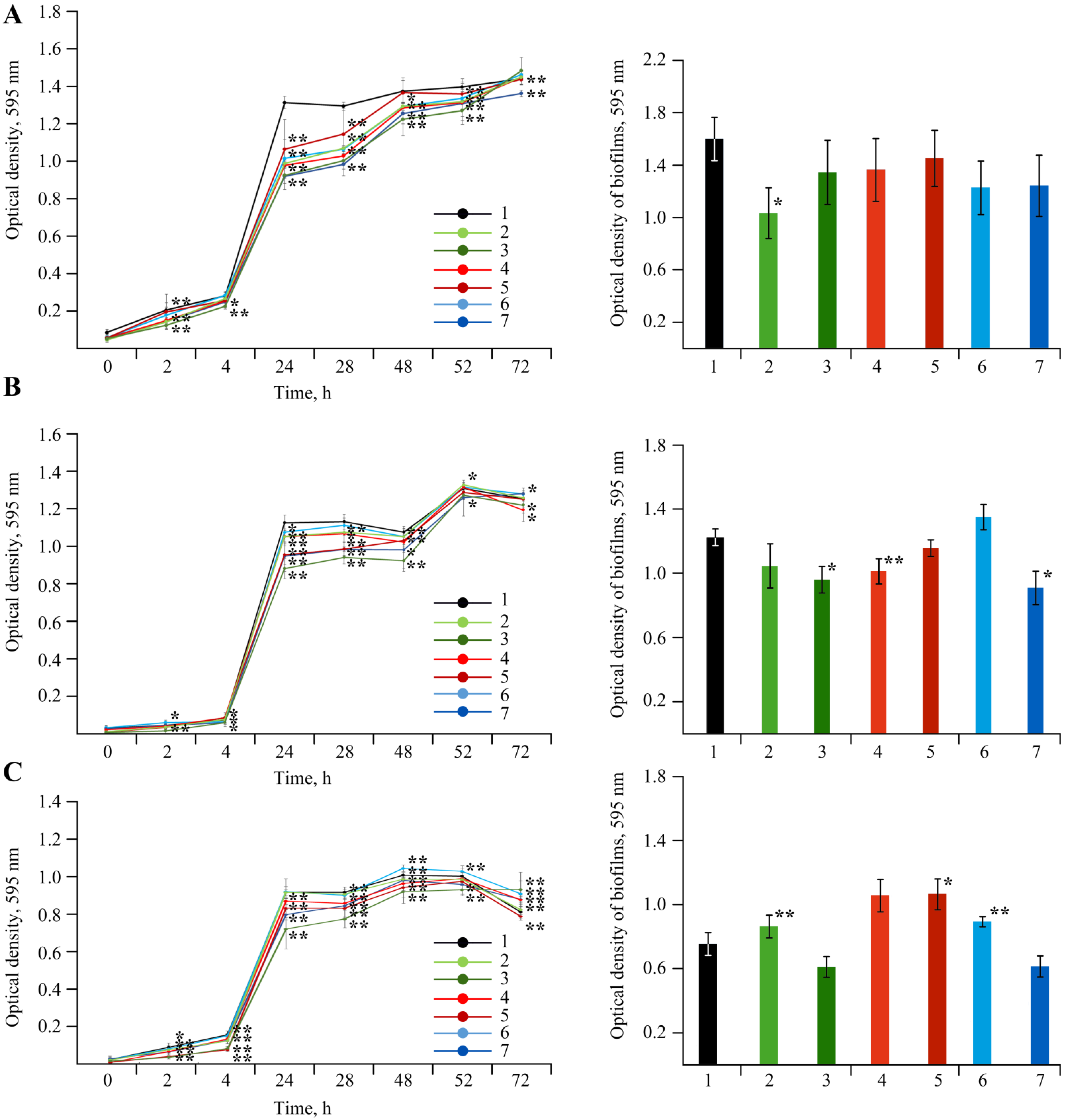
2.6. Bactericidal Effect of NCs on Soil Bacteria
3. Discussion
4. Materials and Methods
4.1. Synthesis of Manganese-Containing NCs
4.2. Physicochemical Measurements
4.3. Plant Material
4.4. Stress Resistance Experiments
4.5. Bactericidal and Bacteriostatic Effect of the NCs
4.6. Experiments with Soil Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giroto, A.S.; Guimarães, G.G.F.; Foschini, M.; Ribeiro, C. Role of slow-release nanocomposite fertilizers on nitrogen and phosphate availability in soil. Sci. Rep. 2017, 7, 46032. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.S.; Kumar, A.; Verma, N. Bacterial homoserine lactones as a nanocomposite fertilizer and defense regulator for chickpeas. Environ. Sci. Nano 2019, 6, 1246–1258. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, G.; Xiang, Y.; Cai, D.; Wu, Z. Fabrication of reusable temperature-controlled-released fertilizer using a palygorskite-based magnetic nanocomposite. Appl. Clay Sci. 2018, 161, 194–202. [Google Scholar] [CrossRef]
- Cholapandian, K.; Mythily, B. Development of nanocomposites bio-organic fertilizer. In Proceedings of the 2nd International Conference on Advances in Electrical, Electronics, Information, Communication and Bio-Informatics (AEEICB), Chennai, India, 27–28 February 2016; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2016; pp. 460–463. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Ganenko, T.V.; Graskova, I.A.; Sukhov, B.G.; Artem’ev, A.V.; Trofimov, B.A.; Krutovsky, K.V. Selenium nanocomposites in natural matrices as potato recovery agent. Int. J. Mol. Sci. 2021, 22, 4576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, M.; Chen, Y.; Lu, J.; Li, H.; Sun, Q.; Nechitaylo, G.S.; Zhigach, A.N.; Leypunsky, I.O.; Rakhmetova, A.A.; et al. The Method for Cultivation of Plants Using Metal Nanoparticles and the Nutrient Medium for Its. Implementation. Patent WO 2017101691 A1, 22 June 2017. [Google Scholar]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Nechitailo, G.S.; Bogoslovskaya, O.A.; Ol’khovskaya, I.P.; Glushchenko, N.N. Influence of iron, zinc, and copper nanoparticles on some growth indices of pepper plants. Nanotechnol. Russ. 2018, 13, 161–167. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Müller, A.; Cheetham, A.K. The Chemistry of Nanomaterials; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Landau, U.; Anselm, K. Bactericidal and Oligodynamic Action of Silver and Copper in Hygiene, Medicine and Water Treatment; Finishing Publications Ltd.: Stevenage, UK, 2007. [Google Scholar]
- Khutsishvili, S.S.; Toidze, P.; Donadze, M.; Gabrichidze, M.; Makhaldiani, N.; Agladze, T. Structural surface features of paramagnetic multifunctional nanohybrids based on silver oleic acid. J. Clust. Sci. 2020, 32, 1351–1359. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yu, H.J.; Wang, L.; Deng, Z.; Vatsadze, S.Z. In-situ preparation of palladium nanoparticles loaded ferrocene based metal-organic framework and its application in oxidation of benzyl alchogol. J. Mol. Struct. 2019, 1198, 126895. [Google Scholar] [CrossRef]
