Glycyrrhizin Attenuates Portal Hypertension and Collateral Shunting via Inhibition of Extrahepatic Angiogenesis in Cirrhotic Rats
Abstract
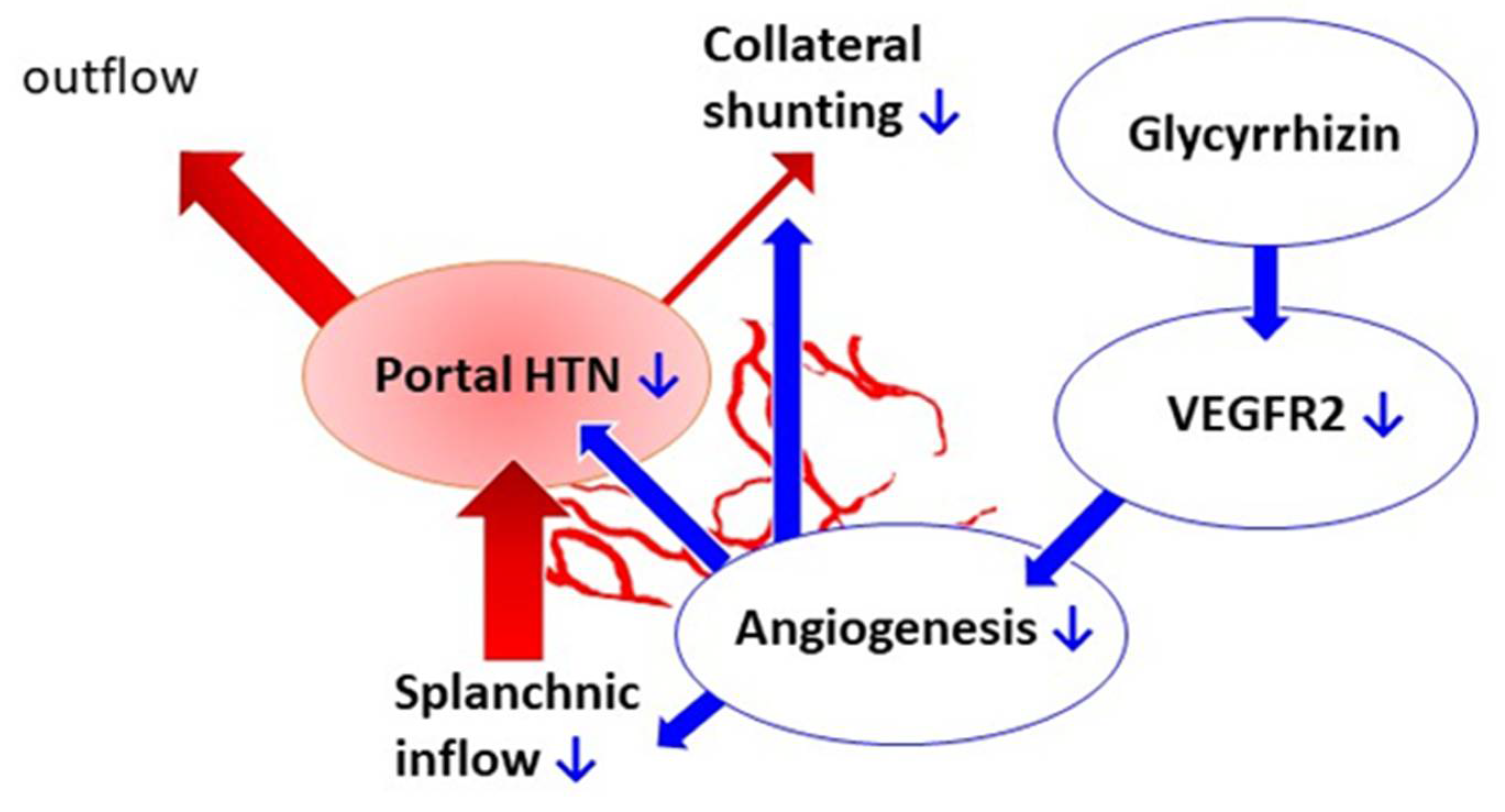
:1. Introduction
2. Results
2.1. Effects of Glycyrrhizin on Body Weight and Systemic Circulation
2.2. Effects of Glycyrrhizin on Portal Hypertension
2.3. Effects of Glycyrrhizin on Extrahepatic Systems and Mesenteric Angiogenesis
2.4. Effects of Glycyrrhizin on Hepatic System
3. Discussion
4. Materials and Methods
4.1. Animal Model: Common Bile Duct Ligation (BDL)
4.2. Experiment Design
4.3. Measurement of Systemic and Portal Hemodynamics
4.4. Immunofluorescent Study for the Mesenteric Vascular Density
4.5. Color Microsphere Method for Portosystemic Shunting Degree Analysis
4.6. Western Blot
4.7. Hepatic Fibrosis Determination with Sirius Red Staining
4.8. Drugs
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bosch, J.; Pizcueta, P.; Feu, F.; Fernández, M.; Garcia-Pagan, J.C. Pathophysiology of portal hypertension. Gastroenterol. Clin. N. Am. 1992, 21, 1–14. [Google Scholar] [CrossRef]
- Fernandez, M. Molecular pathophysiology of portal hypertension. Hepatology 2015, 61, 1406–1415. [Google Scholar] [CrossRef]
- Mejias, M.; García-Pras, E.; Tiani, C.; Miquel, R.; Bosch, J.; Fernandez, M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 2008, 49, 1245–1256. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Hautaniemi, E.J.; Tahvanainen, A.M.; Koskela, J.K.; Tikkakoski, A.J.; Kähönen, M.; Uitto, M.; Sipilä, K.; Niemelä, O.; Mustonen, J.; Pörsti, I.H. Voluntary liquorice ingestion increases blood pressure via increased volume load, elevated peripheral arterial resistance, and decreased aortic compliance. Sci. Rep. 2017, 7, 10947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumpricht, E.; Dahl, R.; Devereaux, M.W.; Sokol, R.J. Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J. Biol. Chem. 2005, 280, 10556–10563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, M.; Iqbal, J.; Malik, A.; Ramzan, H.S.; Qureshi, M.S.; Asif, M.; Qazi, M.H.; Kamal, M.A.; Chaudhary, A.G.A.; Al-Qahtani, M.H.; et al. Hepatoprotective Effects of Silybum marianum (Silymarin) and Glycyrrhiza glabra (Glycyrrhizin) in Combination: A Possible Synergy. Evid. Based Complement. Altern. Med. 2014, 2014, 641597. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Nnane, I.P.; Cheng, T.Y. The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity in rats. Toxicon 1999, 37, 1259–1270. [Google Scholar] [CrossRef]
