Differential Expression of the Host Lipid Regulators ANGPTL-3 and ANGPTL-4 in HCV Infection and Treatment
Abstract
1. Introduction
2. Results
2.1. Demographical and Clinical Characteristics of Study Participants
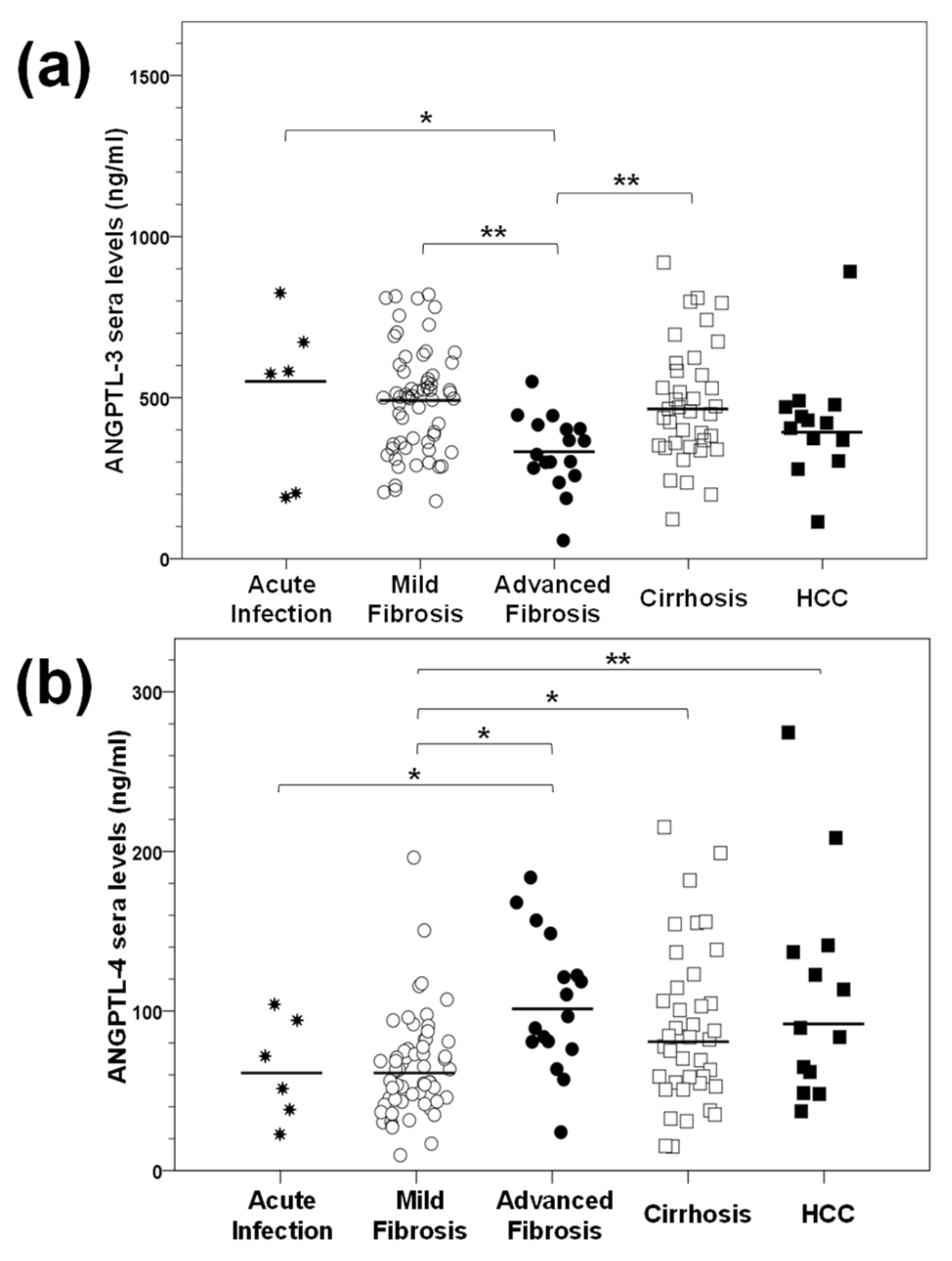
2.2. Determination of ANGPTL-3 and ANGPTL-4 Serum Levels in HCV Infection In Vivo
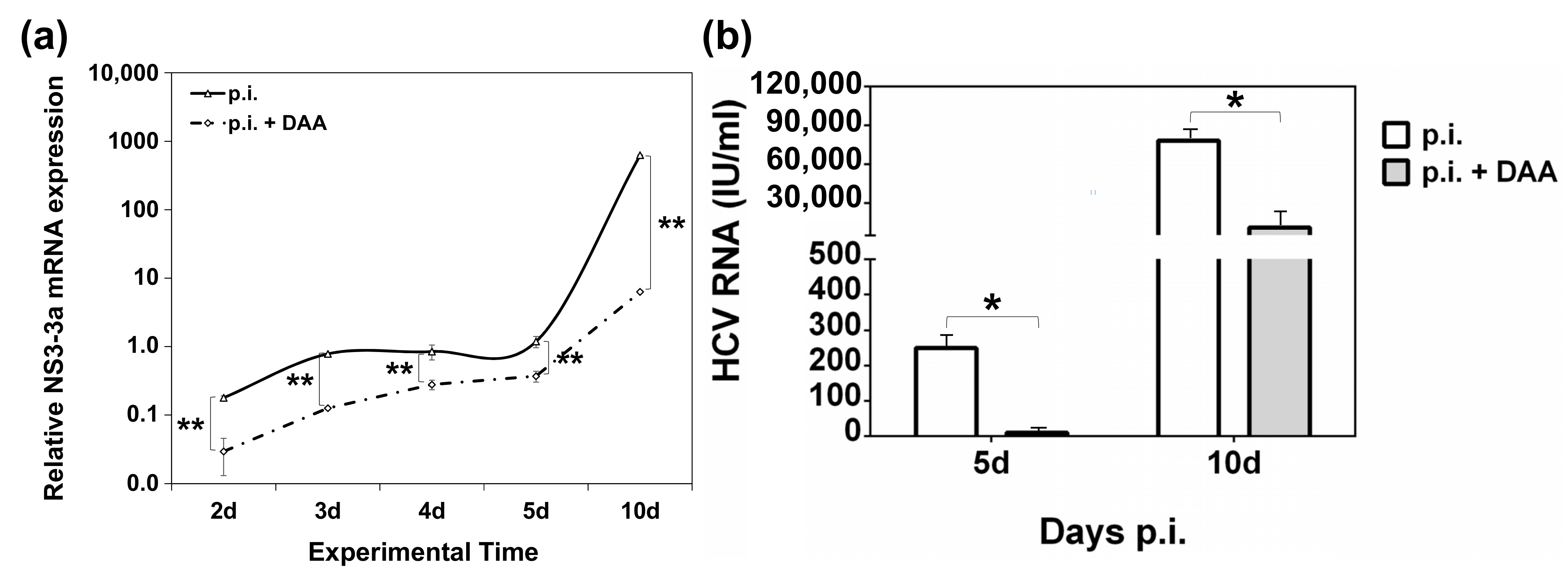
2.3. ANGPTL-3 and ANGPTL-4 Gene Expression Patterns during Long-Term HCV Infection In Vitro
