HIV-Associated Cancer Biomarkers: A Requirement for Early Diagnosis
Abstract
1. Introduction
2. Cancer Prevalence in HIV-Positive Patients
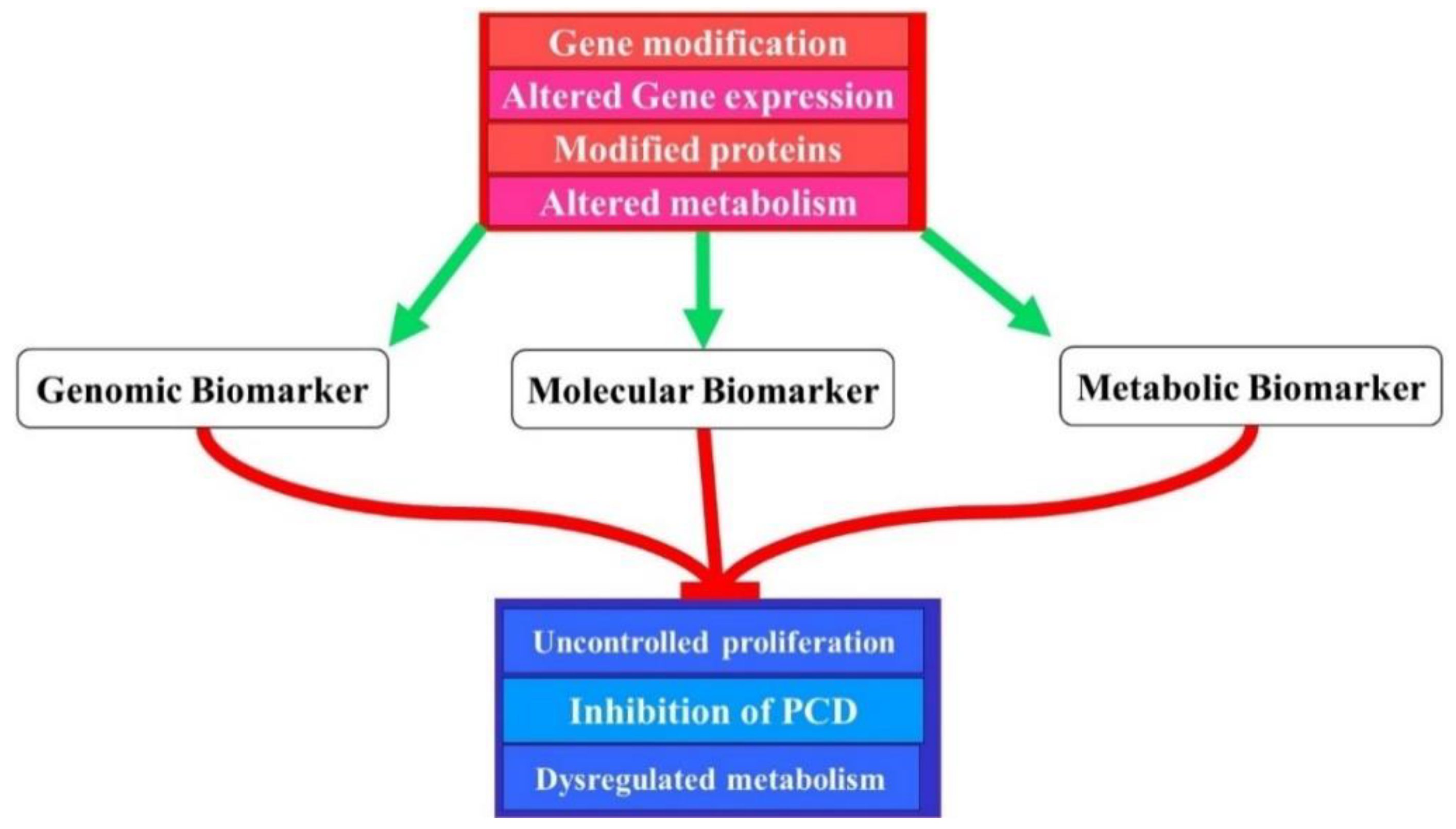
3. The Genetics of Molecular Biomarkers
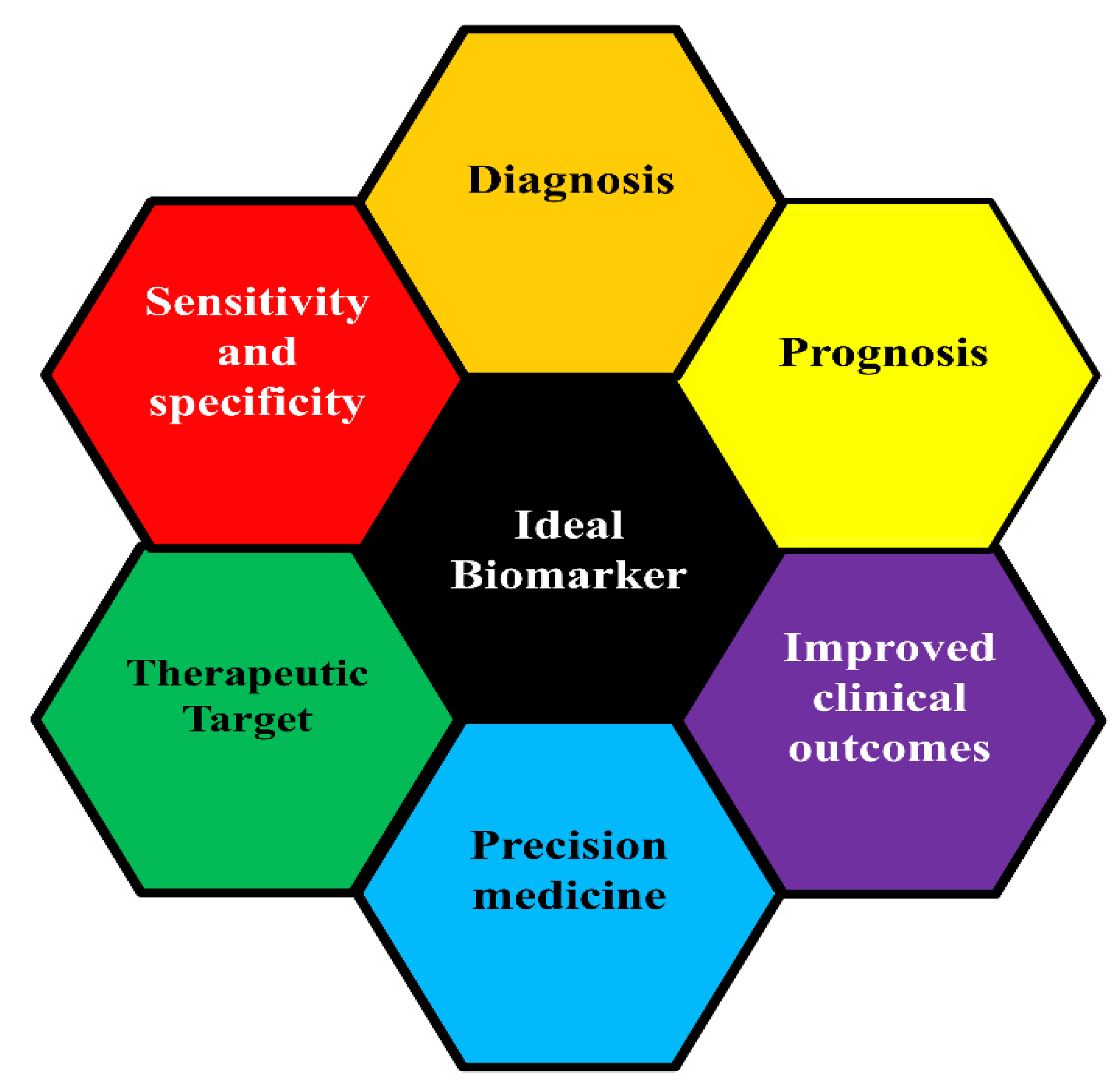
3.1. Classification of Cancer Biomarker
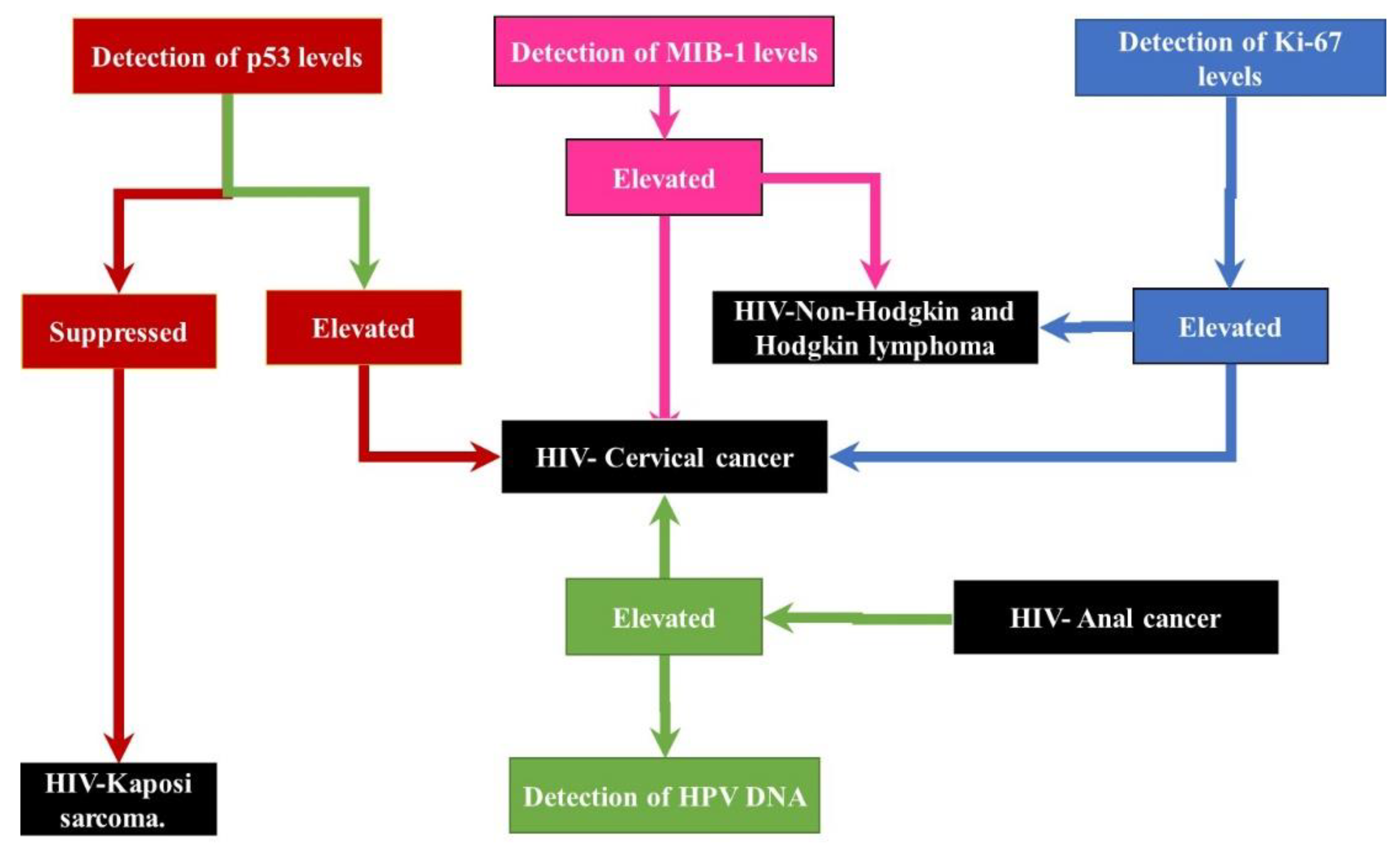
3.2. Biomarkers Used in HIV-Associated Cancer Diagnosis and Prognosis
3.2.1. Diagnosis and Prognosis Challenges of HIV-Associated Cervical Cancer
| HIV-Associated Cervical Cancer | Changes in HIV-Cervical Cancer | References |
|---|---|---|
| HPV DNA | Elevated | [40,63] |
| HPVE6/E7 | Elevated | [47,50,64] |
| Ki-67 | Elevated | [65,66] |
| P16 | Elevated | [40,65] |
| CK17 | Elevated | [40,67] |
| MCM | Elevated | [68,69] |
| CDC6 | Elevated | [70,71] |
| Ribosomal protein S12 | Elevated | [72] |
| P53 | Elevated | [43,73] |
| PCNA | Elevated | [74,75] |
| MIB-1 | Elevated | [75] |
| P63 | Suppressed | [40,76] |
| CD44 | Elevated | [77] |
3.2.2. Diagnosis/Prognosis Challenges of HIV-Associated Non-Hodgkin Lymphoma
| HIV-Associated NHL Biomarkers | Changes in HIV-NHL | References |
|---|---|---|
| LDH | Elevated | [93,100,101] |
| Ki-67/MIB-1 | Elevated | [102,103] |
| CD19, CD20, CD22 | Elevated | [79,104] |
| PAX-5 | Elevated | [79,104] |
| CD10 | Elevated | [17,102] |
| bcl6 | Elevated | [1,105] |
| MUM-1 | Elevated | [17,102] |
| cMYC | Elevated | [106,107] |
| IL-6 | Elevated | [39,108] |
| IL-10 | Elevated | [39,108,109] |
| TNF-α | Elevated | [39,110] |
| CRP | Elevated | [93,108] |
| sCD23, sCD27, sCD30, sCD44 | Elevated | [92,94] |
| EBV DNA | Elevated | [95] |
| CXCL13 | Elevated | [39,94] |
| FLC | Elevated | [39,111] |
| FOXP1 | Elevated | [1,112] |
| B2M | Elevated | [1,100] |
3.2.3. Diagnosis/Prognosis Challenges of HIV-Associated Kaposi’s Sarcoma
4. HIV-Associated Cancer Mechanisms
5. Treatment for HIV-Associated Cancers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flepisi, B.T.; Bouic, P.; Sissolak, G.; Rosenkranz, B. Biomarkers of HIV-associated Cancer. Biomark. Cancer 2014, 6. [Google Scholar] [CrossRef]
- Casper, C. The increasing burden of HIV-associated malignancies in resource-limited regions. Annu. Rev. Med. 2011, 62, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Sasco, A.J.; Jaquet, A.; Boidin, E.; Ekouevi, D.K.; Thouillot, F.; Lemabec, T.; Forstin, M.A.; Renaudier, P.; N’Dom, P.; Malvy, D.; et al. The challenge of AIDS-related malignancies in sub-Saharan Africa. PLoS ONE 2010, 5, e8621. [Google Scholar] [CrossRef]
- Chinula, L.; Moses, A.; Gopal, S. HIV-associated malignancies in sub-Saharan Africa: Progress, challenges, and opportunities. Curr. Opin. HIV AIDS 2017, 12, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.A.; Pfeiffer, R.M.; Shiels, M.S.; Li, J.; Hall, H.I.; Engels, E.A. Excess cancers among HIV-infected people in the United States. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Ambinder, R.F.; Bhatia, K.; Martinez-Maza, O.; Mitsuyasu, R. Cancer biomarkers in HIV patients. Curr. Opin. HIV AIDS 2010, 5, 531. [Google Scholar] [CrossRef]
- Hathout, Y.; Brody, E.; Clemens, P.R.; Cripe, L.; DeLisle, R.K.; Furlong, P.; Gordish-Dressman, H.; Hache, L.; Henricson, E.; Hoffman, E.P.; et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 2015, 112, 7153–7158. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.H.; Yates, J.R., III. Shotgun proteomics and biomarker discovery. Dis. Markers 2002, 18, 99–105. [Google Scholar] [CrossRef]
