Dysregulation of microRNA and Intracerebral Hemorrhage: Roles in Neuroinflammation
Abstract
1. Introduction
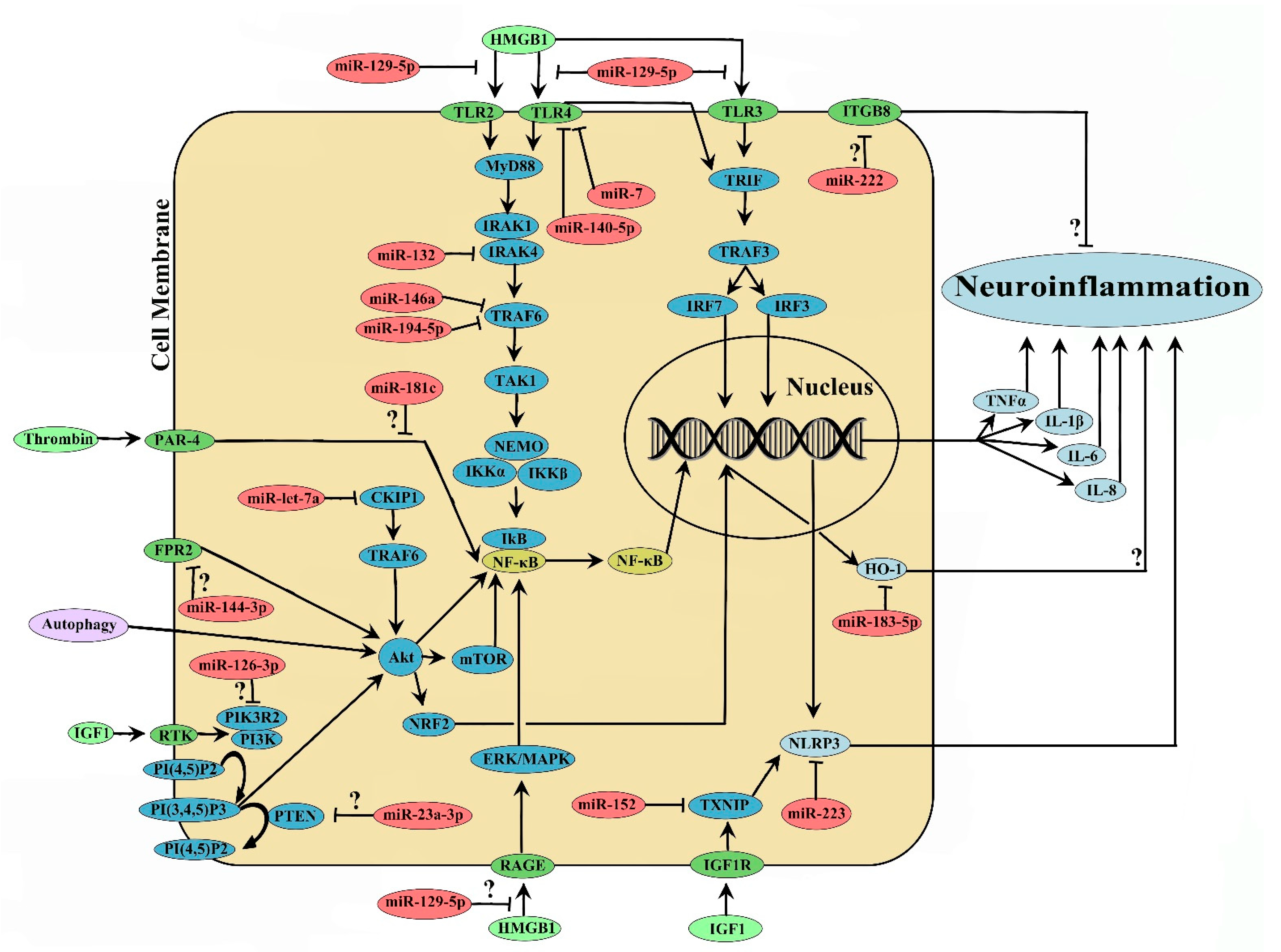
2. MicroRNA and ICH-Induced Neuroinflammation
2.1. miR-144
2.2. miR-155
2.3. miR-222
2.4. miR-145
2.5. miR-494
2.6. miR-223
2.7. miR-7
2.8. miR-let-7a
2.9. miR-21-5p
2.10. miR-23a-3p
2.11. miR-23b
2.12. miR-124
2.13. miR-126-3p
2.14. miR-129-5p
2.15. miR-132
2.16. miR-140-5p
2.17. miR-146a
2.18. miR-152
2.19. miR-181c
2.20. miR-183-5p
2.21. miR-194-5p
3. Therapeutic and Diagnostic Implications of microRNA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| Akt | protein kinase B |
| BBB | blood brain barrier |
| CEBP-α | CCAAT/enhancer-binding protein alpha |
| CKIP-1 | Casein Kinase 2 Interacting Protein-1, also known as PLEKHO1 |
| CNS | central nervous system |
| ERK | extracellular signal-regulated kinase |
| FPR2 | formyl peptide receptor 2 |
| Hb | hemoglobin |
| HMGB1 | high mobility group box-1 |
| HO-1 | heme oxygenase 1 |
| ICH | intracerebral hemorrhage |
| IFNβ | interferon beta |
| IGF1 | Insulin Like Growth Factor 1 |
| IGF1R | Insulin Like Growth Factor 1 Receptor |
| IkB | inhibitory Kappa B |
| IKK | inhibitor of nuclear factor-κB (IκB) kinase |
| IL-1β | interleukin 1 beta |
| IL-4 | interleukin 4 |
| IL-6 | interleukin 6 |
| IPMK | inositol polyphosphate multikinase |
| IRAK1 | interleukin 1 Receptor Associated Kinase 1 |
| IRAK4 | interleukin 1 Receptor Associated Kinase 4 |
| IRF3 | Interferon Regulatory Factor 3 |
| IRF7 | Interferon Regulatory Factor 7 |
| ITGB8 | integrin subunit β8 |
| LNA | locked nucleic acid |
| MAPK | mitogen activated protein kinase |
| MCAO | middle cerebral artery occlusion |
| miR | microRNA(s) |
| MPP | 1-methyl-4-phenylpyridinium |
| mTOR | mammalian target of Rapamycin |
| MyD88 | Myeloid differentiation primary response 88 |
| NEMO | NF-kappa-B essential modulator |
| NF-κB | nuclear factor kappa light chain enhancer of activated B cells |
| NFE2L2 | nuclear factor, erythroid 2 like 2 |
| NLR | nod like receptor |
| NLRP3 | NLR family pyrin domain containing 3 |
| NRDP1 | neuregulin receptor degradation protein-1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PI(3,4,5)P3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| PI(4,5)P2 | Phosphatidylinositol 4,5-bisphosphate |
| PI3K | phosphoinositide 3 kinase |
| PIK3R2 | phosphoinositide 3 kinase regulatory subunit 2 |
| pri-miRNA | primary long miRNA transcript |
| PTEN | phosphatase and tensin homolog |
| RAGE | Receptor for advanced glycation endproducts |
| RNA | ribonucleic acid |
| RNA Pol II | RNA polymerase II |
| RNA Pol III | RNA polymerase III |
| ROS | reactive oxygen species |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| RTK | receptor tyrosine kinase |
| Th17 | T helper 17 cells |
| TLR | toll like receptor |
| TLR2 | toll like receptor 2 |
| TLR3 | toll like receptor 3 |
| TLR4 | toll like receptor 4 |
| TNF-α | tumor necrosis factor alpha |
| TRAF3 | TNF receptor-associated factor 3 |
| TRAF6 | tumor necrosis factor receptor-associated factor 6 |
| TRIF | (Toll/interleukin-1 receptor) domain-containing adaptor inducing IFNβ |
| TXNIP | thioredoxin interacting protein |
| UTR | untranslated region |
References
- Hostettler, I.C.; Seiffge, D.J.; Werring, D.J. Intracerebral hemorrhage: An update on diagnosis and treatment. Expert Rev. Neurother. 2019, 19, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.M.; Qureshi, D.; Talarico, R.; Tanuseputro, P.; Dowlatshahi, D.; Sood, M.M.; Smith, E.E.; Hill, M.D.; McCredie, V.A.; Scales, D.C.; et al. Intracerebral Hemorrhage Incidence, Mortality, and Association With Oral Anticoagulation Use: A Population Study. Stroke 2021, 52, 1673–1681. [Google Scholar] [CrossRef]
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 2010, 92, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef]
- Rost, N.S.; Greenberg, S.M.; Rosand, J. The genetic architecture of intracerebral hemorrhage. Stroke 2008, 39, 2166–2173. [Google Scholar] [CrossRef]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Borchert, G.M.; Lanier, W.; Davidson, B.L. RNA polymerase III transcribes human microRNAs. Nat. Struct. Mol. Biol. 2006, 13, 1097–1101. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Li, L. Identification and analysis of the proximal promoters of microRNA genes in Arabidopsis. Genomics 2013, 101, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeon, K.; Lee, J.T.; Kim, S.; Kim, V.N. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO J. 2002, 21, 4663–4670. [Google Scholar] [CrossRef]
- Zeng, Y.; Cullen, B.R. Sequence requirements for micro RNA processing and function in human cells. RNA 2003, 9, 112–123. [Google Scholar] [CrossRef]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Ketting, R.F.; Fischer, S.E.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Brondani, V.; Billy, E.; Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002, 21, 5875–5885. [Google Scholar] [CrossRef]
- Xie, Z.; Kasschau, K.D.; Carrington, J.C. Negative feedback regulation of Dicer-Like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr. Biol. 2003, 13, 784–789. [Google Scholar] [CrossRef]
