Toll-Like Receptors: Expression and Roles in Otitis Media
Abstract
:1. Introduction
1.1. Overview of Otitis Media
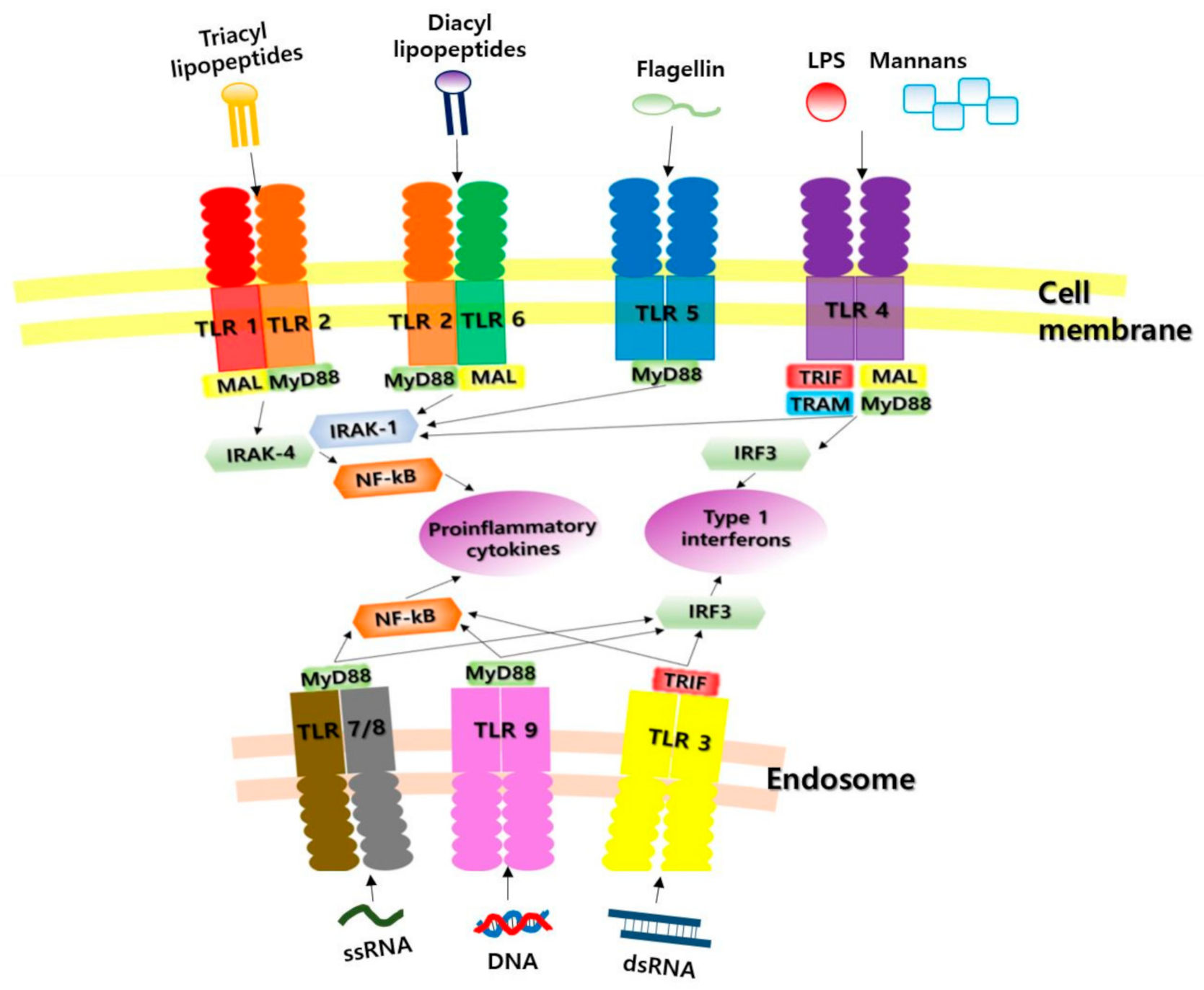
1.2. Toll-Like Receptors as Pattern Recognition Receptors (PRRs)
2. TLRs and Otitis Media
2.1. TLR1
2.2. TLR2
2.3. TLR3
2.4. TLR4
2.5. TLR5
2.6. TLR6
2.7. TLR7
2.8. TLR8
2.9. TLR9
2.10. TLR10
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rovers, M.M.; Schilder, A.G.; Zielhuis, G.A.; Rosenfeld, R.M. Otitis media. Lancet 2004, 363, 465–473. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.S.; Kim, Y.I.; Jung, S.Y.; Kim, S.H.; Yeo, S.G. Decreased Aquaporin 4 and 6 mRNAs in Patients with Chronic Otitis Media with Otorrhea. Clin. Exp. Otorhinolaryngol. 2019, 12, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.H.; Shan, Y.J.; Han, Y.; Zhu, L.W.; Ma, Z.X. Pathological study of otitis media with effusion after treatment with intranasal pulmonary surfactant. Laryngoscope 2013, 123, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tsuprun, V.; Kawano, H.; Paparella, M.M.; Zhang, Z.; Anway, R.; Ho, S.B. Characterization of mucins in human middle ear and Eustachian tube. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, M.; van der Veen, E.L.; Rovers, M.M.; Sanders, E.A.; Schilder, A.G. Chronic suppurative otitis media: A review. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wiatr, M.; Skladzien, J.; Strek, P.; Przeklasa-Muszynska, A.; Wiatr, A. Chronic Otitis Media with Granulation Is a Poor Prognostic Factor for Hearing Improvement and Development of Intracranial Complications. J. Int. Adv. Otol. 2019, 15, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, D.; Park, D.C.; Lee, E.H.; Choi, Y.S.; Ryu, J.; Kim, S.H.; Yeo, S.G. Immunoglobulins and Transcription Factors in Otitis Media. Int. J. Mol. Sci. 2021, 22, 3201. [Google Scholar] [CrossRef]
- Choi, S.A.; Kang, H.M.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Analysis of differences in facial nerve dehiscence and ossicular injury in chronic otitis media and cholesteatoma. Acta Otolaryngol. 2014, 134, 455–461. [Google Scholar] [CrossRef]
- Kim, M.J.; Cho, Y.K.; Sung, Y.C. Pattern recognition receptors in immune modulation. BioWave 2006, 12, 1–22. [Google Scholar]
- Opitz, B.; Eitel, J.; Meixenberger, K.; Suttorp, N. Role of Toll-like receptors, NOD-like receptors and RIG-I-like receptors in endothelial cells and systemic infections. Thromb. Haemost. 2009, 102, 1103–1109. [Google Scholar]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 2019, 89, 24–33. [Google Scholar]
- Kawai, T.; Akira, S. The role of TLRs, RLRs and NLRs in pathogen recogniton. Int. Immunol. 2009, 4, 317–337. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptor. Ann. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligdeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [Green Version]
- Sasai, M.; Yamamoto, M. Pathogen recognition receptors: Ligands and signaling pathways by Toll-like receptors. Int. Rev. Immunol. 2013, 32, 116–133. [Google Scholar] [CrossRef]
- Lee, H.H. Role of Innate Immunity in Otitis Media. Review. Korean J. Otorhinolaryngol. Head Neck Surg. 2016, 59, 483–489. [Google Scholar] [CrossRef]
- Song, D.H.; Lee, J.O. Sensing of microbial molecular patterns by Toll-like receptors. Immunol. Rev. 2012, 250, 216–229. [Google Scholar] [CrossRef]