- Rozenberg, B.A.; Tenne, R. Polymer-assisted fabrication of nanoparticles and nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Ochsner, A.; Shokuhfar, A. New Frontiers of Nanoparticles and Nanocomposite Materials; Novel Principles and Techniques; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotech. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Lesnichaya, M.V.; Vakul’skaya, T.I.; Dolmaa, G.; Aleksandrova, G.P.; Rakevich, A.L.; Sukhov, B.G. Humic-based bionanocomposites containing stable paramagnetic gold nanoparticles for prospective use in pharmaceuticals. Spectrosc. Lett. 2018, 51, 169–173. [Google Scholar] [CrossRef]
- Marradi, M.; Chiodo, F.; García, I.; Penadés, S. Glyconano particles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013, 42, 4728–4745. [Google Scholar] [CrossRef] [PubMed]
- Khutsishvili, S.S.; Vakul’skaya, T.I.; Aleksandrova, G.P.; Sukhov, B.G. Stabilized silver nanoparticles and clusters Agn of humic-based bioactive nanocomposites. J. Clust. Sci. 2017, 28, 3067–3074. [Google Scholar] [CrossRef]
- Lesnichaya, M.V.; Sukhov, B.G.; Aleksandrova, G.P.; Gasilova, E.R.; Vakul’skaya, T.I.; Khutsishvili, S.S.; Sapozhnikov, A.N.; Klimenkov, I.V.; Trofimov, B.A. Chiroplasmonic magnetic gold nanocomposites produced by one-step aqueous method using κ-carrageenan. Carbohydr. Polym. 2017, 175, 18–26. [Google Scholar] [CrossRef]
- Ganenko, T.V.; Tantsyrev, A.P.; Sapozhnikov, A.N.; Khutsishvili, S.S.; Vakul’skaya, T.I.; Fadeeva, T.V.; Sukhov, B.G.; Trofimov, B.A. Nanocomposites of silver with arabinogalactan sulfate: Preparation, structure, and antimicrobial activity. Russ. J. Gen. Chem. 2015, 85, 477–484. [Google Scholar] [CrossRef]
- Mulder, E.G. Mineral nutrition in relation to the biochemistry and physiology of potatoes. Plant Soil 1949, 2, 59–121. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Huner, N.P.A. Introduction to Plant Physiology, 4th ed.; Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Burnell, J.N. The biochemistry of manganese in plants. In Manganese in Soils and Plants; Developments in Plant and Soil Sciences; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; The Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; Volume 33. [Google Scholar]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese deficiency in plants: The impact on photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef]
- Wallace, T. The Diagnosis of Mineral Deficiencies in Plants by Visual Symptoms; A Colour Atlas and Guide; H.M. Stationery Office: London, UK, 1943. [Google Scholar]