2.4. The Influence of DAA Treatment on ANGPTL-3 and ANGPTL-4 Serum Levels Depends upon the Pre-Treatment Liver Disease Stage
2.5. Establishment of an In Vitro HCV Clearance Model
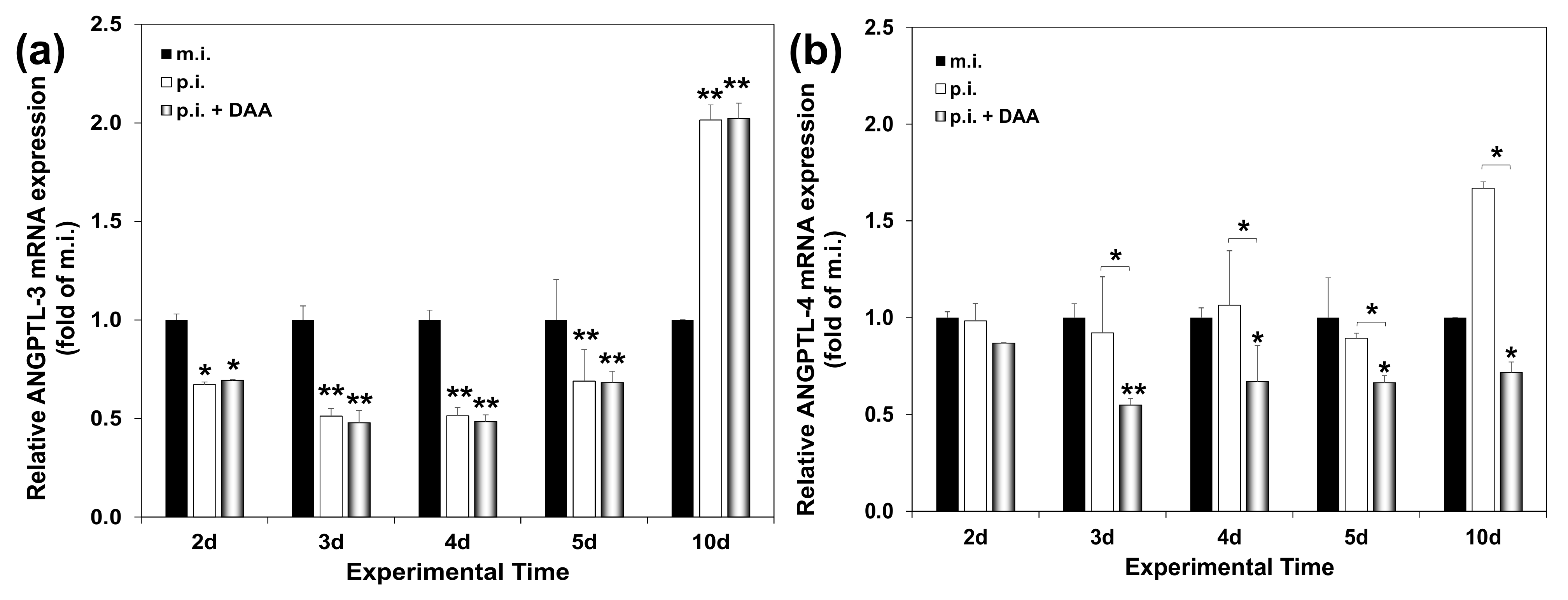
2.6. Modelling the Effect of DAA Treatment on ANGPTL-3 and ANGPTL-4 Gene Expression of HCV-Infected Hepatocytes In Vitro
2.7. Does Serum Expression of ANGPTLs during HCV Infection Correlate with Specific Demographic, Clinical and Viral Parameters?