- Trzpis, K.; Kasprzycka, E.; Skotnicka, B.; Hassmann-Poznańska, E.; Wysocka, J. Expression of Toll-like receptors on peripheral blood white cells in acute otitis media. Otolaryngol. Pol. 2014, 68, 77–82. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chung, J.H.; Lee, S.K.; Byun, J.Y.; Kim, Y.I.; Yeo, S.G. Toll-like receptors, cytokines & nitric oxide synthase in patients with otitis media with effusion. Indian J. Med. Res. 2013, 138, 523–530. [Google Scholar] [PubMed]
- Huang, Y.; Wang, Z.; Jin, C.; Wang, L.; Zhang, X.; Xu, W.; Xiang, Y.; Wang, W.; He, X.; Yin, Y.; et al. TLR2 promotes macrophage recruitment and Streptococcus pneumoniae clearance during mouse otitis media. Pediatr. Res. 2016, 80, 886–893. [Google Scholar] [CrossRef]
- Han, F.; Yu, H.; Tian, C.; Li, S.; Jacobs, M.R.; Benedict-Alderfer, C.; Zheng, Q.Y. Role for Toll-like receptor 2 in the immune response to Streptococcus pneumoniae infection in mouse otitis media. Infect. Immun. 2009, 77, 3100–3108. [Google Scholar] [CrossRef] [Green Version]
- Song, J.J.; Cho, J.G.; Woo, J.S.; Lee, H.M.; Hwang, S.J.; Chae, S.W. Differential expression of toll-like receptors 2 and 4 in rat middle ear. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 821–824. [Google Scholar] [CrossRef]
- Leichtle, A.; Hernandez, M.; Pak, K.; Yamasaki, K.; Cheng, C.F.; Webster, N.J.; Ryan, A.F.; Wasserman, S.I. TLR4-mediated induction of TLR2 signaling is critical in the pathogenesis and resolution of otitis media. Innate Immun. 2009, 15, 205–215. [Google Scholar] [CrossRef]
- Jesic, S.; Jotic, A.; Tomanovic, N.; Zivkovic, M. Expression of toll-like receptors 2, 4 and nuclear factor kappa B in mucosal lesions of human otitis: Pattern and relationship in a clinical immunohistochemical study. Ann. Otol. Rhinol. Laryngol. 2014, 123, 434–441. [Google Scholar] [CrossRef]
- Kaur, R.; Casey, J.; Pichichero, M. Differences in innate immune response gene regulation in the middle ear of children who are otitis prone and in those not otitis prone. Am. J. Rhinol. Allergy 2016, 30, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Casey, J.; Pichichero, M. Cytokine, chemokine, and Toll-like receptor expression in middle ear fluids of children with acute otitis media. Laryngoscope 2015, 125, E39–E44. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Ryu, E.W.; Kim, J.B.; Yeo, S.G. Clinical approaches for understanding the expression levels of pattern recognition receptors in otitis media with effusion. Clin. Exp. Otorhinolaryngol. 2011, 4, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Zhang, Z.G.; Chen, S.J.; Zheng, Y.Q.; Chen, Y.B.; Liu, Y.; Jiang, H.; Feng, L.Q.; Huang, X. Attenuated TLRs in middle ear mucosa contributes to susceptibility of chronic suppurative otitis media. Hum. Immunol. 2014, 75, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Kariya, S.; Okano, M.; Fukushima, K.; Kataoka, Y.; Maeda, Y.; Nishizaki, K. Expression of toll-like receptors in chronic otitis media and cholesteatoma. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 674–676. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Z.G.; Huang, C.Y.; He, J.F.; Feng, L.Q.; Chen, Y.B.; Chen, T. Differential expression of toll-like receptors in chronic suppurative otitis media and cholesteatoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012, 47, 388–393. [Google Scholar]
