Photodynamic Inactivation of an Endodontic Bacteria Using Diode Laser and Indocyanine Green-Loaded Nanosphere
Abstract
1. Introduction
2. Results
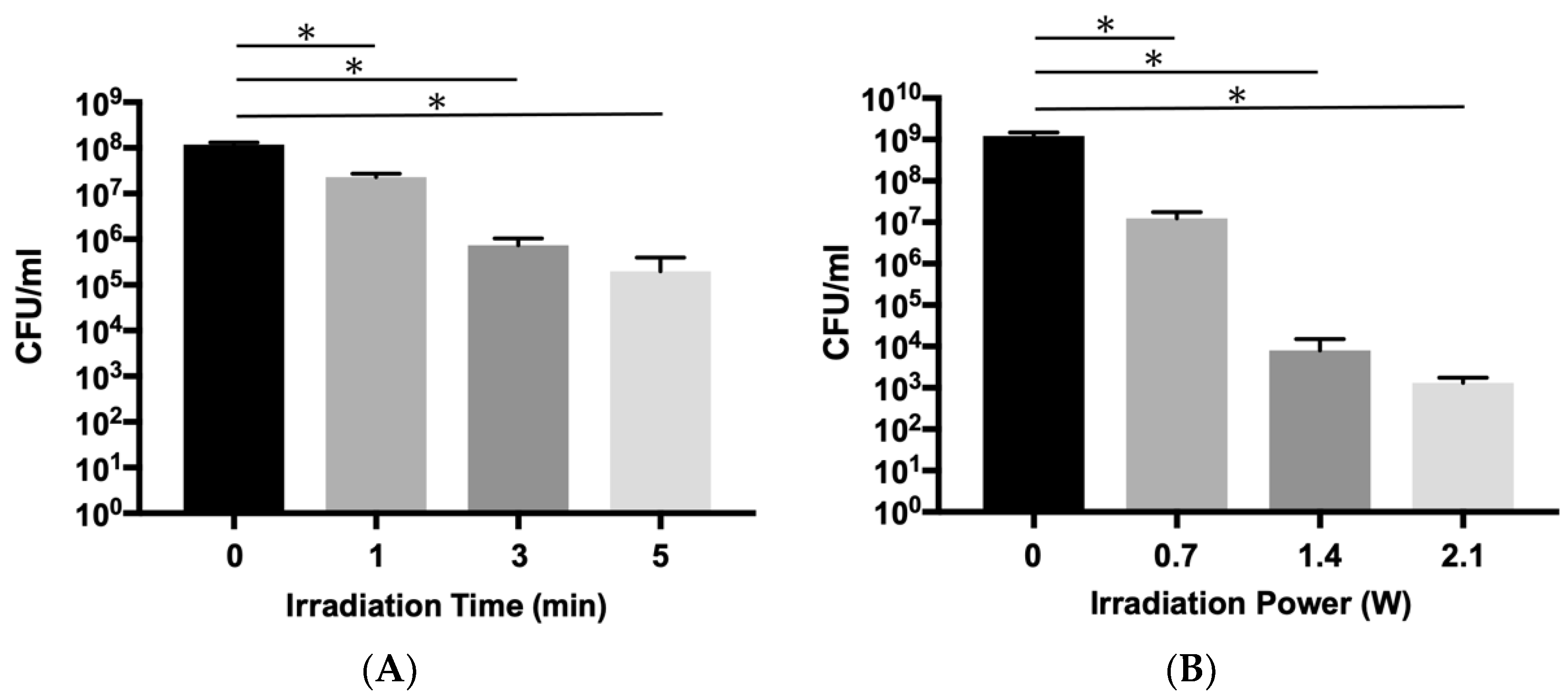
2.1. Bactericidal Effects aPDT/PACT with ICG-Nano/c on Planktonic E. faecalis
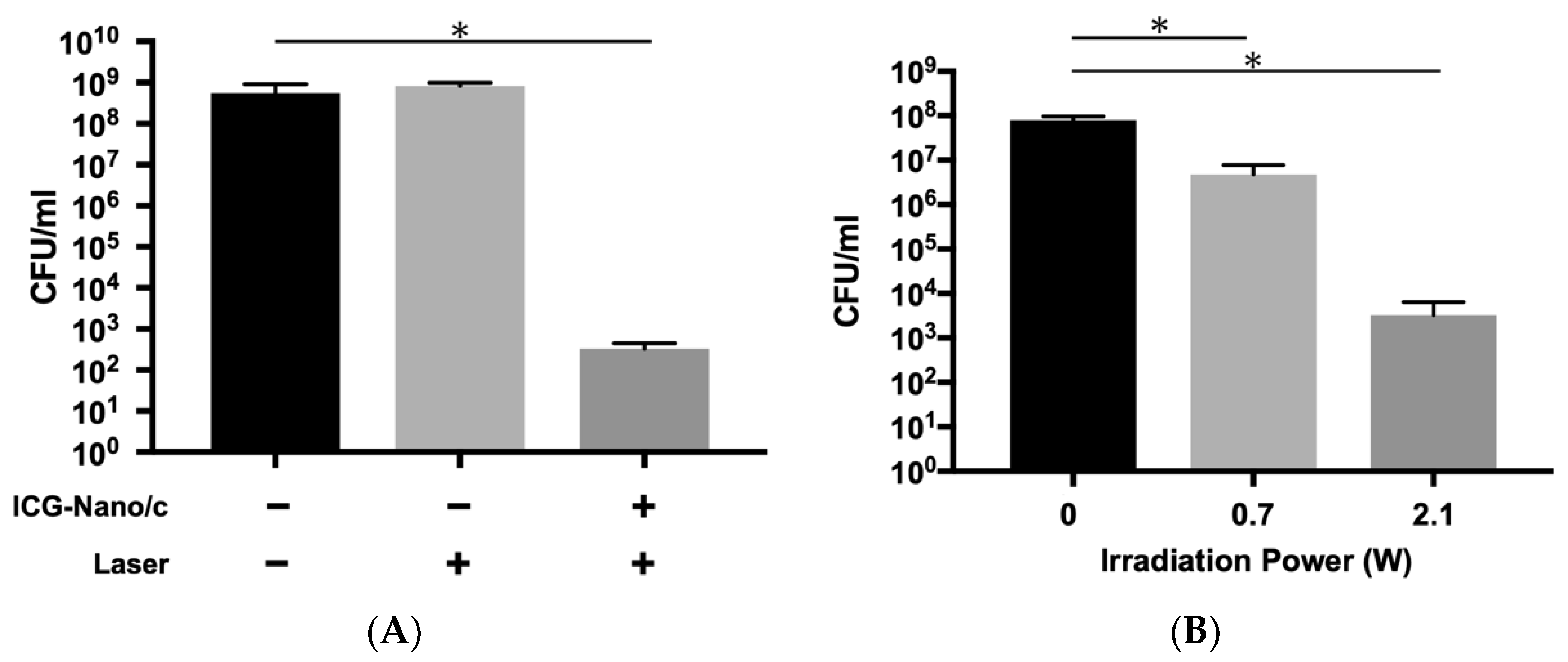
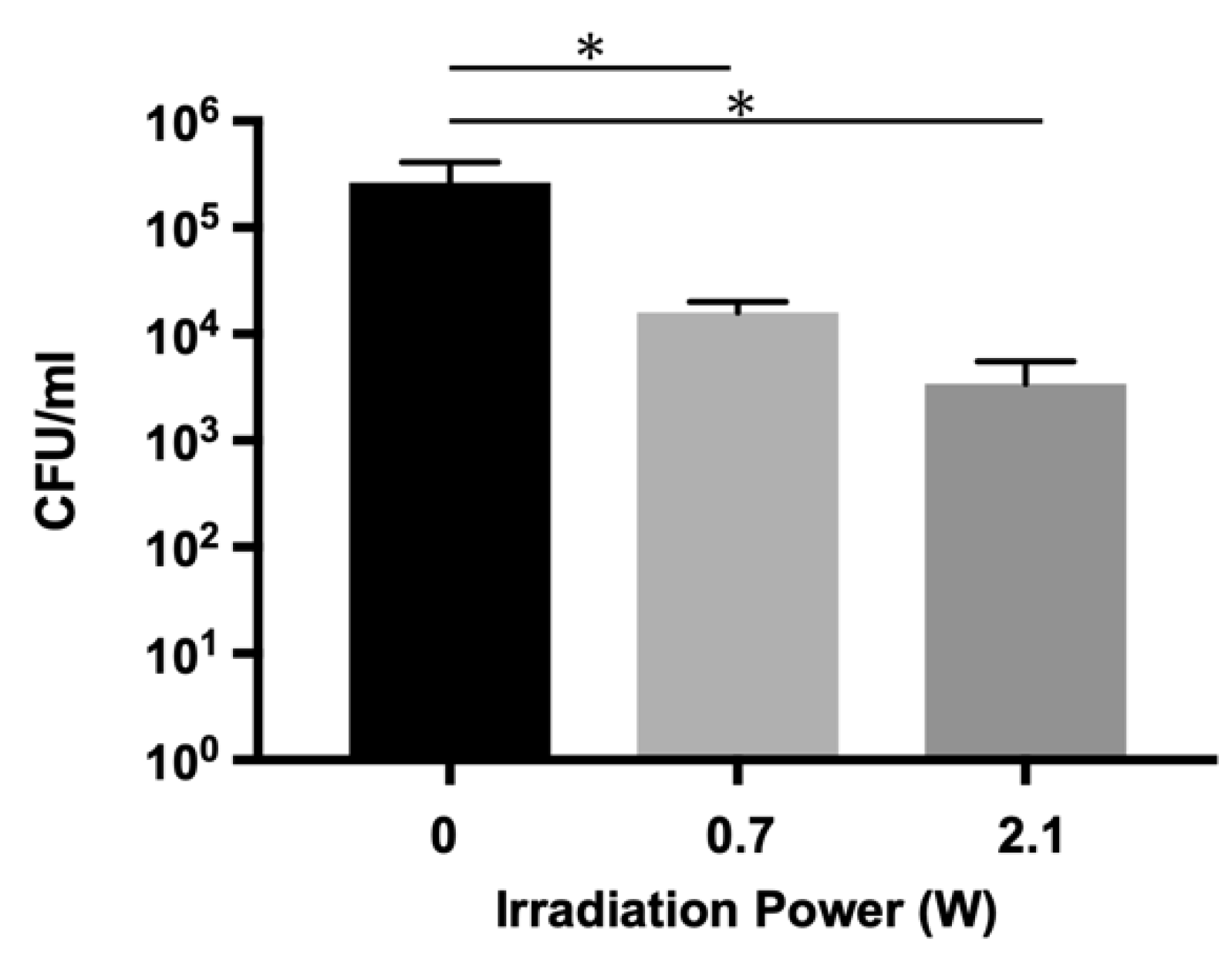
2.2. Bactericidal Effects on E. faecalis Biofilm in the Infected Root Canal Model
2.3. Scanning Electron Microscopy (SEM) Observations of E. faecalis Biofilm on Dentin Blocks after Treatment
2.4. Effect of Cooling on Temperature Elevation of the Root Surface during Laser Irradiation
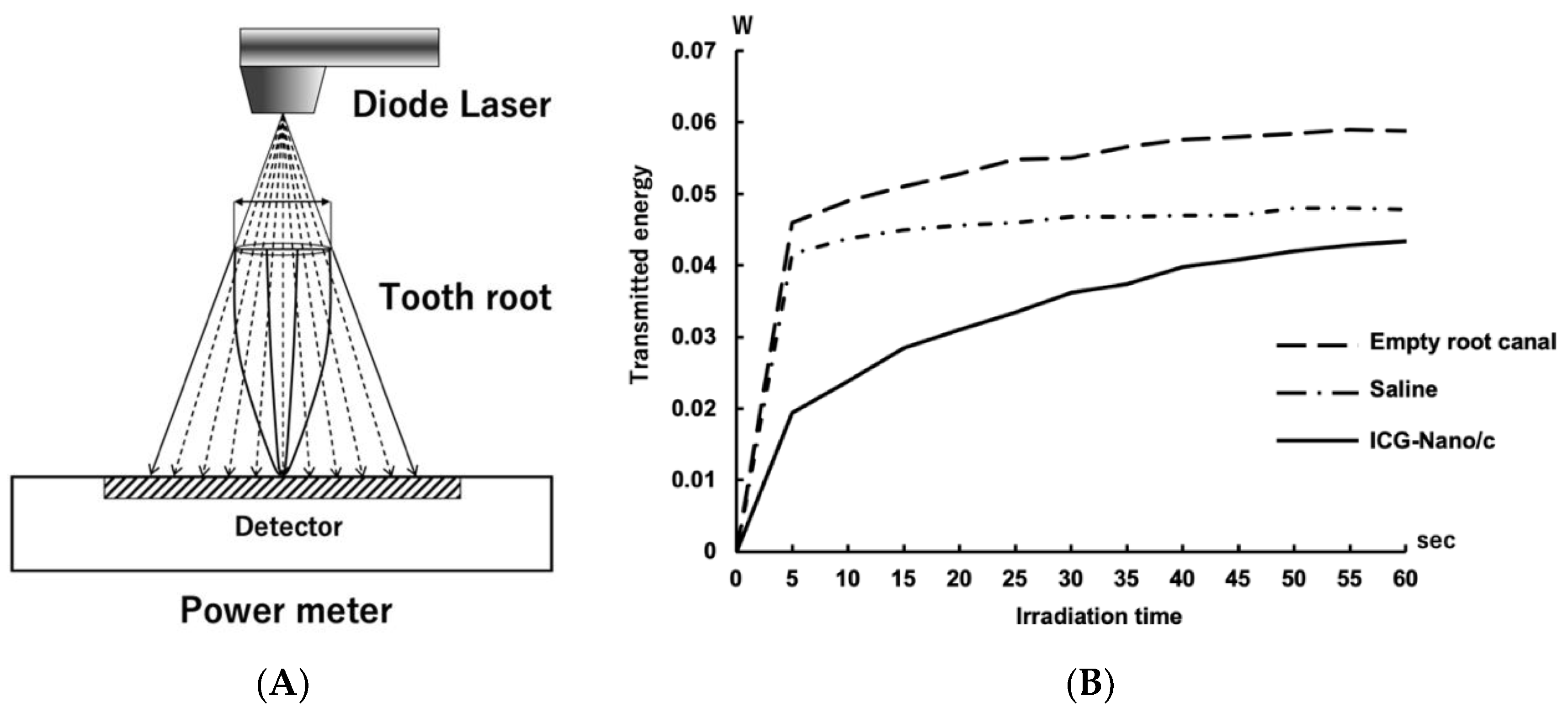
2.5. Measurement of the Light Energy Transmitted through the Tooth Root
3. Discussion
4. Materials and Methods
4.1. Preparation of ICG-Nano/c
4.2. Bacterial Strain
4.3. Laser Application
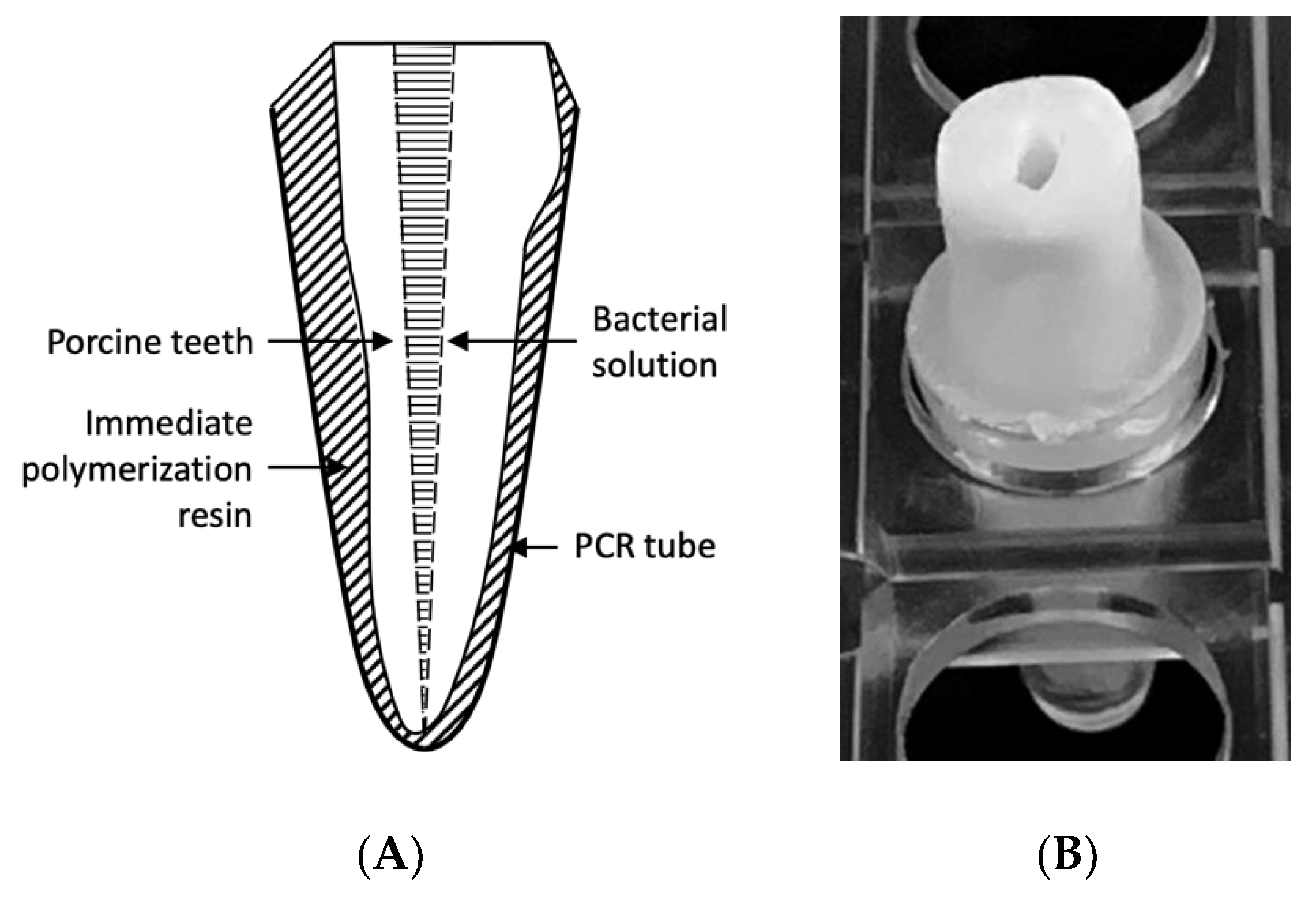
4.4. Preparation of the Infected Root Canal Model
4.5. Bactericidal Assay on Planktonic Cells
4.6. Bactericidal Assay on the Biofilm in the Infected Root Canal Model
4.7. SEM Observations of Biofilm on Dentin Blocks after aPDT/PACT Treatment
4.8. Confirmation of the Cooling Effect on the Root Surface during Laser Irradiation
4.9. Measurement of the Light Energy Transmitted through the Tooth Root
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| a | Control | 1 min, 0.7 W | 3 min, 0.7 W | 5 min, 0.7 W |
|---|---|---|---|---|
| CFU/mL Average | 1.19 × 108 | 2.31 × 107 | 7.33 × 105 | 2.00 × 105 |
| SD | 0.18 × 108 | 0.41 × 107 | 3.05 × 105 | 2.00 × 105 |
| log10 | 8.07 | 7.36 | 5.87 | 5.3 |
| log reduction | 0.71 | 2.21 | 2.77 | |
| % reduction | 80.59 | 99.38 | 99.83 | |
| b | Control | 0.7 W, 5 min | 1.4 W, 5 min | 2.1 W, 5 min |
| CFU/mL Average | 1.21 × 109 | 2.31 × 107 | 8.00 × 103 | 1.33 × 103 |
| SD | 0.26 × 109 | 0.41 × 107 | 7.21 × 103 | 0.41 × 103 |
| log10 | 9.09 | 7.10 | 3.90 | 3.12 |
| log reduction | 1.99 | 5.18 | 5.96 | |
| % reduction | 98.98 | 99.9993 | 99.99989 |
