Interleukin-4 Promotes Tuft Cell Differentiation and Acetylcholine Production in Intestinal Organoids of Non-Human Primate
Abstract
:1. Introduction
2. Results
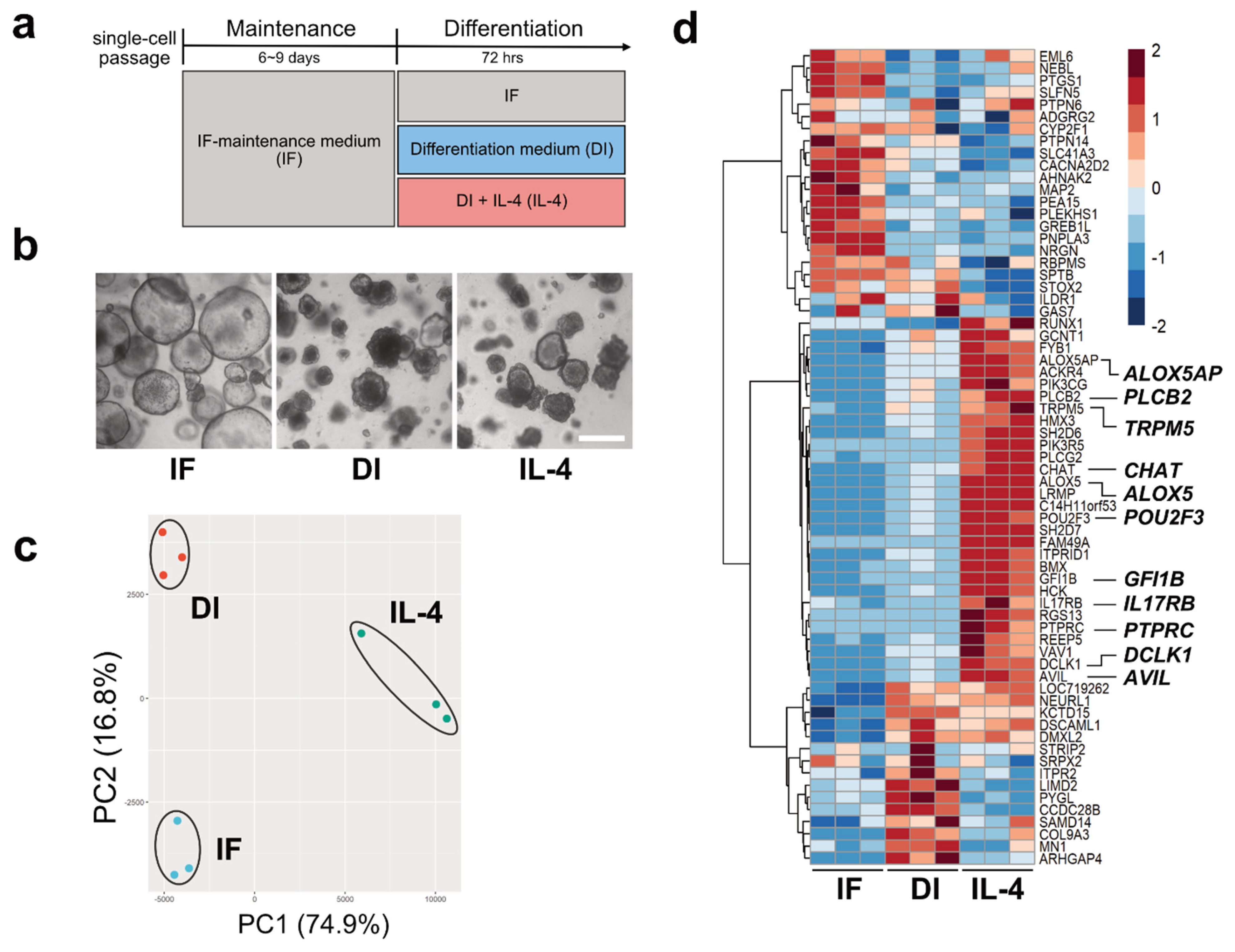
2.1. Transcriptome Analysis of Macaque Intestinal Organoids after 72 h Culture in Differentiation Medium
2.2. Confirmation of RNA-Seq Results by Semi-Quantitative RT-PCR
2.3. ChAT and Ach Are Upregulated by IL-4
2.4. Granule Secretion Induced by ACh in Organoid Cultures
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of Organoid Culture Media
4.3. Crypt Isolation and Organoid Culture from Macaques
4.4. Immunostaining of Tissues and Organoids
4.5. RNA-Seq Analysis
4.6. Semi-Quantitative Reverse Transcription-Polymerase Chain Reaction
4.7. Preparation of the Organoid Samples for the Quantification of Acetylcholine Using HPLC/MS
4.8. Calibration Standard Preparation for HPLC/MS Analysis
4.9. Pretreatment of Organoid Samples for HPLC/MS Analysis
4.10. Quantification of Acetylcholine Using HPLC/MS
4.11. Imaging of Granule Secretion from Paneth Cells Using Organoids
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwatsuki, K.; Torii, K. Peripheral chemosensing system for tastants and nutrients. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 19–25. [Google Scholar] [CrossRef]
- Bezencon, C.; le Coutre, J.; Damak, S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses 2007, 32, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Billipp, T.E.; Nadjsombati, M.S.; von Moltke, J. Tuning tuft cells: New ligands and effector functions reveal tissue-specific function. Curr. Opin. Immunol. 2021, 68, 98–106. [Google Scholar] [CrossRef]
- Ting, H.A.; von Moltke, J. The Immune Function of Tuft Cells at Gut Mucosal Surfaces and Beyond. J. Immunol. 2019, 202, 1321–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of immune responses by tuft cells. Nat. Rev. Immunol. 2019, 19, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Herring, C.A.; Chen, B.; Kim, H.; Simmons, A.J.; Southard-Smith, A.N.; Allaman, M.M.; White, J.R.; Macedonia, M.C.; McKinley, E.T.; et al. Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology 2020, 159, 2101–2115.e5. [Google Scholar] [CrossRef]
- Schutz, B.; Ruppert, A.L.; Strobel, O.; Lazarus, M.; Urade, Y.; Buchler, M.W.; Weihe, E. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci. Rep. 2019, 9, 17466. [Google Scholar] [CrossRef]
- Krasteva, G.; Canning, B.J.; Hartmann, P.; Veres, T.Z.; Papadakis, T.; Muhlfeld, C.; Schliecker, K.; Tallini, Y.N.; Braun, A.; Hackstein, H.; et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc. Natl. Acad. Sci. USA 2011, 108, 9478–9483. [Google Scholar] [CrossRef] [Green Version]