- Felekkis, K.; Touvana, E.; Stefanou, C.; Deltas, C. microRNAs: A newly described class of encoded molecules that play a role in health and disease. Hippokratia 2010, 14, 236–240. [Google Scholar]
- Shyu, A.B.; Wilkinson, M.F.; van Hoof, A. Messenger RNA regulation: To translate or to degrade. EMBO J. 2008, 27, 471–481. [Google Scholar] [CrossRef]
- Valinezhad Orang, A.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug Des. Devel. Ther. 2021, 15, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Schwanhausser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef]
- Turchinovich, A.; Tonevitsky, A.G.; Burwinkel, B. Extracellular miRNA: A Collision of Two Paradigms. Trends Biochem. Sci. 2016, 41, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Samatov, T.R.; Tonevitsky, A.G.; Burwinkel, B. Circulating miRNAs: Cell-cell communication function? Front. Genet. 2013, 4, 119. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef]
- Bhalala, O.G.; Srikanth, M.; Kessler, J.A. The emerging roles of microRNAs in CNS injuries. Nat. Rev. Neurol. 2013, 9, 328–339. [Google Scholar] [CrossRef]
- Moon, J.M.; Xu, L.; Giffard, R.G. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J. Cereb. Blood Flow Metab. 2013, 33, 1976–1982. [Google Scholar] [CrossRef]
- Stuve, O.; Zettl, U. Neuroinflammation of the central and peripheral nervous system: An update. Clin. Exp. Immunol. 2014, 175, 333–335. [Google Scholar] [CrossRef]
- Freilich, R.W.; Woodbury, M.E.; Ikezu, T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS ONE 2013, 8, e79416. [Google Scholar] [CrossRef]
- Guo, Y.; Hong, W.; Wang, X.; Zhang, P.; Korner, H.; Tu, J.; Wei, W. MicroRNAs in Microglia: How do MicroRNAs Affect Activation, Inflammation, Polarization of Microglia and Mediate the Interaction Between Microglia and Glioma? Front. Mol. Neurosci. 2019, 12, 125. [Google Scholar] [CrossRef]
- Varol, D.; Mildner, A.; Blank, T.; Shemer, A.; Barashi, N.; Yona, S.; David, E.; Boura-Halfon, S.; Segal-Hayoun, Y.; Chappell-Maor, L.; et al. Dicer Deficiency Differentially Impacts Microglia of the Developing and Adult Brain. Immunity 2017, 46, 1030–1044.e8. [Google Scholar] [CrossRef] [PubMed]
- Blandford, S.N.; Galloway, D.A.; Moore, C.S. The roles of extracellular vesicle microRNAs in the central nervous system. Glia 2018, 66, 2267–2278. [Google Scholar] [CrossRef]
- Morton, M.C.; Neckles, V.N.; Seluzicki, C.M.; Holmberg, J.C.; Feliciano, D.M. Neonatal Subventricular Zone Neural Stem Cells Release Extracellular Vesicles that Act as a Microglial Morphogen. Cell Rep. 2018, 23, 78–89. [Google Scholar] [CrossRef]
- Pinto, S.; Cunha, C.; Barbosa, M.; Vaz, A.R.; Brites, D. Exosomes from NSC-34 Cells Transfected with hSOD1-G93A Are Enriched in miR-124 and Drive Alterations in Microglia Phenotype. Front. Neurosci. 2017, 11, 273. [Google Scholar] [CrossRef]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, Q.; Hu, M.; Qian, D.; Wang, B.; He, N. The Inhibition of miR-144-3p on Cell Proliferation and Metastasis by Targeting TOP2A in HCMV-Positive Glioblastoma Cells. Molecules 2018, 23, 3259. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, T.; Zhong, W.; Duan, H.; Wang, S.; Ye, P.; Wang, J.; Zhong, S.; Yang, Z. miRNA-144 induces microglial autophagy and inflammation following intracerebral hemorrhage. Immunol. Lett. 2017, 182, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, B.; Fu, F.; Huang, S.; Yang, Z. Hemoglobin enhances miRNA-144 expression and autophagic activation mediated inflammation of microglia via mTOR pathway. Sci. Rep. 2017, 7, 11861. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, M.; Zhang, J.; Liu, A.; Ji, W.; Li, Y.; Yang, X.; Wu, Z. MiR-144 promotes beta-amyloid accumulation-induced cognitive impairments by targeting ADAM10 following traumatic brain injury. Oncotarget 2017, 8, 59181–59203. [Google Scholar] [CrossRef]
- Fan, W.; Li, X.; Zhang, D.; Li, H.; Shen, H.; Liu, Y.; Chen, G. Detrimental Role of miRNA-144-3p in Intracerebral Hemorrhage Induced Secondary Brain Injury is Mediated by Formyl Peptide Receptor 2 Downregulation Both In Vivo and In Vitro. Cell Transplant. 2019, 28, 723–738. [Google Scholar] [CrossRef]
- Sadrzadeh, S.M.; Anderson, D.K.; Panter, S.S.; Hallaway, P.E.; Eaton, J.W. Hemoglobin potentiates central nervous system damage. J. Clin. Investig. 1987, 79, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.P.; Xi, G.; Keep, R.F.; Hua, Y.; Nemoianu, A.; Hoff, J.T. Brain edema after experimental intracerebral hemorrhage: Role of hemoglobin degradation products. J. Neurosurg. 2002, 96, 287–293. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Wu, L.; Duan, X.; Hu, Y.; Sun, Y.; Wei, Y.; Dong, Z.; Wu, C.; Yu, D.; et al. Deletion of MicroRNA-144/451 Cluster Aggravated Brain Injury in Intracerebral Hemorrhage Mice by Targeting 14-3-3zeta. Front. Neurol. 2020, 11, 551411. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Huang, C.; Zhang, B.; Feng, Y.Q. Association of circulating miR-155 expression level and inflammatory markers with white coat hypertension. J. Hum. Hypertens. 2020, 34, 397–403. [Google Scholar] [CrossRef]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon. Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.; Jurj, A.; Petrushev, B.; Tomuleasa, C.; Matei, D. MicroRNA-155 Implication in M1 Polarization and the Impact in Inflammatory Diseases. Front. Immunol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Maimaijian, A.G.D.; Yimamu, Y.; Lin, L.; Li, D.; Zhang, Y. Prognostic value of serum miR-155 in intracerebral hemorrhage. Int. J. Clin. Exp. Pathol. 2017, 10, 3845–3850. [Google Scholar]