- Trofimov, B.A.; Sukhov, B.G.; Aleksandrova, G.P.; Medvedeva, S.A.; Grishchenko, L.A.; Mal’kina, A.G.; Feoktistova, L.P.; Sapozhnikov, A.N.; Dubrovina, V.I.; Martynovich, E.F.; et al. Nanocomposites with magnetic, optical, catalytic, and biologically active properties based on arabinogalactan. Dokl. Chem. 2003, 393, 287–288. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A. Small AgNP in the biopolymer nanocomposite system. Int. J. Mol. Sci. 2020, 21, 9388. [Google Scholar] [CrossRef]
- Langille, A.R.; Batteese, R.I., Jr. Influence of manganese concentration in nutrient solution on the growth and elemental content of the “Katahdin” potato plant. Can. J. Plant Sci. 1974, 54, 375–381. [Google Scholar] [CrossRef]
- Ingram, D.J.E. Biologycal and Biochemical Applications of Electron Spin Resonance; Adam Hilder LTD: London, UK, 1969. [Google Scholar]
- Labanowska, M.; Filek, M.; Kurdziel, M.; Bednarska, E.; Dlubacz, A.; Hartikainen, H. Electron paramagnetic resonance (EPR) spectroscopy characterization of wheat grains from plants of different water stress tolerance. J. Plant Physiol. 2012, 169, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.P.; Farach, H.A. Line shapes in electron spin resonance. Bull. Magn. Reson. 1979, 1, 162–194. [Google Scholar]
- Polat, M.; Korkmaz, M. Detection of irradiated black tea (Camellia sinensis) and rooibos tea (Aspalathuslinearis) by ESR spectroscopy. Food Chem. 2008, 107, 956–961. [Google Scholar] [CrossRef]
- Zaafarany, I.; Gobouri, A.; Hassan, R. Oxidation of some sulfated carbohydrates: Kinetics and mechanism of oxidation of chondroitin-4-sulfate by alkaline permanganate with novel synthesis of coordination biopolymer precursor. J. Mater. Sci. Res. 2013, 2, 23–36. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Ganenko, T.V.; Sukhov, B.G. Formation and paramagnetic properties of manganese-containing bionanocomposites based on natural polysaccharide matrices. J. Carbohydr. Chem. 2021. [Google Scholar] [CrossRef]
- Tajmir-Riahi, H.A. Carbohydrate metal ion complexes. Interaction of D-glucono-1,5-lactone with Zn(II), Cd(II), and Hg(II) ions in the solid and aqueous solution, studied by 13C-NMR, FT-IR, and X-ray powder diffraction measurements. Can. J. Chem. 1989, 67, 651–654. [Google Scholar] [CrossRef]
- De Souza, R.F.V.; De Giovani, W.F. Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim. Acta A 2005, 61, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, G.S.; Cakić, M.D. Analysis of bioactive oligosaccharide-metal complexes. In Fourier Transforms—New Analytical Aproaches and FTIR Strategies; Nikolić, G.S., Ed.; InTech: Rijeka, Croatia, 2011; p. 15. [Google Scholar]
- Lis, T.; Matuszewski, J. Manganese(II) malonate dihydrate: A reinvestigation. ActaCryst. B 1979, 35, 2212–2214. [Google Scholar] [CrossRef]
- Möncke, D.; Ehrt, D.; Kamitsos, E.I. Spectroscopic study of manganese-containing borate and borosilicate glasses: Cluster formation and phase separation. Phys. Chem. Glasses B 2013, 54, 42–51. [Google Scholar]