| a | Control | Laser Only | ICG-Nano/C with Laser |
|---|---|---|---|
| CFU/mL Average | 5.58 × 108 | 8.29 × 108 | 3.33 × 102 |
| SD | 3.50 × 108 | 1.65 × 108 | 1.15 × 102 |
| log10 | 8.74 | 8.92 | 2.52 |
| log reduction | −0.17 | 6.22 | |
| % reduction | −48.4 | 99.99994 | |
| b | Control | 0.7 W, 5 min | 2.1 W, 5 min |
| CFU/mL Average | 8.00 × 107 | 4.80 × 106 | 3.27 × 103 |
| SD | 1.69 × 107 | 2.98 × 106 | 3.12 × 103 |
| log10 | 7.9 | 6.88 | 3.51 |
| log reduction | 1.22 | 4.38 | |
| % reduction | 94.0 | 99.9959 |
| Control | 0.7 W, 5 min | 2.1 W, 5 min | |
|---|---|---|---|
| CFU/mL Average | 2.63 × 105 | 1.60 × 104 | 3.40 × 103 |
| SD | 1.46 × 105 | 0.4 × 108 | 2.15 × 103 |
| log10 | 5.42 | 4.2 | 3.53 |
| log reduction | 1.21 | 1.88 | |
| % reduction | 93.93 | 98.70 |
Appendix B

References
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Ragàs, X.; He, X.; Agut, M.; Roxo-Rosa, M.; Gonsalves, A.R.; Serra, A.C.; Nonell, S. Singlet oxygen in antimicrobial photodynamic therapy: Photosensitizer-dependent production and decay in E. coli. Molecules 2013, 18, 2712–2725. [Google Scholar] [CrossRef]
- Akhtar, F.; Khan, A.U.; Misba, L.; Akhtar, K.; Ali, A. Antimicrobial and antibiofilm photodynamic therapy against vancomycin resistant Staphylococcus aureus (VRSA) induced infection in vitro and in vivo. Eur. J. Pharm. Biopharm. 2021, 160, 65–76. [Google Scholar] [CrossRef]
- Carrera, E.T.; Dias, H.B.; Corbi, S.C.T.; Marcantonio, R.A.C.; Bernardi, A.C.A.; Bagnato, V.S.; Hamblin, M.R.; Rastelli, A.N.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016, 26, 123001. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.G.; de Carvalho, V.F.; Godoy-Miranda, B.A.; Kassa, C.T.; Horliana, A.C.R.T.; Prates, R.A. Efficacy of antimicrobial photodynamic therapy (aPDT) for nonsurgical treatment of periodontal disease: A systematic review. Lasers Med. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef]