- Xu, H.F.; Fang, X.Y.; Zhu, S.H.; Xu, X.H.; Zhang, Z.X.; Wang, Z.F.; Zhao, Z.Q.; Ding, Y.J.; Tao, L.Y. Glucocorticoid treatment inhibits intracerebral hemorrhageinduced inflammation by targeting the microRNA155/SOCS1 signaling pathway. Mol. Med. Rep. 2016, 14, 3798–3804. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Wang, R.; Duan, Z.; Wang, H. A blockade of microRNA-155 signal pathway has a beneficial effect on neural injury after intracerebral haemorrhage via reduction in neuroinflammation and oxidative stress. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Xing, G.; Luo, Z.; Zhong, C.; Pan, X.; Xu, X. Influence of miR-155 on Cell Apoptosis in Rats with Ischemic Stroke: Role of the Ras Homolog Enriched in Brain (Rheb)/mTOR Pathway. Med. Sci. Monit. 2016, 22, 5141–5153. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Shi, H.; Lai, N.; Liao, K.; Zhang, S.; Lu, X. Overexpression of microRNA-155 predicts poor prognosis in glioma patients. Med. Oncol. 2014, 31, 911. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.R.; Custodia, C.M.; Silva, R.J.; de Almeida, L.P.; Pedroso de Lima, M.C.; Cardoso, A.L. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum. Mol. Genet. 2014, 23, 6286–6301. [Google Scholar] [CrossRef] [PubMed]
- Thome, A.D.; Harms, A.S.; Volpicelli-Daley, L.A.; Standaert, D.G. microRNA-155 Regulates Alpha-Synuclein-Induced Inflammatory Responses in Models of Parkinson Disease. J. Neurosci. 2016, 36, 2383–2390. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Niu, J.Z. miR222 regulates brain injury and inflammation following intracerebral hemorrhage by targeting ITGB8. Mol. Med. Rep. 2020, 21, 1145–1153. [Google Scholar]
- Su, H.; Kim, H.; Pawlikowska, L.; Kitamura, H.; Shen, F.; Cambier, S.; Markovics, J.; Lawton, M.T.; Sidney, S.; Bollen, A.W.; et al. Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am. J. Pathol. 2010, 176, 1018–1027. [Google Scholar] [CrossRef]
- Ma, L.; Shen, F.; Jun, K.; Bao, C.; Kuo, R.; Young, W.L.; Nishimura, S.L.; Su, H. Integrin beta8 Deletion Enhances Vascular Dysplasia and Hemorrhage in the Brain of Adult Alk1 Heterozygous Mice. Transl. Stroke Res. 2016, 7, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Yang, G.; Sun, J.; Beylerli, O.; Chen, X.; Zhang, D.; Zhao, B.; Zhang, R.; Sun, Z.; Yang, Q.; et al. Circulating MicroRNAs as Potential Noninvasive Biomarkers of Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2020, 133, e369–e375. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.B.; Rathod, S.S.; Karthik, S.; Kaur, N.; Muzumdar, D.; Shiras, A.S. MiR-145 functions as a tumor-suppressive RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro Oncol. 2013, 15, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Sisk, J.M.; Gama, L.; Clements, J.E. MicroRNA regulation of IFN-beta protein expression: Rapid and sensitive modulation of the innate immune response. J. Immunol. 2010, 184, 2369–2376. [Google Scholar] [CrossRef]
- Lorente-Cebrian, S.; Mejhert, N.; Kulyte, A.; Laurencikiene, J.; Astrom, G.; Heden, P.; Ryden, M.; Arner, P. MicroRNAs regulate human adipocyte lipolysis: Effects of miR-145 are linked to TNF-alpha. PLoS ONE 2014, 9, e86800. [Google Scholar] [CrossRef]
- Wu, X.; Luo, J.; Liu, H.; Cui, W.; Guo, W.; Zhao, L.; Guo, H.; Bai, H.; Guo, K.; Feng, D.; et al. Recombinant adiponectin peptide promotes neuronal survival after intracerebral haemorrhage by suppressing mitochondrial and ATF4-CHOP apoptosis pathways in diabetic mice via Smad3 signalling inhibition. Cell Prolif. 2020, 53, e12759. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Hu, Q.; Shou, J.; Zhu, L.; Tian, N.; Sun, L.; Luo, H.; Zuo, F.; Li, F.; et al. Immune changes in peripheral blood and hematoma of patients with intracerebral hemorrhage. FASEB J. 2020, 34, 2774–2791. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; Bagley, J.H.; Di, C.; Friedman, A.H.; Adamson, C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg. Focus 2012, 32, E8. [Google Scholar] [CrossRef]
- Bonsack, F.T.; Alleyne, C.H., Jr.; Sukumari-Ramesh, S. Augmented expression of TSPO after intracerebral hemorrhage: A role in inflammation? J. Neuroinflamm. 2016, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Zhou, C.; Ma, K.; Zhao, W.; Xiong, Q.; Yang, L.; Huang, Z.; Yang, Z. MiRNA-494 enhances M1 macrophage polarization via Nrdp1 in ICH mice model. J. Inflamm. 2020, 17, 17. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Q.; Zhao, W.; Yang, L.; Huang, Z.; Yang, Z. Nrdp1 increases neuron apoptosis via downregulation of Bruce following intracerebral haemorrhage. J. Inflamm. 2019, 16, 24. [Google Scholar] [CrossRef]
- Vian, L.; Di Carlo, M.; Pelosi, E.; Fazi, F.; Santoro, S.; Cerio, A.M.; Boe, A.; Rotilio, V.; Billi, M.; Racanicchi, S.; et al. Transcriptional fine-tuning of microRNA-223 levels directs lineage choice of human hematopoietic progenitors. Cell Death Differ. 2014, 21, 290–301. [Google Scholar] [CrossRef]