- Hung, C.-H.; Kee, K.-M.; Chen, C.-H.; Tseng, P.-L.; Tsai, M.-C.; Chen, C.-H.; Wang, J.-H.; Chang, K.-C.; Kuo-Chin, C.; Yen, Y.-H.; et al. A Randomized Controlled Trial of Glycyrrhizin Plus Tenofovir vs. Tenofovir in Chronic Hepatitis B with Severe Acute Exacerbation. Clin. Transl. Gastroenterol. 2017, 8, e104. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Cichoń, T.; Matuszczak, S.; Mitrus, I.; Lesiak, M.; Kobusińska, M.; Kamysz, W.; Jarosz, M.; Sieroń, A.; Szala, S. The Role of Glycyrrhizin, an Inhibitor of HMGB1 Protein, in Anticancer Therapy. Arch. Immunol. Ther. Exp. 2012, 60, 391–399. [Google Scholar] [CrossRef]
- Kim, K.J.; Choi, J.S.; Kim, K.W.; Jeong, J.W. The anti-angiogenic activities of glycyrrhizic acid in tumor progression. Phytother. Res. 2013, 27, 841–846. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, B.; Xu, X.-Y.; Yang, S.-J.; Zhang, T.; Song, Y.-J.; Liu, X.-T.; Wang, Y.-Q.; Cai, D.-Y. Glycyrrhizinate reduces portal hypertension in isolated perfused rat livers with chronic hepatitis. World J. Gastroenterol. 2013, 19, 6069–6076. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Liang, B.; Wang, X.W.; Fan, F.G.; Jin, J.; Lan, R.; Yang, J.H.; Wang, X.C.; Jin, L.; Cao, Q. Glycyrrhizic acid attenuates CCl₄-induced hepatocyte apoptosis in rats via a p53-mediated pathway. World J. Gastroenterol. 2013, 19, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Guo, X.-L.; Jin, J.; Ma, Y.-C.; Feng, Z.-Q. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J. Gastroenterol. 2015, 21, 5271–5280. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-T.; Li, J.; Wang, F.-P.; Li, L.; Wang, J.-Y.; Jiang, W. Glycyrrhizin regulates CD4+T cell response during liver fibrogenesis via JNK, ERK and PI3K/AKT pathway. Int. Immunopharmacol. 2012, 14, 410–421. [Google Scholar] [CrossRef]
- Wallace, K.; Burt, A.; Wright, M.C. Liver fibrosis. Biochem. J. 2008, 411, 1–18. [Google Scholar] [CrossRef]
- Fernandez, M.; Vizzutti, F.; Garcia-Pagan, J.C.; Rodes, J.; Bosch, J. Anti-VEGF receptor-2 monoclonal antibody prevents por-tal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology 2004, 126, 886–894. [Google Scholar] [CrossRef]
- Khan, R.; Khan, A.Q.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Sultana, S. Glycyrrhizic acid suppresses the develop-ment of precancerous lesions via regulating the hyperproliferation, inflammation, angiogenesis and apoptosis in the colon of Wistar rats. PLoS ONE 2013, 8, e56020. [Google Scholar]
- Hsu, S.-J.; Lee, F.-Y.; Wang, S.-S.; Hsin, I.-F.; Lin, T.-Y.; Huang, H.-C.; Chang, C.-C.; Chuang, C.-L.; Ho, H.-L.; Lin, H.-C.; et al. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology 2014, 61, 1672–1684. [Google Scholar] [CrossRef]
- Lee, J.-J.; Hsiao, C.-C.; Yang, I.-H.; Chou, M.-H.; Wu, C.-L.; Wei, Y.-C.; Chen, C.-H.; Chuang, J.-H. High-mobility group box 1 protein is implicated in advanced glycation end products–induced vascular endothelial growth factor A production in the rat retinal ganglion cell line RGC-5. Mol. Vis. 2012, 18, 838–850. [Google Scholar]
