Development of [18F]LU14 for PET Imaging of Cannabinoid Receptor Type 2 in the Brain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Organic Chemistry
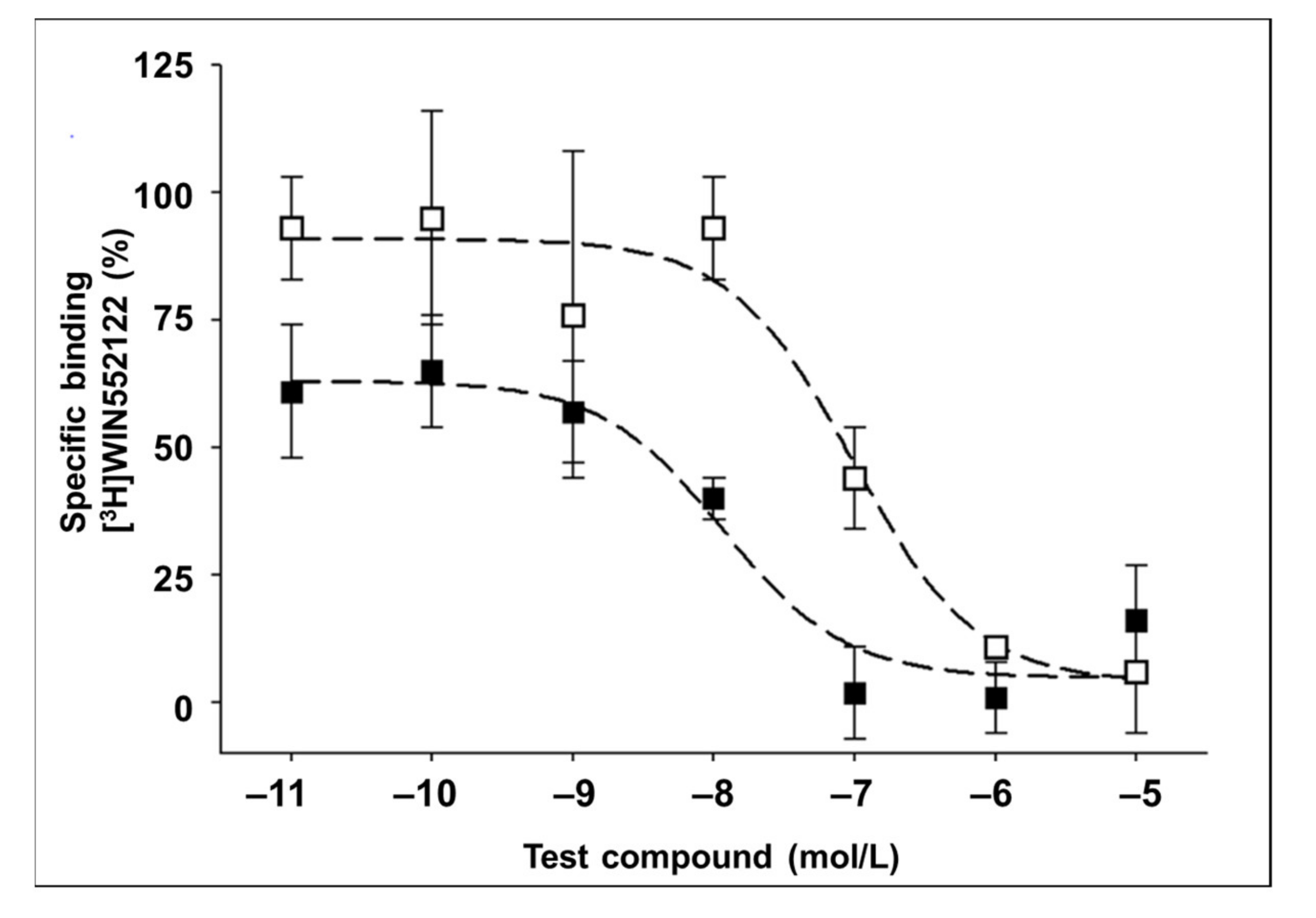
2.2. In Vitro Binding Assay
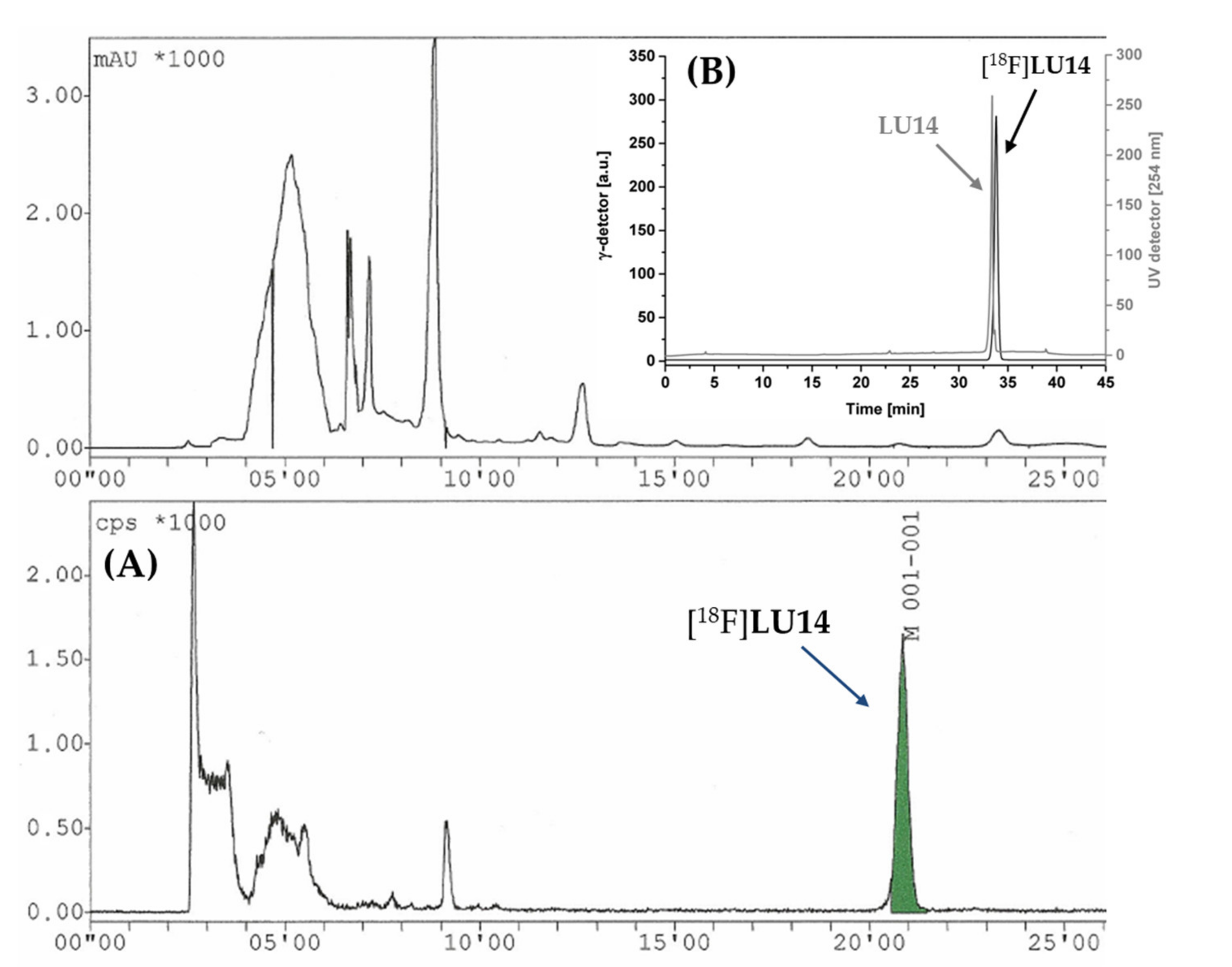
2.3. Radiochemistry
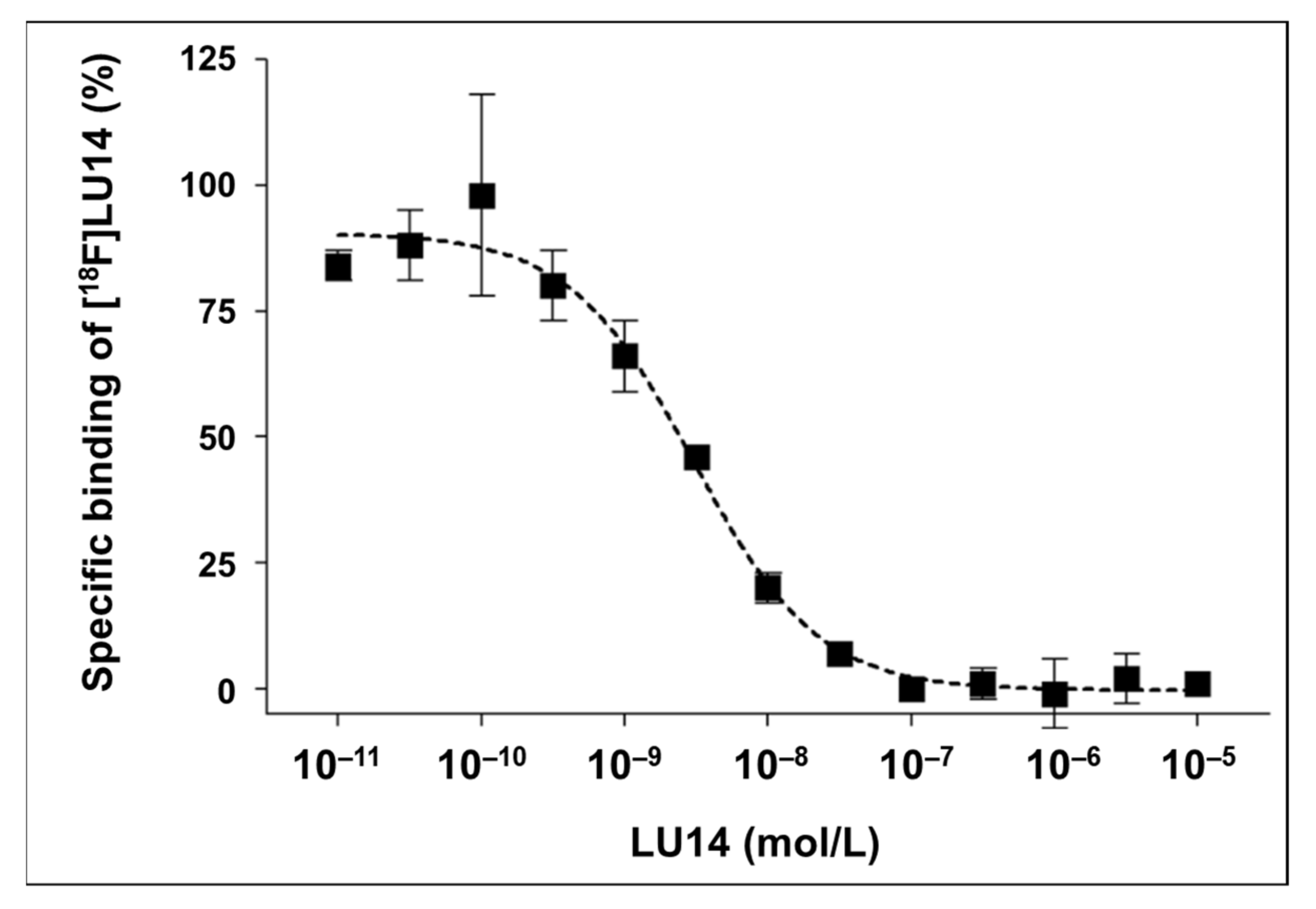
2.4. In Vitro Evaluation of [18F]LU14
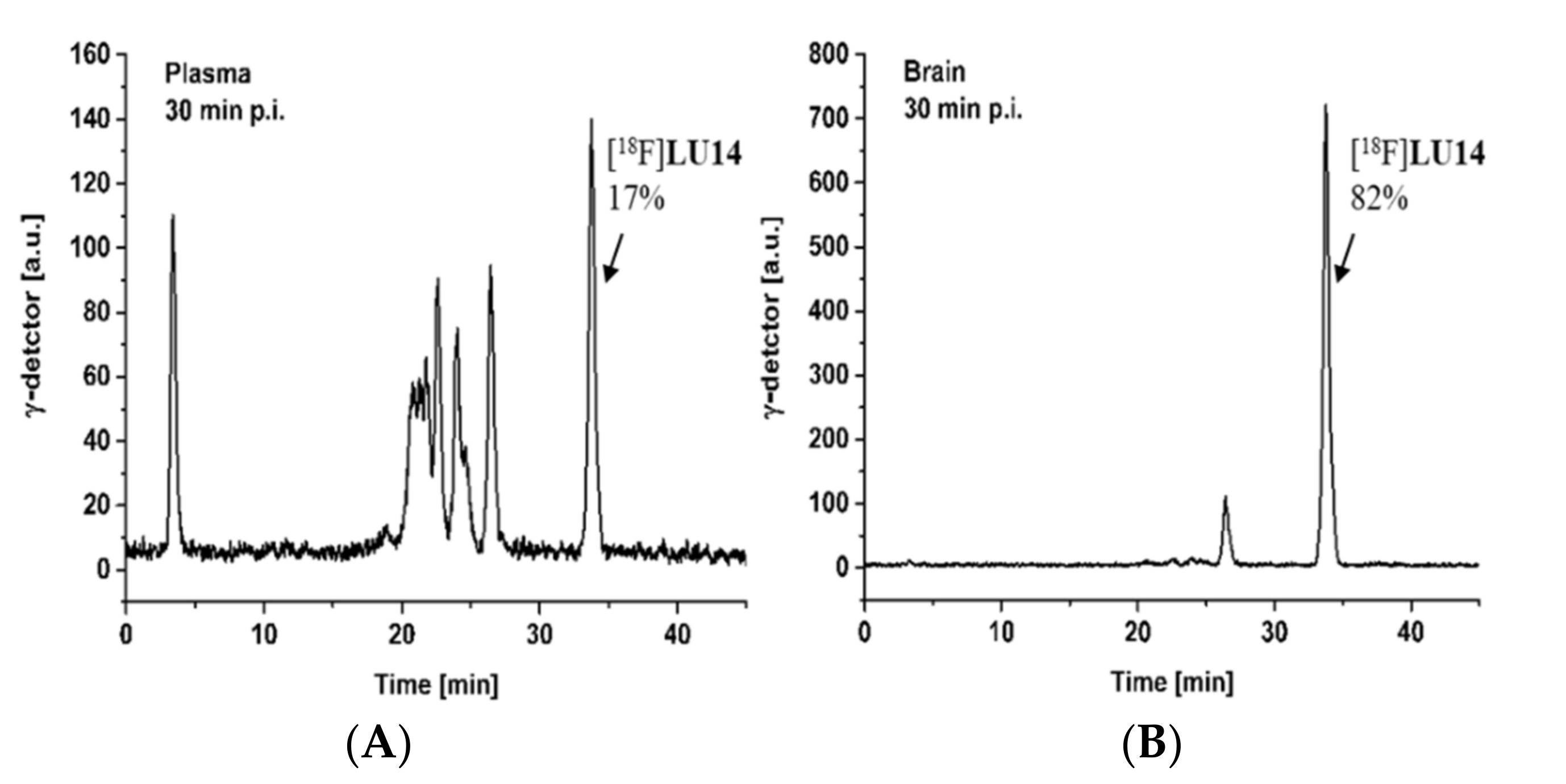
2.5. In Vivo Metabolism
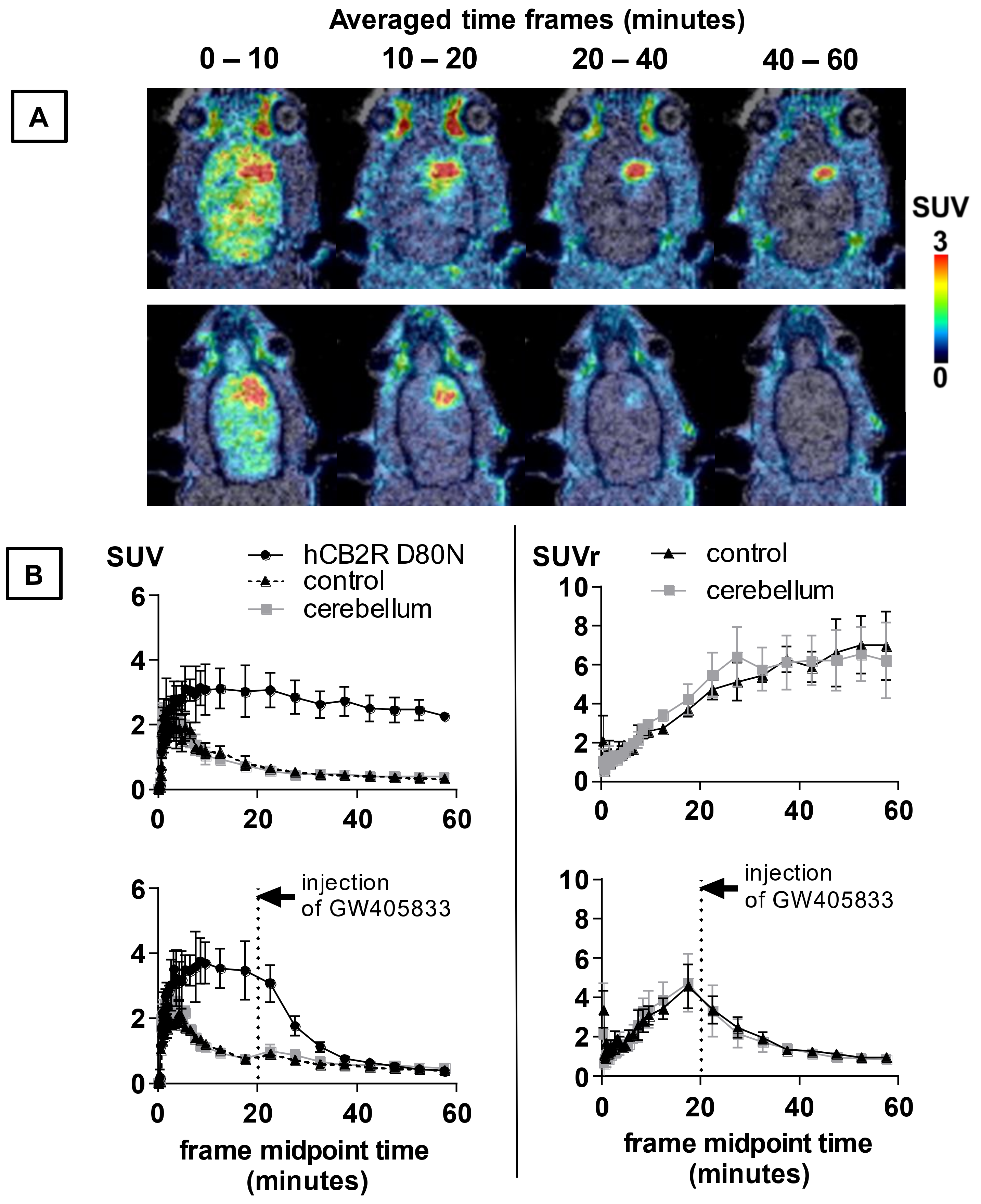
2.6. PET Imaging
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. Chemical Synthesis
3.2. Radiochemistry
3.2.1. Automated Radiosynthesis of [18F]LU14
3.2.2. Quality Control
3.2.3. Determination of Lipophilicity (LogD7.4)
3.3. Biological Experiments
3.3.1. Determination of Binding Affinities by Homogenate Assays
3.3.2. In Vitro Autoradiography
3.3.3. Quantification of Radiometabolites
3.3.4. PET Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm J. 2020, 25, 1–3. [Google Scholar]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Stella, N.; Schweitzer, P.; Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 1997, 388, 773–778. [Google Scholar] [CrossRef] [Green Version]
- Hua, T.; Li, X.; Wu, L.; Iliopoulos-Tsoutsouvas, C.; Wang, Y.; Wu, M.; Shen, L.; Brust, C.A.; Nikas, S.P.; Song, F.; et al. Activation and signaling mechanism revealed by cannabinoid receptor-G(i) complex structures. Cell 2020, 180, 655–665.e18. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The endocannabinoid system as a target in cancer diseases: Are we there yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.J. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Childers, S.R.; Deadwyler, S.A. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem. Pharmacol. 1996, 52, 819–827. [Google Scholar] [CrossRef]
- Mensching, L.; Rading, S.; Nikolaev, V.; Karsak, M. Monitoring cannabinoid CB2-receptor mediated cAMP dynamics by FRET-based live cell imaging. Int. J. Mol. Sci. 2020, 21, 7880. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology 2006, 54, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci. Lett. 2006, 396, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.S.; Hu, S.; Min, X.; Cabral, G.A.; Lokensgard, J.R.; Peterson, P.K. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia 2005, 49, 211–219. [Google Scholar] [CrossRef]
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef]
- Benito, C.; Tolón, R.M.; Pazos, M.R. Cannabinoid CB2 receptors in human brain inflammation. Br. J. Pharmacol. 2008, 153, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef] [Green Version]
