The Effect of Castration on Peripheral Autonomic Neurons Supplying Mammalian Male Genitourinary System
Abstract
:1. Introduction
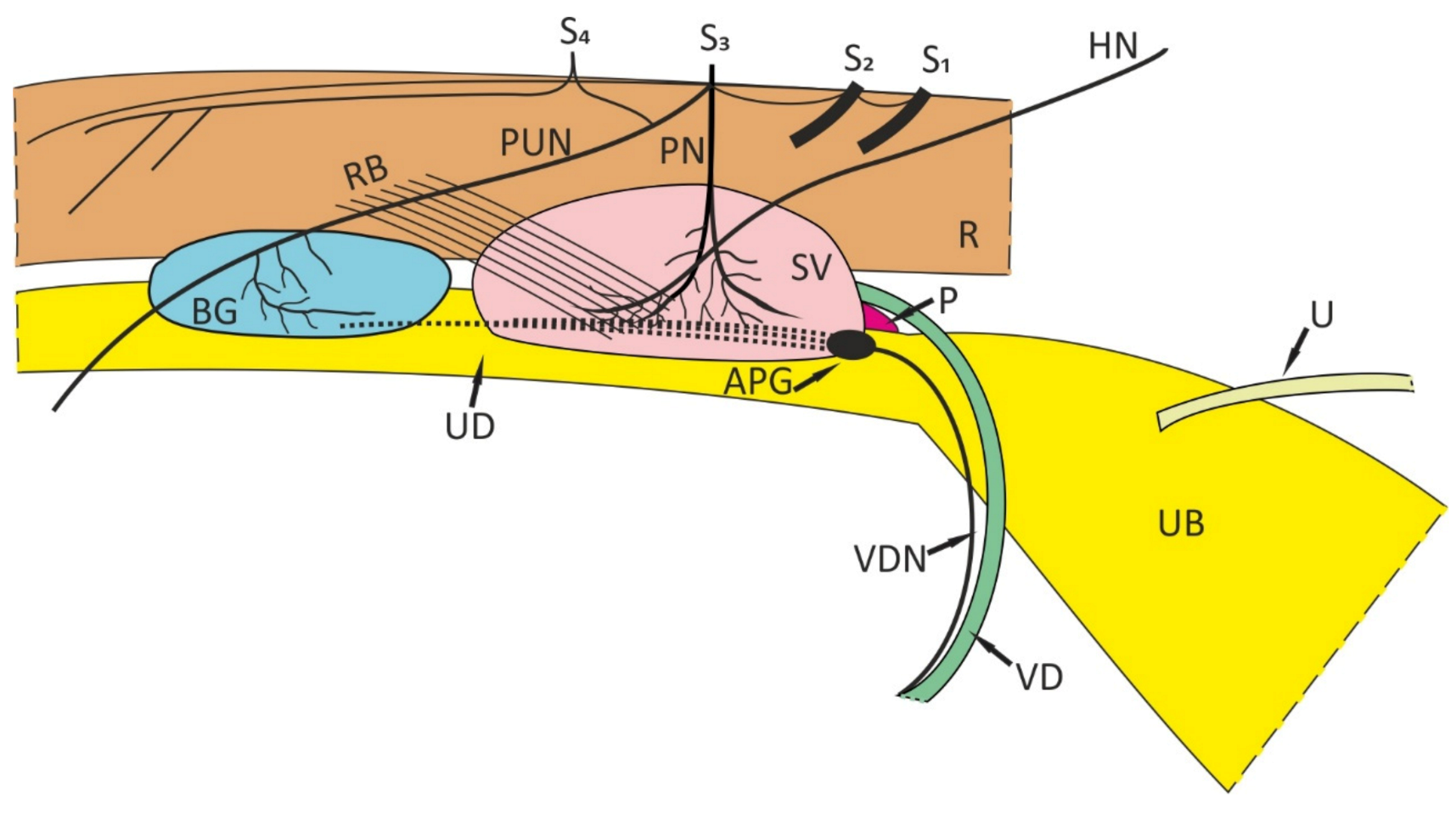
2. Some General Remarks
3. Androgen-Dependent Peripheral Neurons in Male Rats and Pigs
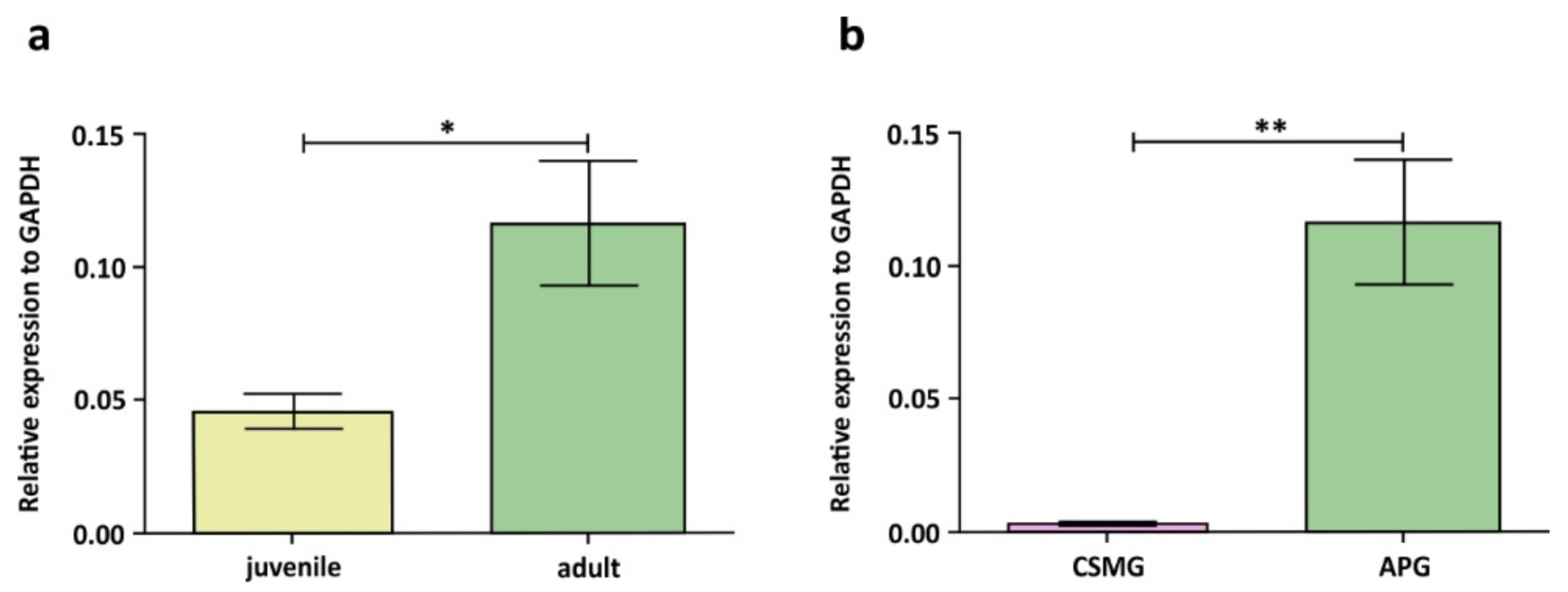
4. Effect of Testosterone Deprivation (Castration) on