Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity
Abstract
:1. Introduction
2. Materials and Methods
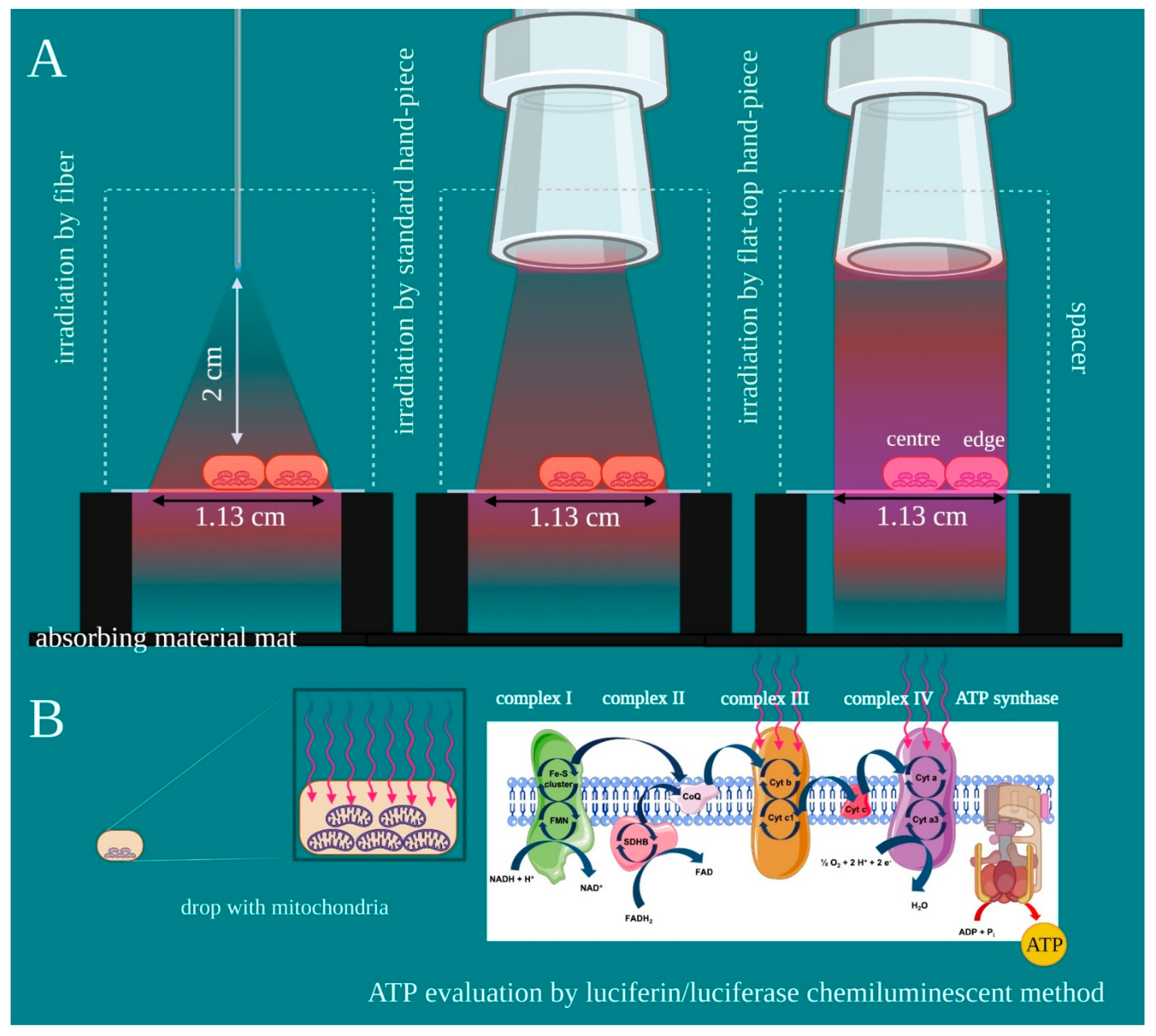
2.1. Experimental Design and Purpose
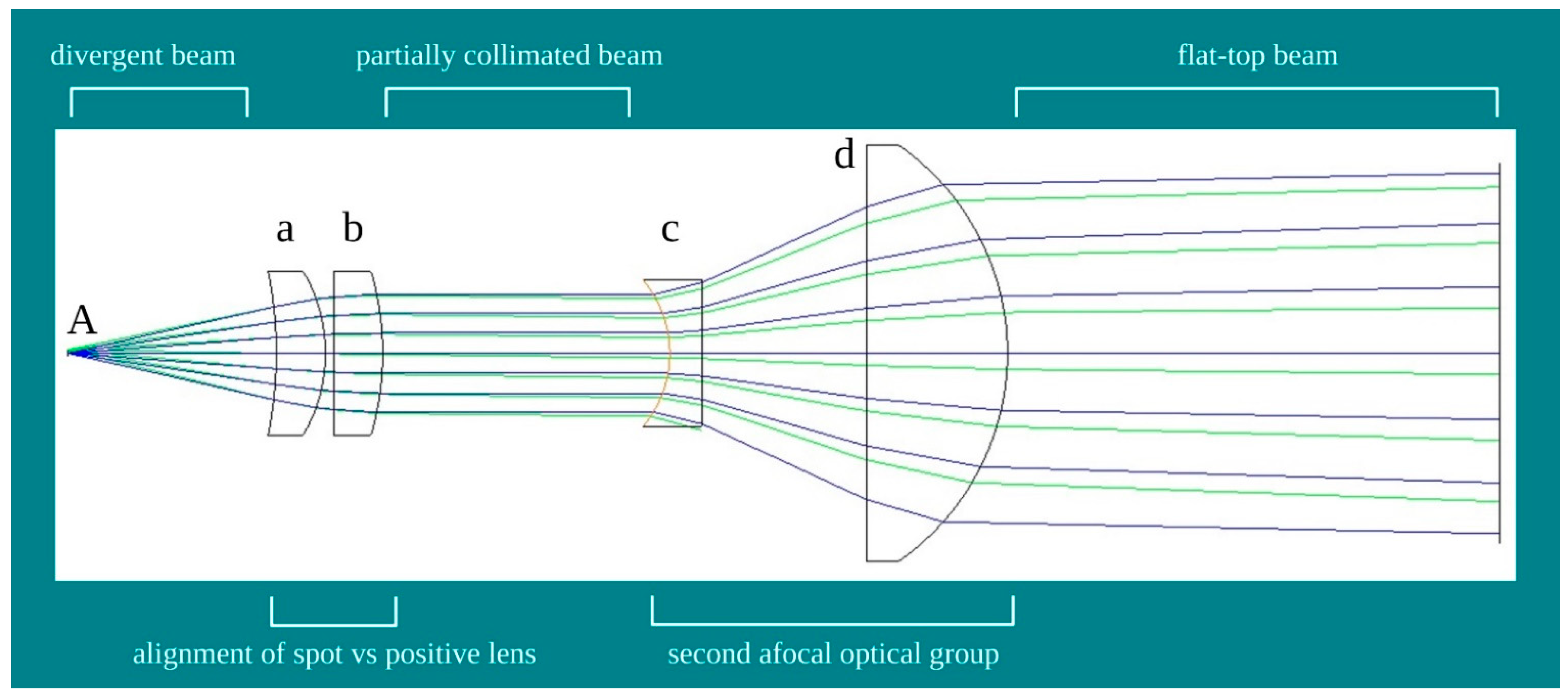
2.2. Design of the Flat-Top Hand-Piece
2.3. Characterization of the Probes Beam Profile
2.4. Mitochondria Enriched-Fraction Isolation
2.5. Evaluation of Mitochondrial ATP Synthesis
2.6. Statistical Analysis
3. Results
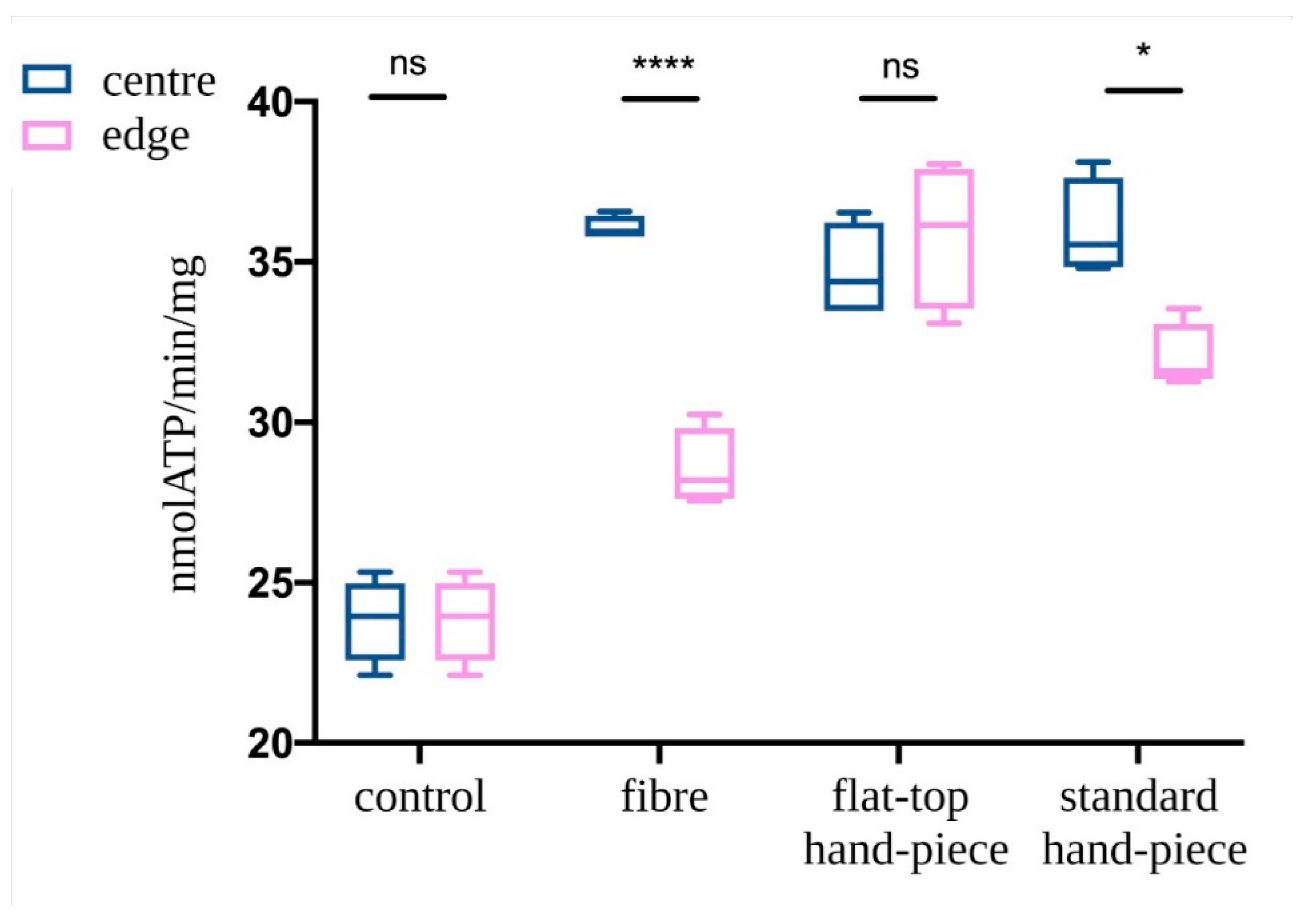
3.1. Characterization of the Probes Beam Profile and Irradiation
3.2. Evaluation of Mitochondrial ATP Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosca, R.C.; Ong, A.A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv. Skin Wound Care 2019, 32, 157–167. [Google Scholar] [CrossRef]
- Mester, E.; Spiry, T.; Szende, B.; Tota, J.G. Effect of laser rays on wound healing. Am. J. Surg. 1971, 122, 532–535. [Google Scholar] [CrossRef]
- Mester, E.; Szende, B.; Gartner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Mester, E.; Szende, B.; Spiry, T.; Scher, A. Stimulation of wound healing by laser rays. Acta Chir. Acad. Sci. Hung. 1972, 13, 315–324. [Google Scholar]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Niklas, K.J. The Evolutionary Biology of Plant; University of Chicago: Chicago, IL, USA, 1997; p. 449. [Google Scholar]
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef] [Green Version]
- Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Colombo, E.; Zekiy, A.; Aicardi, S.; Benedicenti, S.; De Angelis, N. Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation-A Review. Biology 2020, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and Oxidative Stress: 980 nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxid. Med. Cell. Longev. 2021, 3, 6626286. [Google Scholar]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef] [Green Version]
- Arany, P.R. Craniofacial Wound Healing with Photobiomodulation Therapy: New Insights and Current Challenges. J. Dent. Res. 2016, 95, 977–984. [Google Scholar] [CrossRef]
- Fornaini, C.; Arany, P.; Rocca, J.P.; Merigo, E. Photobiomodulation in Pediatric Dentistry: A Current State-of-the-Art. Photobiomodul. Photomed. Laser Surg. 2019, 3, 7798–7813. [Google Scholar] [CrossRef]
- Pasquale, C.; Colombo, E.; Benedicenti, S.; Signore, A.; Amaroli, A. 808-Nm Near-Infrared Laser Photobiomodulation versus Switched-Off Laser Placebo in Major Aphthae Management: A Randomized Double-Blind Controlled Trial. Appl. Sci. 2021, 11, 4717. [Google Scholar] [CrossRef]
- Salehpour, F.; Cassano, P.; Rouhi, N.; Hamblin, M.R.; De Taboada, L.; Farajdokht, F.; Mahmoudi, J. Penetration Profiles of Visible and Near-Infrared Lasers and Light-Emitting Diode Light Through the Head Tissues in Animal and Human Species: A Review of Literature. Photobiomodul. Photomed. Laser Surg. 2019, 37, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Hochman-Elam, L.N.; Heidel, R.E.; Shmalberg, J.W. Effects of laser power, wavelength, coat length, and coat color on tissue penetration using photobiomodulation in healthy dogs. Can. J. Vet. Res. 2020, 84, 131–137. [Google Scholar]