2.8. Differential Association of ANGPTL-3 and ANGPTL-4 Pre-Treatment Levels with the Profibrotic Cytokine TGF-β
3. Discussion
4. Materials and Methods
4.1. Clinical Samples and Other Materials
4.2. Cell Lines and Viral Infections
4.3. In Vitro HCV Clearance Model
4.4. mRNA Analysis
4.5. ELISA Analysis
4.6. Viral Infectivity Measurements
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Institutional Ethics Committee Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
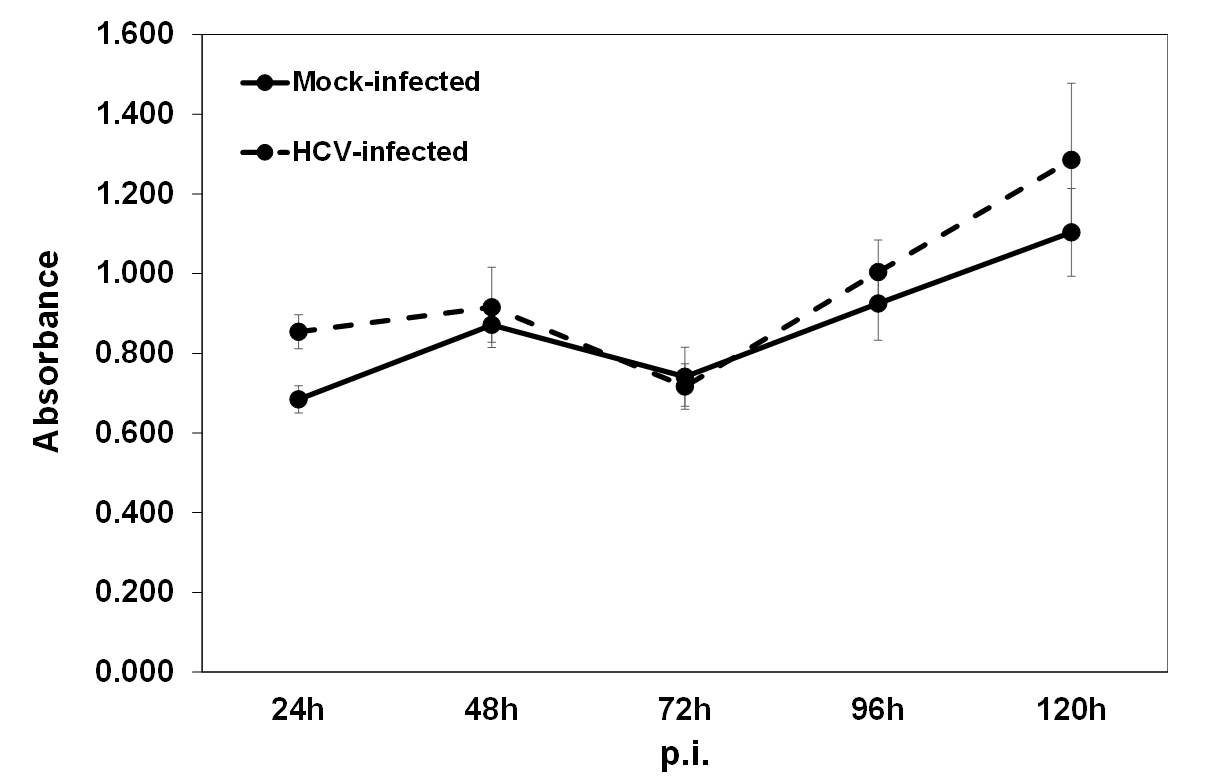
Appendix A.1. ELISA Validation
Appendix A.2. Alterations in ANGPTL Expression Levels Depend on Fibrosis Stage Following DAA Treatment
| Disease Stage | % HCV Patients with Altered ANGPTL-3 Levels AT | % HCV Patients with Altered ANGPTL-4 Levels AT | ||||
|---|---|---|---|---|---|---|
| Decreased | Increased | p-Value | Decreased | Increased | p-Value | |
| Mild Fibrosis | 76.3 | 23.7 | 0.001 | 71.4 | 28.6 | 0.011 |
| Advanced Fibrosis | 53.8 | 46.2 | 0.782 | 87.5 | 12.5 | 0.003 |
| Cirrhosis | 59.1 | 40.9 | 0.394 | 70.8 | 29.2 | 0.041 |
| Clinical Parameter | Male | Female | ||
|---|---|---|---|---|
| Mean ± SD | p-Value | |||
| Age | 51 ± 14 | 54 ± 13 | 0.317 | |
| ALT (IU/mL) | 64 ± 57 | 129 ± 69 | 0.014 | |
| Clinical Characteristic | n * (%) | p-value | n * (%) | p-value |
| VRL-low (<1 × 106 IU/mL) | 21 (30.4%) | 0.02 | 35 (48.6%) | 0.808 |
| VRL-high (≥1 × 106 IU/mL) | 39 (56.5%) | 33 (45.8%) | ||
| Not determined | 9 (13.1%) | 4 (5.6%) | ||
| Genotype | 0.07 | 0.339 | ||
| HCV1a | 10 (14.5%) | 15 (20.9%) | ||
| HCV1b | 13 (18.8%) | 20 (27.8%) | ||
| HCV2a | 6 (8.7%) | 9 (12.5%) | ||
| HCV3 | 21 (30.5%) | 14 (19.4%) | ||
| HCV4 | 13 (18.8%) | 14 (19.4%) | ||
| Not determined | 6 (8.7%) | - | ||
References
- Lombardi, A.; Mondelli, M.U.; ESCMID Study Group for Viral Hepatitis (ESGVH). Hepatitis C: Is eradication possible? Liver Int. 2019, 39, 416–426. [Google Scholar] [CrossRef]
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Lingala, S.; Ghany, M.G. Natural History of Hepatitis C. Gastroenterol. Clin. N. Am. 2015, 44, 717–734. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Romero-Gómez, M. Steatosis and insulin resistance in hepatitis C: A way out for the virus? World J. Gastroenterol. 2009, 15, 5014–5019. [Google Scholar] [CrossRef]
- Garcia-Mediavilla, V.; Pisonero-Vaquero, S.; Cabello, M.E.L.; Benedicto, I.; Majano, P.L.; Jorquera, F.; González-Gallego, J.; Sánchez-Campos, S. Liver X receptor α-mediated regulation of lipogenesis by core and NS5A proteins contributes to HCV-induced liver steatosis and HCV replication. Lab. Investig. 2012, 92, 1191–1202. [Google Scholar] [CrossRef]
- Carmona, I.; Cordero, P.; Ampuero, J.; Rojas, A.; Romero-Gómez, M. Role of assessing liver fibrosis in management of chronic hepatitis C virus infection. Clin. Microbiol. Infect. 2016, 22, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Wobser, H.; Dorn, C.; Weiss, T.; Amann, T.; Bollheimer, C.; Büttner, R.; Schölmerich, J.; Hellerbrand, C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009, 19, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 11033–11053. [Google Scholar] [CrossRef]
- Dash, S.; Aydin, Y.; Widmer, K.E.; Nayak, L. Hepatocellular Carcinoma Mechanisms Associated with Chronic HCV Infection and the Impact of Direct-Acting Antiviral Treatment. J. Hepatocell. Carcinoma 2020, 7, 45–76. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Suarez, A.A.R.; et al. HCV-Induced Epigenetic Changes Associated with Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e7. [Google Scholar] [CrossRef] [PubMed]
- Virzì, A.; Suarez, A.A.R.; Baumert, T.F.; Lupberger, J. Oncogenic Signalling Induced by HCV Infection. Viruses 2018, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef]
- Liu, H.-R.; Li, W.-M. Treg-specific demethylated region activity in isolated regulatory t lymphocytes is a surrogate for disease severity in hepatocellular carcinoma. IUBMB Life 2015, 67, 355–360. [Google Scholar] [CrossRef]
- Hu, B.; Lin, J.; Yang, X.; Sang, X. Aberrant lipid metabolism in hepatocellular carcinoma cells as well as immune microenvironment: A review. Cell Prolif. 2020, 53, e12772. [Google Scholar] [CrossRef]
- Pope, E.D.; Kimbrough, E.O.; Vemireddy, L.P.; Surapaneni, P.K.; Copland, J.A.; Mody, K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin. Ther. Targets 2019, 23, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sangineto, M.; Villani, R.; Cavallone, F.; Romano, A.; Loizzi, D.; Serviddio, G. Lipid Metabolism in Development and Progression of Hepatocellular Carcinoma. Cancers 2020, 12, 1419. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, G.; Cui, X.; Xu, Y. Transcriptomic Data Analyses Reveal a Reprogramed Lipid Metabolism in HCV-Derived Hepatocellular Cancer. Front. Cell Dev. Biol. 2020, 8, 581863. [Google Scholar] [CrossRef]
