High-Fat Diet Impairs Mouse Median Eminence: A Study by Transmission and Scanning Electron Microscopy Coupled with Raman Spectroscopy
Abstract
:1. Introduction
2. Results
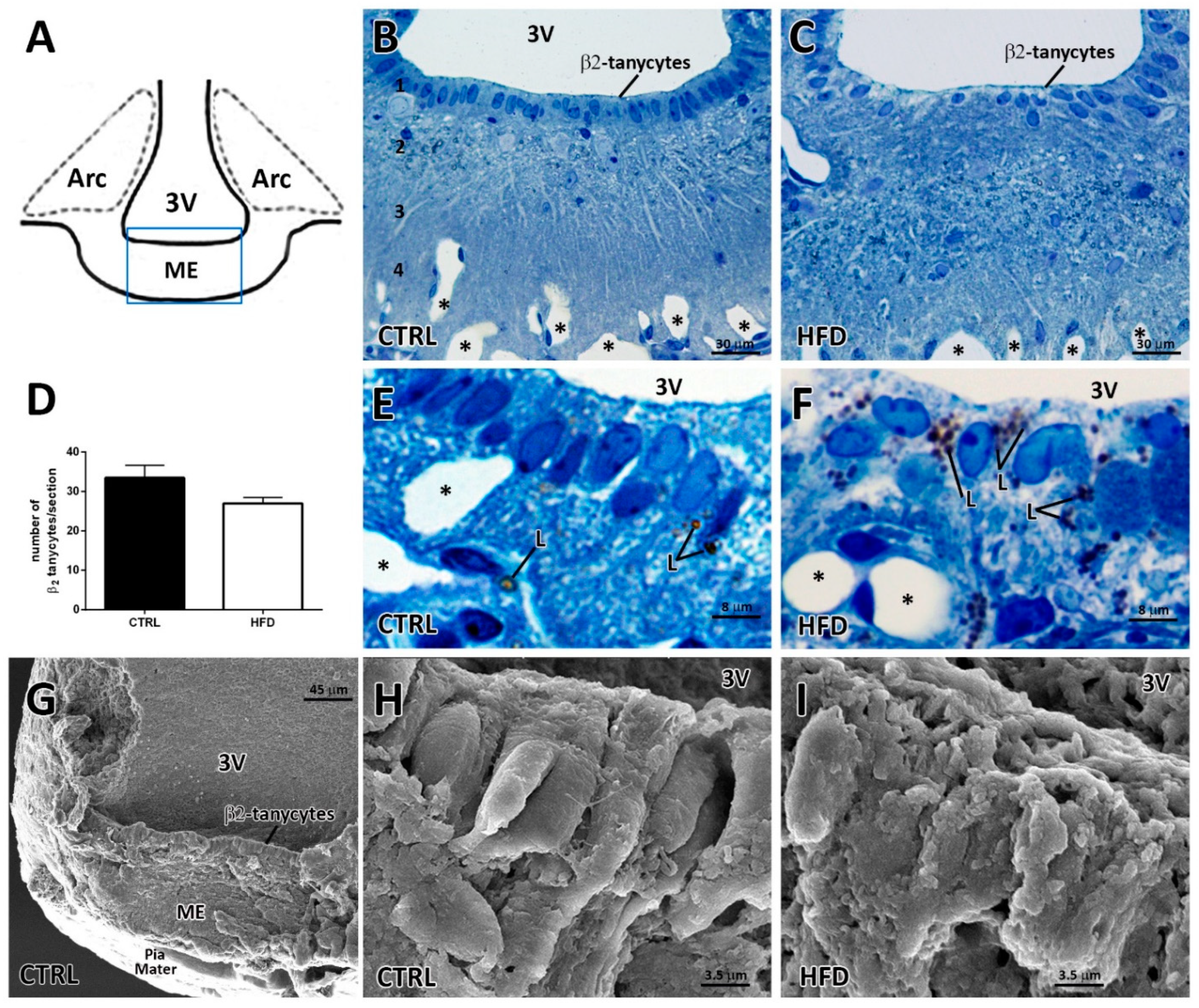
2.1. High-Fat Diet Induces Prominent Alterations in the Median Eminence β2-tanycyte Layer
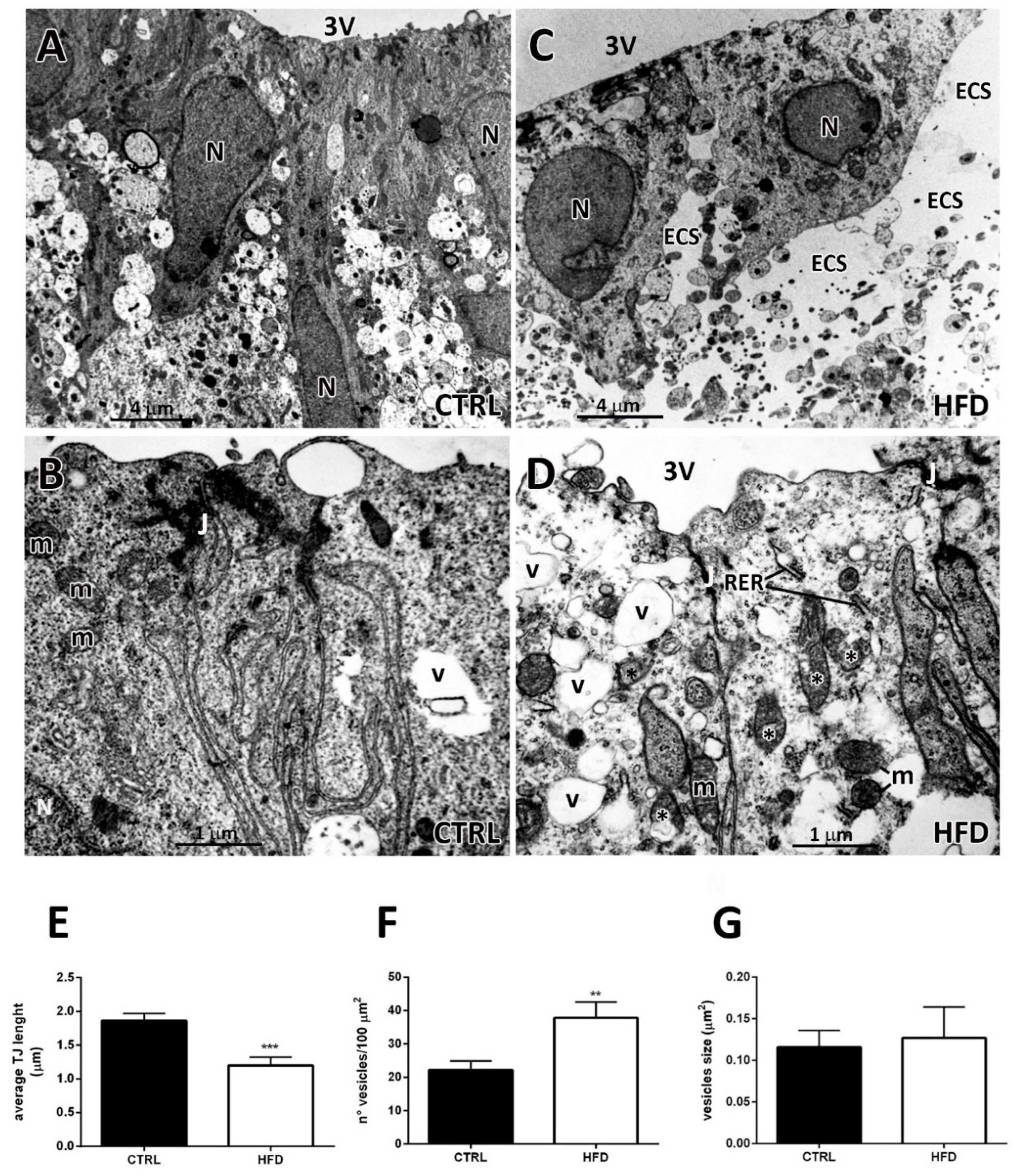
2.2. Transmission Electron Microscopy Discloses Numerous Degenerating Aspects in β2-tanycytes from HFD Mice