- Verma, M. Personalized medicine and cancer. J. Pers. Med. 2012, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Shiels, M.S.; Modur, S.P.; Land, S.R.; Crothers, K.A.; Kitahata, M.M.; Thorne, J.E.; Mathews, W.C.; Fernández-Santos, D.M.; Mayor, A.M.; et al. Cancer burden attributable to cigarette smoking among HIV-infected people in North America. AIDS 2018, 32, 513–521. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Gail, M.H.; Hall, H.I.; Li, J.; Chaturvedi, A.K.; Bhatia, K.; Uldrick, T.S.; Yarchoan, R.; Goedert, J.J.; et al. Cancer burden in the HIV-infected population in the United States. J. Natl. Cancer Inst. 2011, 103, 753–762. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef]
- Rubinstein, P.G.; Aboulafia, D.M.; Zloza, A. Malignancies in HIV/AIDS: From epidemiology to therapeutic challenges. AIDS 2014, 28, 453–465. [Google Scholar] [CrossRef]
- Justice, A.C.; Erlandson, K.M.; Hunt, P.W.; Landay, A.; Miotti, P.; Tracy, R.P. Can Biomarkers Advance HIV Research and Care in the Antiretroviral Therapy Era? J. Infect. Dis. 2018, 217, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Chiao, E.Y.; Hartman, C.M.; El-Serag, H.B.; Giordano, T.P. The impact of HIV viral control on the incidence of HIV-associated anal cancer. J. Acquir. Immune Defic. Syndr. 2013, 63, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Davison, D.D.; Adams, J.; Lopez-Beltran, A.; Wang, L.; Montironi, R.; Zhang, S. Biomarkers in bladder cancer: Translational and clinical implications. Crit. Rev. Oncol. Hematol. 2014, 89, 73–111. [Google Scholar] [CrossRef]
- Barreto, L.; Azambuja, D.; Morais, J.C. Expression of immunohistochemical markers in patients with AIDS-related lymphoma. Braz. J. Infect. Dis. 2012, 16, 74–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borges, A.H.; Dubrow, R.; Silverberg, M.J. Factors contributing to risk for cancer among HIV-infected individuals, and evidence that earlier combination antiretroviral therapy will alter this risk. Curr. Opin. HIV AIDS 2014, 9, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Dryden-Peterson, S.; Medhin, H.; Kebabonye-Pusoentsi, M.; Seage, G.R., III; Suneja, G.; Kayembe, M.K.; Mmalane, M.; Rebbeck, T.; Rider, J.R.; Essex, M.J.P.O. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE 2015, 10, e0135602. [Google Scholar] [CrossRef]
- Engels, E.A. Non-AIDS-defining malignancies in HIV-infected persons: Etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS 2009, 23, 875–885. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 2145. [Google Scholar] [CrossRef]
- Miller, K.D.; Goding Sauer, A.; Ortiz, A.P.; Fedewa, S.A.; Pinheiro, P.S.; Tortolero-Luna, G.; Martinez-Tyson, D.; Jemal, A.; Siegel, R.L. Cancer statistics for hispanics/latinos. CA Cancer J. Clin. 2018, 68, 425–445. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Goedert, J.J.; Virgo, P.; McNeel, T.S.; Scoppa, S.M.; Biggar, R.J. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS 2006, 20, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Park, L.S.; Hernández-Ramírez, R.U.; Silverberg, M.J.; Crothers, K.; Dubrow, R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: A meta-analysis. AIDS 2016, 30, 273–291. [Google Scholar] [CrossRef]