- Chiniforush, N.; Pourhajibagher, M.; Shahabi, S.; Kosarieh, E.; Bahador, A. Can Antimicrobial Photodynamic Therapy (aPDT) Enhance the Endodontic Treatment? J. Lasers Med. Sci. 2016, 7, 76–85. [Google Scholar] [CrossRef]
- Ervolino, E.; Statkievicz, C.; Toro, L.F.; de Mello-Neto, J.M.; Cavazana, T.P.; Issa, J.P.M.; Dornelles, R.C.M.; de Almeida, J.M.; Nagata, M.J.H.; Okamoto, R.; et al. Antimicrobial photodynamic therapy improves the alveolar repair process and prevents the occurrence of osteonecrosis of the jaws after tooth extraction in senile rats treated with zoledronate. Bone 2019, 120, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Tortamano, A.C.A.; Anselmo, G.G.; Kassa, C.T.; Godoy-Miranda, B.; Pavani, C.; Kato, I.T.; Wainwright, M.; Prates, R.A. Antimicrobial photodynamic therapy mediated by methylene blue in surfactant vehicle on periodontopathogens. Photodiagn. Photodyn. Ther. 2020, 31, 101784. [Google Scholar] [CrossRef]
- Nagai, Y.; Suzuki, A.; Katsuragi, H.; Shinkai, K. Effect of antimicrobial photodynamic therapy (aPDT) on the sterilization of infected dentin in vitro. Odontology 2018, 106, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, M.A.A.; Wainwright, M.; Miessi, D.M.J.; Scalet, V.; Olivo, M.B.; Ervolino, E.; Garcia, V.G.; Theodoro, L.H. Effects of butyl toluidine blue photosensitizer on antimicrobial photodynamic therapy for experimental periodontitis treatment in rats. Photodiagn. Photodyn. Ther. 2020, 31, 101868. [Google Scholar] [CrossRef] [PubMed]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue-Mediated Antimicrobial Photodynamic Therapy on. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef]
- Wu, M.K.; Shemesh, H.; Wesselink, P.R. Limitations of previously published systematic reviews evaluating the outcome of endodontic treatment. Int. Endod. J. 2009, 42, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Suriyanarayanan, T.; Swarup, S.; Chia, K.H.B.; Nagarajan, N.; Zhang, C. Transcriptomics Analysis Reveals Putative Genes Involved in Biofilm Formation and Biofilm-associated Drug Resistance of Enterococcus faecalis. J. Endod. 