1. Introduction
Chronic non-healing wounds of the skin, such as venous or diabetic foot ulcers, are globally a major cause of morbidity and burden to health services, the incidence of which is rising due to the increase in co-morbidities such as diabetes and vascular diseases [
1]. They have a complex etiology and closure continues to be challenging with current guideline treatments that include compression or pressure offloading, debridement, infection control, and local ulcer care with various multilayer wound dressings [
2,
3]. These treatments are not effective in a large percentage of patients and relapse occurs, thus additional advanced care therapeutic approaches are required to induce healing or shorten healing time.
Extracorporeal shockwave therapy (ESWT), has been used clinically for several conditions including urological lithotripsy [
4], scars, tendonitis, non-union fractures, plantar fasciitis and osteonecrosis, with few side effects [
5,
6,
7,
8]. Potential for such a therapy, using comparatively low shockwave intensities, has been recognized clinically for diabetic and venous ulcer management and is a feasible non-invasive method for improving chronic wound healing [
9,
10]. ESWT also improves skin flap survival, burn wound healing, and blood flow perfusion in animal studies [
11]. Much of the effects induced by ESWT relate to induced neovascularization that improves blood flow perfusion and enhanced cell proliferation to accelerate tissue regeneration [
12]. It has also been suggested that ESWT dampens the release of pro-inflammatory mediators to improve healing in experimental models [
13]; however, the full molecular and cellular effects have not been characterized.
Chronic wounds are characterized by persistent inflammation. Macrophages are heterogeneous immune cells that play key roles in inflammation and all stages of the wound healing process, as well as in host defense responses and restoring and maintaining homeostasis following challenges from injury, infection, or malignancy [
14]. Macrophages facilitate healing directly by promotion and resolution of inflammation and phagocytosis of cell debris, apoptotic cells, and microbes [
15]. They secrete a variety of factors, including cytokines, which support angiogenesis, extracellular matrix synthesis, fibroblast proliferation, and epithelialization, therefore facilitating regeneration of the injured tissue. In chronic non-healing wounds, there is a stalling of the inflammatory phase with excess proinflammatory macrophages and consequently an inability to resolve the wound [
16]. Targeting and correcting cellular and molecular causes of prolonged and unrestrained inflammation in chronic wounds represents a potential therapeutic strategy to return them to a healing state and restart wound resolution.
The phenotype and functions that macrophages develop are determined by integration of multiple signals from their microenvironment [
14]. In vitro, microbial stimuli, for example, LPS (M1 activation), can drive the proinflammatory, tissue-destructive properties of macrophages, whereas IL-4, IL-10 or uptake of apoptotic cells (M2 activation) induces polarization to a more tissue-reparative phenotype [
14]. This M1 and M2 macrophage classification, based on in vitro activation studies, however, does not accurately represent macrophage function in vivo, where there is a continuum of phenotypes due to the complexity of the activating environment and where the cells modify their role based on the needs of the tissue [
17]. The microenvironmental stimuli driving the different macrophage phenotypes and functions have been predominantly characterized as biologic and chemical stimuli, such as infectious products, cytokines, and metabolic factors. However, recent studies have shown macrophages are mechanosensitive and can also respond to physical stimuli e.g., stretch, matrix topography and electrical stimulation [
18,
19].
In light of the clinical evidence that ESWT promotes resolution of inflammatory processes and tissue repair and the key role of macrophages in these events, the purpose of the work was to determine how low intensity shockwave stimulation influences the response and functional properties of macrophages, both in patient biopsies and in cells in vitro, and to identify potential underlying mechanisms of any responses generated. We provide a new insight into the role of shockwaves as important contributors to the co-ordination and regulation of macrophage wound resolution functions that could have important clinical implications for other macrophage-mediated disorders.