- Inaba, A.; Kumaki, S.; Arinaga, A.; Tanaka, K.; Aihara, E.; Yamane, T.; Oishi, Y.; Imai, H.; Iwatsuki, K. Generation of intestinal chemosensory cells from nonhuman primate organoids. Biochem. Biophys. Res. Commun. 2021, 536, 20–25. [Google Scholar] [CrossRef]
- Bjerknes, M.; Khandanpour, C.; Moroy, T.; Fujiyama, T.; Hoshino, M.; Klisch, T.J.; Ding, Q.; Gan, L.; Wang, J.; Martin, M.G.; et al. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev. Biol. 2012, 362, 194–218. [Google Scholar] [CrossRef] [Green Version]
- Gerbe, F.; Brulin, B.; Makrini, L.; Legraverend, C.; Jay, P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 2009, 137, 2179–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezencon, C.; Furholz, A.; Raymond, F.; Mansourian, R.; Metairon, S.; Le Coutre, J.; Damak, S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J. Comp. Neurol. 2008, 509, 514–525. [Google Scholar] [CrossRef]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Nagana Gowda, G.A.; Fontana, M.F.; et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49, 33–41.e7. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, C.E.; Schneider, C.; Locksley, R.M. Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu. Rev. Immunol. 2019, 37, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y. Choline acetyltransferase: The structure, distribution and pathologic changes in the central nervous system. Pathol. Int. 1999, 49, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Middelhoff, M.; Nienhuser, H.; Valenti, G.; Maurer, H.C.; Hayakawa, Y.; Takahashi, R.; Kim, W.; Jiang, Z.; Malagola, E.; Cuti, K.; et al. Prox1-positive cells monitor and sustain the murine intestinal epithelial cholinergic niche. Nat. Commun. 2020, 11, 111. [Google Scholar] [CrossRef]
- Yokoi, Y.; Nakamura, K.; Yoneda, T.; Kikuchi, M.; Sugimoto, R.; Shimizu, Y.; Ayabe, T. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci. Rep. 2019, 9, 2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinty, J.W.; Ting, H.A.; Billipp, T.E.; Nadjsombati, M.S.; Khan, D.M.; Barrett, N.A.; Liang, H.E.; Matsumoto, I.; von Moltke, J. Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity. Immunity 2020, 52, 528–541.e7. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Iwatsuki, K.; Inaba, A.; Sato, M.; Tadaishi, M.; Shimizu, M.; Kobayashi-Hattori, K. Interleukin-4 suppresses the proliferation and alters the gene expression in enteroids. Cytotechnology 2020, 72, 479–488. [Google Scholar] [CrossRef]
- Hollenhorst, M.I.; Jurastow, I.; Nandigama, R.; Appenzeller, S.; Li, L.; Vogel, J.; Wiederhold, S.; Althaus, M.; Empting, M.; Altmuller, J.; et al. Tracheal brush cells release acetylcholine in response to bitter tastants for paracrine and autocrine signaling. FASEB J. 2020, 34, 316–332. [Google Scholar] [CrossRef] [Green Version]