- Park, S.Y.; Kwon, S.J.; Lim, S.S.; Kim, J.-K.; Lee, K.W.; Park, J.H.Y. Licoricidin, an Active Compound in the Hexane/Ethanol Extract of Glycyrrhiza uralensis, Inhibits Lung Metastasis of 4T1 Murine Mammary Carcinoma Cells. Int. J. Mol. Sci. 2016, 17, 934. [Google Scholar] [CrossRef] [Green Version]
- Bolognesi, M.; Merkel, C.; Sacerdoti, D.; Nava, V.; Gatta, A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig. Liver Dis. 2002, 34, 144–150. [Google Scholar] [CrossRef]
- Merkel, C.; Gatta, A.; Bolognesi, M.; Finucci, G.; Battaglia, G.; Angeli, P.; Zuin, R. Hemodynamic changes of systemic, hepatic, and splenic circulation following triglycyl-lysin-vasopressin administration in alcoholic cirrhosis. Dig. Dis. Sci. 1988, 33, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Gigou, M.; Szekely, A.M.; Bismuth, H. Portal hypertension after bile duct obstruction. Effect of the bile diversion on portal pressure in the rat. Arch. Surg. 1979, 114, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Cameron, G.R.; Hasan, S.M. Disturbances of structure and function in the liver as the result of biliary obstruction. J. Pathol. Bacteriol. 1958, 75, 333–349. [Google Scholar] [CrossRef]
- Kountouras, J.; Billing, B.H.; Scheuer, P.J. Prolonged bile duct ligation obstruction: A new experimental model of cirrhosis in the rat. Br. J. Exp. Pathol. 1984, 65, 305–311. [Google Scholar] [PubMed]
- Dhingra, D.; Parle, M.; Kulkarni, S.K. Memory enhancing activity of Glycyrrhiza glabra in mice. J. Ethnopharmacol. 2004, 91, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.-Y.; Colombato, L.A.; Albillos, A.; Groszmann, R.J. Administration of Nω-nitro-l-arginine ameliorates portal-systemic shunting in portal-hypertensive rats. Gastroenterology 1993, 105, 1464–1470. [Google Scholar] [CrossRef]
- Huang, H.-C.; Wang, S.-S.; Hsin, I.-F.; Chang, C.-C.; Lee, F.-Y.; Lin, H.-C.; Chuang, C.-L.; Lee, J.-Y.; Hsieh, H.-G.; Lee, S.-D. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology 2012, 56, 248–258. [Google Scholar] [CrossRef]
- Albillos, A.; Colombato, L.A.; Groszmann, R.J. Vasodilatation and sodium retention in prehepatic portal hypertension. Gastroenterology 1992, 102, 931–935. [Google Scholar] [CrossRef]
- Chojkier, M.; Groszmann, R.J. Measurement of portal-systemic shunting in the rat by using γ-labeled microspheres. Am. J. Physiol. 1981, 240, G371–G375. [Google Scholar] [CrossRef] [PubMed]
- Hodeige, D.; De Pauw, M.; Eechaute, W.; Weyne, J.; Heyndrickx, G.R. On the validity of blood flow measurement using colored microspheres. Am. J. Physiol. Content 1999, 276, H1150–H1158. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- SPSS Statistics, Version 21.0, Software for Statistics Analysis; IBM Corp: Armonk, NY, USA, 2012.