- Gilicze, A.B.; Wiener, Z.; Toth, S.; Buzas, E.; Pallinger, E.; Falcone, F.H.; Falus, A. Myeloid-derived microRNAs, miR-223, miR27a, and miR-652, are dominant players in myeloid regulation. Biomed. Res. Int. 2014, 2014, 870267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhong, L.; Xian, R.; Yuan, B. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol. Immunol. 2015, 65, 267–276. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, S.; Hu, Q.; Feng, H.; Zhang, J.H.; Tang, J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann. Neurol. 2014, 75, 209–219. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.Y. Increased Circulating Exosomal miRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, Y.; Guo, M.; Yue, D.; Chen, C.; Liang, G.; Xu, L. MicroRNA-7: Expression and function in brain physiological and pathological processes. Cell Biosci. 2020, 10, 77. [Google Scholar] [CrossRef]
- Kalinowski, F.C.; Brown, R.A.; Ganda, C.; Giles, K.M.; Epis, M.R.; Horsham, J.; Leedman, P.J. microRNA-7: A tumor suppressor miRNA with therapeutic potential. Int. J. Biochem. Cell Biol. 2014, 54, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Lee, K.W.; Jeong, B.S.; Chan, T.W.; Im, J.Y.; Mouradian, M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef]
- Zhao, Y.; Alexandrov, P.N.; Jaber, V.; Lukiw, W.J. Deficiency in the Ubiquitin Conjugating Enzyme UBE2A in Alzheimer’s Disease (AD) is Linked to Deficits in a Natural Circular miRNA-7 Sponge (circRNA; ciRS-7). Genes 2016, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lv, X.; Zhai, K.; Xu, R.; Zhang, Y.; Zhao, S.; Qin, X.; Yin, L.; Lou, J. MicroRNA-7 inhibits neuronal apoptosis in a cellular Parkinson’s disease model by targeting Bax and Sirt2. Am. J. Transl. Res. 2016, 8, 993–1004. [Google Scholar] [PubMed]
- Zhang, X.D.; Fan, Q.Y.; Qiu, Z.; Chen, S. MiR-7 alleviates secondary inflammatory response of microglia caused by cerebral hemorrhage through inhibiting TLR4 expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5597–5604. [Google Scholar]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Hu, K.; Xie, M.; Wu, H.; Li, W.; Wu, B.; Man, R.; Nie, M. Intracerebroventricular injection of miR-7 inhibits secondary brain injury induced by intracerebral hemorrhage via EGFR/STAT3 pathway in rats. Chin. J. Cell. Mol. Immunol. 2018, 34, 141–147. [Google Scholar]
- Choi, D.C.; Chae, Y.J.; Kabaria, S.; Chaudhuri, A.D.; Jain, M.R.; Li, H.; Mouradian, M.M.; Junn, E. MicroRNA-7 protects against 1-methyl-4-phenylpyridinium-induced cell death by targeting RelA. J. Neurosci. 2014, 34, 12725–12737. [Google Scholar] [CrossRef] [PubMed]
- Fragkouli, A.; Doxakis, E. miR-7 and miR-153 protect neurons against MPP(+)-induced cell death via upregulation of mTOR pathway. Front. Cell Neurosci. 2014, 8, 182. [Google Scholar] [CrossRef]
- Cho, K.J.; Song, J.; Oh, Y.; Lee, J.E. MicroRNA-Let-7a regulates the function of microglia in inflammation. Mol. Cell Neurosci. 2015, 68, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, X.; Zhang, J.; Huang, X.; Zhang, X.; Wang, J.; Shi, H.; Yu, A. Let-7a promotes microglia M2 polarization by targeting CKIP-1 following ICH. Immunol. Lett. 2018, 202, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, G.; Tie, Y.; Tang, Y.; Tian, C.; Li, L.; Sun, L.; Wei, H.; Zhu, Y.; He, F. Role for the pleckstrin homology domain-containing protein CKIP-1 in AP-1 regulation and apoptosis. EMBO J. 2005, 24, 766–778. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Wang, Y.; Zhang, L.; Wei, J.; Zhang, X.; He, F.; Zhang, L. Casein Kinase 2 Interacting Protein-1 regulates M1 and M2 inflammatory macrophage polarization. Cell Signal. 2017, 33, 107–121. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.L.; He, Z.Y.; Jin, F.; Tang, L. Association of Altered Serum MicroRNAs with Perihematomal Edema after Acute Intracerebral Hemorrhage. PLoS ONE 2015, 10, e0133783. [Google Scholar] [CrossRef]
- Feng, Y.H.; Tsao, C.J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef]
- Ji, R.; Cheng, Y.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007, 100, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Hong, C.; Chen, I.Y.; Lypowy, J.; Abdellatif, M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ. Res. 2007, 100, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Thum, T. MicroRNA-21: From cancer to cardiovascular disease. Curr. Drug Targets 2010, 11, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Z.; Wang, J.; Ji, X.; Yao, Z.; Wang, X. miR21 promotes osteoclastogenesis through activation of PI3K/Akt signaling by targeting Pten in RAW264.7 cells. Mol. Med. Rep. 2020, 21, 1125–1132. [Google Scholar] [PubMed]
- Liu, F.; Zheng, S.; Liu, T.; Liu, Q.; Liang, M.; Li, X.; Sheyhidin, I.; Lu, X.; Liu, W. MicroRNA-21 promotes the proliferation and inhibits apoptosis in Eca109 via activating ERK1/2/MAPK pathway. Mol. Cell Biochem. 2013, 381, 115–125. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Y.; Sun, X.; Jiang, M.; Ma, Y.; Yang, X.; Guo, Z. Propofol regulates the expression of TLR4 through miR21 in human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 16, 9074–9080. [Google Scholar] [CrossRef]