3. Discussion
In this study, we show for the first time that low intensity shockwave treatment is an important physical stimulus that regulates macrophage functions in vivo and in vitro and this is conducive to wound healing. As established from analysis of chronic venous ulcers and biopsy data, one exposure of ESWT improves healing in the majority of patients as demonstrated by a decrease in wound area, increased angiogenesis and importantly a decrease in macrophage number per biopsy area Low intensity shockwave treatment of macrophages in vitro resulted in enhanced macrophage activation as determined by increased expressions of TNF, IL-1, PDGF, and TGFβ and the overall percentage of elongated macrophages. However, the greater effect was the significantly enhanced uptake of apoptotic cells by macrophages following shockwave treatment, an effect potentially mediated, in part, through mechanotransduction via ERK activation. Collectively, these shockwave-induced biological processes have potential as a non-invasive process to reengage macrophages in the progression of healing in chronic wounds.
Low intensity shockwaves are used clinically to accelerate healing, an effect largely ascribed to increased angiogenesis and reduced inflammation in wounded tissue [
25,
26] although the exact underlying cellular and molecular mechanism of how this therapy aids repair, is still poorly understood. Chronic wounds fail to heal because they are stalled in the early inflammatory stage of healing and subsequent cell proliferative and tissue remodeling phases do not readily occur [
2,
15]. Our demonstration that ESWT restarts wound closure in patients where biopsies show a decrease in macrophage number but not in those where the number of macrophages per biopsy area remain unchanged or increase, suggests this is an important alteration induced by ESWT to encourage the in vivo healing process. It is not clear if the shockwave-induced decrease in macrophage number per biopsy area is due to increased apoptosis and clearance or a change in their migratory potential into or out of the wound area [
26]. A decrease in general leukocyte infiltration was observed in the skin wounds of diabetic rats three days post-ESWT as compared with no ESWT treatment controls [
11]; however, these were experimental acute wounds, unlike our human chronic ulcers.
High levels of CD68 macrophages in the dermis and wound edges in chronic leg ulcers have previously been detected [
27] yet, as in our pre-ESWT ulcer biopsies, levels of macrophage activation markers remained generally low [
28], suggesting potential senescence. ESWT treatment increased activation marker expression in the majority of our post-ESWT biopsies but not explicitly discriminating between M1 and M2 activation phenotypes. This lack of a specific M1 or M1 macrophage bias is not unexpected given the heterogeneity of macrophages previously identified in chronic wounds [
15].
The concept of shockwave-induced macrophage activation in vivo was followed up in macrophage cultures in vitro. Activation of macrophages is recognized through a change in cell metabolism and secretion of inflammatory mediators or a change in cell morphology [
14,
21,
22]. Chronic wound macrophages have impaired growth factor production and dysregulated inflammatory activity [
15] that prevents their healing. We demonstrate here that isolated macrophages in vitro are sensitive to shockwave exposure with significantly enhanced gene expression of wound healing growth factors and cytokines, similar to those secreted by activated wound macrophages that stimulate adjacent cells to engage in repair [
29]. A previous study reported shockwaves of a comparable intensity to that used in our study did not induce activation of resting macrophages [
13]. However, in their study, gene expressions of conventional M1(LPS) and M2 (IL-4) activation markers were analyzed. In our study, up-regulation of a completely different set of genes relating to healing and induced in macrophages in a complex wound environment [
29] signified activation.
The significant increase in activation. as determined by gene expression in our study, may be reflected in a shockwave-induced increase (up to 20%) in the percentage of elongated cells that have been reported to be indicative of an M2 activated phenotype [
21,
22].
Macrophages in chronic wounds have a reduced capability to phagocytose and clear apoptotic neutrophils, contributing to the failure to heal [
16,
30]. A key result from our in vitro studies is that shockwave treatment significantly enhanced phagocytic uptake of apoptotic cells but not polystyrene beads. The reasons for the differences in uptake by the different substrates were not explored but potentially results from their engagement of different classes of cell surface receptors on macrophages [
31]. Apoptotic cell-specific receptors including phosphatidylserine receptors, integrins, and Gas6 receptors e.g., MerTK and Axl drive apoptotic cell uptake by macrophages, while scavenger receptors are important in bead uptake. Integrins are known to be major mechanosensors and low-energy shockwaves interact with integrins [
24] and activate FAK by phosphorylation, thereby triggering a series of cellular signaling including ERK1/2 activation and actin remodeling, known to be important in phagocytosis and migration [
32] It is of interest that low intensity ultrasound can enhance uptake of opsonized
E. coli that engages completely different uptake receptors from that of apoptotic cells and this is mediated through enhanced actin remodeling [
33] via ERK activation. Electric fields, another physical stimulus, also up-regulates macrophage phagocytosis of apoptotic cells; however, uptake of carboxylate beads and fungal pathogens were also enhanced [
34].