the Number of Peripheral Neurons Supplying Male Urogenital Tract
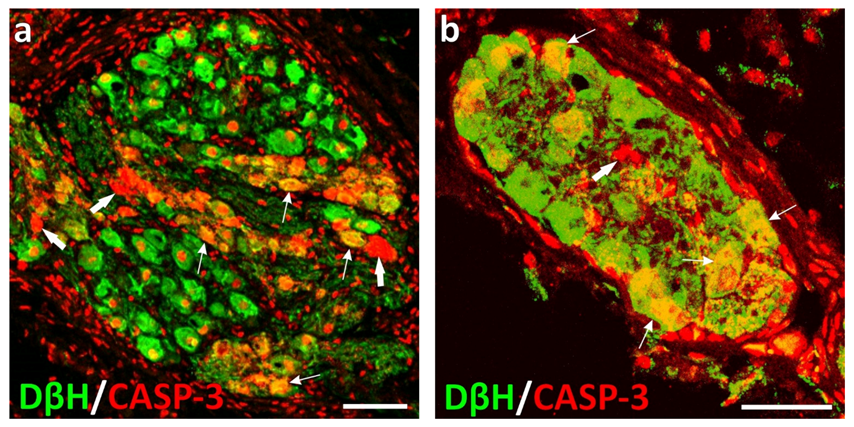
5. Effect of Castration on the Chemical Coding of Peripheral Neurons Supplying Male Urogenital Tract
6. Conclusions
- -
- Would the neuronal loss found in male pigs castrated a few days after birth be observed also in the castrated adult animals?