- Sheedy, F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19. [Google Scholar] [CrossRef]
- Ma, X.; Conklin, D.J.; Li, F.; Dai, Z.; Hua, X.; Li, Y.; Xu-Monette, Z.Y.; Young, K.H.; Xiong, W.; Wysoczynski, M.; et al. The oncogenic microRNA miR-21 promotes regulated necrosis in mice. Nat. Commun. 2015, 6, 7151. [Google Scholar] [CrossRef]
- Zille, M.; Karuppagounder, S.S.; Chen, Y.; Gough, P.J.; Bertin, J.; Finger, J.; Milner, T.A.; Jonas, E.A.; Ratan, R.R. Neuronal Death After Hemorrhagic Stroke In Vitro and In Vivo Shares Features of Ferroptosis and Necroptosis. Stroke 2017, 48, 1033–1043. [Google Scholar] [CrossRef]
- Wang, M.; Mungur, R.; Lan, P.; Wang, P.; Wan, S. MicroRNA-21 and microRNA-146a negatively regulate the secondary inflammatory response of microglia after intracerebral hemorrhage. Int. J. Clin. Exp. Pathol. 2018, 11, 3348–3356. [Google Scholar] [PubMed]
- Ouyang, Y.; Li, D.; Wang, H.; Wan, Z.; Luo, Q.; Zhong, Y.; Yin, M.; Qing, Z.; Li, Z.; Bao, B.; et al. MiR-21-5p/dual-specificity phosphatase 8 signalling mediates the anti-inflammatory effect of haem oxygenase-1 in aged intracerebral haemorrhage rats. Aging Cell 2019, 18, e13022. [Google Scholar] [CrossRef]
- Chen-Roetling, J.; Regan, R.F. Targeting the Nrf2-Heme Oxygenase-1 Axis after Intracerebral Hemorrhage. Curr. Pharm. Des. 2017, 23, 2226–2237. [Google Scholar] [CrossRef]
- Gao, Z.G.; Yang, P.; Huang, J.; Ding, Y.Q. CircFBXW7 alleviates glioma progression through regulating miR-23a-3p/PTEN axis. Anat. Rec. 2021, 304, 279–290. [Google Scholar] [CrossRef]
- Rojo, A.I.; Rada, P.; Mendiola, M.; Ortega-Molina, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Hardisson, D.; Serrano, M.; Cuadrado, A. The PTEN/NRF2 axis promotes human carcinogenesis. Antioxid. Redox. Signal. 2014, 21, 2498–2514. [Google Scholar] [CrossRef]
- Kong, Y.; Li, S.; Zhang, M.; Xu, W.; Chen, Q.; Zheng, L.; Liu, P.; Zou, W. Acupuncture Ameliorates Neuronal Cell Death, Inflammation, and Ferroptosis and Downregulated miR-23a-3p After Intracerebral Hemorrhage in Rats. J. Mol. Neurosci. 2021. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.C.; Ross, J.T.; Paquette, K.M.; Paulino, V.M.; Nasser, S.; Yang, Z.; Kloss, J.; Kim, S.; Berens, M.E.; Tran, N.L. miRNA expression profiling in migrating glioblastoma cells: Regulation of cell migration and invasion by miR-23b via targeting of Pyk2. PLoS ONE 2012, 7, e39818. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Luo, H.; Pu, Y.; Zhou, Z.; Wu, X.; Xu, W.; Yang, Z. Methylation mediated silencing of miR-23b expression and its role in glioma stem cells. Neurosci. Lett. 2012, 528, 185–189. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Liu, W.; van Wijnen, A.J. Regulation and biological roles of the multifaceted miRNA-23b (MIR23B). Gene 2018, 642, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Aghaee-Bakhtiari, S.H.; Arefian, E.; Naderi, M.; Noorbakhsh, F.; Nodouzi, V.; Asgari, M.; Fard-Esfahani, P.; Mahdian, R.; Soleimani, M. MAPK and JAK/STAT pathways targeted by miR-23a and miR-23b in prostate cancer: Computational and in vitro approaches. Tumour Biol. 2015, 36, 4203–4212. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, H.; Wang, B.; Ao, Q.; Shi, J.; He, Z. MicroRNA-23b alleviates neuroinflammation and brain injury in intracerebral hemorrhage by targeting inositol polyphosphate multikinase. Int. Immunopharmacol. 2019, 76, 105887. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Makeyev, E.V.; Zhang, J.; Carrasco, M.A.; Maniatis, T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell 2007, 27, 435–448. [Google Scholar] [CrossRef]
- Sanuki, R.; Onishi, A.; Koike, C.; Muramatsu, R.; Watanabe, S.; Muranishi, Y.; Irie, S.; Uneo, S.; Koyasu, T.; Matsui, R.; et al. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat. Neurosci. 2011, 14, 1125–1134. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C. miR-124 and Parkinson’s disease: A biomarker with therapeutic potential. Pharmacol. Res. 2019, 150, 104515. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, Y.; Zhu, Z.; Mo, L.; Lin, C.; Wang, Q.; Wang, H.; Gong, X.; He, X.; Lu, G.; et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol. 2016, 26, 167–176. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, Z.; Wu, J.; Zhang, Y.; Zhang, H.; Sun, X.; Qian, C.; Wang, B.; Xie, L.; Zhang, S.; et al. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J. 2019, 33, 8648–8665. [Google Scholar] [CrossRef] [PubMed]
- Hamzei Taj, S.; Kho, W.; Aswendt, M.; Collmann, F.M.; Green, C.; Adamczak, J.; Tennstaedt, A.; Hoehn, M. Dynamic Modulation of Microglia/Macrophage Polarization by miR-124 after Focal Cerebral Ischemia. J. Neuroimmune Pharmacol. 2016, 11, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, N.; Beiping, H.; Dheen, S.T.; Tay, S.S. Downregulation of miR-124 in MPTP-treated mouse model of Parkinson’s disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins. Neuroscience 2014, 272, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Laterza, O.F.; Lim, L.; Garrett-Engele, P.W.; Vlasakova, K.; Muniappa, N.; Tanaka, W.K.; Johnson, J.M.; Sina, J.F.; Fare, T.L.; Sistare, F.D.; et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin. Chem. 2009, 55, 1977–1983. [Google Scholar] [CrossRef]