2017, 43, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Gernhardt, C.R.; Eppendorf, K.; Kozlowski, A.; Brandt, M. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int. Endod. J. 2004, 37, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.R.; Ethunandan, M.; Brennan, P.A. Life-threatening airway obstruction secondary to hypochlorite extrusion during root canal treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 402–404. [Google Scholar] [CrossRef]
- Sharma, S.; Hackett, R.; Webb, R.; Macpherson, D.; Wilson, A. Severe tissue necrosis following intra-arterial injection of endodontic calcium hydroxide: A case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 666–669. [Google Scholar] [CrossRef]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial Photodynamic Therapy against Endodontic Enterococcus faecalis and Candida albicans Mono and Mixed Biofilms in the Presence of Photosensitizers: A Comparative Study with Classical Endodontic Irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef]
- De Freitas, L.M.; Lorenzón, E.N.; Cilli, E.M.; de Oliveira, K.T.; Fontana, C.R.; Mang, T.S. Photodynamic and peptide-based strategy to inhibit Gram-positive bacterial biofilm formation. Biofouling 2019, 35, 742–757. [Google Scholar] [CrossRef]
- Er Karaoğlu, G.; Uğur Ydın, Z.; Erdönmez, D.; Göl, C.; Durmuş, M. Efficacy of antimicrobial photodynamic therapy administered using methylene blue, toluidine blue and tetra 2-mercaptopyridine substituted zinc phthalocyanine in root canals contaminated with Enterococcusaecalis. Photodiagn. Photodyn. Ther. 2020, 32, 102038. [Google Scholar] [CrossRef]
- Beltes, C.; Sakkas, H.; Economides, N.; Papadopoulou, C. Antimicrobial photodynamic therapy using Indocyanine green and near-infrared diode laser in reducing Entrerococcus faecalis. Photodiagn. Photodyn. Ther. 2017, 17, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Kazemian, H.; Chiniforush, N.; Hosseini, N.; Pourakbari, B.; Azizollahi, A.; Rezaei, F.; Bahador, A. Exploring