- -
- Which structures are innervated by the porcine male apoptotic neurons?
- -
- Is castration followed by significant loss of nerve fibers in the organs of the porcine male urogenital system (in either juvenile or adult individuals)?
- -
- In adult individuals of both sexes of other species (including humans), can any significant loss of pelvic or UB intramural neurons be observed after castration (female and male cats, male horses) or following/during natural (menopausal women) or therapeutic (prostate cancer patients) sex steroid deprivation? The same question applies to nerve fibers, especially those supplying the pelvic organs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ford, J.J.; D’Occhio, M.J. Differentiation of sexual behavior in cattle, sheep and swine. J. Anim. Sci. 1989, 67, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Kawata, M. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci. Res. 1995, 24, 1–46. [Google Scholar] [CrossRef]
- Panzica, G.C.; Balthazart, J.; Frye, C.A.; Garcia-Segura, L.M.; Herbison, A.E.; Mensah-Nyagan, A.G.; Panzica, G.C.; Balthazart, J.; Frye, C.A.; Garcia-Segura, L.M.; et al. Milestones on steroids and the nervous system: 10 years of basic and translational research. J. Neuroendocrinol. 2012, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrocco, J.; McEwen, B.S. Sex in the brain: Hormones and sex differences. Dialogues Clin. Neurosci. 2016, 18, 373–383. [Google Scholar] [CrossRef]
- Heberden, C. Sex steroids and neurogenesis. Biochem. Pharmacol. 2017, 141, 56–62. [Google Scholar] [CrossRef]
- Keast, J.R. The autonomic nerve supply of male sex organs-An important target of circulating androgens. Behav. Brain Res. 1999, 105, 81–92. [Google Scholar] [CrossRef]
- Keast, J.R. Unusual autonomic ganglia: Connections, chemistry, and plasticity of pelvic ganglia. Int. Rev. Cytol. 1999, 193, 1–69. [Google Scholar] [CrossRef]
- Keast, J.R. Effects of testosterone on pelvic autonomic pathways: Progress and pitfalls. J. Auton. Nerv. Syst. 2000, 79, 67–73. [Google Scholar] [CrossRef]
- Keast, J.R. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int. Rev. Cytol. 2006, 248, 141–208. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Timmermans, J.P.; Majewski, M.; Lakomy, M.; Scheuermann, D.W. Immunohistochemical properties of nerve fibres supplying accessory male genital glands in the pig. A colocalisation study. Histochem. Cell Biol. 1999, 111, 217–228. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Kasica-Jarosz, N.; Pidsudko, Z.; Dudek, A.; Klimczuk, M.; Sienkiewicz, W. Effect of castration on pelvic neurons in the male pig. Histochem. Cell Biol. 2020, 53, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Kaleczyc, J.; Sienkiewicz, W.; Lepiarczyk, E.; Kasica-Jarosz, N.; Pidsudko, Z. The influence of castration on intramural neurons of the urinary bladder trigone in male pigs. J. Anat. 2021. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Patrol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. What is neural plasticity? Adv. Exp. Med. Biol. 2017, 1015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Keast, J.R. Visualization and immunohistochemical characterization of sympathetic and parasympathetic neurons in the male rat major pelvic ganglion. Neuroscience 1995, 66, 655–662. [Google Scholar] [CrossRef]