- Komori, M.; Nakamura, Y.; Ping, J.; Feng, L.; Toyama, K.; Kim, Y.; Ferrieri, P.; Lin, J. Pneumococcal peptidoglycan-polysaccharides regulate Toll-like receptor 2 in the mouse middle ear epithelial cells. Pediatr. Res. 2011, 69, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, L.; Vuononvirta, J.; Mertsola, J.; Waris, M.; He, Q.; Peltola, V. Polymorphisms of Mannose-binding Lectin and Toll-like Receptors 2, 3, 4, 7 and 8 and the Risk of Respiratory Infections and Acute Otitis Media in Children. Pediatr. Infect. Dis. J. 2017, 36, e114–e122. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kim, C.; Shim, J.S.; Byun, J.Y.; Park, M.S.; Cha, C.I.; Kim, Y.I.; Lee, J.W.; Yeo, S.G. Toll-like receptors 2 and 4 and their mutations in patients with otitis media and middle ear effusion. Clin. Exp. Otorhinolaryngol. 2008, 1, 189–195. [Google Scholar] [CrossRef]
- Szczepański, M.; Szyfter, W.; Jenek, R.; Wróbel, M.; Lisewska, I.M.; Zeromski, J. Toll-like receptors 2, 3 and 4 (TLR-2, TLR-3 and TLR-4) are expressed in the microenvironment of human acquired cholesteatoma. Eur. Arch. Otorhinolaryngol. 2006, 263, 603–607. [Google Scholar] [CrossRef]
- Hirano, T.; Kodama, S.; Fujita, K.; Maeda, K.; Suzuki, M. Role of Toll-like receptor 4 in innate immune responses in a mouse model of acute otitis media. FEMS Immunol. Med. Microbiol. 2007, 49, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Leichtle, A.; Hernandez, M.; Pak, K.; Webster, N.J.; Wasserman, S.I.; Ryan, A.F. The toll-Like receptor adaptor TRIF contributes to otitis media pathogenesis and recovery. BMC Immunol. 2009, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Emonts, M.; Veenhoven, R.H.; Wiertsema, S.P.; Houwing-Duistermaat, J.J.; Walraven, V.; de Groot, R.; Hermans, P.W.; Sanders, E.A. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics 2007, 120, 814–823. [Google Scholar] [CrossRef] [Green Version]
- Tuoheti, A.; Gu, X.; Cheng, X.; Zhang, H. Silencing Nrf2 attenuates chronic suppurative otitis media by inhibiting pro-inflammatory cytokine secretion through up-regulating TLR4. Innate Immun. 2021, 27, 70–80. [Google Scholar] [CrossRef]
- Hafrén, L.; Einarsdottir, E.; Kentala, E.; Hammarén-Malmi, S.; Bhutta, M.F.; MacArthur, C.J.; Wilmot, B.; Casselbrant, M.; Conley, Y.P.; Weeks, D.E.; et al. Predisposition to Childhood Otitis Media and Genetic Polymorphisms within the Toll-Like Receptor 4 (TLR4) Locus. PLoS ONE 2015, 10, e0132551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granath, A.; Uddman, R.; Cardell, L.O. Increased TLR7 expression in the adenoids among children with otitis media with effusion. Acta Otolaryngol. 2010, 130, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Leichtle, A.; Hernandez, M.; Lee, J.; Pak, K.; Webster, N.J.; Wollenberg, B.; Wasserman, S.I.; Ryan, A.F. The role of DNA sensing and innate immune receptor TLR9 in otitis media. Innate Immun. 2012, 18, 3–13. [Google Scholar] [CrossRef]