- Pulitzer, M. Molecular diagnosis of infection-related cancers in dermatopathology. Semin. Cutan. Med. Surg. 2012, 31, 247–257. [Google Scholar] [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Chyla, B.; Daver, N.; Doyle, K.; McKeegan, E.; Huang, X.; Ruvolo, V.; Wang, Z.; Chen, K.; Souers, A.; Leverson, J.; et al. Genetic Biomarkers of Sensitivity and Resistance to Venetoclax Monotherapy in Patients with Relapsed Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, E202–E205. [Google Scholar] [CrossRef]
- Banerjee, J.; Pradhan, R.; Gupta, A.; Kumar, R.; Sahu, V.; Upadhyay, A.D.; Chaterjee, P.; Dwivedi, S.; Dey, S.; Dey, A.B. CDK4 in lung, and head and neck cancers in old age: Evaluation as a biomarker. Clin. Transl. Oncol. 2017, 19, 571–578. [Google Scholar] [CrossRef]
- Sahutoglu, T.; Sakaci, T.; Hasbal, N.B.; Ahbap, E.; Kara, E.; Sumerkan, M.C.; Sevinc, M.; Akgol, C.; Koc, Y.; Basturk, T.; et al. Serum VEGF-C levels as a candidate biomarker of hypervolemia in chronic kidney disease. Medicine 2017, 96, e6543. [Google Scholar] [CrossRef] [PubMed]
- Heckman-Stoddard, B.M. Oncology biomarkers: Discovery, validation, and clinical use. Semin. Oncol. Nurs. 2012, 28, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Ahmad, M.K.; Srivatava, V.K.; Rastogi, N.; Serajuddin, M.; Kumar, S.; Mishra, D.P.; Sankhwar, S.N.; Mahdi, A.A. Evaluation of miR-711 as Novel Biomarker in Prostate Cancer Progression. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 2185–2191. [Google Scholar] [CrossRef]
- Lesko, L.J.; Atkinson, A.J., Jr. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: Criteria, validation, strategies. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 347–366. [Google Scholar] [CrossRef]
- Maisel, A.S.; Katz, N.; Hillege, H.L.; Shaw, A.; Zanco, P.; Bellomo, R.; Anand, I.; Anker, S.D.; Aspromonte, N.; Bagshaw, S.M. Biomarkers in kidney and heart disease. Nephrol. Dial. Transplant. 2011, 26, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Cai, Q.; Verma, S.C.; Choi, J.Y.; Ma, M.; Robertson, E.S. Kaposi’s sarcoma-associated herpesvirus inhibits interleukin-4-mediated STAT6 phosphorylation to regulate apoptosis and maintain latency. J. Virol. 2010, 84, 11134–11144. [Google Scholar] [CrossRef]
- Cai, Q.; Verma, S.C.; Kumar, P.; Ma, M.; Robertson, E.S. Hypoxia inactivates the VHL tumor suppressor through PIASy-mediated SUMO modification. PLoS ONE 2010, 5, e9720. [Google Scholar] [CrossRef]
- Verma, M. Molecular profiling and companion diagnostics: Where is personalized medicine in cancer heading? Pers. Med. 2014, 11, 761–771. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.; Masel, R.I.; Kenis, P.J. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Vendrame, E.; Hussain, S.K.; Breen, E.C.; Magpantay, L.I.; Widney, D.P.; Jacobson, L.P.; Variakojis, D.; Knowlton, E.R.; Bream, J.H.; Ambinder, R.F.; et al. Serum levels of cytokines and biomarkers for inflammation and immune activation, and HIV-associated non-Hodgkin B-cell lymphoma risk. Cancer Epidemiol. Biomark. Prev. 2014, 23, 343–349. [Google Scholar] [CrossRef]
- Selvi, K.; Badhe, B.A.; Papa, D.; Ganesh, R.N. Role of p16, CK17, p63, and human papillomavirus in diagnosis of cervical intraepithelial neoplasia and distinction from its mimics. Int. J. Surg. Pathol. 2014, 22, 221–230. [Google Scholar] [CrossRef]
- Izadi-Mood, N.; Sarmadi, S.; Eftekhar, Z.; Jahanteegh, H.A.; Sanii, S. Immunohistochemical expression of p16 and HPV L1 capsid proteins as predictive markers in cervical lesions. Arch. Gynecol. Obstet. 2014, 289, 1287–1292. [Google Scholar] [CrossRef]