Regardless of the exact mechanism for shockwave-induced uptake of apoptotic cells in murine or human primary macrophages, enhancement of their phagocytic clearance would be critical for restoring tissue homeostasis, not only by preventing proinflammatory responses but also by switching on the production of anti-inflammatory, wound-resolving mediators, such as VEGF, TGF-β, and PDGF and reducing the excessive abundance of M1 macrophages found in chronic wounds [
15,
16]. Restoration of healing in chronic wounds requires an orchestrated interaction of cell types, extracellular matrix and cytokines. Shockwave application to endothelial cells, fibroblasts and keratinocytes has previously been shown to stimulate pro-healing cytokine secretion, morphological changes and increased cell migration of keratinocytes, proliferation of fibroblasts and a pro-angiogenic activity of endothelial cells [
35]. We now add macrophages to the cohort of important cells that respond to shockwaves and produce signals that interplay and collectively induce the healing process.
Shockwave treatment activates intracellular signaling cascades in other cell types including p38, AKT, ERK1/2, FAK3, Wnt, ATP/P2X7 and PERK/ATF [
24,
32,
36]. The effect of shockwave treatment on macrophage signaling pathways remains to be fully elucidated; however, we show ERK1/2 was activated and this process can drive actin remodeling, phagocytosis and activation processes, providing one potential pathway central to the regulation of macrophage function. Previously, stimulation of macrophages by electric fields reported an activation of ERK1/2 and PI3K (AKT) and phagocytosis and migration [
34]. We did not, however, observe a change in the phosphorylation of Akt in macrophages in our study, suggesting specific effects by different physical stimuli.
Patients with chronic ulcers experience significant discomfort, risk of infection, and impaired quality of life. Treatment is still sub-optimal and the success uncertain. Targeting and correcting cellular and molecular causes of prolonged inflammation and cell senescence in chronic wounds has potential to return them to healing states. ESWT, even following one round [
5], can improve the outcome in many patients as shown in the current study; however, for some patients this is not effective. Our study may help explain mechanisms underlying those non-responding patients through the inability of ESWT to favorably change macrophage abundance and activity crucial in the wound healing process.
In conclusion, our findings shed new light on the underlying mechanisms by which ESWT can potentially exert its clinical effects, namely by modulating macrophage behavior to initiate wound healing processes. This suggests shockwave therapy, as an easily administered treatment with few side effects, could also be exploited as an adjuvant therapy for other macrophage-mediated disorders.
4. Materials and Methods
4.1. Patient Recruitment
Ten patient volunteers from Aberdeen Royal Infirmary Vascular Outpatient’s Department were recruited to the study after approval was granted by the National Research Ethics Service, NOSRES Committee, reference number 16/NS/0062 and screening for eligibility by the care team. All eligible patients referred to the vascular department with treatment-resistant venous ulceration and selected to undergo ESWT were given the opportunity to participate in this study. The inclusion criteria consisted of patients with reference ulcers in excess of 1.5 cm in one axis of measurement persisting despite at least 6 weeks of treatment with 4-layer compression bandaging. The exclusion criteria included patients with ankle brachial pressure index less than 0.8, wounds being actively treated with antibiotics, patients with diabetes, suspicion of malignancy within the ulcer, acute deep vein thrombosis or neuropathy. Evaluations included clinical assessment of ulcer severity status, local blood flow perfusion, wound area size and biopsy for histopathological examination and immunohistochemical analysis. Biopsies of ulcers were collected immediately before ESWT and 2 weeks post-treatment. Of the 10 patients recruited one deteriorated and a second biopsy was not obtained so was excluded from the analysis.