- Kim, M.G.; Park, D.C.; Shim, J.S.; Jung, H.; Park, M.S.; Kim, Y.I.; Lee, J.W.; Yeo, S.G. TLR-9, NOD-1, NOD-2, RIG-I and immunoglobulins in recurrent otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1425–1429. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, Y.I.; Lee, J.W.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Decreased Expression of TLR-9 and Cytokines in the Presence of Bacteria in Patients with Otitis Media with Effusion. Clin. Exp. Otorhinolaryngol. 2013, 6, 195–200. [Google Scholar] [CrossRef]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Raieli, S.; Trichot, C.; Korniotis, S.; Pattarini, L.; Soumelis, V. TLR1/2 orchestrate human plasmacytoid predendritic cell response to gram+ bacteria. PLoS Biol. 2019, 17, e3000209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, M.; Nishitani, C.; Mitsuzawa, H.; Shimizu, T.; Sano, H.; Harimaya, A.; Fujii, N.; Himi, T.; Kuroki, Y. Alloiococcus otitidis is a ligand for collectins and Toll-like receptor 2, and its phagocytosis is enhanced by collectins. Eur. J. Immunol. 2006, 36, 1527–1536. [Google Scholar]
- Jun, E.J.; Kim, Y.K. Activation of Innate Immune System during Viral Infection: Role of Pattern-recognition Receptors (PRRs) in Viral Infection. J. Bacteriol. Virol. 2009, 39, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Borges, P.V.; Moret, K.H.; Maya-Monteiro, C.M.; Souza-Silva, F.; Alves, C.R.; Batista, P.R.; Caffarena, E.R.; Pacheco, P.; Henriques, M.; Penido, C. Gedunin Binds to Myeloid Differentiation Protein 2 and Impairs Lipopolysaccharide-Induced Toll-Like Receptor 4 Signaling in Macrophages. Mol. Pharmacol. 2015, 88, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor-5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Navas, T.A.; Lyons, S.; Godowski, P.J.; Madara, J.L. Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 2001, 167, 1882–1885. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front Immunol. 2012, 3, 79. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, L.A.; Macedo, G.C.; Marinho, F.A.; Gomes, M.T.; Corsetti, P.P.; Silva, A.M.; Cassataro, J.; Giambartolomei, G.H.; Oliveira, S.C. Toll-like receptor 6 plays an important role in host innate resistance to Brucella abortus infection in mice. Infect. Immun. 2013, 81, 1654–1662. [Google Scholar] [CrossRef] [Green Version]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [Green Version]
- de Marcken, M.; Dhaliwal, K.; Danielsen, A.C.; Gautron, A.S.; Dominguez-Villar, M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci. Signal. 2019, 12, eaaw1347. [Google Scholar] [CrossRef]
- Bauer, S.; Kirschning, C.J.; Hacker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef] [Green Version]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Jang, S.; Li, X.; Hess, N.J.; Guan, Y.; Tapping, R.I. TLR10 is a Negative Regulator of Both MyD88-Dependent and Independent TLR Signaling. J. Immunol. 2016, 196, 3834–3841. [Google Scholar] [CrossRef] [Green Version]
- Hess, N.J.; Jang, S.; Li, X.; Guan, Y.; Tapping, R.I. TLR10 is a B-cell Intrinsic Suppressor of Adaptive Immune Responses. J. Immunol. 2017, 198, 699–707. [Google Scholar] [CrossRef] [Green Version]
| TLR | Species | Components |
|---|---|---|
| TLR1 | Bacteria and mycobacteria | Triacyl lipopeptides |
| Neisseria meningitidis | Soluble factors released by live bacteria | |
| TLR2 | Mycoplasma | Diacyl lipopeptides |
| Bacteria and mycobacteria | Triacyl lipopeptides | |
| Group B Streptococcus | LTA | |