- Soliman, P.T.; Langley, G.; Munsell, M.F.; Vaniya, H.A.; Frumovitz, M.; Ramirez, P.T. Analgesic and antiemetic requirements after minimally invasive surgery for early cervical cancer: A comparison between laparoscopy and robotic surgery. Ann. Surg. Oncol. 2013, 20, 1355–1359. [Google Scholar] [CrossRef]
- Madhumati, G.; Kavita, S.; Anju, M.; Uma, S.; Raj, M. Immunohistochemical Expression of Cell Proliferating Nuclear Antigen (PCNA) and p53 Protein in Cervical Cancer. J. Obstet. Gynaecol. India 2012, 62, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhu, Y.; Yang, L.; Zhang, X.; Liu, L.; Wang, Z.; Jiang, D. Prognostic and diagnostic validity of p16/Ki-67, HPV E6/E7 mRNA, and HPV DNA in women with ASCUS: A follow-up study. Virol. J. 2019, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhu, Y.; Yang, L.; Zhang, X.; Liu, L.; Ren, C. Diagnostic performance of HPV E6/E7 mRNA assay for detection of cervical high-grade intraepithelial neoplasia and cancer among women with ASCUS Papanicolaou smears. Arch. Gynecol. Obstet. 2018, 297, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Derbie, A.; Mekonnen, D.; Woldeamanuel, Y.; Van Ostade, X.; Abebe, T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): A systematic review. Infect. Agents Cancer 2020, 15, 9. [Google Scholar] [CrossRef]
- Campbell, L.M.; Pitta, D.R.; De Assis, A.M.; Derchain, S.F.; Campos, E.A.; Sarian, L.O. Retrieval of HPV oncogenes E6 and E7 mRNA from cervical specimens using a manual open technology protocol. SpringerPlus 2013, 2, 473. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, D. The precision prevention and therapy of HPV-related cervical cancer: New concepts and clinical implications. Cancer Med. 2018, 7, 5217–5236. [Google Scholar] [CrossRef]
- Basu, P.; Banerjee, D.; Mittal, S.; Dutta, S.; Ghosh, I.; Chowdhury, N.; Abraham, P.; Chandna, P.; Ratnam, S. Sensitivity of APTIMA HPV E6/E7 mRNA test in comparison with hybrid capture 2 HPV DNA test for detection of high risk oncogenic human papillomavirus in 396 biopsy confirmed cervical cancers. J. Med. Virol. 2016, 88, 1271–1278. [Google Scholar] [CrossRef]
- Ratnam, S.; Coutlee, F.; Fontaine, D.; Bentley, J.; Escott, N.; Ghatage, P.; Gadag, V.; Holloway, G.; Bartellas, E.; Kum, N.; et al. Aptima HPV E6/E7 mRNA test is as sensitive as Hybrid Capture 2 Assay but more specific at detecting cervical precancer and cancer. J. Clin. Microbiol. 2011, 49, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Xu, W.; Xu, M.; Wang, L.; Zhou, W.; Xiang, R.; Shi, Y.; Zhang, Y.; Piao, Y. Integrative analysis of DNA methylation and gene expression identified cervical cancer-specific diagnostic biomarkers. Signal Transduct. Target. Ther. 2019, 4, 55. [Google Scholar] [CrossRef]
- Dashti, N.; Mahmoudi, M.; Gharibdoost, F.; Kavosi, H.; Rezaei, R.; Imeni, V.; Jamshidi, A.; Aslani, S.; Mostafaei, S.; Vodjgani, M. Evaluation of ITGB2 (CD18) and SELL (CD62L) genes expression and methylation of ITGB2 promoter region in patients with systemic sclerosis. Rheumatol. Int. 2018, 38, 489–498. [Google Scholar] [CrossRef]
- Nyman, J.; Mercke, C.; Lindström, J. Prognostic factors for local control and survival of cancer of the oral tongue. A retrospective analysis of 230 cases in western Sweden. Acta Oncol. 1993, 32, 667–673. [Google Scholar] [CrossRef]