- Weng, H.; Shen, C.; Hirokawa, G.; Ji, X.; Takahashi, R.; Shimada, K.; Kishimoto, C.; Iwai, N. Plasma miR-124 as a biomarker for cerebral infarction. Biomed. Res. 2011, 32, 135–141. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, G.; Sze, J.; Liu, Y.; Lin, S.; Yao, H.; Zhang, J.; Xie, D.; Liu, Q.; Kung, H.F.; et al. Plasma miR-124 Is a Promising Candidate Biomarker for Human Intracerebral Hemorrhage Stroke. Mol. Neurobiol. 2018, 55, 5879–5888. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhang, T.; Duan, H.; Pan, Y.; Zhang, X.; Yang, G.; Wang, J.; Deng, Y.; Yang, Z. MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-alpha pathway in intracerebral hemorrhage. Immunol. Lett. 2017, 182, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.D.; Zhou, X.T.; Zhou, L.T.; Wang, F.; Yin, X.; Lu, Y.; Zhu, L.Q.; Liu, D. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell 2020, 19, e13235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Chen, Z.; Zhang, Y.; Li, J.; Liu, S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Jin, F.; Zhu, Y.; Wang, J.; Tang, L.; Wang, Y.; Liebeskind, D.S.; He, Z. MicroRNA-126-3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem Biophys. Res. Commun. 2017, 494, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Li, S.Y.; Shang, F. Effect of microRNA-129-5p targeting HMGB1-RAGE signaling pathway on revascularization in a collagenase-induced intracerebral hemorrhage rat model. Biomed. Pharmacother. 2017, 93, 238–244. [Google Scholar] [CrossRef]
- Lei, C.; Zhang, S.; Cao, T.; Tao, W.; Liu, M.; Wu, B. HMGB1 may act via RAGE to promote angiogenesis in the later phase after intracerebral hemorrhage. Neuroscience 2015, 295, 39–47. [Google Scholar] [CrossRef]
- Lei, C.; Lin, S.; Zhang, C.; Tao, W.; Dong, W.; Hao, Z.; Liu, M.; Wu, B. High-mobility group box1 protein promotes neuroinflammation after intracerebral hemorrhage in rats. Neuroscience 2013, 228, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Wu, Y.T.; Wang, Y.P. MicroRNA-129-5p inhibits the development of autoimmune encephalomyelitis-related epilepsy by targeting HMGB1 through the TLR4/NF-kB signaling pathway. Brain Res. Bull. 2017, 132, 139–149. [Google Scholar] [CrossRef]
- Li, C.; Peng, S.; Liu, X.; Han, C.; Wang, X.; Jin, T.; Liu, S.; Wang, W.; Xie, X.; He, X.; et al. Glycyrrhizin, a Direct HMGB1 Antagonist, Ameliorates Inflammatory Infiltration in a Model of Autoimmune Thyroiditis via Inhibition of TLR2-HMGB1 Signaling. Thyroid 2017, 27, 722–731. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, F.S.; Tan, W.F.; Fang, B.; Zhang, Z.L.; Ma, H. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J. Neuroinflamm. 2017, 14, 205. [Google Scholar] [CrossRef]
- Soreq, H.; Wolf, Y. NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol. Med. 2011, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Saba, R.; Schratt, G. microRNAs in neurons: Manifold regulatory roles at the synapse. Curr. Opin. Genet. Dev. 2011, 21, 491–497. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Aromolaran, K.A.; Zukin, R.S. Epigenetic mechanisms in stroke and epilepsy. Neuropsychopharmacology 2013, 38, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Shaked, I.; Meerson, A.; Wolf, Y.; Avni, R.; Greenberg, D.; Gilboa-Geffen, A.; Soreq, H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 2009, 31, 965–973. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, B.; He, Y.; Li, D.; Ma, X.; Liu, Q.; Hao, J. MicroRNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. Neurochem. Int. 2017, 107, 182–190. [Google Scholar] [CrossRef]
- Kong, H.; Yin, F.; He, F.; Omran, A.; Li, L.; Wu, T.; Wang, Y.; Peng, J. The Effect of miR-132, miR-146a, and miR-155 on MRP8/TLR4-Induced Astrocyte-Related Inflammation. J. Mol. Neurosci. 2015, 57, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Yanez, M.; Brea, D.; Arias, S.; Blanco, M.; Pumar, J.M.; Castillo, J.; Sobrino, T. Increased expression of Toll-like receptors 2 and 4 is associated with poor outcome in intracerebral hemorrhage. J. Neuroimmunol. 2012, 247, 75–80. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, P.F.; Fang, H.; Chen, J.; Xiong, X.Y.; Yang, Q.W. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 2013, 44, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yin, S.; Sun, R.; Zhang, S.; Fu, M.; Wu, Y.; Zhang, T.; Khaliq, J.; Li, Y. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol. Cancer 2017, 16, 139. [Google Scholar] [CrossRef]
- Yang, H.; Fang, F.; Chang, R.; Yang, L. MicroRNA-140-5p suppresses tumor growth and metastasis by targeting transforming growth factor beta receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology 2013, 58, 205–217. [Google Scholar] [CrossRef]
- Wang, S.; Cui, Y.; Xu, J.; Gao, H. miR-140-5p Attenuates Neuroinflammation and Brain Injury in Rats Following Intracerebral Hemorrhage by Targeting TLR4. Inflammation 2019, 42, 1869–1877. [Google Scholar] [CrossRef]