- Huang, C.-F.; Yu, M.-L. Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy. Clin. Mol. Hepatol. 2020, 26, 251–260. [Google Scholar] [CrossRef]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Beste, L.A.; Green, P.K.; Singal, A.G.; Tapper, E.B.; Waljee, A.K.; Sterling, R.K.; Feld, J.J.; Kaplan, D.E.; Taddei, T.H.; et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients with Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology 2019, 157, 1264–1278.e4. [Google Scholar] [CrossRef]
- Perez, S.; Kaspi, A.; Domovitz, T.; Davidovich, A.; Lavi-Itzkovitz, A.; Meirson, T.; Holmes, J.A.; Dai, C.-Y.; Huang, C.-F.; Chung, R.T.; et al. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019, 15, e1008181. [Google Scholar] [CrossRef]
- Polyak, S.J.; Crispe, I.N.; Baumert, T.F. Liver Abnormalities after Elimination of HCV Infection: Persistent Epigenetic and Immunological Perturbations Post-Cure. Pathology 2021, 10, 44. [Google Scholar] [CrossRef]
- Rinaldi, L.; Nevola, R.; Franci, G.; Perrella, A.; Corvino, G.; Marrone, A.; Berretta, M.; Morone, M.V.; Galdiero, M.; Giordano, M.; et al. Risk of Hepatocellular Carcinoma after HCV Clearance by Direct-Acting Antivirals Treatment Predictive Factors and Role of Epigenetics. Cancers 2020, 12, 1351. [Google Scholar] [CrossRef]
- Köster, A.; Chao, Y.B.; Mosior, M.; Ford, A.; Gonzalez-DeWhitt, P.A.; Hale, J.E.; Li, D.; Qiu, Y.; Fraser, C.C.; Yang, D.D.; et al. Transgenic Angiopoietin-Like (Angptl)4 Overexpression and Targeted Disruption of Angptl4 and Angptl3: Regulation of Triglyceride Metabolism. Endocrinology 2005, 146, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Shimizugawa, T.; Ono, M.; Shimamura, M.; Yoshida, K.; Ando, Y.; Koishi, R.; Ueda, K.; Inaba, T.; Minekura, H.; Kohama, T.; et al. ANGPTL3 Decreases Very Low Density Lipoprotein Triglyceride Clearance by Inhibition of Lipoprotein Lipase. J. Biol. Chem. 2002, 277, 33742–33748. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, L.; Berbée, J.F.; Van Dijk, S.J.; Van Dijk, K.W.; Bensadoun, A.; Kema, I.P.; Voshol, P.J.; Müller, M.; Rensen, P.C.; Kersten, S. Angptl4 Up-regulates Cholesterol Synthesis in Liver via Inhibition of LPL- and HL-Dependent Hepatic Cholesterol Uptake. Arter. Thromb. Vasc. Biol. 2007, 27, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Koishi, R.; Shimizugawa, T.; Ando, Y. Angptl3-null Mice Show Low Plasma Lipid Concentrations by Enhanced Lipoprotein Lipase Activity. Exp. Anim. 2006, 55, 27–34. [Google Scholar] [CrossRef]
- Olivecrona, G. Role of lipoprotein lipase in lipid metabolism. Curr. Opin. Lipidol. 2016, 27, 233–241. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakajima, T.; Inoue, I. Molecular modelling of the dimeric structure of human lipoprotein lipase and functional studies of the carboxyl-terminal domain. JBIC J. Biol. Inorg. Chem. 2002, 269, 4701–4710. [Google Scholar] [CrossRef]
- Adam, R.C.; Mintah, I.J.; Alexa-Braun, C.A.; Shihanian, L.M.; Lee, J.S.; Banerjee, P.; Hamon, S.C.; Kim, H.I.; Cohen, J.C.; Hobbs, H.H.; et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J. Lipid Res. 2020, 61, 1271–1286. [Google Scholar] [CrossRef]
- Oteng, A.-B.; Ruppert, P.M.M.; Boutens, L.; Dijk, W.; van Dierendonck, X.; Olivecrona, G.; Stienstra, R.; Kersten, S. Characterization of ANGPTL4 function in macrophages and adipocytes using Angptl4-knockout and Angptl4-hypomorphic mice. J. Lipid Res. 2019, 60, 1741–1754. [Google Scholar] [CrossRef]
- Santulli, G. Angiopoietin-Like Proteins: A Comprehensive Look. Front. Endocrinol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Ge, H.; Cha, J.-Y.; Gopal, H.; Harp, C.; Yu, X.; Repa, J.; Li, C. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J. Lipid Res. 2005, 46, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Sukonina, V.; Lookene, A.; Olivecrona, T. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA 2006, 103, 17450–17455. [Google Scholar] [CrossRef] [PubMed]
- Foka, P.; Karamichali, E.; Dalagiorgou, G.; Serti, E.; Doumba, P.P.; Pissas, G.; Kakkanas, A.; Kazazi, D.; Kochlios, E.; Gaitanou, M.; et al. Hepatitis C virus modulates lipid regulatory factor Angiopoietin-like 3 gene expression by repressing HNF-1α activity. J. Hepatol. 2014, 60, 30–38. [Google Scholar] [CrossRef]
- Endo, M. The Roles of ANGPTL Families in Cancer Progression. J. UOEH 2019, 41, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Ogawara, K.; Kasamatsu, A.; Okamoto, A.; Kasama, H.; Minakawa, Y.; Shimada, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer. Cancer Med. 2015, 4, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liang, X.; Chen, J.; Bao, Y.; Wang, A.; Gan, X.; Lu, X.; Wang, L. ANGPTL3 inhibits renal cell carcinoma metastasis by inhibiting VASP phosphorylation. Biochem. Biophys. Res. Commun. 2019, 516, 880–887. [Google Scholar] [CrossRef]