- Casteels, C.; Ahmad, R.; Vandenbulcke, M.; Vandenberghe, W.; Van Laere, K. Chapter 4—Cannabinoids and Huntington’s disease. In Cannabinoids in Neurologic and Mental Disease; Academic Press: San Diego, CA, USA, 2015; pp. 61–97. [Google Scholar]
- Fernandez-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and Neurodegenerative Disorders: Parkinson’s Disease, Huntington’s Chorea, Alzheimer’s Disease, and Others. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 231, pp. 233–259. [Google Scholar]
- Bisogno, T.; Oddi, S.; Piccoli, A.; Fazio, D.; Maccarrone, M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016, 111, 721–730. [Google Scholar] [CrossRef]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 2009, 132, 3152–3164. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, G.; Lupi, M.; Sarchione, F.; Matarazzo, I.; Santacroce, R.; Petruccelli, F.; Martinotti, G.; Di Giannantonio, M. The endocannabinoid system: A putative role in neurodegenerative diseases. Int. J. High Risk Behav. Addict. 2013, 2, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Yiangou, Y.; Facer, P.; Banati, R.B.; O’Shaughnessy, C.T.; Chessell, I.P.; Anand, P. Cannabinoid receptor CB2 expression in activated microglia of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1228. [Google Scholar]
- Roche, M.; Finn, D.P. Brain CB2 receptors: Implications for neuropsychiatric disorders. Pharmaceuticals 2010, 3, 2517–2553. [Google Scholar] [CrossRef] [Green Version]
- Kucerova, J.; Tabiova, K.; Drago, F.; Micale, V. Therapeutic potential of cannabinoids in schizophrenia. Recent Pat. CNS Drug Discov. 2014, 9, 13–25. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Ciechomska, I.; Kaminska, B. Cannabinoid signaling in glioma cells. Adv. Exp. Med. Biol. 2013, 986, 209–220. [Google Scholar] [PubMed]
- Jia, N.; Zhang, S.; Shao, P.; Bagia, C.; Janjic, J.M.; Ding, Y.; Bai, M. Cannabinoid CB2 receptor as a new phototherapy target for the inhibition of tumor growth. Mol. Pharm. 2014, 11, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Nikan, M.; Nabavi, S.M.; Manayi, A. Ligands for cannabinoid receptors, promising anticancer agents. Life Sci. 2016, 146, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Afaq, F.; Mukhtar, H. Cannabinoids for cancer treatment: Progress and promise. Cancer Res. 2008, 68, 339–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meccariello, R. Endocannabinoid system in health and disease: Current situation and future perspectives. Int. J. Mol. Sci. 2020, 21, 3549. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Shen, H.; Gao, M.; Ma, Z.; Hempel, B.J.; Bi, G.H.; Gardner, E.L.; Wu, J.; Xi, Z.X. Cannabinoid CB(2) receptors are expressed in glutamate neurons in the red nucleus and functionally modulate motor behavior in mice. Neuropharmacology 2021, 189, 108538. [Google Scholar] [CrossRef]
- Kienzl, M.; Kargl, J.; Schicho, R. The immune endocannabinoid system of the tumor microenvironment. Int. J. Mol. Sci. 2020, 21, 8929. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in glioblastoma therapy: New applications for old drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Capozzi, A.; Mattei, V.; Martellucci, S.; Manganelli, V.; Saccomanni, G.; Garofalo, T.; Sorice, M.; Manera, C.; Misasi, R. Anti-proliferative properties and proapoptotic function of new CB2 selective cannabinoid receptor agonist in jurkat leukemia cells. Int. J. Mol. Sci. 2018, 19, 1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malan, T.P., Jr.; Ibrahim, M.M.; Lai, J.; Vanderah, T.W.; Makriyannis, A.; Porreca, F. CB2 cannabinoid receptor agonists: Pain relief without psychoactive effects? Cur. Opin. Pharmacol. 2003, 3, 62–67. [Google Scholar] [CrossRef]
- Murineddu, G.; Deligia, F.; Dore, A.; Pinna, G.; Asproni, B.; Pinna, G.A. Different classes of CB2 ligands potentially useful in the treatment of pain. Recent Pat. CNS Drug Discov. 2013, 8, 42–69. [Google Scholar] [CrossRef] [Green Version]
- Komorowska-Müller, J.A.; Schmöle, A.C. CB2 receptor in microglia: The guardian of self-control. Int. J. Mol. Sci. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tao, Y.; Feng, Z.; Yin, N.; Tan, Q.; Zheng, H.; Chen, Q.; Tang, J.; Zhang, Y.; Zhu, G.; et al. Inflammatory regulation by driving microglial M2 polarization: Neuroprotective effects of cannabinoid receptor-2 activation in intracerebral hemorrhage. Front. Immunol. 2017, 8, 112. [Google Scholar]
- Tanaka, M.; Sackett, S.; Zhang, Y. Endocannabinoid modulation of microglial phenotypes in neuropathology. Front. Neurol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Spinelli, F.; Capparelli, E.; Abate, C.; Colabufo, N.A.; Contino, M. Perspectives of cannabinoid type 2 receptor (CB2R) ligands in neurodegenerative disorders: Structure–affinity relationship (SAfiR) and structure-activity relationship (SAR) studies. J. Med. Chem. 2017, 60, 9913–9931. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal structure of the human cannabinoid receptor CB2. Cell 2019, 176, 459–467.e13. [Google Scholar] [CrossRef] [Green Version]