- Carozzi, F.; Gillio-Tos, A.; Confortini, M.; Del Mistro, A.; Sani, C.; De Marco, L.; Girlando, S.; Rosso, S.; Naldoni, C.; Dalla Palma, P.; et al. Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results: A prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2013, 14, 168–176. [Google Scholar] [CrossRef]
- Cecchini, S.; Carozzi, F.; Confortini, M.; Zappa, M.; Ciatto, S. Persistent human papilloma virus infection as an indicator of risk of recurrence of high-grade cervical intraepithelial neoplasia treated by the loop electrosurgical excision procedure. Tumori J. 2004, 90, 225–228. [Google Scholar] [CrossRef]
- Martens, J.E.; Arends, J.; Van der Linden, P.J.; De Boer, B.A.; Helmerhorst, T.J. Cytokeratin 17 and p63 are markers of the HPV target cell, the cervical stem cell. Anticancer Res. 2004, 24, 771–775. [Google Scholar]
- Ikeda, K.; Tate, G.; Suzuki, T.; Mitsuya, T. Coordinate expression of cytokeratin 8 and cytokeratin 17 immunohistochemical staining in cervical intraepithelial neoplasia and cervical squamous cell carcinoma: An immunohistochemical analysis and review of the literature. Gynecol. Oncol. 2008, 108, 598–602. [Google Scholar] [CrossRef]
- Garima; Pandey, S.; Pandey, L.K.; Saxena, A.K.; Patel, N. The Role of p53 Gene in Cervical Carcinogenesis. J. Obstet. Gynaecol. India 2016, 66, 383–388. [Google Scholar] [CrossRef][Green Version]
- Romus, I.; Triningsih, F.E.; Mangunsudirdjo, S.; Harijadi, A. Clinicopathology significance of p53 and p63 expression in Indonesian cervical squamous cell carcinomas. Asian Pac. J. Cancer Prev. 2013, 14, 7737–7741. [Google Scholar] [CrossRef]
- Zajkowska, M.; Zbucka-Krętowska, M.; Sidorkiewicz, I.; Lubowicka, E.; Gacuta, E.; Szmitkowski, M.; Chrostek, L.; Ławicki, S. Plasma levels and diagnostic utility of macrophage-colony stimulating factor, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 as tumor markers in cervical cancer patients. Tumour Biol. 2018, 40, 1010428318790363. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Mishra, A.; Verma, M. Cancer biomarkers: Are we ready for the prime time? Cancers 2010, 2, 190–208. [Google Scholar] [CrossRef]
- Roncaglia, M.T.; Fregnani, J.H.; Tacla, M.; De Campos, S.G.; Caiaffa, H.H.; Ab’saber, A.; Da Motta, E.V.; Alves, V.A.; Baracat, E.C.; Longatto Filho, A. Characterization of p16 and E6 HPV-related proteins in uterine cervix high-grade lesions of patients treated by conization with large loop excision. Oncol. Lett. 2013, 6, 63–68. [Google Scholar] [CrossRef]
- Sari Aslani, F.; Safaei, A.; Pourjabali, M.; Momtahan, M. Evaluation of Ki67, p16 and CK17 Markers in Differentiating Cervical Intraepithelial Neoplasia and Benign Lesions. Iran. J. Med. Sci. 2013, 38, 15–21. [Google Scholar]
- Iaconis, L.; Hyjek, E.; Ellenson, L.H.; Pirog, E.C. p16 and Ki-67 immunostaining in atypical immature squamous metaplasia of the uterine cervix: Correlation with human papillomavirus detection. Arch. Pathol. Lab. Med. 2007, 131, 1343–1349. [Google Scholar] [CrossRef]
- Regauer, S.; Reich, O. CK17 and p16 expression patterns distinguish (atypical) immature squamous metaplasia from high-grade cervical intraepithelial neoplasia (CIN III). Histopathology 2007, 50, 629–635. [Google Scholar] [CrossRef]
- Ishimi, Y.; Okayasu, I.; Kato, C.; Kwon, H.J.; Kimura, H.; Yamada, K.; Song, S.Y. Enhanced expression of Mcm proteins in cancer cells derived from uterine cervix. Eur. J. Biochem. 2003, 270, 1089–1101. [Google Scholar] [CrossRef]