- Guo, L.; Li, S.-Y.; Ji, F.-Y.; Zhao, Y.-F.; Zhong, Y.; Lv, X.-J.; Wu, X.-L.; Qian, G.-S. Role of Angptl4 in vascular permeability and inflammation. Inflamm. Res. 2013, 63, 13–22. [Google Scholar] [CrossRef]
- Carbone, C.; Piro, G.; Merz, V.; Simionato, F.; Santoro, R.; Zecchetto, C.; Tortora, G.; Melisi, D. Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer. Int. J. Mol. Sci. 2018, 19, 431. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Teo, Z.; Sng, M.K.; Zhu, P.; Tan, N.S. Emerging Roles of Angiopoietin-like 4 in Human Cancer. Mol. Cancer Res. 2012, 10, 677–688. [Google Scholar] [CrossRef]
- Foka, P.; Pourchet, A.; Hernandez-Alcoceba, R.; Doumba, P.P.; Pissas, G.; Kouvatsis, V.; Dalagiorgou, G.; Kazazi, D.; Marconi, P.C.R.; Foschini, M.; et al. Novel tumour-specific promoters for transcriptional targeting of hepatocellular carcinoma by herpes simplex virus vectors. J. Gene Med. 2010, 12, 956–967. [Google Scholar] [CrossRef]
- Ando, Y.; Shimizugawa, T.; Takeshita, S.; Ono, M.; Shimamura, M.; Koishi, R.; Furukawa, H. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J. Lipid Res. 2003, 44, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Forns, X.; Costa, J. HCV virological assessment. J. Hepatol. 2006, 44, S35–S39. [Google Scholar] [CrossRef]
- Massard, J.; Ratziu, V.; Thabut, D.; Moussalli, J.; Lebray, P.; Benhamou, Y.; Poynard, T. Natural history and predictors of disease severity in chronic hepatitis C. J. Hepatol. 2006, 44, S19–S24. [Google Scholar] [CrossRef]
- Poynard, T.; Ratziu, V.; Benhamou, Y.; Opolon, P.; Cacoub, P.; Bedossa, P. Natural history of HCV infection. Best Pract. Res. Clin. Gastroenterol. 2000, 14, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Ziada, D.; Salama, M.; Hamisa, M.; Badawi, R.; Hawash, N.; Selim, A.; Abd-Elsalam, S. Predictors for Fibrosis Regression in Chronic HCV Patients after the Treatment with DAAS: Results of a Real-world Cohort Study. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.-C.; Deng, R.; Wang, M.-L.; Lv, D.-D.; Yuan, M.; Wang, Y.-H.; Chen, E.-Q.; Tang, H. Satisfactory virological response and fibrosis improvement of sofosbuvir-based regimens for Chinese patients with hepatitis C virus genotype 3 infection: Results of a real-world cohort study. Virol. J. 2018, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sakai, T. Biological Significance of Local TGF-β Activation in Liver Diseases. Front. Physiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.W.; Thompson, R.K.; Raccor, B. Hepatitis C Virus-Genotype 3: Update on Current and Emergent Therapeutic Interventions. Curr. Infect. Dis. Rep. 2017, 19, 1333. [Google Scholar] [CrossRef] [PubMed]
- Shimotohno, K. HCV Assembly and Egress via Modifications in Host Lipid Metabolic Systems. Cold Spring Harb. Perspect. Med. 2021, 11, a036814. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, E.; Galmozzi, E.; Pelusi, S.; D’Ambrosio, R.; Soffredini, R.; Borghi, M.; Perbellini, R.; Facchetti, F.; Iavarone, M.; SanGiovanni, A.; et al. Hepatic Fat—Genetic Risk Score Predicts Hepatocellular Carcinoma in Patients With Cirrhotic HCV Treated With DAAs. Hepatology 2020, 72, 1912–1923. [Google Scholar] [CrossRef]
- Ziol, M.; Handra-Luca, A.; Kettaneh, A.; Christidis, C.; Mal, F.; Kazemi, F.; De Lédinghen, V.; Marcellin, P.; Dhumeaux, D.; Trinchet, J.-C.; et al. Non-invasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2004, 41, 48–54. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, S.-D.; Wu, L.; Wang, S.-Q.; Chen, Y.; Liu, L.-L.; Li, J.; Yang, C.-Q.; Wang, J.-Y.; Jiang, W. The prognostic role of liver stiffness in patients with chronic liver disease: A systematic review and dose–response meta-analysis. Hepatol. Int. 2019, 13, 560–572. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Axley, P.; Mudumbi, S.; Sarker, S.; Kuo, Y.-F.; Singal, A. Patients with stage 3 compared to stage 4 liver fibrosis have lower frequency of and longer time to liver disease complications. PLoS ONE 2018, 13, e0197117. [Google Scholar] [CrossRef]
- Dooley, S.; ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Khan, R.; Velpari, S.; Koppe, S. All Patients with Advanced Fibrosis Should Continue to Be Screened for Hepatocellular Carcinoma After Sustained Virological Response of Hepatitis C Virus. Clin. Liver Dis. 2018, 12, 137–139. [Google Scholar] [CrossRef]
- El-Shal, A.S.; Zidan, H.E.; Rashad, N.M.; Wadea, F.M. Angiopoietin-like protein 3 and 4 expression 4 and their serum levels in hepatocellular carcinoma. Cytokine 2017, 96, 75–86. [Google Scholar] [CrossRef]