- Tizzano, M.; Gulbransen, B.D.; Vandenbeuch, A.; Clapp, T.R.; Herman, J.P.; Sibhatu, H.M.; Churchill, M.E.; Silver, W.L.; Kinnamon, S.C.; Finger, T.E. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl. Acad. Sci. USA 2010, 107, 3210–3215. [Google Scholar] [CrossRef] [Green Version]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Shao, X.; Huang, J. Acetylcholine From Tuft Cells: The Updated Insights Beyond Its Immune and Chemosensory Functions. Front. Cell Dev. Biol. 2020, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Ayabe, T.; Bainbridge, B.; Guina, T.; Ernst, R.K.; Darveau, R.P.; Miller, S.I.; Ouellette, A.J. Mouse paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect. Immun. 2005, 73, 2312–2320. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, S.; Cailotto, C.; Harthoorn, L.F.; de Jonge, W.J. Cholinergic signalling in gut immunity. Life Sci. 2012, 91, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Schreiner, C.M.; Wert, S.E.; Mucenski, M.L.; Scott, W.J.; Whitsett, J.A. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development 2008, 135, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liang, X.; Xuan, Y.; Geng, C.; Li, Y.; Lu, H.; Qu, S.; Mei, X.; Chen, H.; Yu, T.; et al. A reference human genome dataset of the BGISEQ-500 sequencer. Gigascience 2017, 6, gix024. [Google Scholar] [CrossRef] [Green Version]
- Ohki, J.; Sakashita, A.; Aihara, E.; Inaba, A.; Uchiyama, H.; Matsumoto, M.; Ninomiya, Y.; Yamane, T.; Oishi, Y.; Iwatsuki, K. Comparative analysis of enteroendocrine cells and their hormones between mouse intestinal organoids and native tissues. Biosci. Biotechnol. Biochem. 2020, 84, 936–942. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inaba, A.; Arinaga, A.; Tanaka, K.; Endo, T.; Hayatsu, N.; Okazaki, Y.; Yamane, T.; Oishi, Y.; Imai, H.; Iwatsuki, K. Interleukin-4 Promotes Tuft Cell Differentiation and Acetylcholine Production in Intestinal Organoids of Non-Human Primate. Int. J. Mol. Sci. 2021, 22, 7921. https://doi.org/10.3390/ijms22157921
Inaba A, Arinaga A, Tanaka K, Endo T, Hayatsu N, Okazaki Y, Yamane T, Oishi Y, Imai H, Iwatsuki K. Interleukin-4 Promotes Tuft Cell Differentiation and Acetylcholine Production in Intestinal Organoids of Non-Human Primate. International Journal of Molecular Sciences. 2021; 22(15):7921. https://doi.org/10.3390/ijms22157921
Chicago/Turabian StyleInaba, Akihiko, Ayane Arinaga, Keisuke Tanaka, Takaho Endo, Norihito Hayatsu, Yasushi Okazaki, Takumi Yamane, Yuichi Oishi, Hiroo Imai, and Ken Iwatsuki. 2021. "Interleukin-4 Promotes Tuft Cell Differentiation and Acetylcholine Production in Intestinal Organoids of Non-Human Primate" International Journal of Molecular Sciences 22, no. 15: 7921. https://doi.org/10.3390/ijms22157921