4.2. ESWT Application to Chronic Ulcers
ESWT was performed on patients at NHS Grampian by a trained professional vascular nurse. Theshockwave source used was Dermagold100 and an OP155 applicator (Tissue Regeneration Technologies, LLC, manufactured by MTS Europe GmbH, Baden-Württemberg, Germany). The treatment dosage was dependent on the ulcer size; the number of impulses equaled the treatment area in cm2 × 8, but at least 500 shocks at 4 Hz (equivalent to 0.11 mJ/mm2 energy flux density) were delivered fortnightly for three treatments and a further three if the wound was not entirely healed.
4.3. Tissue Biopsy Collection
Prior to biopsy, 1% lidocaine was injected for local anesthesia and full thickness punch biopsies 3mm in diameter and 3–4 mm in depth were taken immediately before shockwave therapy and two weeks post-treatment to assess the inflammatory response and the cellular/molecular healing parameters. Biopsies were collected from the ulcer tissue adjacent to intact peri-ulcer skin (wound margin) and were taken at opposite poles of the wound for the first and second biopsy. All biopsies were immediately fixed in formalin and processed and cut to 5 µm by the Pathology Department at Aberdeen Royal Infirmary. Sections were stained, with H&E for histological analysis or Masson’s trichrome for analysis of collagen fibers, by the NHS Pathology Department. Wound healing from pre- and post-shockwave biopsies was assessed and scored histologically in H&E stained sections by a consultant histopathologist, Dr E Husain, NHS Grampian in a blinded fashion by the state of the epidermis, presence of fibrin, inflammation and hemosiderin, scarring/fibrosis, capillary proliferation and edema presence. Before ESWT, samples showed signs of chronic inflammation including intracorneal neutrophils, epidermal exocytosis, spongiosis, dermal edema, vascular ectasia, and a predominantly lymphocytic and neutrophilic dermal inflammatory cell infiltrate.
4.4. Immunohistochemistry
Sections were deparaffinized, microwaved in citrate buffer (pH 6.0) and endogenous peroxidase activity quenched by incubation with 3% H
2O
2. Macrophages were detected by anti-CD68 antibodies (Agilent Technologies. Cheshire, UK M0814, clone KP1) and the DAKO Envision Detection Kit K5007 [
19]. Angiogenesis and cell proliferation were determined using mouse anti-CD31, 1:1200, ab9498 Abcam, Cambridge UK), anti-SMC (clone1A4, ab7817 Abcam) and anti Ki67 (Ab16667) followed by a reaction with biotinylated secondary antibody and streptavidin–biotin–peroxidase complex. 3,3-diaminobenzidine (DAB) was used as a chromogen, and hematoxylin solution as a counterstain and nuclei blued with Scots tap water. The DAKO G2-Double staining kit, K5361 (DakoCytomation) was used for staining with anti-CD68 (as above) and the M1-macrophage markers anti HLA DR (TAI.1B5) and SOCS3 (AB16030, Abcam) and the M2-macrophage marker, anti CD163 (EDHu-1, AbD Serotec, Kidlington, UK), according to the manufacturer’s instructions. Positive staining for activation markers was detected using diaminobenzidine/Liquid Permanent-Red (DakoCytomation) [
19] as shown in
Supplementary Figure S2. A negative control where the primary antibody was replaced by a non-specific IgG equivalent was included in all analyses.
4.5. Morphometric Measurements
Entire stained sections were photographed using the slide scanner (Zeiss Axioscan Z1, Zeiss, Cambridge, UK) 20× magnification and biopsy tissue areas were calculated using Zeiss ZEN Blue software. Intact CD68-positive cells were counted and expressed as the number of positive cells /biopsy area (mm2). Counts were repeated until three consecutive values were obtained with a margin of error of ±5%. Both the intra-observer and inter-observer variations were found to be less than 5% (p < 0.002). The number of double-positive cells was counted and expressed as a percentage of total macrophages. For CD31, SMC actin and Ki67 quantification, sections were imaged using a slide scanner and analyzed using either semiautomated computerized ImageJ software or Zeiss ZEN Blue software. The stained area was divided by the total biopsy area to obtain a relative value. For the Masson’s trichrome stained sections, the mean blue intensity measurement was taken as a relative measurement for the presence of stained collagen. To normalize the data sets and obtain values relative to the pre-treatment values, the post-treatment value in each set was expressed as a percentage.