| Gram-positive bacteria | PG | |
| Neisseria | Porins | |
| Neisseria meningitidis | Soluble factors released by live bacteria | |
| Mycobacteria | Lipoarabinomannan | |
| Saccharomyces cerevisiae | Zymosan | |
| Yeasts | Zymosan | |
| Candida albicans | Phospholipomannan | |
| Cryptococcus neoformans | Glucuronoxylomannan | |
| Trypanosoma | tGPI-mutin | |
| Trypanosoma cruzi | Glycosylphosphatidylinositol anchors | |
| Measles virus | Hemagglutinin protein | |
| HCMV, HSV1 | ND (Not determined) | |
| Several bacterial species | Lipoprotein | |
| Staphylococcus aureus | Lipoteichoic acid | |
| Phosphatidylinositol dimannoside | ||
| Soluble phenol modulin | ||
| Leptospira interrogans | Endotoxin (LPS) | |
| Porphyromonas gingivalis | Endotoxin (LPS) | |
| Mycoplasma fermentans | Macrophage activating lipopeptide-2(MALP-2) | |
| TLR3 | Viruses | dsRNA |
| Gram-positive bacteria | Peptidoglycan | |
| Staphylococcus aureus | Soluble phenol modulin | |
| Yeasts | Zymosan | |
| Mycoplasma fermentans | Macrophage activating lipopeptide-2(MALP-2) | |
| TLR4 | Gram-negative bacteria | LPS |
| Candida albicans | Mannan | |
| Trypanosoma | Glycoinositolphospholipids | |
| RSV, MMTV | Envelope proteins | |
| Gram positive bacteria | Peptidoglycan | |
| Staphylococcus aureus | Soluble phenol modulin | |
| Yeasts | Zymosan | |
| Mycoplasma fermentans | Macrophage activating lipopeptide-2 (MALP-2) | |
| Plants | Taxol | |
| Heat-shock protein 60,70 | - | |
| Fibrinogen | - | |
| TLR5 | Flagellated bacteria | Flagellin |
| Gram-positive bacteria | Peptidoglycan | |
| Staphylococcus aureus | Soluble phenol modulin | |
| Yeasts | Zymosan | |
| Mycoplasma fermentans | Macrophage activating lipopeptide-2 (MALP-2) | |
| TLR6 | Mycoplasma | Diacyl lipopeptides |
| Group B Streptococcus | LTA | |
| Saccharomyces cerevisiae | Zymosan | |
| Yeasts | Zymosan | |
| Gram-positive bacteria | Peptidoglycan | |
| Staphylococcus aureus | Soluble phenol modulin | |
| Mycoplasma fermentans | Macrophage activating lipopeptide-2 (MALP-2) | |
| TLR7 | RNA viruses | ssRNA |
| Chemical compounds | Imidazoquinoline antiviral compounds (imiquimod and R-848) | |
| TLR8 | RNA viruses | ssRNA |
| TLR9 | Bacteria and mycobacteria | CpG-DNA |
| Plasmodium | Hemozoin | |
| Viruses | DNA | |
| TLR10 | ? | ? |
| Author [Reference] | Associated Diseases | Study Design | Species and/or Sample | Detection Method | Target Gene(s) or Pathway(s) Associated with TLRs | Results/Conclusion |
|---|---|---|---|---|---|---|
| Trzpis K et al. [20] | rAOM | Prospective study | Human: Peripheral blood | flow cytometric analysis | TLR1, TLR2, TLR4 | Expression of all examined TLRs on monocytes was significantly higher in the AOM group. Peripheral blood monocytes are characterized by increased expression of TLRs in the course of recurrent AOM. |
| Lee HY et al. [21] | OME | Prospective study | Human: Middle ear fluids | RT- PCR | TLR1, TLR2, TLR4, TLR5, TLR6, TLR9 | Expression levels of TLR-2, -4, -6, and -9 mRNA were significantly lower in the otitis-prone than in the non-otitis-prone group. Decreased expression of TLRs may be associated with increased susceptibility to OME. |