- Ni, R.; Mu, L.; Ametamey, S. Positron emission tomography of type 2 cannabinoid receptors for detecting inflammation in the central nervous system. Acta Pharmacol. Sin. 2019, 40, 351–357. [Google Scholar] [CrossRef]
- Spinelli, F.; Mu, L.; Ametamey, S.M. Radioligands for positron emission tomography imaging of cannabinoid type 2 receptor. J. Label. Comp. Radiopharm. 2018, 61, 299–308. [Google Scholar] [CrossRef]
- Ory, D.; Celen, S.; Verbruggen, A.; Bormans, G. PET radioligands for in vivo visualization of neuroinflammation. Curr. Pharm. Des. 2014, 20, 5897–5913. [Google Scholar] [CrossRef] [Green Version]
- Evens, N.; Bormans, G.M. Non-invasive imaging of the type 2 cannabinoid receptor, focus on positron emission tomography. Curr. Top. Med. Chem. 2010, 10, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Terry, G.E.; Raymont, V.; Horti, A.G. PET Imaging of the Endocannabinoid System. In PET and SPECT of Neurobiological Systems; Dierckx, R.A.J.O., Otte, A., de Vries, E.F.J., van Waarde, A., Lammertsma, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 319–426. [Google Scholar]
- Hou, L.; Rong, J.; Haider, A.; Ogasawara, D.; Varlow, C.; Schafroth, M.A.; Mu, L.; Gan, J.; Xu, H.; Fowler, C.J.; et al. Positron emission tomography imaging of the endocannabinoid system: Opportunities and challenges in radiotracer development. J. Med. Chem. 2021, 64, 123–149. [Google Scholar] [CrossRef]
- Brutlag, A.; Hommerding, H. Toxicology of marijuana, synthetic cannabinoids, and cannabidiol in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 1087–1102. [Google Scholar] [CrossRef]
- Thomas, B.F.; Compton, D.R.; Martin, B.R. Characterization of the lipophilicity of natural and synthetic analogs of delta 9-tetrahydrocannabinol and its relationship to pharmacological potency. J. Pharmacol. Exp. Ther. 1990, 255, 624–630. [Google Scholar] [PubMed]
- Evens, N.; Vandeputte, C.; Coolen, C. Preclinical evaluation of [11C]NE40, a type 2 cannabinoid receptor PET tracer. Nucl. Med. Biol. 2012, 39, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Postnov, A.; Ahmad, R.; Evens, N.; Versijpt, J.; Vandenbulcke, M.; Yaqub, M.; Verbruggen, A.; Bormans, G.; Vandenberghe, W.; Van Laere, K. Quantification of [11C]NE40, a novel PET radioligand for CB2 receptor imaging. J. Nucl. Med. 2013, 54, 188. [Google Scholar]