4.6. Cell Culture
The murine macrophage cell line J774A.1 (ECACC 91051511) and Jurkat cell line (ATCC TIB-152, clone E6-1) was cultured in DMEM and RPMI 1640 (Lonza 12-167F) respectively, supplemented or with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine. Passage number was kept below 20 to ensure cell line integrity. Human monocyte-derived macrophages were isolated from blood of healthy adult-consenting donors, as approved by the Ethics Review Board of the College of Life Science & Medicine, University of Aberdeen. Peripheral blood mononuclear cells (PBMCs) were fractionated by density gradient centrifugation. Monocytes were isolated from PBMCs by positive selection using human CD14 microbeads to ensure a highly purified population (Miltenyi Biotec) and differentiated into macrophages over 7 d in DMEM (Lonza, Basel, Switzerland), supplemented with 1% L-glutamine, 2% penicillin/streptomycin (GE Healthcare Life Sciences, Buckinghamshire, United Kingdom) and 10% human AB+ serum [
34].
4.7. Shockwave Application to Cultured Macrophages
Shockwave treatment was performed on macrophages in T25 or T12.5 culture flasks or Ibidi µ-slides using electrohydraulic-generated shockwaves via the DermaGold 100 (Tissue Regeneration Technologies) at the parameters of 150–500 impulses, 5Hz, and 0.1 mJ/mm
2. To allow the unhampered physical propagation and reproducible application of shockwaves to the sample in vitro, shockwave treatment was performed using a water bath setup under uniform treatment conditions in terms of temperature and distance to the shockwave applicator, as was previously optimized [
20]. This most closely mimicked the in vivo application [
20]. After treatment, the number of viable cells was determined using the trypan blue exclusion method.
4.8. Phagocytosis Assay
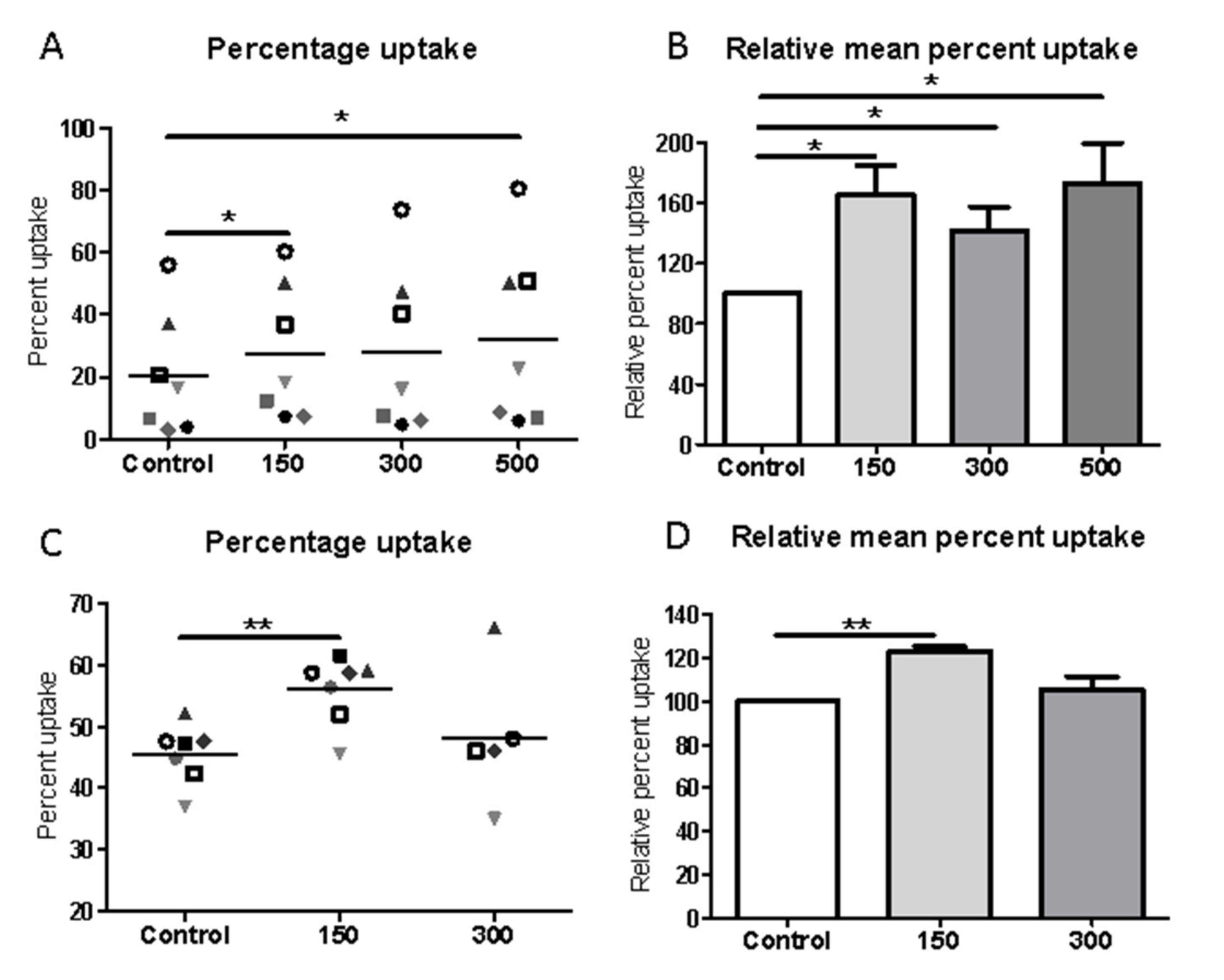