different photosensitizers to optimize elimination of planktonic and biofilm forms of Enterococcus faecalis from infected root canal during antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 24, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Bilici, K.; Atac, N.; Muti, A.; Baylam, I.; Dogan, O.; Sennaroglu, A.; Can, F.; Yagci Acar, H. Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation. Biomater. Sci. 2020, 8, 4616–4625. [Google Scholar] [CrossRef] [PubMed]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagn. Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef]
- Nagahara, A.; Mitani, A.; Fukuda, M.; Yamamoto, H.; Tahara, K.; Morita, I.; Ting, C.C.; Watanabe, T.; Fujimura, T.; Osawa, K.; et al. Antimicrobial photodynamic therapy using a diode laser with a potential new photosensitizer, indocyanine green-loaded nanospheres, may be effective for the clearance of Porphyromonas gingivalis. J. Periodontal Res. 2013, 48, 591–599. [Google Scholar] [CrossRef]
- Fujimura, T.; Mitani, A.; Fukuda, M.; Mogi, M.; Osawa, K.; Takahashi, S.; Aino, M.; Iwamura, Y.; Miyajima, S.; Yamamoto, H.; et al. Irradiation with a low-level diode laser induces the developmental endothelial locus-1 gene and reduces proinflammatory cytokines in epithelial cells. Lasers Med. Sci. 2014, 29, 987–994. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hayashi, J.I.; Fujimura, T.; Iwamura, Y.; Yamamoto, G.; Nishida, E.; Ohno, T.; Okada, K.; Yamamoto, H.; Kikuchi, T.; et al. New Irradiation Method with Indocyanine Green-Loaded Nanospheres for Inactivating Periodontal Pathogens. Int. J. Mol. Sci. 2017, 18, 154. [Google Scholar] [CrossRef]
- Abat, C.; Huart, M.; Garcia, V.; Dubourg, G.; Raoult, D. Enterococcus faecalis urinary-tract infections: Do they have a zoonotic origin? J. Infect. 2016, 73, 305–313. [Google Scholar] [CrossRef]