2.3. Effects of HFD on Other ME Cell Types
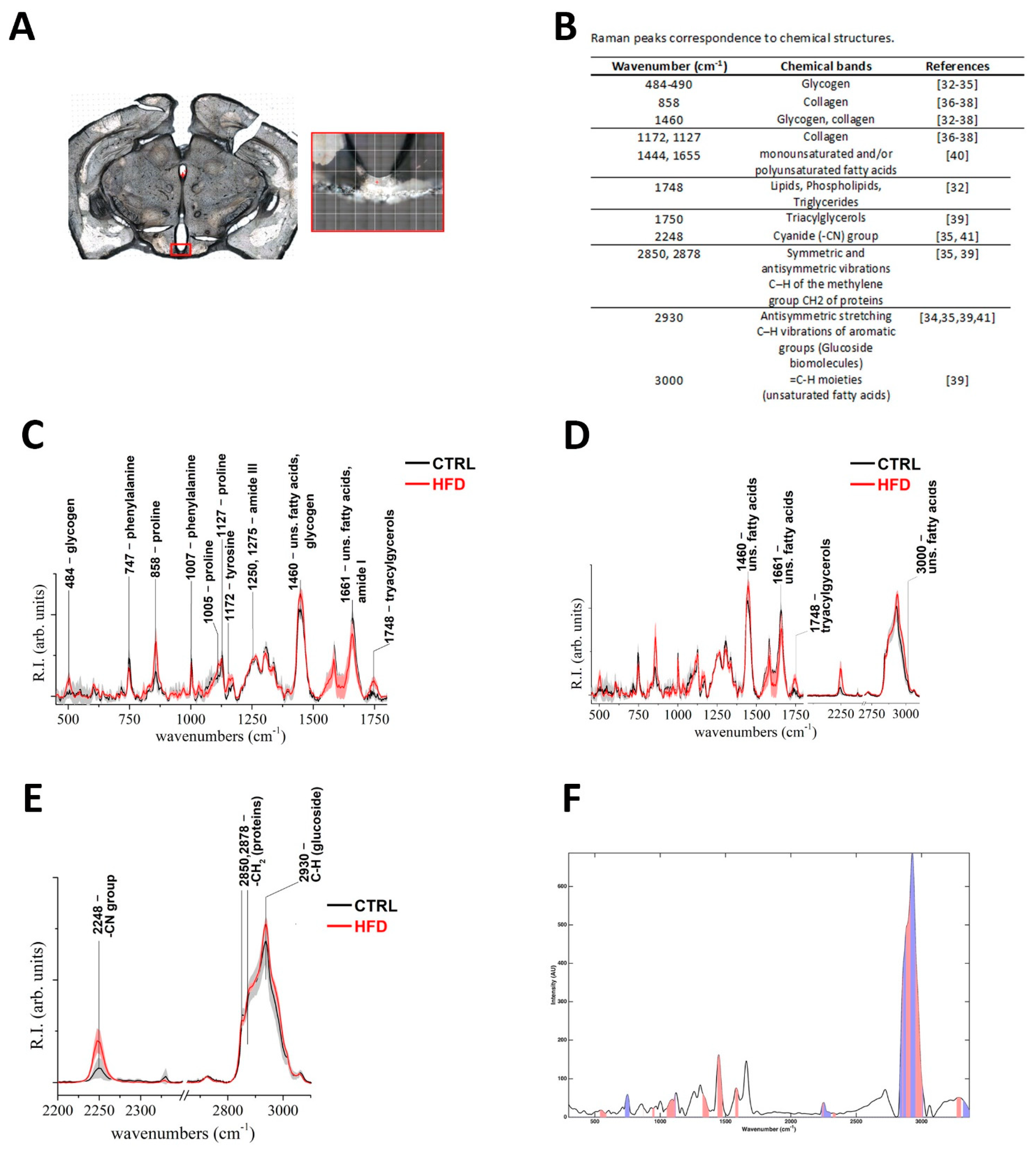
2.4. HFD Induces Distinctive Biochemical Alterations in the Hypothalamic Arc-ME
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals, Experimental Conditions, and Tissue Processing
5.2. Transmission Electron Microscopy
5.3. Scanning Electron Microscopy
5.4. Sirius Red and PAS Staining
5.5. Morphometric Analysis
5.6. Raman Spectroscopy Measurements
5.7. Statistical Analysis of Raman Spectroscopy Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFD | high-fat diet; |
| CTRL | normo-fed control mice; |
| ME | median eminence; |
| Arc | arcuate nucleus; |
| TEM | transmission electron microscopy; |
| SEM | scanning electron microscopy. |
References
- WHO. Obesity Report. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 January 2020).