- Fang, H.; Wang, P.F.; Zhou, Y.; Wang, Y.C.; Yang, Q.W. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J. Neuroinflamm. 2013, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yin, Q.; Zhong, Q.; Lv, F.L.; Zhou, Y.; Li, J.Q.; Wang, J.Z.; Su, B.Y.; Yang, Q.W. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J. Neuroinflamm. 2012, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liang, C.; Ou, M.; Zou, T.; Sun, F.; Zhou, H.; Cui, L. MicroRNA-146a Is a Wide-Reaching Neuroinflammatory Regulator and Potential Treatment Target in Neurological Diseases. Front. Mol. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef]
- Qu, X.; Wang, N.; Cheng, W.; Xue, Y.; Chen, W.; Qi, M. MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress. Exp. Ther. Med. 2019, 18, 3920–3928. [Google Scholar] [CrossRef]
- Cai, J.; Guan, H.; Jiao, X.; Yang, J.; Chen, X.; Zhang, H.; Zheng, Y.; Zhu, Y.; Liu, Q.; Zhang, Z. NLRP3 inflammasome mediated pyroptosis is involved in cadmium exposure-induced neuroinflammation through the IL-1beta/IkB-alpha-NF-kappaB-NLRP3 feedback loop in swine. Toxicology 2021, 453, 152720. [Google Scholar] [CrossRef] [PubMed]
- Huan, S.; Jin, J.; Shi, C.X.; Li, T.; Dai, Z.; Fu, X.J. Overexpression of miR-146a inhibits the apoptosis of hippocampal neurons of rats with cerebral hemorrhage by regulating autophagy. Hum. Exp. Toxicol. 2020, 39, 1178–1189. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Y.; Wang, P.; Liu, Y.; Zhao, L.; Li, Z.; Li, Z.; Xue, Y. MiR-152 functions as a tumor suppressor in glioblastoma stem cells by targeting Kruppel-like factor 4. Cancer Lett. 2014, 355, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ma, J.; Xue, Y.; Wang, P.; Li, Z.; Liu, J.; Chen, L.; Xi, Z.; Teng, H.; Wang, Z.; et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015, 359, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Qin, F.; Dai, S. miR-152 as a tumor suppressor microRNA: Target recognition and regulation in cancer. Oncol. Lett. 2016, 11, 3911–3916. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, H.; Wang, B.; Ao, Q.; He, Z. MicroRNA-152 attenuates neuroinflammation in intracerebral hemorrhage by inhibiting thioredoxin interacting protein (TXNIP)-mediated NLRP3 inflammasome activation. Int. Immunopharmacol. 2020, 80, 106141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Y.; Jin, F.; Tang, L.; He, Z.; He, Z. Differential expression of circulating microRNAs in blood and haematoma samples from patients with intracerebral haemorrhage. J. Int. Med. Res. 2016, 44, 419–432. [Google Scholar] [CrossRef]
- Ruan, J.; Lou, S.; Dai, Q.; Mao, D.; Ji, J.; Sun, X. Tumor suppressor miR-181c attenuates proliferation, invasion, and self-renewal abilities in glioblastoma. Neuroreport 2015, 26, 66–73. [Google Scholar] [CrossRef]
- Olde Loohuis, N.F.; Kole, K.; Glennon, J.C.; Karel, P.; Van der Borg, G.; Van Gemert, Y.; Van den Bosch, D.; Meinhardt, J.; Kos, A.; Shahabipour, F.; et al. Elevated microRNA-181c and microRNA-30d levels in the enlarged amygdala of the valproic acid rat model of autism. Neurobiol. Dis. 2015, 80, 42–53. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, P.H. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim. Biophys. Acta 2016, 1862, 1617–1627. [Google Scholar] [CrossRef]
- Haghikia, A.; Haghikia, A.; Hellwig, K.; Baraniskin, A.; Holzmann, A.; Decard, B.F.; Thum, T.; Gold, R. Regulated microRNAs in the CSF of patients with multiple sclerosis: A case-control study. Neurology 2012, 79, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Z.; Peng, Y.; Yu, C. MicroRNA-181c inhibits glioblastoma cell invasion, migration and mesenchymal transition by targeting TGF-beta pathway. Biochem. Biophys. Res. Commun. 2016, 469, 1041–1048. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Z.; Liu, Y.; Liu, H.; Guo, X.; Li, Y.; Yang, H.; Zhang, L.; Da, Y.; Yao, Z.; et al. MicroRNA-181c promotes Th17 cell differentiation and mediates experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2018, 70, 305–314. [Google Scholar] [CrossRef]
- Schonrock, N.; Humphreys, D.T.; Preiss, T.; Gotz, J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-beta. J. Mol. Neurosci. 2012, 46, 324–335. [Google Scholar] [CrossRef]
- Yin, M.; Chen, Z.; Ouyang, Y.; Zhang, H.; Wan, Z.; Wang, H.; Wu, W.; Yin, X. Thrombin-induced, TNFR-dependent miR-181c downregulation promotes MLL1 and NF-kappaB target gene expression in human microglia. J. Neuroinflamm. 2017, 14, 132. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.Y.; He, Z.Y. MicroRNA-181c provides neuroprotection in an intracerebral hemorrhage model. Neural Regen. Res. 2020, 15, 1274–1282. [Google Scholar]
- Wang, Y.; Song, Y.; Pang, Y.; Yu, Z.; Hua, W.; Gu, Y.; Qi, J.; Wu, H. miR-183-5p alleviates early injury after intracerebral hemorrhage by inhibiting heme oxygenase-1 expression. Aging 2020, 12, 12869–12895. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dore, S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain 2007, 130 Pt 6, 1643–1652. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Chen, X.; Wei, Q.; Ou, R.; Gu, X.; Cao, B.; Shang, H. MicroRNA-183-5p is stress-inducible and protects neurons against cell death in amyotrophic lateral sclerosis. J. Cell. Mol. Med. 2020, 24, 8614–8622. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, Y.; Wang, L.; Zhang, W.; Liu, C.; Yin, A. MicroRNA-194 overexpression protects against hypoxia/reperfusion-induced HK-2 cell injury through direct targeting Rheb. J. Cell Biochem. 2018, 120, 8311–8318. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Zuo, Q. miR-194-5p inhibits LPS-induced astrocytes activation by directly targeting neurexophilin 1. Mol. Cell Biochem. 2020, 471, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Y.; Li, G.S.; Tu, C.; Chen, W.L.; Wang, X.W.; Wang, Y.N.; Peng, L.B.; Tan, F. MicroNAR-194-5p hinders the activation of NLRP3 inflammasomes and alleviates neuroinflammation during intracerebral hemorrhage by blocking the interaction between TRAF6 and NLRP3. Brain Res. 2021, 1752, 147228. [Google Scholar] [CrossRef]