Phagocytosis assays of polystyrene beads or apoptotic Jurkat cells (immortalized line of human T lymphocyte cells) were performed using the J774 mouse macrophage cell line or human monocyte-derived macrophages, with or without exposure to shockwave treatment (150–500 impulses, 5 Hz, 0.1 mJ/mm
2). Polystyrene beads, 6 µm (Polysciences, Eppelheim, Germany) were used as simplified non-specific targets. Apoptotic Jurkat cells were obtained by exposure to UV light, which typically resulted in >70% apoptotic cells, as determined by Annexin V and propidium iodide staining. Apoptotic cells were labeled using CellTrace Far Red (ThermoFisher, Paisley, UK) as recommended by the manufacturer. For uptake assays, apoptotic cells were added to macrophages at a ratio of 10:1 and polystyrene beads (Polysciences, Eppelheim, Germany) were added to macrophages at a ratio of 3:1 [
34]. At the end of the incubation period, typically 2 h after bead/apoptotic cell addition, non-engulfed cells were removed by washing. Macrophages were fixed in 4% paraformaldehyde and the percentage of macrophages phagocytosing at least 1 bead/apoptotic cell (red fluorescence) was determined by imaging using a Zeiss Axio Observer Z1 inverted microscope (20× objective) with an MRm camera for fluorescence (Brightfield and Far Red 633). At least 100 macrophages were designated for analysis. The phagocytic index of engulfed beads was calculated using the following formula: Phagocytic index = (macrophages phagocytosing/total macrophage number) × (total beads taken up by cells) [
34].
4.9. Cell Area and Shape Analysis
Macrophages were fixed in 4% paraformaldehyde and cells observed using a light microscope (Zeiss) and the cell area and aspect ratio were calculated using Image J with >90 cells analyzed for each group per independent preparation from 5 random fields of view where every cell in the field of view was considered [
34]. Representation of F-actin and nuclear staining images of elongated and round cell morphologies are shown in
Supplementary Figure S3.