- RisC. Adult Body-Mass Index. Available online: www.ncdrisc.org/data-visualisations-adiposity.html (accessed on 1 March 2021).
- CDC. Defining Adult Overweight and Obesity. Center for Disease Control and Prevention. 2017. Available online: https://www.cdc.gov/obesity/adult/defining.html (accessed on 26 January 2020).
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease patho-genesis and treatment. J. Lipid. Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, R.; Onodera, T.; Scherer, P.E. Lipotoxicity and beta Cell Maintenance in Obesity and Type 2 Diabetes. J. Endocr. Soc. 2019, 3, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Frontini, A.; Cinti, S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 2016, 15, 405–424. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Giordano, A.; Nisoli, E. Neuroendocrinology of Energy Balance. Obesity 2018, 31–50. [Google Scholar]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.; Giordano, A. Leptin, the brain and energy homeostasis: From an apparently simple to a highly complex neuronal system. Rev. Endocr. Metab. Disord. 2021, 1–15. [Google Scholar] [CrossRef]
- Flament-Durand, J.; Brion, J. Tanycytes: Morphology and Functions: A Review. Adv. Clin. Chem. 1985, 96, 121–155. [Google Scholar] [CrossRef]
- Rodríguez, E.; Guerra, M.; Peruzzo, B.; Blázquez, J.L. Tanycytes: A rich morphological history to underpin future molecular and physiological investigations. J. Neuroendocr. 2019, 31, e12690. [Google Scholar] [CrossRef]
- García-Cáceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.-X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, A.F.; Bombassaro, B.; Dragano, N.; Solon, C.; Morari, J.; Fioravante, M.; Barbizan, R.; Velloso, L.A.; Araujo, E.P. Dietary fats promote functional and structural changes in the median eminence blood/spinal fluid interface—The protective role for BDNF. J. Neuroinflamm. 2018, 15, 1–18. [Google Scholar] [CrossRef] [Green Version]
- de Souza, G.F.P.; Solon, C.; Nascimento, L.F.; De-Lima-Junior, J.C.; Nogueira, G.; Moura, R.; Rocha, G.; Fioravante, M.; Bobbo, V.; Morari, J.; et al. Defective regulation of POMC precedes hypothalamic inflammation in diet-induced obesity. Sci. Rep. 2016, 6, 29290. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Ma, Y.; Liu, D. High-Fat Diet-Induced Adiposity, Adipose Inflammation, Hepatic Steatosis and Hyperinsulinemia in Outbred CD-1 Mice. PLoS ONE 2015, 10, e0119784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, A.; Fosca, M.; Tabacco, G.; Marini, F.; Graziani, V.; Santarsia, M.C.; Longo, F.; Lauria, A.; Cesareo, R.; Giovannoni, I.; et al. Raman Spectroscopy Applied to Parathyroid Tissues: A New Diagnostic Tool to Discriminate Normal Tissue from Adenoma. Anal. Chem. 2018, 90, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Marini, F.; Fosca, M.; Cippitelli, C.; Rocchia, M.; Di Napoli, A. Raman spectroscopy discriminates malignant follicular lymphoma from benign follicular hyperplasia and from tumour metastasis. Talanta 2019, 194, 763–770. [Google Scholar] [CrossRef]
- Guo, S.; Beleites, C.; Neugebauer, U.; Abalde-Cela, S.; Afseth, N.K.; Alsamad, F.; Anand, S.; Araujo-Andrade, C.; Askrabic, S.; Avci, E.; et al. Comparability of Raman Spectroscopic Con-figurations: A Large Scale Cross-Laboratory Study. Anal. Chem. 2020, 92, 15745–15756. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Wisniewski, H. Histological and Ultrastructural Changes with Experimental Hydrocephalus in Adult Rabbits. Brain 1969, 92, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Go, K.G.; Stokroos, I.; Blaauw, E.H.; Zuidervee, F.; Molenaar, I. Changes of ventricular ependyma and choroid plexus in ex-perimental hydrocephalus, as observed by scanning electron microscopy. Acta Neuropathol. 1976, 34, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wittkowski, W. Tanycytes and pituicytes: Morphological and functional aspects of neuroglial interaction. Microsc. Res. Tech. 1998, 41, 29–42. [Google Scholar] [CrossRef]