- Ahmad, R.; Koole, M.; Evens, N.; Serdons, K.; Verbruggen, A.; Bormans, G.; Van Laere, K. Whole-body biodistribution and radiation dosimetry of the cannabinoid type 2 receptor ligand [11C]NE40 in healthy subjects. Mol. Imaging Biol. 2013, 15, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horti, A.G.; Gao, Y.; Ravert, H.T.; Finley, P.; Valentine, H.; Wong, D.F.; Endres, C.J.; Savonenko, A.V.; Dannals, R.F. Synthesis and biodistribution of [11C]A-836339, a new potential radioligand for PET imaging of cannabinoid type 2 receptors (CB2). Bioorg. Med. Chem. 2010, 18, 5202–5207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldovan, R.-P.; Teodoro, R.; Gao, Y.; Deuther-Conrad, W.; Kranz, M.; Wang, Y.; Kuwabara, H.; Nakano, M.; Valentine, H.; Fischer, S.; et al. Development of a high-affinity PET radioligand for imaging cannabinoid subtype 2 receptor. J. Med. Chem. 2016, 59, 7840–7855. [Google Scholar] [CrossRef]
- Moldovan, R.-P.; Deuther-Conrad, W.; Teodoro, R.; Wang, Y.; Fischer, S.; Pomper, M.; Wong, D.; Dannals, R.; Brust, P.; Horti, A. 18F-JHU94620, a high affinity PET radioligand for imaging of cannabinoid subtype 2 receptors (CB2R). J. Nucl. Med. 2015, 56, 1048. [Google Scholar]
- Caillé, F.; Cacheux, F.; Peyronneau, M.-A.; Jego, B.; Jaumain, E.; Pottier, G.; Ullmer, C.; Grether, U.; Winkeler, A.; Dollé, F.; et al. From structure–activity relationships on thiazole derivatives to the in vivo evaluation of a new radiotracer for cannabinoid subtype 2 PET imaging. Mol. Pharm. 2017, 14, 4064–4078. [Google Scholar] [CrossRef] [PubMed]
- Caillé, F.; Cacheux, F.; Jego, B.; Jaumain, E.; Peyronneau, M.A.; Pottier, G.; Ullmer, C.; Grether, U.; Dollé, F.; Damont, A.; et al. Synthesis, radiolabeling and biodistribution of [18F]FC0324, a new PET tracer to image CB2 receptors. J. Label. Compd. Radiopharm. 2017, 60, S55. [Google Scholar]
- Mu, L.; Bieri, D.; Slavik, R.; Drandarov, K.; Muller, A.; Cermak, S.; Weber, M.; Schibli, R.; Kramer, S.D.; Ametamey, S.M. Radiolabeling and in vitro/in vivo evaluation of N-(1-adamantyl)-8-methoxy-4-oxo-1-phenyl-1,4-dihydroquinoline-3-carboxamide as a PET probe for imaging cannabinoid type 2 receptor. J. Neurochem. 2013, 126, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Müller Herde, A.; Slavik, R.; Weber, M.; Mugnaini, C.; Ligresti, A.; Schibli, R.; Mu, L.; Ametamey, S.M. Synthesis and biological evaluation of thiophene-based cannabinoid receptor type 2 radiotracers for PET imaging. Front. Neurosci. 2016, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; van Veghel, D.; Ullmer, C.; Van Laere, K.; Verbruggen, A.; Bormans, G.M. Synthesis, Biodistribution and in vitro evaluation of brain permeable high affinity type 2 cannabinoid receptor agonists [11C]MA2 and [18F]MA3. Front. Neurosci. 2016, 10, 431. [Google Scholar] [CrossRef]