| Huang Y et al. [22] | AOM | Animal study | Mice | qRT-PCR, immunofluorescence | TLR2 | TLR2 expression in ME mucosa was markedly enhanced following infection with Streptococcus pneumoniae in wild-type mice. TLR2 signaling is critical for bacterial clearance and timely resolution of inflammation in AOM induced by Streptococcus pneumoniae. |
| Han F et al. [23] | AOM | Animal study | Mice | RT-PCR, Hematoxylin/eosin-stain | TLR2 | The histological pathology was characterized by effusion and tissue damage in the middle ear and, in TLR2−/− mice, the outcome of infection became more severe at 7 days. At both 3 and 7 days postchallenge, TLR2−/− mice had higher blood bacterial titers than WT mice. TLR2 is important in the molecular pathogenesis and host response to AOM. |
| Song JJ et al. [24] | Normal tubotympanum | Animal study | Mice | RT-PCR, Western blot analysis | TLR2, TLR4 | Expression of TLR2 and TLR4 in the middle ear was increased more than in other anatomical areas. Differential expression of subtypes of the TLR in the normal physiology of the tubotympanum and upper aerodigestive tract also suggests that they may play a role in the pathophysiology of OM. |
| Leichtle A at al. [25] | OM | Animal study | Mice | DNA Microarray, Immunohistochemistry, Quantitative PCR | TLR2, TLR4 | TLR2−/− and TLR4−/− mice exhibited more profound, persistent inflammation with impaired bacterial clearance compared to controls. TLR4 signaling appears to induce TLR2 expression, and TLR2 activation is critical for bacterial clearance and timely resolution of OM. |
| Jesic S et al. [26] | Chole OM | Prospective study | Human: Middle ear mucosa | Semiquantitative immunohistochemical methods | TLR 2, TLR 4 | Stronger expression of TLR2 and -4 was found in inflamed mucosa than in controls in children and adults, in cholesteatoma perimatrix compared to tubotympanic lesions in children and adults. TLR2 and TLR4 mediate inflammation in cholesteatoma and mucosal lesions of tubotympanic otitis in children and adults. |
| Kaur R et al. [27] | AOM | Prospective, longitudinal study | Human | RT-PCR | TLR2 | Expression of all examined TLRs on monocytes was significantly higher in the AOM group. Peripheral blood monocytes are characterized by increased expression of TLRs in the course of recurrent AOM. |
| Kaur R et al. [28] | OME | Prospective study | Human: MEF | RT-PCR | TLR2, TLR4, TLR9 | Expression levels of TLR2, -4, -6, and -9 mRNA were significantly lower in the otitis-prone than in the non-otitis-prone group. Decreased expression of TLRs may be associated with increased susceptibility to OME. |
| Lee SY et al. [29] | OME | Prospective study | Human: MEF | RT-PCR | TLR2, TLR4, TLR5, TLR9 | All effusion fluid samples collected from patients with OME showed expression of TLR2, -4, -5, -9 mRNA by PCR. Exudates of OME patients show TLR expression levels that are related to the innate immune response regardless of the characteristics of effusion fluid, presence of bacteria in exudates, or frequency of ventilation tube insertion. |
| Si Y et al. [30] | COM, CSOM | Prospective study | Human: ME mucosa | RT-PCR, Western blot | TLR2, TLR4, TLR5, TLR9 | mRNA and protein levels of TLR2, -4, and -5 exhibited no difference between the non-OM and COM groups but were significantly lower in the CSOM group. Reduced TLR levels in the middle-ear mucosa might cause weak host response to bacteria, persistent inflammation and susceptibility to CSOM. |