- Souza-Barros, L.; Dhaidan, G.; Maunula, M.; Solomon, V.; Gabison, S.; Lilge, L.; Nussbaum, E.L. Skin color and tissue thickness effects on transmittance, reflectance, and skin temperature when using 635 and 808 nm lasers in low intensity therapeutics. Lasers Surg. Med. 2018, 50, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A Comparative Study Between the Effectiveness of 980 nm Photobiomodulation Delivered by Hand-Piece with Gaussian vs. Flat-Top Profiles on Osteoblasts Maturation. Front Endocrinol. 2019, 20, 92. [Google Scholar] [CrossRef] [Green Version]
- Ravera, S.; Ferrando, S.; Agas, D.; De Angelis, N.; Raffetto, M.; Sabbieti, M.G.; Signore, A.; Benedicenti, S.; Amaroli, A. 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes. J. Biophotonics 2019, 12, 201900101. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Amaroli, A.; Benedicenti, S.; Bianco, B.; Bosco, A.; Clemente Vargas, M.R.; Hanna, R.; Kalarickel Ramakrishnan, P.; Raffetto, M.; Ravera, S. Electromagnetic Dosimetry for Isolated Mitochondria Exposed to Near-Infrared Continuous-Wave Illumination in Photobiomodulation Experiments. Bioelectromagnetics 2021, 18, 22342. [Google Scholar]
- Selting, W. Atlas of Laser Therapy: State of the Art, 4th ed.; Teamwork Media Srl: Villa Carcina, Italy, 2016; pp. 225–236. [Google Scholar]
- Lescuyer, P. Progress in the Definition of a Reference Human Mitochondrial Proteome. Proteomics 2003, 3, 157–167. [Google Scholar] [CrossRef]