- Galyametdinov, Y.G.; Sagdeev, D.O.; Sukhanov, A.A.; Voronkova, V.K.; Shamilov, R.R. Monitoring of the mechanism of Mn ions incorporation into quantum dots by optical and EPR spectroscopy. Photonics 2019, 6, 107. [Google Scholar] [CrossRef]
- Anderson, P.W.; Weiss, P.R. Exchange narrowing in paramagnetic resonance. Rev. Mod. Phys. 1953, 25, 269–276. [Google Scholar] [CrossRef]
- Elsi, S.; Pushpanathan, K. Role of Cu and Mn dopants on d0 ferromagnetism of ZnS nanoparticles. J. Mater. Sci. Mater. 2019, 30, 10792–10807. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci. Plant Nutr. 2009, 55, 698–704. [Google Scholar] [CrossRef]
- Baysal, F.G.; Tipirdamaz, R. Physiological and antioxidant response of three cultivars of cucumber (Cucumissativus L.) to salinity. Turk. J. Biol. 2010, 34, 287–296. [Google Scholar] [CrossRef]
- Sharlaeva, E.A.; Chirkova, V.Y. The impact of short-wave UV radiation on peroxidase activity in soft wheat seeds. IOP Conf. Ser. Earth Environ. Sci. 2021, 670, 012008. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 2018, 122, 21–27. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Kamalova, G.V.; Akulov, A.N.; Rumyantseva, N.I. Comparison of redox state of cells of tatar buckwheat morphogenic calluses and non-morphogenic calluses obtained from them. Biochemistry 2009, 74, 686–694. [Google Scholar] [CrossRef]
- Huang, Q.; Jin, Y.; Zhang, L.; Cheung, P.C.K.; Kennedy, J.F. Structure, molecular size and antitumor activities of polysaccharides from Poriacocos mycelia produced in fermenter. Carbohydr. Polym. 2007, 70, 324–333. [Google Scholar] [CrossRef]
- Tong, H.; Xia, F.; Feng, K.; Sun, G.; Gao, X.; Sun, L.; Jiang, R.; Tian, D.; Sun, X. Structural characterization and in vitro antitumor activity of a novel polysaccharide isolated from the fruiting bodies of Pleurotusostreatus. Bioresour. Technol. 2009, 100, 1682–1686. [Google Scholar] [CrossRef]
- Moradali, M.-F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.-A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharm. 2007, 7, 701–724. [Google Scholar] [CrossRef]
- Eichenlaub, R.; Gartemann, K.-H. The Clavibacter michiganensis subspecies: Molecular investigation of gramm-positive bacterial plant pathogens. Annu. Rev. Phytopathol. 2011, 49, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tambong, J.; Yuan, K.X.; Chen, W.; Xu, H.; Lévesque, C.A.; De Boer, S.H. Re-classification of Clavibacter michiganensis subspecies on the basis of whole-genome and multi-locus sequence analyses. Int. J. Syst. Evol. Microbiol. 2018, 68, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Nozhevnikova, A.N.; Botchkova, E.A.; Plakunov, V.K. Multi-species biofilms in ecology, medicine, and biotechnology. Microbiology 2015, 84, 731–750. [Google Scholar] [CrossRef]
- Velmourougane, K.; Prasanna, R.; Saxena, A.K. Agriculturally important microbial biofilms: Present status and future prospects. J. Basic Microbiol. 2017, 57, 548–573. [Google Scholar] [CrossRef]