| Hirai H et al. [31] | COM, chole OM | Prospective study | Human:ME tissue | Immunohistochemistry | TLR2, TLR4 | Both TLR2 and -4 were markedly expressed in COM and chole OM. There was a significant difference between COM and normal controls in the expression of both TLRs. TLRs may play a principal role in human COM and chole OM. |
| Si Y et al. [32] | CSOM | Prospective study | Human: normal canal skin, mucosa and granulation tissue | RT- PCR, Western blot, Immunohistochemistry | TLR2, TLR4 | Both mRNA and protein levels of TLR2 and -4 in mucosa of CSOM and chole OM were higher than those in normal canal skin, but lower than those in chole OM epithelium. There was no significant difference in mucosa of the two OM groups. Differential expression of TLR2 and -4 in mucosa suggests that they may play a different role in the pathophysiology of COM and chole OM. |
| Komori M et al. [33] | OME | Human and animal study | Human and animal (rat and mouse) specimens | Quantitative PCR, Immunohistochemistry, Western blot | TLR2 | Expression of TLR2 was activated in ME epithelial cells through the NF-κB cytokine signaling pathway, while the I kappa B alpha mutant (IκBαM), a dominant negative inhibitor of NF-κB, abrogated the expression of TLR2 induced by PGPS. |
| Toivonen L et al. [34] | AOM | Prospective cohort study | Human | PCR | TLR2 Arg753Gln, TLR3 Leu412Phe, TLR4 Asp299Gly, TLR7 Gln11Leu and TLR8 Leu651Leu. | TLR2 polymorphisms were associated with recurrent AOM. TLR7 polymorphisms were associated with a decreased risk of rhinovirus-associated AOM. Genetic polymorphisms in TLRs promote susceptibility to or protection against respiratory infections. |
| Lee YC et al. [35] | OME | Prospective study | Human: MEF | RT-PCR | TLR2, TLR4 | TLR2 and -4 were expressed in the MEF and the expression of TLR2 was higher than that of TLR4. TLR2 and -4 were expressed in all MEF samples of OME, but the mutations of TLR 2 and 4 were not detected. |
| Szczepański M et al. [36] | Chole OM | Prospective study | Human: cholesteatoma and normal external auditory canal skin | Immunohistochemistry | TLR2, TLR3, TLR4 | All TLRs tested were demonstrated in matrix (layer of keratinizing epithelium) and perimatrix (granulation tissue) of this inflammatory tumor. Weak expression of these receptors on normal skin may also suggest the important role of TLRs in the etiopathogenesis of cholesteatoma. |
| Hirano T et al. [37] | AOM | Animal study | C3H/HeJ mice: ME mucosa | H&E staining, Confocal laser scanning microscopy | TLR4 | In WT mice, PMNs that had infiltrated the ME mucosa showed strong immunostaining of both TLR2 and -4 24 h after NTHi injection. In TLR4 deficient mice, PMNs showed hardly any staining of TLR2 and -4. TLR 4 plays a part in the early accumulation and functional promotion of PMNs in the ME for eradicating NTHi infection. |
| Leichtle A at al. [38] | OM | Animal study | Mice | Quantitative PCR, DNA Microarray | TLR adaptor TRIF | Expression of TRIF mRNA was only modestly enhanced during OM. TRIF-deficient mice showed reduced but more persistent mucosal hyperplasia and less leukocyte infiltration into the ME in response to NTHi infection than did WT animals. Activation of TRIF/type I IFN responses is important in both the pathogenesis and resolution of NTHi-induced OM. |