- Teodoro, R.; Moldovan, R.-P.; Lueg, C.; Günther, R.; Donat, C.K.; Ludwig, F.-A.; Fischer, S.; Deuther-Conrad, W.; Wünsch, B.; Brust, P. Radiofluorination and biological evaluation of N-aryl-oxadiazolyl-propionamides as potential radioligands for PET imaging of cannabinoid CB2 receptors. Org. Med. Chem. Lett. 2013, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Slavik, R.; Grether, U.; Herde, A.M.; Gobbi, L.; Fingerle, J.; Ullmer, C.; Krämer, S.D.; Schibli, R.; Mu, L.; Ametamey, S.M. Discovery of a high affinity and selective pyridine analog as a potential positron emission tomography imaging agent for cannabinoid type 2 receptor. J. Med. Chem. 2015, 58, 4266–4277. [Google Scholar] [CrossRef]
- Haider, A.; Kretz, J.; Gobbi, L.; Ahmed, H.; Atz, K.; Bürkler, M.; Bartelmus, C.; Fingerle, J.; Guba, W.; Ullmer, C.; et al. Structure–activity relationship studies of pyridine-based ligands and identification of a fluorinated derivative for positron emission tomography imaging of cannabinoid type 2 receptors. J. Med. Chem. 2019, 62, 11165–11181. [Google Scholar] [CrossRef]
- Haider, A.; Gobbi, L.; Kretz, J.; Ullmer, C.; Brink, A.; Honer, M.; Woltering, T.J.; Muri, D.; Iding, H.; Bürkler, M.; et al. Identification and preclinical development of a 2,5,6-trisubstituted fluorinated pyridine derivative as a radioligand for the positron emission tomography imaging of cannabinoid type 2 receptors. J. Med. Chem. 2020, 63, 10287–10306. [Google Scholar] [CrossRef] [PubMed]
- Saccomanni, G.; Pascali, G.; Carlo, S.D.; Panetta, D.; De Simone, M.; Bertini, S.; Burchielli, S.; Digiacomo, M.; Macchia, M.; Manera, C.; et al. Design, synthesis and preliminary evaluation of 18F-labelled 1,8-naphthyridin- and quinolin-2-one-3-carboxamide derivatives for PET imaging of CB2 cannabinoid receptor. Bioorg. Med. Chem. Lett. 2015, 25, 2532–2535. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, V.; Hurst, D.P.; Shore, D.M.; Bertini, S.; Ehrmann, B.M.; Allarà, M.; Lawrence, L.; Ligresti, A.; Minutolo, F.; Saccomanni, G.; et al. CB2-selective cannabinoid receptor ligands: Synthesis, pharmacological evaluation, and molecular modeling investigation of 1,8-naphthyridin-2(1H)-one-3-carboxamides. J. Med. Chem. 2014, 57, 8777–8791. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, C.; Evens, N.; Toelen, J.; Deroose, C.M.; Bosier, B.; Ibrahimi, A.; Van der Perren, A.; Gijsbers, R.; Janssen, P.; Lambert, D.M.; et al. A PET brain reporter gene system based on type 2 cannabinoid receptors. J. Nucl. Med. 2011, 52, 1102–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attili, B.; Celen, S.; Ahamed, M.; Koole, M.; Haute, C.V.D.; Vanduffel, W.; Bormans, G. Preclinical evaluation of [18F]MA3: A CB2 receptor agonist radiotracer for PET. Br. J. Pharmacol. 2019, 176, 1481–1491. [Google Scholar] [CrossRef]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef]
- Lynn, A.B.; Herkenham, M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: Implications for receptor-mediated immune modulation by cannabinoids. J. Pharmacol. Exp. Ther. 1994, 268, 1612–1623. [Google Scholar] [PubMed]
- Govaerts, S.J.; Hermans, E.; Lambert, D.M. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur. J. Pharm. Sci. 2004, 23, 233–243. [Google Scholar] [CrossRef]
- Turkman, N.; Shavrin, A.; Paolillo, V.; Yeh, H.H.; Flores, L.; Soghomonian, S.; Rabinovich, B.; Volgin, A.; Gelovani, J.; Alauddin, M. Synthesis and preliminary evaluation of [18F]-labelled 2-oxoquinoline derivatives for PET imaging of cannabinoid CB2 receptor. Nucl. Med. Biol. 2012, 39, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Yrjölä, S.; Sarparanta, M.; Airaksinen, A.J.; Hytti, M.; Kauppinen, A.; Pasonen-Seppanen, S.; Adinolfi, B.; Nieri, P.; Manera, C.; Keinanen, O.; et al. Synthesis, in vitro and in vivo evaluation of 1,3,5-triazines as cannabinoid CB2 receptor agonists. Eur. J. Pharm. Sci. 2015, 67, 85–96. [Google Scholar] [CrossRef]
- Evens, N.; Vandeputte, C.; Muccioli, G.G.; Lambert, D.M.; Baekelandt, V.; Verbruggen, A.M.; Debyser, Z.; Van Laere, K.; Bormans, G.M. Synthesis, in vitro and in vivo evaluation of fluorine-18 labelled FE-GW405833 as a PET tracer for type 2 cannabinoid receptor imaging. Bioorg. Med. Chem. 2011, 19, 4499–4505. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.P.; Wenzel, B.; Teodoro, R.; Neumann, W.; Dukic-Stefanovic, S.; Kraus, W.; Rong, P.; Deuther-Conrad, W.; Hey-Hawkins, E.; Krugel, U.; et al. Studies towards the development of a PET radiotracer for imaging of the P2Y1 receptors in the brain: Synthesis, [18F]-labeling and preliminary biological evaluation. Eur. J. Med. Chem. 2019, 165, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Rühl, T.; Deuther-Conrad, W.; Fischer, S.; Günther, R.; Hennig, L.; Krautscheid, H.; Brust, P. Cannabinoid receptor type 2 (CB2)-selective N-aryl-oxadiazolyl-propionamides: Synthesis, radiolabelling, molecular modelling and biological evaluation. Org. Med. Chem. Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| TAC Parameter | hCB2R(D80N) | Contralateral | p-Value | Cerebellum | p-Value |