- Secor, G.A.; DeBuhr, L.; Gudmestad, N.C. Susceptibility of Corynebacterium sepedonicum to disinfectants in vitro. Plant Dis. 1988, 72, 585–588. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.-H.; Deep, A. Recent advancements in sensing techniques based on functional materials for organophosphate pesticides. Biosens. Bioelectron. 2015, 70, 469–481. [Google Scholar] [CrossRef]
- Bempah, C.K.; Donkor, A.; Yeboah, P.O.; Dubey, B.; Osei-Fosu, P. A preliminary assessment of consumer’s exposure to organochlorine pesticides in fruits and vegetables and the potential health risk in Accra Metropolis, Ghana. Food Chem. 2011, 128, 1058–1065. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Bécares, E.; Fernández-Aláez, C.; Fernández-Aláez, M.; Mayo, R.; Jiménez, J.J. Chemical pollution in inland shallow lakes in the Mediterranean region (NW Spain): PAHs, insecticides and herbicides in water and sediments. Sci. Total Environ. 2016, 544, 797–810. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Smreczak, B. The impact of organic matter on polycyclic aromatic hydrocarbon (PAH) availability and persistentence in soils. Molecules 2020, 25, 2470. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidat activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant activity of metal nanoparticles coated with tocopherol-like residues—The importance of studies in homo- and heterogeneous systems. Antioxidants 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Mahlangeni, N.T.; Moodley, R. Biosynthesis of manganese oxide nanoparticles using Urginea sanguinea and their effects on cytotoxicity and antioxidant activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 015015. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Vakul’skaya, T.I.; Aleksandrova, G.P.; Sukhov, B.G. Strong stabilization properties of humic substance matrixes for silver bionanocomposites. Micro Nano Lett. 2017, 12, 418–421. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Moty’leva, S.M.; Klimenkov, I.V.; Arsent’ev, K.Y.; Graskova, A.I.; Sukhov, B.G.; Trofimov, B.A. Development of antimicrobial nano-selenium biocomposite for protecting potatoes from bacterial phytopathogens. Nanotechnol. Russ. 2017, 12, 553–558. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Liu. Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef]
- Kolouchová, I.; Schreiberová, O.; Masák, J.; Sigler, K.; Rezanka, T. Structural analysis of mycolic acids from phenol-degrading strain of Rhodococcus erythropolis by liquid chromatography-tandem mass spectrometry. Folia Microbiol. 2012, 57, 473–483. [Google Scholar] [CrossRef]
- Nahar, A.; Baker, A.L.; Nichols, D.S.; Bowman, J.P.; Britz, M.L. Benchmarking DNA extraction methods for phylogenomic analysis of sub-antarctic Rhodococcus and Williamsia species. Microorganisms 2021, 9, 1253. [Google Scholar] [CrossRef]