- Peruzzo, B.; Pastor, F.E.; Blazquez, J.L.; Schobitz, K.; Pelaez, B.; Amat, P.; Rodriguez, E.M. A second look at the barriers of the medial basal hy-pothalamus. Exp. Brain. Res. 2000, 132, 10–26. [Google Scholar] [CrossRef]
- Ghadially, F.N.; More, I.A.R. Ultrastructural Pathology of the Cell and Matrix 3rd edition. Histopathology 1989, 15, 102. [Google Scholar] [CrossRef]
- Wang, K.; Klionsky, D.J. Mitochondria removal by autophagy. Autophagy 2011, 7, 297–300. [Google Scholar] [CrossRef]
- Yi, C.-X.; Gericke, M.; Krüger, M.; Alkemade, A.; Kabra, D.G.; Hanske, S.; Filosa, J.; Pfluger, P.; Bingham, N.; Woods, S.C.; et al. High calorie diet triggers hypothalamic angiopathy. Mol. Metab. 2012, 1, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbo-hydrates: A review. Spectrochim. Acta Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Gałat, A. Study of the Raman scattering and infrared absorption spectra of branched polysaccharides. Acta Biochim. Pol. 1980, 27, 135–142. [Google Scholar] [PubMed]
- De Gelder, J.D.G.; Vandenabeele, P.; Moens, L. Reference Database of Raman Spectra of Biological Molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Talari, A.M.Z.; Rehman, S.; Rehman, I. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2014, 50, 46–111. [Google Scholar] [CrossRef]
- Ramser, K.; Malinina, E.; Candefjord, S. Resonance Micro-Raman Investigations of the Rat Medial Preoptic Nucleus: Effects of a Low-Iron Diet on the Neuroglobin Content. Appl. Spectrosc. 2012, 66, 1454–1460. [Google Scholar] [CrossRef] [Green Version]
- Cárcamo, J.J.; Aliaga, A.E.; Clavijo, R.E.; Brañes, M.R.; Campos-Vallette, M.M. Raman study of the shockwave effect on collagens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.R.S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Czamara, K.M.K.; Pacia, M.C.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2014, 46, 4–20. [Google Scholar] [CrossRef]
- Samek, O.; Jonáš, A.; Pilát, Z.; Zemanek, P.; Nedbal, L.; Tříska, J.; Kotas, P.; Trtílek, M. Raman Microspectroscopy of Individual Algal Cells: Sensing Unsaturation of Storage Lipids in vivo. Sensors 2010, 10, 8635–8651. [Google Scholar] [CrossRef] [PubMed]
- Heraud, P.; Cowan, M.F.; Marzec, K.M.; Møller, B.L.; Blomstedt, C.K.; Gleadow, R. Label-free Raman hyperspectral imaging analysis localizes the cyanogenic glucoside dhurrin to the cytoplasm in sorghum cells. Sci. Rep. 2018, 8, 2691. [Google Scholar] [CrossRef]
- Hofmann, K.; Lamberz, C.; Piotrowitz, K.; Offermann, N.; But, D.; Scheller, A.; Al-Amoudi, A.; Kuerschner, L. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia 2016, 65, 231–249. [Google Scholar] [CrossRef]
- McLean, F.H.; Campbell, F.M.; Langston, R.F.; Sergi, D.; Resch, C.; Grant, C.; Morris, A.C.; Mayer, C.D.; Williams, L.M. A high-fat diet induces rapid changes in the mouse hypothalamic proteome. Nutr. Metab. 2019, 16, 1–16. [Google Scholar] [CrossRef]
- Gunasekar, P.; Borowitz, J.; Turek, J.; Van Horn, D.; Isom, G. Endogenous generation of cyanide in neuronal tissue: Involvement of a peroxidase system. J. Neurosci. Res. 2000, 61, 570–575. [Google Scholar] [CrossRef]
- Pettersen, J.C.; Cohen, S.D. The effects of cyanide on brain mitochondrial cytochrome oxidase and respiratory activities. J. Appl. Toxicol. 1993, 13, 9–14. [Google Scholar] [CrossRef]