- Keast, J.R.; Luckensmeyer, G.B.; Schemann, M. All pelvic neurons in male rats contain immunoreactivity for the synthetic enzymes of either noradrenaline or acetylcholine. Neurosci. Lett. 1995, 196, 209–212. [Google Scholar] [CrossRef]
- Keast, J.R.; de Groat, W.C. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. J. Comp. Neurol. 1989, 288, 387–400. [Google Scholar] [CrossRef]
- Keast, J.R.; Saunders, R.J. Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neuroscience 1998, 85, 543–556. [Google Scholar] [CrossRef]
- Melvin, J.E.; McNeill, T.H.; Hamill, R.W. Biochemical and morphological effects of castration on the postorganizational development of the hypogastric ganglion. Brain Res. 1988, 466, 131–139. [Google Scholar] [CrossRef]
- Melvin, J.E.; McNeill, T.H.; Hervonen, A.; Hamill, R.W. Organizational role of testosterone on the biochemical and morphological development of the hypogastric ganglion. Brain Res. 1989, 485, 1–10. [Google Scholar] [CrossRef]
- Kaleczyc, J. Origin and neurochemical characteristics of nerve fibres supplying the mammalian vas deferens. Microsc. Res. Tech. 1998, 42, 409–422. [Google Scholar] [CrossRef]
- Sienkiewicz, W. Sources of the porcine testis innervation. Andrologia 2010, 42, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Pidsudko, Z. Immunohistochemical characteristics and distribution of neurons in the paravertebral, prevertebral and pelvic ganglia supplying the urinary bladder in the male pig. J. Mol. Neurosci. 2014, 52, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.S.; Gilpin, S.-A.; Gilpin, C.J.; Gosling, J.A. Intramural ganglia of the human urinary bladder. Br. J. Urol. 1983, 55, 195–198. [Google Scholar] [CrossRef]
- Fehér, E.; Csányi, K.; Vajda, J. Ultrastructure of the nerve cells and fibres in the urinary bladder wall of the cat. Acta Anat. 1979, 103, 109–118. [Google Scholar] [CrossRef]
- Gabella, G. Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res. 1990, 261, 231–237. [Google Scholar] [CrossRef]
- Gilpin, C.J.; Dixon, J.S.; Gilpin, S.A.; Gosling, J.A. The fine structure of autonomic neurons in the wall of the human urinary bladder. J. Anat. 1983, 137, 705–713. [Google Scholar] [PubMed]
- Lincoln, J.; Burnstock, G. Autonomic innervation of the urinary bladder and urethra. In Nervous Control of the Urogenital System, 1st ed.; Maggi, C.A., Ed.; Harwood Academic Publishers: London, UK, 1993; pp. 33–68. [Google Scholar]
- Pirker, M.E.; Montedonico, S.; Rolle, U.; Austvoll, H.; Puri, P. Regional differences in nitrergic neuronal density in the developing porcine urinary bladder. Pediatric Surg. Int. 2005, 21, 161–168. [Google Scholar] [CrossRef]
- Gabella, G.; Uvelius, B. Urinary bladder of rat: Fine structure of normal and hypertrophic musculature. Cell Tissue Res. 1990, 262, 67–79. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Kasica-Jarosz, N.; Pidsudko, Z.; Przyborowska, A.; Sienkiewicz, W. The expression of androgen receptor in neurons of the anterior pelvic ganglion and celiac-superior mesenteric ganglion in the male pig. Pol. J. Vet. Sci. 2019, 22, 151–155. [Google Scholar] [CrossRef]
- Watkins, T.W.; Keast, J.R. Androgen-sensitive preganglionic neurons innervate the male rat pelvic ganglion. Neuroscience 1999, 93, 1147–1157. [Google Scholar] [CrossRef]
- Keast, J.R.; Gleeson, R.J. Androgen receptor immunoreactivity is present in primary sensory neurons of male rats. Neuroreport 1998, 9, 4137–4140. [Google Scholar] [CrossRef] [PubMed]