- De Souza, M.V.N.; Ferreira, M.L.; Pinheiro, A.C.; Saraiva, M.F.; de Almeida, M.V.; Valle, M.S. Synthesis and biological aspects of mycolic acids: An important target against Mycobacterium tuberculosis. Sci. World J. 2008, 8, 720–751. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Wang, C.; Besra, G.S. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2005, 18, 81–101. [Google Scholar] [CrossRef]
- Bhatt, A.; Molle, V.; Besra, G.S.; Jacobs, W.R.; Kremer, L. The Mycobacterium tuberculosis FAS-II condensing enzymes: Their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Mol. Microbiol. 2007, 64, 1442–1454. [Google Scholar] [CrossRef]
- Retamal-Morales, G.; Heine, T.; Tischler, J.S.; Erler, B.; Gröning, J.A.D.; Kaschabek, S.R.; Schlömann, M.; Levicán, G.; Tischler, D. Draft genome sequence of Rhodococcus erythropolis B7g, a biosurfactant producing actinobacterium. J. Biotechnol. 2018, 280, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Abdul Khalil, K.; Zahri, K.N.M.; Gomez-Fuentes, C.; Convey, P.; Zulkharnain, A.; Sabri, S.; Alias, S.A.; González-Rocha, G.; Ahmad, S.A. Biosurfactant production and growth kinetics studies of the waste canola oil-degrading bacterium Rhodococcus erythropolis AQ5-07 from Antarctica. Molecules 2020, 25, 3878. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.D.; Lavrov, K.V.; Kasianov, A.S.; Korzhenkov, A.A.; Gubanova, T.A.; Yanenko, A.S. Draft genome sequence of Rhodococcus erythropolis HX7, a psychrotolerant soil-derived oil degrader. Microbiol. Resour. Announc. 2021, 10, e01353-20. [Google Scholar] [CrossRef]
- Cao, J.; Chande, C.; Kalensee, F.; Schüler, T.; Köhler, M. Microfluidically supported characterization of responses of Rhodococcus erythropolis strains isolated from different soils on Cu-, Ni-, and Co-stress. Braz. J. Microbiol. 2021, 52, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Retamal-Morales, G.; Senges, C.H.R.; Stapf, M.; Olguín, A.; Modak, B.; Bandow, J.E.; Tischler, D.; Schlömann, M.; Levicán, G. Isolation and characterization of arsenic-binding siderophores from Rhodococcus erythropolis S43: Role of heterobactin B and other heterobactin variants. Appl. Microbiol. Biotechnol. 2021, 105, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Yoshida, N. Functional analysis of putative transporters involved in oligotrophic growth of Rhodococcus erythropolis N9T-4. Appl. Microbiol. Biotechnol. 2019, 103, 4167–4175. [Google Scholar] [CrossRef]
- Kostyro, J.A.; Ganenko, T.V.; Medvedeva, S.A.; Sukhov, B.G.; Trofimov, B.A. Method for Preparing Sulfated Derivates of Arabinogalactan Possessing Anticoagulating and Hypolipidemic. Activity. Patent RU 2319707 C1, 9 January 2007. [Google Scholar]