- Sparo, M.; Delpech, G.; García Allende, N. Impact on Public Health of the Spread of High-Level Resistance to Gentamicin and Vancomycin in Enterococci. Front. Microbiol. 2018, 9, 3073. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Banerjei, L.; Myers, G.S.; Nelson, K.E.; Seshadri, R.; Read, T.D.; Fouts, D.E.; Eisen, J.A.; Gill, S.R.; Heidelberg, J.F.; et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 2003, 299, 2071–2074. [Google Scholar] [CrossRef]
- Szemes, T.; Vlkova, B.; Minarik, G.; Tothova, L.; Drahovska, H.; Turna, J.; Celec, P. On the origin of reactive oxygen species and antioxidative mechanisms in Enterococcus faecalis. Redox Rep. 2010, 15, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Peppoloni, S.; Posteraro, B.; Colombari, B.; Manca, L.; Hartke, A.; Giard, J.C.; Sanguinetti, M.; Fadda, G.; Blasi, E. Role of the (Mn)superoxide dismutase of Enterococcus faecalis in the in vitro interaction with microglia. Microbiology 2011, 157, 1816–1822. [Google Scholar] [CrossRef]
- Frankenberg, L.; Brugna, M.; Hederstedt, L. Enterococcus faecalis heme-dependent catalase. J. Bacteriol. 2002, 184, 6351–6356. [Google Scholar] [CrossRef]
- Vlková, B.; Celec, P. Does Enterococcus faecalis contribute to salivary thiobarbituric acid-reacting substances? In Vivo 2009, 23, 343–345. [Google Scholar] [PubMed]
- Zand, V.; Milani, A.S.; Amini, M.; Barhaghi, M.H.; Lotfi, M.; Rikhtegaran, S.; Sohrabi, A. Antimicrobial efficacy of photodynamic therapy and sodium hypochlorite on monoculture biofilms of Enterococcus faecalis at different stages of development. Photomed. Laser Surg. 2014, 32, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kishen, T.J.; Mohapatra, B.; Diwan, A.D.; Etherington, G. Post-traumatic thoracic scoliosis with rib head dislocation and intrusion into the spinal canal: A case report and review of literature. Eur. Spine J. 2010, 19 (Suppl. 2), S183–S186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oda, D.F.; Duarte, M.A.H.; Andrade, F.B.; Moriyama, L.T.; Bagnato, V.S.; de Moraes, I.G. Antimicrobial action of photodynamic therapy in root canals using LED curing light, curcumin and carbopol gel. Int. Endod. J. 2019, 52, 1010–1019. [Google Scholar] [CrossRef]