- Keast, J.R. Patterns of co-existence of peptides and differences of nerve fibre types associated with noradrenergic and non-noradrenergic (putative cholinergic) neurons in the major pelvic ganglion of the male rat. Cell Tissue Res. 1991, 266, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Schirar, A.; Chang, C.; Rousseau, J.P. Localization of androgen receptor in nitric oxide synthase- and vasoactive intestinal peptide-containing neurons of the major pelvic ganglion innervating the rat penis. J. Neuroendocrinol. 1997, 9, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kaleczyc, J.; Wąsowicz, K.; Klimczuk, M.; Czaja, K.; Łakomy, M. Immunohistochemical characterisation of cholinergic neurons in the anterior pelvic ganglion of the male pig. Folia Histochem. Cytobiol. 2003, 41, 65–72. [Google Scholar]
- Schirar, A.; Bonnefond, C.; Meusnier, C.; Devinoy, E. Androgens modulate nitric oxide synthase messenger ribonucleic acid expression in neurons of the major pelvic ganglion in the rat. Endocrinology 1997, 138, 3093–3102. [Google Scholar] [CrossRef]
- Hisasue, S.; Kato, R.; Suetomi, T.; Kato, K.; Suzuki, K.; Kobayashi, K.; Itoh, N.; Kiyama, H.; Tsukamoto, T. Age-related alteration of neurturin receptor GFRa2 and nNOS in pelvic ganglia. Neurobiol. Aging 2006, 27, 1524–1530. [Google Scholar] [CrossRef]
- Huang, X.Z.; Park, J.T.; Kim, H.G.; Lee, C.K.; Won, Y.J.; Park, B.G.; Jeong, S.W. Phenotype-specific down-regulation of nicotinic acetylcholine receptors in the pelvic ganglia of castrated rats: Implications for neurogenic erectile dysfunction. Neurosci. Lett. 2011, 501, 55–59. [Google Scholar] [CrossRef]
- Meusburger, S.M.; Keast, J.R. Testosterone and nerve growth factor have distinct but interacting effects on structure and neurotransmitter expression of adult pelvic ganglion cells in vitro. Neuroscience 2001, 108, 331–340. [Google Scholar] [CrossRef]
- Purves-Tyson, T.D.; Arshi, M.S.; Handelsman, D.J.; Cheng, Y.; Keast, J.R. Androgen and estrogen receptor-mediated mechanisms of testosterone action in male rat pelvic autonomic ganglia. Neuroscience 2007, 148, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melvin, J.E.; Hamill, R.W. Androgen-specific critical periods for the organization of the major pelvic ganglion. J. Neurosci. 1989, 9, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melvin, J.E.; Hamill, R.W. Hypogastric ganglion perinatal development: Evidence for androgen specificity via androgen receptors. Brain Res. 1989, 485, 11–19. [Google Scholar] [CrossRef]
- Dubreuil, P.; Pelletier, G.; Couture, Y.; Lapierre, H.; Petitclerc, D.; Morisset, J.; Gaudreau, P.; Brazeau, P. Castration and testosterone effects on endogenous and somatocrinin-induced growth hormone release in intact and castrated male pigs. Domest. Anim. Endocrinol. 1989, 6, 15–24. [Google Scholar] [CrossRef]
- Lacorn, M.; Bauer, A.; Claus, R. Is the early postnatal rise of testosterone responsible for a later male pattern of growth hormone secretion in pigs? Theriogenology 2009, 72, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, W. Immunohistochemical properties of caudal mesenteric ganglion and anterior pelvic ganglion neurons projecting to the porcine testes subjected to hemicastration, castration and testosterone supplementation. Bull. Vet. Inst. Pulawy 2010, 54, 357–367. [Google Scholar]