- Das, M.; Prasad, S.B.; Yadav, S.S.; Govardhan, H.B.; Pandey, L.K.; Singh, S.; Pradhan, S.; Narayan, G. Over expression of minichromosome maintenance genes is clinically correlated to cervical carcinogenesis. PLoS ONE 2013, 8, e69607. [Google Scholar] [CrossRef]
- Bonds, L.; Baker, P.; Gup, C.; Shroyer, K.R. Immunohistochemical localization of cdc6 in squamous and glandular neoplasia of the uterine cervix. Arch. Pathol. Lab. Med. 2002, 126, 1164–1168. [Google Scholar] [CrossRef]
- Murphy, N.; Ring, M.; Heffron, C.C.; Martin, C.M.; McGuinness, E.; Sheils, O.; O’Leary, J.J. Quantitation of CDC6 and MCM5 mRNA in cervical intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix. Mod. Pathol. 2005, 18, 844–849. [Google Scholar] [CrossRef]
- Cheng, Q.; Lau, W.M.; Chew, S.H.; Ho, T.H.; Tay, S.K.; Hui, K.M. Identification of molecular markers for the early detection of human squamous cell carcinoma of the uterine cervix. Br. J. Cancer 2002, 86, 274–281. [Google Scholar] [CrossRef]
- Portari, E.A.; Russomano, F.B.; de Camargo, M.J.; Machado Gayer, C.R.; da Rocha Guillobel, H.C.; Santos-Rebouças, C.B.; Brito Macedo, J.M. Immunohistochemical expression of cyclin D1, p16Ink4a, p21WAF1, and Ki-67 correlates with the severity of cervical neoplasia. Int. J. Gynecol. Pathol. 2013, 32, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Branca, M.; Ciotti, M.; Giorgi, C.; Santini, D.; Di Bonito, L.; Costa, S.; Benedetto, A.; Bonifacio, D.; Di Bonito, P.; Paba, P.; et al. Up-regulation of proliferating cell nuclear antigen (PCNA) is closely associated with high-risk human papillomavirus (HPV) and progression of cervical intraepithelial neoplasia (CIN), but does not predict disease outcome in cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 130, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.M.; Mehrotra, A. Immunohistochemical expression of MIB-1 and PCNA in precancerous and cancerous lesions of uterine cervix. Indian J. Cancer 2013, 50, 200–205. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Q.; Ling, B.; Xiao, W.; Liu, P. Reduced expression of ΔΝp63α in cervical squamous cell carcinoma. Clin. Investig. Med. Med. Clin. Exp. 2011, 34, E184–E191. [Google Scholar] [CrossRef]
- Speiser, P.; Wanner, C.; Tempfer, C.; Mittelböck, M.; Hanzal, E.; Bancher-Todesca, D.; Gitsch, G.; Reinthaller, A.; Kainz, C. CD44 is an independent prognostic factor in early-stage cervical cancer. Int. J. Cancer 1997, 74, 185–188. [Google Scholar] [CrossRef]
- Emmanuel, B.; Anderson, W.F. Non-Hodgkin lymphoma in early life. J. Natl. Cancer Inst. 2012, 104, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Sangle, N.A.; Agarwal, A.M.; Smock, K.J.; Leavitt, M.O.; Warnke, R.; Bahler, D.; Perkins, S.L. Diffuse large B-cell lymphoma with aberrant expression of the T-cell antigens CD2 and CD7. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 579–583. [Google Scholar] [CrossRef]
- Kim, M.K.; Bae, S.H.; Bae, Y.K.; Kum, Y.S.; Ryoo, H.M.; Cho, H.S.; Lee, K.H.; Koh, S.A.; Lee, H.Y.; Yun, S.Y.; et al. Biological characterization of nodal versus extranodal presentation of diffuse large B-Cell lymphoma using immunohistochemistry. Clin. Lymphoma Myeloma Leuk. 2011, 11, 403–408. [Google Scholar] [CrossRef]
- Pörtner, L.M.; Schönberg, K.; Hejazi, M.; Brünnert, D.; Neumann, F.; Galonska, L.; Reusch, U.; Little, M.; Haas, R.; Uhrberg, M. T and NK cells of B cell NHL patients exert cytotoxicity against lymphoma cells following binding of bispecific tetravalent antibody CD19 × CD3 or CD19 × CD16. Cancer Immunol. Immunother. 2012, 61, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.J. Will we find biomarkers of aging? Sci. Aging Knowl. Environ. SAGE KE 2005, 26, nf48. [Google Scholar] [CrossRef] [PubMed]