- Golmohamadpour, A.; Bahramian, B.; Khoobi, M.; Pourhajibagher, M.; Barikani, H.R.; Bahador, A. Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against Enterococcus faecalis. Photodiagn. Photodyn. Ther. 2018, 23, 331–338. [Google Scholar] [CrossRef]
- Li, R.; Yuan, L.; Jia, W.; Qin, M.; Wang, Y. Effects of Rose Bengal- and Methylene Blue-Mediated Potassium Iodide-Potentiated Photodynamic Therapy on Enterococcus faecalis: A Comparative Study. Lasers Surg. Med. 2021, 53, 400–410. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.; Bahador, A.; Sarraf, P.; Ayar, R.; Fekrazad, R.; Asefi, S. Ex vivo comparison of antibacterial efficacy of conventional chemomechanical debridement alone and in combination with light-activated disinfection and laser irradiation against Enterococcus faecalis biofilm. Photodiagn. Photodyn. Ther. 2020, 29, 101648. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núñez, S.C.; Azambuja, N.; Fregnani, E.R.; Rodriguez, H.M.; Hamblin, M.R.; Suzuki, H.; Ribeiro, M.S. Effects of photodynamic therapy on Gram-positive and Gram-negative bacterial biofilms by bioluminescence imaging and scanning electron microscopic analysis. Photomed. Laser Surg. 2013, 31, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, A.; Manchanda, R.; Lei, T.; Carvajal, D.A.; Tang, Y.; Kazmi, S.Z.; McGoron, A.J. Comparative study of the optical and heat generation properties of IR820 and indocyanine green. Mol. Imaging 2012, 11, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Wei, X.; Liu, L.; Zhang, F.; Shi, Y.; Wu, W. Heat transfers to periodontal tissues and gutta-percha during thermoplasticized root canal obturation in a finite element analysis model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 257–263. [Google Scholar] [CrossRef]