- Gonzalez-Crespo, S.; Levine, M. Related target enhancers for dorsal and NF-kappa B signaling pathways. Science 1994, 264, 255–258. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-kappaB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Carmody, R.J. NF-kappaB and the Transcriptional Control of Inflammation. Int. Rev. Cell. Mol. Biol. 2018, 335, 41–84. [Google Scholar]
- Napetschnig, J.; Wu, H. Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-kappaB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Huang, Q.; Su, Y.; Zhang, Y.; Wang, G.; Li, F. NF-kappaB activation and cell death after intracerebral hemorrhage in patients. Neurol. Sci. 2014, 35, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Liu, Y.G.; Huang, Q.B.; Wang, H.W.; Song, Y.; Xu, Z.K.; Li, F. Nuclear factor-kappaB activation in perihematomal brain tissue correlates with outcome in patients with intracerebral hemorrhage. J. Neuroinflamm. 2015, 12, 53. [Google Scholar] [CrossRef]
- Rhyasen, G.W.; Starczynowski, D.T. IRAK signalling in cancer. Br. J. Cancer 2015, 112, 232–237. [Google Scholar] [CrossRef]
- Sakai, J.; Cammarota, E.; Wright, J.A.; Cicuta, P.; Gottschalk, R.A.; Li, N.; Fraser, I.D.C.; Bryant, C.E. Lipopolysaccharide-induced NF-kappaB nuclear translocation is primarily dependent on MyD88, but TNFalpha expression requires TRIF and MyD88. Sci. Rep. 2017, 7, 1428. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, Y.; Ding, R.; Feng, L.; Fu, Z.; Yang, S.; Deng, X.; Xie, Z.; Zheng, S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-kappaB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J. Neuroinflamm. 2017, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xiao, F.; Wang, S.; Xing, G.; Li, Y.; Yin, X.; Lu, K.; Wei, R.; Fan, J.; et al. CKIP-1 regulates macrophage proliferation by inhibiting TRAF6-mediated Akt activation. Cell Res. 2014, 24, 742–761. [Google Scholar] [CrossRef]
- Camacho, E.; LoPresti, M.A.; Bruce, S.; Lin, D.; Abraham, M.; Appelboom, G.; Taylor, B.; McDowell, M.; DuBois, B.; Sathe, M.; et al. The role of age in intracerebral hemorrhages. J. Clin. Neurosci. 2015, 22, 1867–1870. [Google Scholar] [CrossRef]
- Krepelkova, I.; Mrackova, T.; Izakova, J.; Dvorakova, B.; Chalupova, L.; Mikulik, R.; Slaby, O.; Bartos, M.; Ruzicka, V. Evaluation of miRNA detection methods for the analytical characteristic necessary for clinical utilization. Biotechniques 2019, 66, 277–284. [Google Scholar] [CrossRef]
- Jin, J.; Vaud, S.; Zhelkovsky, A.M.; Posfai, J.; McReynolds, L.A. Sensitive and specific miRNA detection method using SplintR Ligase. Nucleic Acids Res. 2016, 44, e116. [Google Scholar] [CrossRef]
- Wood, H. FDA approves patisiran to treat hereditary transthyretin amyloidosis. Nat. Rev. Neurol. 2018, 14, 570. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Givosiran: First Approval. Drugs 2020, 80, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Dirin, M.; Winkler, J. Influence of diverse chemical modifications on the ADME characteristics and toxicology of antisense oligonucleotides. Expert Opin. Biol. Ther. 2013, 13, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Baumann, V.; Winkler, J. miRNA-based therapies: Strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med. Chem. 2014, 6, 1967–1984. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef]
- Lv, Q.; Deng, J.; Chen, Y.; Wang, Y.; Liu, B.; Liu, J. Engineered Human Adipose Stem-Cell-Derived Exosomes Loaded with miR-21-5p to Promote Diabetic Cutaneous Wound Healing. Mol. Pharm. 2020, 17, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashif, H.; Shah, D.; Sukumari-Ramesh, S. Dysregulation of microRNA and Intracerebral Hemorrhage: Roles in Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 8115. https://doi.org/10.3390/ijms22158115
Kashif H, Shah D, Sukumari-Ramesh S. Dysregulation of microRNA and Intracerebral Hemorrhage: Roles in Neuroinflammation. International Journal of Molecular Sciences. 2021; 22(15):8115. https://doi.org/10.3390/ijms22158115
Chicago/Turabian StyleKashif, Hisham, Dilan Shah, and Sangeetha Sukumari-Ramesh. 2021. "Dysregulation of microRNA and Intracerebral Hemorrhage: Roles in Neuroinflammation" International Journal of Molecular Sciences 22, no. 15: 8115. https://doi.org/10.3390/ijms22158115
APA StyleKashif, H., Shah, D., & Sukumari-Ramesh, S. (2021). Dysregulation of microRNA and Intracerebral Hemorrhage: Roles in Neuroinflammation. International Journal of Molecular Sciences, 22(15), 8115. https://doi.org/10.3390/ijms22158115