4.10. RNA Extraction and Quantitative RT-PCR
Total cellular RNA was isolated from untreated or cytokine-stimulated human monocyte-derived macrophages using Trizol extraction reagent (Thermo Fisher Scientific Life Sciences), followed by RNA clean-up using an RNeasy Mini Kit (Qiagen, Germantown, MD, USA), according to the manufacturer’s instructions. A total of 5 μg from each sample was reverse transcribed using the First Strand cDNA Synthesis Kit and Oligo(dT) 15 Primer (Promega, Southampton, United Kingdom) and SuperScript II (Thermo Fisher Scientific Life Sciences), as recommended by the manufacturers. For each gene, the PCR was performed using the following primers: TNF-α forward (CTGTAGCCCACGTCGTAGC)TNF-α reverse (TTGAGATCCATGCCGTTG);IL-6 forward (gatgagtacaaaagtcctgatcca), IL-6 reverse (ctgcagccactggttctgt); PDGF forward (caacctgaacccagaccatc) PDGF reverse (tccttttccggtttttacctg); VEGF forward (aaaaacgaaagcgcaagaaa) VEGF reverse (tttctccgctctgaacaagg) TGFβ1 forward(tggagcaacatgtggaactc) TGFβ1 reverse (gtcagcagccggttacca) IL-1β Forward (agttgacggaccccaaaag) IL-1β reverse (agctggatgctctcatcagg). Quantitative real-time PCR was carried out by LightCycler 480 (Roche Diagnostics, West Sussex, United Kingdom) with the Universal Probe Library system (Roche Diagnostics). Gene expression was analyzed using the comparative threshold method with target gene mRNA levels being normalized to β-actin (Actb) or non-POU domain-containing (Nono). Data are expressed as fold-change differences in gene levels in untreated cells compared with that of those exposed to shockwave therapy.
4.11. Western Blotting
Total macrophage protein lysates were extracted using 1× RIPA buffer (Sigma Aldrich, St-Louis, MO, USA; R0278) containing complete protease inhibitors and Phosphostop (Thermo 1861281). Subsequently, the protein concentration was quantified by the Pierce BCA Protein Assay Kit, Thermo Fisher 10741395 and the sample was boiled at 100˚C for 10 min with a loading buffer (Invitrogen, ThermoFisher, Paisley, UK; NP007). Equal amounts of protein of 20 µg per lane were separated using SDS-PAGE (Invitrogen NuPage 4–12% Bis-Tris NP0321) and transferred onto a HybondTM-P PVDF membrane (Amersham, GE Healthcare, Buckinghamshire, UK). Following blocking with 5% nonfat dairy milk in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 1 h at room temperature, the membrane was incubated with the following primary antibodies: Rabbit anti-ERK1/2 (monoclonal antibody; 1:1000; cat. no. 4695; Cell Signaling Technology, Inc, London, UK.) and rabbit anti-p-ERK1/2 (monoclonal antibody; 1:1000; cat. no. 4370; Cell Signaling Technology, Inc.) at 4°C overnight. The following morning, the membranes were washed four times with TBST for 10 min and incubated with an HRP-conjugated goat anti-rabbit IgG secondary antibody (Cell Signaling 7074; 1:1000) for 1 h. The signal was detected with a chemiluminescence kit containing hydrogen peroxide and Luminol components for Enhanced Chemiluminescence, (Thermo (Pierce) 32106). Subsequently, the bands were semi-quantified using an iBright or LiCor imaging device.
4.12. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 (version 5.04) software. Data sets were tested for normal distribution using the Shapiro–Wilk test and/or the Kolmogorov–Smirnov test (with the Dallal-Wilkinson-Lillie for corrected p-value). In the event of normal distribution, significance was determined using a Students t-test. In the absence of normal distribution, significance was based on the Wilcoxon matched-pairs signed-ranks test or the Mann–Whitney test. All tests were performed with 95% confidence intervals. In some cases, a one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test was used. P-values less than 0.05 were taken as statistically significant, and values are given as the mean ± SEM.