- Lipski, M.; Woźniak, K. In vitro infrared thermographic assessment of root surface temperature rises during thermafil retreatment using system B. J. Endod. 2003, 29, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Uoshima, K.; Oda, K.; Maeda, T. Influence of heat stress to matrix on bone formation. Clin. Oral Implant. Res. 2009, 20, 782–790. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J. Control. Release 2005, 102, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hancock, H.H.; Sigurdsson, A.; Trope, M.; Moiseiwitsch, J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 579–586. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, N.; Hayashi, J.-i.; Fujita, M.; Iwamura, Y.; Sasaki, Y.; Goto, R.; Ohno, T.; Nishida, E.; Yamamoto, G.; Kikuchi, T.; et al. Photodynamic Inactivation of an Endodontic Bacteria Using Diode Laser and Indocyanine Green-Loaded Nanosphere. Int. J. Mol. Sci. 2021, 22, 8384. https://doi.org/10.3390/ijms22168384
Higuchi N, Hayashi J-i, Fujita M, Iwamura Y, Sasaki Y, Goto R, Ohno T, Nishida E, Yamamoto G, Kikuchi T, et al. Photodynamic Inactivation of an Endodontic Bacteria Using Diode Laser and Indocyanine Green-Loaded Nanosphere. International Journal of Molecular Sciences. 2021; 22(16):8384. https://doi.org/10.3390/ijms22168384
Chicago/Turabian StyleHiguchi, Naoya, Jun-ichiro Hayashi, Masanori Fujita, Yuki Iwamura, Yasuyuki Sasaki, Ryoma Goto, Tasuku Ohno, Eisaku Nishida, Genta Yamamoto, Takeshi Kikuchi, and et al. 2021. "Photodynamic Inactivation of an Endodontic Bacteria Using Diode Laser and Indocyanine Green-Loaded Nanosphere" International Journal of Molecular Sciences 22, no. 16: 8384. https://doi.org/10.3390/ijms22168384
APA StyleHiguchi, N., Hayashi, J.-i., Fujita, M., Iwamura, Y., Sasaki, Y., Goto, R., Ohno, T., Nishida, E., Yamamoto, G., Kikuchi, T., Mitani, A., & Fukuda, M. (2021). Photodynamic Inactivation of an Endodontic Bacteria Using Diode Laser and Indocyanine Green-Loaded Nanosphere. International Journal of Molecular Sciences, 22(16), 8384. https://doi.org/10.3390/ijms22168384