| Emonts M et al. [39] | AOM | Randomized, controlled trial | Human DNA | PCR | TLR2, TLR4 | TLR4 299 A/A genotype was associated with an otitis-prone condition. Variation in innate immunoresponse genes contributes to an otitis-prone condition. |
| Tuoheti A et al. [40] | CSOM, COM and non-OM | Prospective study | Human and C57BL/6 mice | qRT-PCR, Western blot | TLR2,TLR4, TLR5, TLR9 | TLR4, instead of other TLRs, showed low expression in the CSOM group compared to the COM and non-COM groups. TLR4 deficiency promoted chronic inflammation in LPS-induced acute otitis media mice models. Knock-down of Nrf2 reversed chronic inflammation to attenuate CSOM by up-regulating TLR4. |
| Hafrén L et al. [41] | rAOM or OME | Cohort study | Human: DNA was extracted from peripheral blood | SNP | TLR4 gene | SNP rs5030717 in the TLR4 gene region showed significant association to OM. The TLR4 gene locus, regulating the innate immune response, influences the genetic predisposition to childhood OM. |
| Granath A et al. [42] | OME | Controlled, prospective study | Human: adenoid tissue | qRT-PCR, Immunohistochemistry | TLR7 | mRNA levels for TLR7 were increased among children with a history of OME. |
| Leichtle A et al. [43] | Otitis media | Animal study | C57Bl/6:CB F1 hybrid mice | qRT-PCR, Immunohistochemistry | TLR9 | TLR9 deletion significantly prolonged the inflammatory response induced by NTHi in the ME and delayed bacterial clearance. The results suggest that DNA sensing via TLR9 plays a role in OM pathogenesis and recovery. |
| Kim MG et al. [44] | OME | Prospective study | Human: MEF | RT-PCR | TLR9 | The levels of TLR9 mRNAs were significantly lower in the otitis-prone than in the non-otitis-prone group. Decreased expression of TLRs may be associated with increased susceptibility to OME. |
| Lee HY et al. [45] | OME | Prospective study | Human: MEF | RT-PCR | TLR9 | Down-regulation of TLR9 was observed in the culture-positive group. The expression of TLR9 significantly decreased in OME with confirmed bacterial pathogens. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.Y.; Kim, D.; Park, D.C.; Kim, S.S.; Oh, T.I.; Kang, D.W.; Kim, S.H.; Yeo, S.G. Toll-Like Receptors: Expression and Roles in Otitis Media. Int. J. Mol. Sci. 2021, 22, 7868. https://doi.org/10.3390/ijms22157868
Jung SY, Kim D, Park DC, Kim SS, Oh TI, Kang DW, Kim SH, Yeo SG. Toll-Like Receptors: Expression and Roles in Otitis Media. International Journal of Molecular Sciences. 2021; 22(15):7868. https://doi.org/10.3390/ijms22157868
Chicago/Turabian StyleJung, Su Young, Dokyoung Kim, Dong Choon Park, Sung Soo Kim, Tong In Oh, Dae Woong Kang, Sang Hoon Kim, and Seung Geun Yeo. 2021. "Toll-Like Receptors: Expression and Roles in Otitis Media" International Journal of Molecular Sciences 22, no. 15: 7868. https://doi.org/10.3390/ijms22157868
APA StyleJung, S. Y., Kim, D., Park, D. C., Kim, S. S., Oh, T. I., Kang, D. W., Kim, S. H., & Yeo, S. G. (2021). Toll-Like Receptors: Expression and Roles in Otitis Media. International Journal of Molecular Sciences, 22(15), 7868. https://doi.org/10.3390/ijms22157868