- Papkina, A.V.; Perfileva, A.I.; Zhivetev, M.A.; Borovskiy, G.B.; Graskova, I.A.; Lesnichaya, M.V.; Klimenkov, I.V.; Sukhov, B.G.; Trofimov, B.A. Effect of selenium and arabinogalactan nanocomposite on viability of the phytopathogen Clavibacter michiganensis subsp. sepedonicus. Dokl. Biol. Sci. 2015, 461, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Nozhkina, O.A.; Perfileva, A.I.; Graskova, I.A.; Dyakova, A.V.; Nurminsky, V.N.; Klimenkov, I.V.; Ganenko, T.V.; Borodina, T.N.; Aleksandrova, G.P.; Sukhov, B.G.; et al. The biological activity of a selenium nanocomposite encapsulated in carrageenan macromolecules with respect to ring rot pathogenesis of potato plants. Nanotechnol. Russ. 2019, 14, 255–262. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Graskova, I.A.; Sidorov, A.V.; Lesnichaya, M.V.; Aleksandrova, G.P.; Dolmaa, G.; Klimenkov, I.V.; Sukhov, B.G. Synthesis of selenium and silver nanobiocomposites and their influence on phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus. Russ. Chem. Bull. 2018, 67, 157–163. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Graskova, I.A.; Sukhov, B.G.; Trofimov, B.A. Carrageenan as polymer matrix for selenium nanocomposites. Russ. Chem. Bull. 2020, 4, 876–878. [Google Scholar] [CrossRef]
- Romanenko, A.S.; Riffel, A.A.; Graskova, I.A.; Rachenko, M.A. The role of extracellular pH-homeostasis in potato resistance to ring rot pathogen. J. Phytopathol. 1999, 147, 679–686. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signaling events in apoplastic oxidative burst in suspension cultured french bean cells involve camp and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bolland, J.L.; Koch, H.P. The course of antioxidant reaction in polyisoprenes and allied compounds. Part IX. The primary thermal oxidation product of ethyl linoleate. J. Chem. Soc. 1945, 7, 445–447. [Google Scholar] [CrossRef]
- Revin, V.V.; Gromova, N.V.; Revina, E.S.; Samonova, A.Y.; Tychkov, A.Y.; Bochkareva, S.S.; Moskovkin, A.A.; Kuzmenko, T.P. The influence of oxidative stress and natural antioxidants on morphometric parameters of red blood cells, the hemoglobin oxygen binding capacity, and the activity of antioxidant enzyme. Biomed. Res. Int. 2019, 2019, 2109269. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Roozen, N.J.M.; Van Vuurde, J.W.L. Development of a semi-selective medium and an immunofluorescence colony-staining procedure for the detection of Clavibacter michiganensis subsp. sepedonicus in cattle manure slurry. Neth. J. Plant. Pathol. 1991, 97, 321–334. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Tsivileva, O.M.; Nozhkina, O.A.; Karepova, M.S.; Graskova, I.A.; Ganenko, T.V.; Sukhov, B.G.; Krutovsky, K.V. Effect of natural polysaccharide matrix-based selenium nanocomposites on Phytophthora cactorum and rhizospheric microorganisms. Nanomaterials 2021, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Sagdic, O.; Aksoy, A.; Ozkan, G. Evaluation of the antibacterial and antioxidant potentials of gilaburu (Viburnum opulus L.) fruit extract. Acta Aliment. 2006, 35, 487–492. [Google Scholar] [CrossRef]
- Yamori, W.; Kogami, H.; Masuzawa, T. Freezing tolerance in alpine plants as assessed by the FDA-staining method. Polar Biosci. 2005, 18, 73–81. [Google Scholar] [CrossRef]