- Passarella, S.; Karu, T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B 2014, 140, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Manteĭfel’, V.M.; Andreĭchuk, T.N.; Karu, T.I. Reaktsiia mitokhondrial’nogo appparata limfotsitov na obluchenie He-Ne-lazerom i na mitogen fitohemaggliutinin [The effect of irradiation by a He-Ne laser and phytohemagglutinin on lymphocyte mitochondria]. Mol. Biol. 1991, 25, 273–280. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaroli, A.; Arany, P.; Pasquale, C.; Benedicenti, S.; Bosco, A.; Ravera, S. Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. Int. J. Mol. Sci. 2021, 22, 7788. https://doi.org/10.3390/ijms22157788
Amaroli A, Arany P, Pasquale C, Benedicenti S, Bosco A, Ravera S. Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. International Journal of Molecular Sciences. 2021; 22(15):7788. https://doi.org/10.3390/ijms22157788
Chicago/Turabian StyleAmaroli, Andrea, Praveen Arany, Claudio Pasquale, Stefano Benedicenti, Alessandro Bosco, and Silvia Ravera. 2021. "Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity" International Journal of Molecular Sciences 22, no. 15: 7788. https://doi.org/10.3390/ijms22157788
APA StyleAmaroli, A., Arany, P., Pasquale, C., Benedicenti, S., Bosco, A., & Ravera, S. (2021). Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. International Journal of Molecular Sciences, 22(15), 7788. https://doi.org/10.3390/ijms22157788