- Lossi, L.; Castagna, C.; Merighi, A. Caspase-3 mediated cell death in the normal development of the mammalian cerebellum. Int. J. Mol. Sci. 2018, 19, 3999. [Google Scholar] [CrossRef] [Green Version]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [Green Version]
- Ford, J.J.; Christenson, R.K.; Maurer, R.R. Serum testosterone concentrations in embryonic and fetal pigs during sexual differentiation. Biol Reprod. 1980, 23, 583–587. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenberger, F.; Toole, G.S.; Christie, H.L.; Raeside, J.I. Plasma levels of several androgens and estrogens from birth to puberty in male domestic pigs. Acta Endocrinol. 1993, 128, 173–177. [Google Scholar] [CrossRef]
- Ford, J.J. Postnatal differentiation of sexual preference in male pigs. Horm. Behav. 1983, 17, 152–162. [Google Scholar] [CrossRef]
- Melvin, J.E.; Hamill, R.W. The major pelvic ganglion: Androgen control of postnatal development. J. Neurosci. 1987, 7, 1607–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squillacioti, C.; De Luca, A.; Paino, G.; Mirabella, N. Effects of castration on the immunoreactivity to NGF, BDNF and their receptors in the pelvic ganglia of the male rat. Eur. J. Histochem. 2008, 52, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanjhan, R.; Osborne, P.B.; Ouyang, M.; Keast, J.R. Postnatal maturational changes in rat pelvic autonomic ganglion cells: A mixture of steroid-dependent and -independent effects. J. Neurophysiol. 2003, 89, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Abushik, P.A.; Bart, G.; Korhonen, P.; Leinonen, H.; Giniatullina, R.; Sibarov, D.A.; Levonen, A.L.; Malm, T.; Antonov, S.M.; Giniatullin, R. Pro-nociceptive migraine mediator CGRP provides neuroprotection of sensory, cortical and cerebellar neurons via multi-kinase signaling. Cephalalgia 2017, 37, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Blesch, A.; Tuszynski, M.H. GDNF gene delivery to injured adult CNS motor neurons promotes axonal growth, expression of the trophic neuropeptide CGRP, and cellular protection. J. Comp. Neurol. 2001, 436, 399–410. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, L.C. Galanin protects amyloid-beta-induced neurotoxicity on primary cultured hippocampal neurons of rats. J. Alzheimers Dis. 2010, 20, 1143–1157. [Google Scholar] [CrossRef]
- Tunçel, N.; Korkmaz, O.T.; Tekin, N.; Şener, E.; Akyüz, F.; Inal, M. Antioxidant and anti-apoptotic activity of vasoactive intestinal peptide (VIP) against 6-hydroxy dopamine toxicity in the rat corpus striatum. J. Mol. Neurosci. 2012, 46, 51–57. [Google Scholar] [CrossRef]
- Deng, G.; Jin, L. The effects of vasoactive intestinal peptide in neurodegenerative disorders. Neurol. Res. 2017, 39, 65–72. [Google Scholar] [CrossRef]
- Hobson, S.A.; Bacon, A.; Elliot-Hunt, C.R.; Holmes, F.E.; Kerr, N.C.; Pope, R.; Vanderplank, P.; Wynick, D. Galanin acts as a trophic factor to the central and peripheral nervous systems. Cell Mol. Life Sci. 2008, 65, 1806–1812. [Google Scholar] [CrossRef]
- Pidsudko, Z. Immunohistochemical characteristics and distribution of neurons in the intramural ganglia supplying the urinary bladder in the male pig. Pol. J. Vet. Sci. 2013, 16, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaleczyc, J.; Sienkiewicz, W.; Klimczuk, M.; Czaja, K.; Lakomy, M. Differences in the chemical coding of nerve fibres supplying major populations of neurons between the caudal mesenteric ganglion and anterior pelvic ganglion in the male pig. Folia Histochem. Cytobiol 2013, 41, 201–211. [Google Scholar]