|---|---|---|---|---|---|
| time to peak (min) | 7.4 ± 2.8 | 3.8 ± 1.6 | 0.063 | 1.6 ± 1.4 | 0.016 |
| TAC peak value (SUV) | 3.3 ± 0.6 | 2.2 ± 0.4 | 0.024 | 2.7 ± 0.0 | 0.067 |
| AUC (SUV × min) | 155 ± 28 | 42.6 ± 6.8 | 0.001 | 41.1 ± 5.6 | 0.001 |
| AUMC (SUV × min2) | 4349 ± 701 | 854 ± 165 | 0.001 | 819 ± 132 | 0.001 |
| MRT (min) | 28.0 ± 0.4 | 20.0 ± 1.1 | <0.001 | 19.9 ± 1.2 | <0.001 |
| hCB2R D80N-to- | Treatment (5 mg/kg GW405833, i.v.) | AUC0–20min (CI95%) in SUVr × min | AUC40–60min (CI95%) in SUVr × min |
|---|---|---|---|
| contralateral | vehicle (10 min pior tracer) | 40 (37 to 42) | 210 (190 to 231) |
| displacement (20 min after tracer) | 48 (41 to 54) | 56 (50 to 62) −73.4% | |
| cerebellum | vehicle (10 min prior tracer) | 44 (39 to 48) | 215 (190 to 241) |
| displacement (20 min after tracer) | 49 (40 to 59) | 51 (42 to 60) −76.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodoro, R.; Gündel, D.; Deuther-Conrad, W.; Ueberham, L.; Toussaint, M.; Bormans, G.; Brust, P.; Moldovan, R.-P. Development of [18F]LU14 for PET Imaging of Cannabinoid Receptor Type 2 in the Brain. Int. J. Mol. Sci. 2021, 22, 8051. https://doi.org/10.3390/ijms22158051
Teodoro R, Gündel D, Deuther-Conrad W, Ueberham L, Toussaint M, Bormans G, Brust P, Moldovan R-P. Development of [18F]LU14 for PET Imaging of Cannabinoid Receptor Type 2 in the Brain. International Journal of Molecular Sciences. 2021; 22(15):8051. https://doi.org/10.3390/ijms22158051
Chicago/Turabian StyleTeodoro, Rodrigo, Daniel Gündel, Winnie Deuther-Conrad, Lea Ueberham, Magali Toussaint, Guy Bormans, Peter Brust, and Rareş-Petru Moldovan. 2021. "Development of [18F]LU14 for PET Imaging of Cannabinoid Receptor Type 2 in the Brain" International Journal of Molecular Sciences 22, no. 15: 8051. https://doi.org/10.3390/ijms22158051
APA StyleTeodoro, R., Gündel, D., Deuther-Conrad, W., Ueberham, L., Toussaint, M., Bormans, G., Brust, P., & Moldovan, R.-P. (2021). Development of [18F]LU14 for PET Imaging of Cannabinoid Receptor Type 2 in the Brain. International Journal of Molecular Sciences, 22(15), 8051. https://doi.org/10.3390/ijms22158051