- Nyman, H.; Adde, M.; Karjalainen-Lindsberg, M.L.; Taskinen, M.; Berglund, M.; Amini, R.M.; Blomqvist, C.; Enblad, G.; Leppä, S. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood 2007, 109, 4930–4935. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Visco, C.; Li, Y.; Xu-Monette, Z.Y.; Miranda, R.N.; Green, T.M.; Li, Y.; Tzankov, A.; Wen, W.; Liu, W.M.; Kahl, B.S.; et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: A report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia 2012, 26, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.T.; Ribera, J.M.; Oriol, A.; Vaquero, M.; Romeu, J.; Batlle, M.; Gómez, J.; Millá, F.; Feliu, E. International prognostic index is the best prognostic factor for survival in patients with AIDS-related non-Hodgkin’s lymphoma treated with CHOP. A multivariate study of 46 patients. Haematologica 1998, 83, 508–513. [Google Scholar]
- Leong, T.L.; Marini, K.D.; Rossello, F.J.; Jayasekara, S.N.; Russell, P.A.; Prodanovic, Z.; Kumar, B.; Ganju, V.; Alamgeer, M.; Irving, L.B.; et al. Genomic characterisation of small cell lung cancer patient-derived xenografts generated from endobronchial ultrasound-guided transbronchial needle aspiration specimens. PLoS ONE 2014, 9, e106862. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Johnson, D.F.; Connell, T.G.; Irving, L.B. Endobronchial ultrasound-guided biopsy in the evaluation of intrathoracic lymphadenopathy in suspected tuberculosis: A minimally invasive technique with a high diagnostic yield. J. Infect. 2009, 58, 309–311. [Google Scholar] [CrossRef]
- Kaplan, L.D. HIV-associated lymphoma. Pract. Res. Clin. Haematol. 2012, 25, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.D. Management of HIV-associated Hodgkin lymphoma: How far we have come. J. Clin. Oncol. 2012, 30, 4056–4058. [Google Scholar] [CrossRef]
- Tang, Y.L.; Zhou, Y.; Cheng, L.L.; Su, Y.Z.; Wang, C.B. BCL2/Ki-67 index predict survival in germinal center B-cell-like diffuse large B-cell lymphoma. Oncol. Lett. 2017, 14, 3767–3773. [Google Scholar] [CrossRef]
- Weissman, D.; Dybul, M.; Daucher, M.B.; Davey, R.T., Jr.; Walker, R.E.; Kovacs, J.A. Interleukin-2 up-regulates expression of the human immunodeficiency virus fusion coreceptor CCR5 by CD4+ lymphocytes in vivo. J. Infect. Dis. 2000, 181, 933–938. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.; Jia, X.; Zeng, Y.; Yao, S.; Lv, Z.; Qin, D.; Fang, X.; Lei, Y.; Lu, C. Human immunodeficiency virus type 1 Tat accelerates Kaposi sarcoma-associated herpesvirus Kaposin A-mediated tumorigenesis of transformed fibroblasts in vitro as well as in nude and immunocompetent mice. Neoplasia 2009, 11, 1272–1284. [Google Scholar] [CrossRef]
- Suzuki, K.; Terui, Y.; Nishimura, N.; Mishima, Y.; Sakajiri, S.; Yokoyama, M.; Takahashi, S.; Tsuyama, N.; Takeuchi, K.; Hatake, K. Prognostic value of C-reactive protein, lactase dehydrogenase and anemia in recurrent or refractory aggressive lymphoma. Jpn. J. Clin. Oncol. 2013, 43, 37–44. [Google Scholar] [CrossRef]