- Long, L.; Huang, M.; Wang, N.; Wu, Y.; Wang, K.; Gong, A.; Zhang, Z.; Sessler, J.L. A Mitochondria-Specific Fluorescent Probe for Visualizing En-dogenous Hydrogen Cyanide Fluctuations in Neurons. J. Am. Chem. Soc. 2018, 140, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Randi, E.B.; Zuhra, K.; Pecze, L.; Panagaki, T.; Szabo, C. Physiological concentrations of cyanide stimulate mitochondrial Complex IV and enhance cellular bioenergetics. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Cipollone, R.; Visca, P. Is there evidence that cyanide can act as a neuromodulator? IUBMB Life 2007, 59, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, J.L.; Gunasekar, P.G.; Isom, G.E. Hydrogen cyanide generation by mu-opiate receptor activation: Possible neuro-modulatory role of endogenous cyanide. Brain Res. 1997, 768, 294–300. [Google Scholar] [CrossRef]
- Aliev, G.; Miller, J.P.; Leifer, D.W.; Obrenovich, M.E.; Shenk, J.C.; Smith, M.A.; Lamanna, J.C.; Perry, G.; Lust, D.W.; Cohen, A.R. Ultrastructural analysis of a murine model of con-genital hydrocephalus produced by overexpression of transforming growth factor-beta1 in the central nervous system. J. Submicrosc. Cytol. Pathol. 2006, 38, 85–91. [Google Scholar] [PubMed]
- Gruber, T.; Pan, C.; Contreras, R.E.; Wiedemann, T.; Morgan, D.A.; Skowronski, A.A.; Lefort, S.; Murat, C.D.B.; Le Thuc, O.; Legutko, B.; et al. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021, 33, 1155–1170.e10. [Google Scholar] [CrossRef]
- Yoo, S.; Cha, D.; Kim, S.; Jiang, L.; Cooke, P.; Adebesin, M.; Wolfe, A.; Riddle, R.; Aja, S.; Blackshaw, S. Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia 2020, 68, 1987–2000. [Google Scholar] [CrossRef]
- Ståhle, L.; Wold, S. Partial least squares analysis with cross-validation for the two-class problem: A Monte Carlo study. J. Chemom. 1987, 1, 185–196. [Google Scholar] [CrossRef]
- Wold, S.; Martens, H.; Wold, H. Proceedings of a Conference Held at Pite Havsbad, Sweden; Ruhe, A., Kagstrøm, B., Eds.; Springer: Heilderberg, Germany, 1983; pp. 286–293. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severi, I.; Fosca, M.; Colleluori, G.; Marini, F.; Imperatori, L.; Senzacqua, M.; Di Vincenzo, A.; Barbatelli, G.; Fiori, F.; Rau, J.V.; et al. High-Fat Diet Impairs Mouse Median Eminence: A Study by Transmission and Scanning Electron Microscopy Coupled with Raman Spectroscopy. Int. J. Mol. Sci. 2021, 22, 8049. https://doi.org/10.3390/ijms22158049
Severi I, Fosca M, Colleluori G, Marini F, Imperatori L, Senzacqua M, Di Vincenzo A, Barbatelli G, Fiori F, Rau JV, et al. High-Fat Diet Impairs Mouse Median Eminence: A Study by Transmission and Scanning Electron Microscopy Coupled with Raman Spectroscopy. International Journal of Molecular Sciences. 2021; 22(15):8049. https://doi.org/10.3390/ijms22158049
Chicago/Turabian StyleSeveri, Ilenia, Marco Fosca, Georgia Colleluori, Federico Marini, Luca Imperatori, Martina Senzacqua, Angelica Di Vincenzo, Giorgio Barbatelli, Fabrizio Fiori, Julietta V. Rau, and et al. 2021. "High-Fat Diet Impairs Mouse Median Eminence: A Study by Transmission and Scanning Electron Microscopy Coupled with Raman Spectroscopy" International Journal of Molecular Sciences 22, no. 15: 8049. https://doi.org/10.3390/ijms22158049
APA StyleSeveri, I., Fosca, M., Colleluori, G., Marini, F., Imperatori, L., Senzacqua, M., Di Vincenzo, A., Barbatelli, G., Fiori, F., Rau, J. V., & Giordano, A. (2021). High-Fat Diet Impairs Mouse Median Eminence: A Study by Transmission and Scanning Electron Microscopy Coupled with Raman Spectroscopy. International Journal of Molecular Sciences, 22(15), 8049. https://doi.org/10.3390/ijms22158049