| Sham Vehicle | Sham Glycyrrhizin | BDL Vehicle | BDL Glycyrrhizin | p Value * | |
|---|---|---|---|---|---|
| n = 6 | n = 6 | n = 6 | n = 6 | ||
| BW (g) | 452 ± 9 | 428 ± 15 | 375 ± 10 † | 386 ± 17 | 0.605 |
| MAP (mmHg) | 141 ± 8 | 142 ± 7 | 114 ± 4 † | 102 ± 4 | 0.075 |
| HR (beats/min) | 337 ± 19 | 304 ± 24 | 322 ± 13 | 302 ± 18 | 0.386 |
| PP (mmHg) | 9.5 ± 0.8 | 8.7 ± 0.4 | 16.8 ± 1.7 † | 12.3 ± 1.0 * | 0.004 |
| Systemic circulation | |||||
| CI (mL/min/100 g) | 31.5 ± 1.4 | 33.2 ± 2.4 | 43.2 ± 1.4 † | 40.8 ± 3.7 | 0.560 |
| SVR (mmHg/mL/min/100 g) | 4.5 ± 0.3 | 4.3 ± 0.3 | 2.6 ± 0.1 † | 2.6 ± 0.3 | 0.976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pun, C.K.; Huang, H.-C.; Chang, C.-C.; Chuang, C.-L.; Yen, C.-H.; Hsu, S.-J.; Lee, F.-Y.; Hou, M.-C.; Huang, Y.-H. Glycyrrhizin Attenuates Portal Hypertension and Collateral Shunting via Inhibition of Extrahepatic Angiogenesis in Cirrhotic Rats. Int. J. Mol. Sci. 2021, 22, 7662. https://doi.org/10.3390/ijms22147662
Pun CK, Huang H-C, Chang C-C, Chuang C-L, Yen C-H, Hsu S-J, Lee F-Y, Hou M-C, Huang Y-H. Glycyrrhizin Attenuates Portal Hypertension and Collateral Shunting via Inhibition of Extrahepatic Angiogenesis in Cirrhotic Rats. International Journal of Molecular Sciences. 2021; 22(14):7662. https://doi.org/10.3390/ijms22147662
Chicago/Turabian StylePun, Chon Kit, Hui-Chun Huang, Ching-Chih Chang, Chiao-Lin Chuang, Chun-Hsien Yen, Shao-Jung Hsu, Fa-Yauh Lee, Ming-Chih Hou, and Yi-Hsiang Huang. 2021. "Glycyrrhizin Attenuates Portal Hypertension and Collateral Shunting via Inhibition of Extrahepatic Angiogenesis in Cirrhotic Rats" International Journal of Molecular Sciences 22, no. 14: 7662. https://doi.org/10.3390/ijms22147662
APA StylePun, C. K., Huang, H.-C., Chang, C.-C., Chuang, C.-L., Yen, C.-H., Hsu, S.-J., Lee, F.-Y., Hou, M.-C., & Huang, Y.-H. (2021). Glycyrrhizin Attenuates Portal Hypertension and Collateral Shunting via Inhibition of Extrahepatic Angiogenesis in Cirrhotic Rats. International Journal of Molecular Sciences, 22(14), 7662. https://doi.org/10.3390/ijms22147662