- Koritsiadis, G.; Stravodimos, K.; Mitropoulos, D.; Doumanis, G.; Fokitis, I.; Koritsiadis, S.; Constantinides, C. Androgens and bladder outlet obstruction: A correlation with pressure-flow variables in a preliminary study. BJU Int. 2008, 101, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Coit, V.A.; Gibson, I.F.; Evans, N.P.; Dowell, F.J. Neutering affects urinary bladder function by different mechanisms in male and female dogs. Eur. J. Pharmacol. 2008, 584, 153–158. [Google Scholar] [CrossRef]
- Coit, V.A.; Dowell, F.J.; Evans, N.P. Neutering affects mRNA expression levels for the LH- and GnRH-receptors in the canine urinary bladder. Teriogenology 2009, 71, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Aboushwareb, T.; Turner, C.; Mathis, C.; Bennett, C.; Sonntag, W.E.; Andersson, K.E.; Christ, G. Impaired bladder function in aging male rats. J. Urol. 2010, 184, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Magari, T.; Shibata, Y.; Arai, S.; Kashiwagi, B.; Suzuki, K. Influence of castration on bladder blood flow and function during the rapid phase of androgen deprivation. Scand. J. Urol. 2013, 47, 236–241. [Google Scholar] [CrossRef]
- Magari, T.; Shibata, Y.; Arai, S.; Kashiwagi, B.; Suzuki, K.; Suzuki, K. Time-dependent effects of castration on the bladder function and histological changes in the bladder and blood vessels. Asian J. Androl. 2014, 16, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Becerra, S.M.; de Oliveira, M.G.; Calmasini, F.B.; Rojas-Moscoso, J.A.; Zanesco, A.; Antunes, E. Micturition dysfunction in four-month-old ovariectomized rats: Effects of testosterone replacement. Life Sci. 2017, 179, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Urfer, S.R.; Kaeberlein, M. Desexing dogs: A review of the current literature. Animals 2019, 9, 1086. [Google Scholar] [CrossRef] [Green Version]
- Alsaid, B.; Moszkowicz, D.; Peschaud, F.; Bessede, T.; Zaitouna, M.; Karam, I.; Droupy, S.; Benoit, G. Autonomic-somatic communications in the human pelvis: Computer-assisted anatomic dissection in male and female fetuses. J. Anat. 2011, 219, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Badawi, A.; Schenk, E.A. A new theory of the innervation of bladder musculature. Part 4. Innervation of the vesicourethral junction and external urethral sphincter. J. Urol. 1974, 111, 613–615. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleczyc, J.; Lepiarczyk, E. The Effect of Castration on Peripheral Autonomic Neurons Supplying Mammalian Male Genitourinary System. Int. J. Mol. Sci. 2021, 22, 7632. https://doi.org/10.3390/ijms22147632
Kaleczyc J, Lepiarczyk E. The Effect of Castration on Peripheral Autonomic Neurons Supplying Mammalian Male Genitourinary System. International Journal of Molecular Sciences. 2021; 22(14):7632. https://doi.org/10.3390/ijms22147632
Chicago/Turabian StyleKaleczyc, Jerzy, and Ewa Lepiarczyk. 2021. "The Effect of Castration on Peripheral Autonomic Neurons Supplying Mammalian Male Genitourinary System" International Journal of Molecular Sciences 22, no. 14: 7632. https://doi.org/10.3390/ijms22147632
APA StyleKaleczyc, J., & Lepiarczyk, E. (2021). The Effect of Castration on Peripheral Autonomic Neurons Supplying Mammalian Male Genitourinary System. International Journal of Molecular Sciences, 22(14), 7632. https://doi.org/10.3390/ijms22147632