- Revie, D.; Salahuddin, S.Z. Role of macrophages and monocytes in hepatitis C virus infections. World J. Gastroenterol. 2014, 20, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Foka, P.; Dimitriadis, A.; Karamichali, E.; Kyratzopoulou, E.; Giannimaras, D.; Koskinas, J.; Varaklioti, A.; Mamalaki, A.; Georgopoulou, U. Alterations in the iron homeostasis network: A driving force for macrophage-mediated hepatitis C virus persistency. Virulence 2016, 7, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.; Hansen, B.E.; Wiesch, J.S.Z.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated with Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3. [Google Scholar] [CrossRef]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef]
- Khera, T.; Du, Y.; Todt, D.; Deterding, K.; Strunz, B.; Hardtke, S.; Aregay, A.; Port, K.; Hardtke-Wolenski, M.; Steinmann, E.; et al. Long-lasting Imprint in the Soluble Inflammatory Milieu despite Early Treatment of Acute Symptomatic Hepatitis C. J. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Graf, C.; Welzel, T.; Bogdanou, D.; Vermehren, J.; Beckel, A.; Bojunga, J.; Friedrich-Rust, M.; Dietz, J.; Kubesch, A.; Mondorf, A.; et al. Hepatitis C Clearance by Direct-Acting Antivirals Impacts Glucose and Lipid Homeostasis. J. Clin. Med. 2020, 9, 2702. [Google Scholar] [CrossRef]
- Huang, C.; Dai, C.; Yeh, M.; Huang, C.; Lee, H.; Lai, W.; Liang, P.; Lin, Y.; Hsieh, M.; Hou, N.; et al. Cure or curd: Modification of lipid profiles and cardio-cerebrovascular events after hepatitis C virus eradication. Kaohsiung J. Med. Sci. 2020, 36, 920–928. [Google Scholar] [CrossRef]
- Iossa, D.; Vitrone, M.; Gagliardi, M.; Falco, E.; Ragone, E.; Zampino, R.; Durante-Mangoni, E. Anthropometric parameters and liver histology influence lipid metabolic changes in HCV chronic hepatitis on direct-acting antiviral treatment. Ann. Transl. Med. 2021, 9, 35. [Google Scholar] [CrossRef]
- Mauss, S.; Berger, F.; Wehmeyer, M.H.; Ingiliz, P.; Hueppe, D.; Lutz, T.; Simon, K.G.; Schewe, K.; Rockstroh, J.K.; Baumgarten, A.; et al. Effect of antiviral therapy for HCV on lipid levels. Antivir. Ther. 2016, 22, 81–88. [Google Scholar] [CrossRef]
- Nevola, R.; Rinaldi, L.; Zeni, L.; Sasso, F.C.; Pafundi, P.C.; Guerrera, B.; Marrone, A.; Giordano, M.; Adinolfi, L.E. Metabolic and renal changes in patients with chronic hepatitis C infection after hepatitis C virus clearance by direct-acting antivirals. JGH Open 2020, 4, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Cheng, M.-L.; Shiao, M.-S.; Yeh, C.-T.; Wang, C.-H.; Fan, C.-M.; Chiu, C.-T.; Chang, M.-L. Recovery of lipid metabolic alterations in hepatitis C patients after viral clearance: Incomplete restoration with accelerated ω-oxidation. J. Clin. Lipidol. 2018, 12, 756–766. [Google Scholar] [CrossRef]
- Cespiati, A.; Petta, S.; Lombardi, R.; Di Marco, V.; Calvaruso, V.; Bertelli, C.; Pisano, G.; Fatta, E.; Sigon, G.; Iuculano, F.; et al. Metabolic comorbidities and male sex influence steatosis in chronic hepatitis C after viral eradication by direct-acting antiviral therapy (DAAs): Evaluation by the controlled attenuation parameter (CAP). Dig. Liver Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.; Nayak, B.; Patel, A.H.; Gunjan, D.; Singh, V.; Kedia, S. Shalimar Therapy with Oral Directly Acting Agents in Hepatitis C Infection Is Associated with Reduction in Fibrosis and Increase in Hepatic Steatosis on Transient Elastography. J. Clin. Exp. Hepatol. 2019, 9, 207–214. [Google Scholar] [CrossRef]
- Cheng, P.-N.; Chen, J.-Y.; Chiu, Y.-C.; Chiu, H.-C.; Tsai, L.-M. Augmenting central arterial stiffness following eradication of HCV by direct acting antivirals in advanced fibrosis patients. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Hengst, J.; Falk, C.S.; Schlaphoff, V.; Deterding, K.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Direct-Acting Antiviral–Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients with Chronic Hepatitis C. J. Infect. Dis. 2016, 214, 1965–1974. [Google Scholar] [CrossRef]
- Robciuc, M.R.; Tahvanainen, E.; Jauhiainen, M.; Ehnholm, C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J. Lipid Res. 2010, 51, 824–831. [Google Scholar] [CrossRef]
- Smol, E.; Kłapcińska, B.; Kempa, K.; Fredyk, A.; Małecki, A. Effects of Regular Recreational Exercise Training on Serum ANGPTL3-Like Protein and Lipid Profile in Young Healthy Adults. J. Hum. Kinet. 2015, 49, 109–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mc Auley, M.T.; Mooney, K.M. Computational Systems Biology for Aging Research. Interdiscip. Top. Gerontol. 2015, 40, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Heller, T.; Seeff, L.B. Viral load as a predictor of progression of chronic hepatitis C? Hepatology 2005, 42, 1261–1263. [Google Scholar] [CrossRef]