- Tretyakova, M.S.; Belovezhets, L.A.; Markova, Y.A.; Makarova, L.E. Capability of crude oil degrading bacteria to reduce oil toxic effect on plants. Agrochemistry 2017, 12, 46–51, (In Russian with English Abstract). [Google Scholar]
- Silva, F. Pseudomonas (Flavimonas) oryzihabitans. Rev. Chil. De Infectol. 2015, 32, 445–446. [Google Scholar] [CrossRef] [PubMed]


| Mn Sulfate, mol/L | Roots | Stems | Leaves |
|---|---|---|---|
| 0 | 0.02 ± 0.01 * | 0.04 ± 0.01 * | 0.14 ± 0.02 |
| 0.1 (control) | 0.11 ± 0.02 | 0.18 ± 0.01 | 0.16 ± 0.04 |
| 0.2 | 0.17 ± 0.04 | 0.21 ± 0.04 | 0.22 ± 0.01 * |
| 0.5 | 0.54 ± 0.01 * | 0.29 ± 0.08 * | 0.20 ± 0.08 |
| 1.0 | 0.79 ± 0.09 * | 0.88 ± 0.28 * | 0.34 ± 0.08 * |
| 2.0 | 0.97 ± 0.08 * | 1.10 ± 0.13 * | 0.74 ± 0.15 * |
| 10.0 | 1.50 ± 0.25 * | 5.34 ± 0.59 * | 3.82 ± 0.17 * |
| Treatment | Roots | Stems | Leaves |
|---|---|---|---|
| Control | 0.17 ± 0.03 | 0.19 ± 0.04 | 0.20 ± 0.13 |
| AG-Mn | 0.28 ± 0.10 * | 0.27 ± 0.01 * | 0.24 ± 0.20 |
| AGS-Mn | 0.05 ± 0.04 * | 0.16 ± 0.11 | 0.17 ± 0.13 |
| κ-CG-Mn | 0.29 ± 0.11 * | 0.38 ± 0.13 * | 0.24 ± 0.11 |
| Treatment | Length, µm | Width, µm | Dead Cell Count, % |
|---|---|---|---|
| Control | 3.13 ± 0.08 | 0.56 ± 0.02 | 0.34 ± 0.01 |
| AG-Mn | 2.12 ± 0.07 * | 0.49 ± 0.01 * | 2.35 ± 0.46 * |
| AG-Mn† | 2.07 ± 0.08 * | 0.65 ± 0.19 | 5.55 ± 0.40 * |
| AGS-Mn | 2.31 ± 0.09 * | 0.53 ± 0.01 | 3.69 ± 0.54 * |
| AGS-Mn† | 2.53 ± 0.34 * | 0.77 ± 0.01 * | 24.77 ± 1.70 * |
| κ-CG-Mn | 2.41 ± 0.63 * | 0.62 ± 0.04 * | 3.07 ± 0.66 |
| κ-CG-Mn† | 2.62 ± 0.17 * | 0.71 ± 0.24 * | 13.68 ± 4.82 * |
| Nanocomposite | C | H | S | Mn |
|---|---|---|---|---|
| AG-Mn | 38.5 | 5.8 | 0.0 | 5.2 |
| AGS-Mn | 28.7 | 5.8 | 7.1 | 4.8 |
| κ-CG-Mn | 26.8 | 5.3 | 2.5 | 20.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khutsishvili, S.S.; Perfileva, A.I.; Nozhkina, O.A.; Ganenko, T.V.; Krutovsky, K.V. Novel Nanobiocomposites Based on Natural Polysaccharides as Universal Trophic Low-Dose Micronutrients. Int. J. Mol. Sci. 2021, 22, 12006. https://doi.org/10.3390/ijms222112006
Khutsishvili SS, Perfileva AI, Nozhkina OA, Ganenko TV, Krutovsky KV. Novel Nanobiocomposites Based on Natural Polysaccharides as Universal Trophic Low-Dose Micronutrients. International Journal of Molecular Sciences. 2021; 22(21):12006. https://doi.org/10.3390/ijms222112006
Chicago/Turabian StyleKhutsishvili, Spartak S., Alla I. Perfileva, Olga A. Nozhkina, Tatjana V. Ganenko, and Konstantin V. Krutovsky. 2021. "Novel Nanobiocomposites Based on Natural Polysaccharides as Universal Trophic Low-Dose Micronutrients" International Journal of Molecular Sciences 22, no. 21: 12006. https://doi.org/10.3390/ijms222112006
APA StyleKhutsishvili, S. S., Perfileva, A. I., Nozhkina, O. A., Ganenko, T. V., & Krutovsky, K. V. (2021). Novel Nanobiocomposites Based on Natural Polysaccharides as Universal Trophic Low-Dose Micronutrients. International Journal of Molecular Sciences, 22(21), 12006. https://doi.org/10.3390/ijms222112006