- Noh, R.; Lee, D.H.; Kwon, B.W.; Kim, Y.H.; Kim, S.B.; Song, I.H. Clinical Impact of Viral Load on the Development of Hepatocellular Carcinoma and Liver-Related Mortality in Patients with Hepatitis C Virus Infection. Gastroenterol. Res. Pract. 2016, 2016, 7476231. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Broor, S.L.; Vaishnav, Y.; Sarkar, C.; Girish, R.; Dar, L.; Seth, P.; Broor, S. Transforming growth factor beta in hepatitis C virus infection: In vivo and in vitro findings. J. Gastroenterol. Hepatol. 2003, 18, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Benzoubir, N.; Lejamtel, C.; Battaglia, S.; Testoni, B.; Benassi, B.; Gondeau, C.; Perrin-Cocon, L.; Desterke, C.; Thiers, V.; Samuel, D.; et al. HCV core-mediated activation of latent TGF-β via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J. Hepatol. 2013, 59, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- El-Meguid, M.A.; Dawood, R.M.; Mokhles, M.A.; El Awady, M.K. Extrahepatic Up-regulation of Transforming Growth Factor Beta 2 in HCV Genotype 4-Induced Liver Fibrosis. J. Interf. Cytokine Res. 2018, 38, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Ramm, G.A.; Nair-Shalliker, V.; Bridle, K.R.; Shepherd, R.W.; Crawford, D.H.G. Contribution of Hepatic Parenchymal and Nonparenchymal Cells to Hepatic Fibrogenesis in Biliary Atresia. Am. J. Pathol. 1998, 153, 527–535. [Google Scholar] [CrossRef]
- Padua, D.; Zhang, X.; Wang, Q.; Nadal, C.; Gerald, W.L.; Gomis, R.; Massagué, J. TGFβ Primes Breast Tumours for Lung Metastasis Seeding through Angiopoietin-like 4. Cell 2008, 133, 66–77. [Google Scholar] [CrossRef]
- Li, B.; Qian, M.; Cao, H.; Jia, Q.; Wu, Z.; Yang, X.; Ma, T.; Wei, H.; Chen, T.; Xiao, J. TGF-β2-induced ANGPTL4 expression promotes tumour progression and osteoclast differentiation in giant cell tumour of bone. Oncotarget 2017, 8, 54966–54977. [Google Scholar] [CrossRef][Green Version]
- Valiakou, V.D.A.; Foka, P. Effect of TGF-β on ANGPTL-3 mRNA expression. 2021. [Google Scholar]
- Kato, T.; Date, T.; Murayama, A.; Morikawa, K.; Akazawa, D.; Wakita, T. Cell culture and infection system for hepatitis C virus. Nat. Protoc. 2006, 1, 2334–2339. [Google Scholar] [CrossRef]
- Ng, T.I.; Krishnan, P.; Pilot-Matias, T.; Kati, W.; Schnell, G.; Beyer, J.; Reisch, T.; Lu, L.; Dekhtyar, T.; Irvin, M.; et al. In Vitro Antiviral Activity and Resistance Profile of the Next-Generation Hepatitis C Virus NS5A Inhibitor Pibrentasvir. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Inoue, T.; Kohro, T.; Tanaka, T.; Kanki, Y.; Li, G.; Poh, H.-M.; Mimura, I.; Kobayashi, M.; Taguchi, A.; Maejima, T.; et al. Cross-enhancement of ANGPTL4 transcription by HIF1 alpha and PPAR beta/delta is the result of the conformational proximity of two response elements. Genome Biol. 2014, 15, R63. [Google Scholar] [CrossRef]
- Tsitoura, E.; Thomas, J.; Cuchet, D.; Thoinet, K.; Mavromara, P.; Epstein, A.L. Infection with herpes simplex type 1-based amplicon vectors results in an IRF3/7-dependent, TLR-independent activation of the innate antiviral response in primary human fibroblasts. J. Gen. Virol. 2009, 90, 2209–2220. [Google Scholar] [CrossRef] [PubMed]



| Clinical Parameter | Mean ± SD | p-Value |
|---|---|---|
| Age (years) | 49 ± 13 | n/a |
| ALT (IU/mL) | 95 ± 78 | n/a |
| Clinical Characteristic | n = 141 (%) | |
| Male/Female | 69 (48.2%)/72 (51.1%) | 0.801 |
| DAA treatment BT–AT § matched pairs | 92 (65.2%) | n/a |
| VRL (BT)—low (<1 × 106 IU/mL) | 56 (39.7%) | 0.157 |
| VRL (BT)—high (≥1 × 106 IU/mL) | 72 (51.1%) | |
| VRL (BT)—Not determined | 13 (9.2%) | |
| Genotype—HCV 1a | 25 (17.7%) | 0.057 |
| Genotype—HCV 1b | 33 (23.4%) | |
| Genotype—HCV 2a | 15 (10.6%) | |
| Genotype—HCV 3 | 35 (24.8%) | |
| Genotype—HCV 4 | 27 (19.2%) | |
| Genotype—Not determined | 6 (4.3%) | |
| Disease Stage (Fibrosis Score *) | ||
| Acute Infection (F0–F1) | 6 (4.2%) | 0.000 |
| Mild Fibrosis (F0/F1/F2) | 63 (44.7%) (12/24/27) | |
| Advanced Fibrosis (F3) | 17 (12.1%) | |
| Cirrhosis (F4) | 42 (29.8%) | |
| HCC (F4) | 13 (9.2%) |
| Clinical Parameter | Acute | Mild Fibrosis | Advanced Fibrosis | Cirrhosis | HCC | p-Value |
|---|---|---|---|---|---|---|
| Mean ± SD | ||||||
| Age | 34.7 ± 8.5 | 49.7 ± 11.0 | 51 ± 9.5 | 56.1 ± 15.5 | 65.2 ± 8.9 | 0 |
| ALT (IU/mL) | 725.4 ± 300.1 | 57.1 ± 43.2 | 73.1 ± 69.0 | 90.7 ± 85.1 | 63.8 ± 47.8 | 0 |
| Clinical | n * (%) | |||||
| Characteristic | ||||||
| Gender (Male) | 6 (100%) | 23 (36.5%) | 9 (52.9%) | 21 (50%) | 10 (76.9%) | 0.006 |
| VRL-low (<1 × 106 IU/mL) | 2 (33.3%) | 28 (44.4%) | 6 (35.3%) | 18 (42.8%) | 2 (15.4%) | - |
| VRL-high (≥1 × 106 IU/mL) | 2 (33.3%) | 33 (52.4%) | 11 64.7%) | 23 (54.8%) | 3 (23.1%) | 0.951 |
| Not determined | 2 (33.4%) | 2 (3.2%) | n/a | 1 (2.4%) | 8 (61.5%) | |
| Genotype | ||||||
| HCV1a | - | 12 (19.1%) | 4 (23.5%) | 9 (21.4%) | - | 0.347 |
| HCV1b | 2 (33.3%) | 13 (20.6%) | 4 (23.5%) | 13 (31.0%) | 1 (7.6%) | |
| HCV2a | - | 11 (17.5%) | 1 (5.9%) | 3 (7.1%) | - | |
| HCV3 | 4 (66.7%) | 14 (22.2%) | 4 (23.5%) | 10 (23.8%) | 3 (23.1%) | |
| HCV4 | - | 13 (20.6%) | 4 (23.5%) | 7 (16.7%) | 3 (23.1%) | |
| Not determined | - | - | - | - | 6 (46.2%) | |
| Disease Stage | Acute Infection | Mild Fibrosis | Advanced Fibrosis | Cirrhosis | HCC | p-Value * |
|---|---|---|---|---|---|---|
| Median (IQR) in ng/mL | ||||||
| ANGPTL-3 BT 1 | 563.6 (185.1–682.8) | 493.3 (361.5–82.8) | 332.1 (228.3–394.4) | 455.3 (347.1–575.9) | 375.8 (289.3–450.3) | 0.002 |
| ANGPTL-4 BT 1 | 60.1 (24.9–89.0) | 60.1 (40.6–74.1) | 102.8 (65.9–129.5) | 78.5 (53.1–113.1) | 86.0 (49.7–133.2) | 0.002 |
| Disease Stage | ANGPTL-3 | ANGPTL-4 | ||||
|---|---|---|---|---|---|---|
| Median (IQR) in ng/mL | ||||||
| BT 1 | AT 2 | p-Value * | BT 1 | AT 2 | p-Value * | |
| All | 409.7 (346.4–548.6) | 377.1 (300.6–483.7) | 0.000 | 68.0 (53.6–102.5) | 58.3 (46.4–81.6) | 0.000 |
| Mild Fibrosis | 464.9 (346.4–548.6) | 396.3 (300.3–483.7) | 0.003 | 63.4 (47.8–75.4) | 54.8 (40.2–68.2) | 0.031 |
| Advanced Fibrosis | 334.3 (225.0–397.9) | 340.6 (249.5–365.7) | 0.756 | 92.9 (64.9–134.6) | 75.5 (51.0–102.0) | 0.003 |
| Cirrhosis | 418.0 (322.2–549.5) | 421.1 (322.0–509.0) | 0.121 | 94.6 (56.7–139.8) | 60.7 (48.6–81.6) | 0.014 |
| Parameter | ANGPTL-3 | ANGPTL-4 | ||||||
|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | |||||
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| VRL H–L * | −0.037 2 | 0.737 | 0.061 2 | 0.578 | 0.124 2 | 0.254 | 0.024 2 | 0.829 |
| Genotype | 0.129 3 | 0.844 | 0.070 3 | 0.982 | 0.248 3 | 0.249 | 0.269 3 | 0.172 |
| Gender | 0.173 2 | 0.108 | 0.2242 | 0.036 | −0.130 2 | 0.227 | −0.2102 | 0.050 |
| Age | 0.3751 | 0.000 | 0.4271 | 0.000 | 0.091 1 | 0.407 | −0.031 1 | 0.771 |
| Fibrosis | 0.3203 | 0.010 | 0.192 3 | 0.320 | 0.4143 | 0.000 | 0.2683 | 0.042 |
| Parameters | ANGPTL-3 | ANGPTL-4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT (R2 = 0.243) | AT (R2 = 0.441) | BT (R2 = 0.113) | AT (R2 = 0.606) | |||||||||
| B | p-Value | 95% C.I. * | B | p-Value | 95% C.I. * | B | p-Value | 95% C.I. * | B | p-Value | 95% C.I. * | |
| Age | 6.5 | 0.000 | 3.6, 9.4 | 1.8 | 0.196 | −0.9, 4.5 | 0.2 | 0.667 | −0.6, 0.9 | 0.6 | 0.022 | 0.1, 1.1 |
| Females (Ref. Males) | −4.2 | 0.904 | −72.9, 64.6 | 39.9 | 0.173 | −17.8, 97.5 | −7.1 | 0.438 | −25.4, 11.1 | −16.2 | 0.011 | −28.5, −3.8 |
| AF (Ref. MF) | −128.4 | 0.005 | −216.7, −40.2 | −4.6 | 0.908 | −82.4, 73.3 | 31.5 | 0.009 | 8.2, 54.9 | −6.8 | 0.413 | −23.3, 9.7 |
| Cirrhosis (Ref. MF) | −62.9 | 0.129 | −144.3, 18.6 | −24.3 | 0.487 | −93.6, 45.0 | 31.7 | 0.004 | 10.3, 53.0 | −8.9 | 0.244 | −24.2, 6.2 |
| All other genotypes (Ref. HCV-3) | −31.2 | 0.424 | −108.6, 46.1 | −15.8 | 0.630 | −81.0, 49.3 | 7.4 | 0.507 | −14.6, 29.4 | −3.2 | 0.674 | −18.1, 11.8 |
| High VRL (Ref. Low VRL) | −22.8 | 0.506 | −90.6, 45.1 | 72.6 | 0.013 | 15.6, 129.7 | 0.6 | 0.948 | −17.4, 18.6 | −15.0 | 0.016 | −27.1, −2.8 |
| BT levels | - | - | - | 0.6 | 0.000 | 0.4, 0.8 | - | - | - | 0.8 | 0.000 | 0.6, 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiakou, V.; Eliadis, P.; Karamichali, E.; Tsitsilonis, O.; Koskinas, J.; Georgopoulou, U.; Foka, P. Differential Expression of the Host Lipid Regulators ANGPTL-3 and ANGPTL-4 in HCV Infection and Treatment. Int. J. Mol. Sci. 2021, 22, 7961. https://doi.org/10.3390/ijms22157961
Valiakou V, Eliadis P, Karamichali E, Tsitsilonis O, Koskinas J, Georgopoulou U, Foka P. Differential Expression of the Host Lipid Regulators ANGPTL-3 and ANGPTL-4 in HCV Infection and Treatment. International Journal of Molecular Sciences. 2021; 22(15):7961. https://doi.org/10.3390/ijms22157961
Chicago/Turabian StyleValiakou, Vaia, Petros Eliadis, Eirini Karamichali, Ourania Tsitsilonis, John Koskinas, Urania Georgopoulou, and Pelagia Foka. 2021. "Differential Expression of the Host Lipid Regulators ANGPTL-3 and ANGPTL-4 in HCV Infection and Treatment" International Journal of Molecular Sciences 22, no. 15: 7961. https://doi.org/10.3390/ijms22157961
APA StyleValiakou, V., Eliadis, P., Karamichali, E., Tsitsilonis, O., Koskinas, J., Georgopoulou, U., & Foka, P. (2021). Differential Expression of the Host Lipid Regulators ANGPTL-3 and ANGPTL-4 in HCV Infection and Treatment. International Journal of Molecular Sciences, 22(15), 7961. https://doi.org/10.3390/ijms22157961