- De Roos, A.J.; Mirick, D.K.; Edlefsen, K.L.; LaCroix, A.Z.; Kopecky, K.J.; Madeleine, M.M.; Magpantay, L.; Martínez-Maza, O. Markers of B-cell activation in relation to risk of non-Hodgkin lymphoma. Cancer Res. 2012, 72, 4733–4743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tedeschi, R.; Bortolin, M.T.; Bidoli, E.; Zanussi, S.; Pratesi, C.; Vaccher, E.; Tirelli, U.; De Paoli, P. Assessment of immunovirological features in HIV related non-Hodgkin lymphoma patients and their impact on outcome. J. Clin. Virol. 2012, 53, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Ilie, M.; Hofman, V.; Havet, K.; Selva, E.; Butori, C.; Lacour, J.P.; Nelson, A.M.; Cathomas, G.; Hofman, P. LANA-1, Bcl-2, Mcl-1 and HIF-1alpha protein expression in HIV-associated Kaposi sarcoma. Virchows Arch. 2009, 455, 159–170. [Google Scholar] [CrossRef]
- Ojala, P.M.; Tiainen, M.; Salven, P.; Veikkola, T.; Castaños-Vélez, E.; Sarid, R.; Biberfeld, P.; Mäkelä, T.P. Kaposi’s sarcoma-associated herpesvirus-encoded v-cyclin triggers apoptosis in cells with high levels of cyclin-dependent kinase 6. Cancer Res. 1999, 59, 4984–4989. [Google Scholar]
- Pantanowitz, L.; Schwartz, E.J.; Dezube, B.J.; Kohler, S.; Dorfman, R.F.; Tahan, S.R. C-Kit (CD117) expression in AIDS-related, classic, and African endemic Kaposi sarcoma. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.J.; Dorfman, R.F.; Kohler, S. Human herpesvirus-8 latent nuclear antigen-1 expression in endemic Kaposi sarcoma: An immunohistochemical study of 16 cases. Am. J. Surg. Pathol. 2003, 27, 1546–1550. [Google Scholar] [CrossRef]
- Milanovic, N.; Matkovic, S.; Ristic, D.; Jelic, S.; Petrovic, M. Significance of tumor burden, vascular endothelial growth factor, lactate dehydrogenase and beta-2 microglobulin serum levels in advanced diffuse large B cell lymphoma. J. BUON 2012, 17, 497–501. [Google Scholar] [PubMed]
- Bairey, O.; Bar-Natan, M.; Shpilberg, O. Early death in patients diagnosed with non-Hodgkin’s lymphoma. Ann. Hematol. 2013, 92, 345–350. [Google Scholar] [CrossRef]
- Chao, C.; Silverberg, M.J.; Martínez-Maza, O.; Chi, M.; Abrams, D.I.; Haque, R.; Zha, H.D.; McGuire, M.; Xu, L.; Said, J. Epstein-Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell Lymphoma. Clin. Cancer Res. 2012, 18, 4702–4712. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Dirnhofer, S.; Sabattini, E.; Alinari, L.; Piccaluga, P.P.; Stefoni, V.; Tani, M.; Musuraca, G.; Marchi, E.; Falini, B.; et al. Identification of outcome predictors in diffuse large B-cell lymphoma. Immunohistochemical profiling of homogeneously treated de novo tumors with nodal presentation on tissue micro-arrays. Haematologica 2005, 90, 341–347. [Google Scholar]
- Miles, R.R.; Arnold, S.; Cairo, M.S. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br. J. Haematol. 2012, 156, 730–743. [Google Scholar] [CrossRef]
- De Mello, C.A.; De Andrade, V.P.; De Lima, V.C.; Carvalho, A.L.; Soares, F.A. Prognostic impact of MUM1 expression by immunohistochemistry on primary mediastinal large B-cell lymphoma. Leuk. Lymphoma 2011, 52, 1495–1503. [Google Scholar] [CrossRef]
- Mead, G.M.; Barrans, S.L.; Qian, W.; Walewski, J.; Radford, J.A.; Wolf, M.; Clawson, S.M.; Stenning, S.P.; Yule, C.L.; Jack, A.S. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood 2008, 112, 2248–2260. [Google Scholar] [CrossRef]
- Ladanyi, M.; Offit, K.; Jhanwar, S.C.; Filippa, D.A.; Chaganti, R.S. MYC rearrangement and translocations involving band 8q24 in diffuse large cell lymphomas. Blood 1991, 77, 1057–1063. [Google Scholar] [CrossRef]
- Breen, E.C.; Hussain, S.K.; Magpantay, L.; Jacobson, L.P.; Detels, R.; Rabkin, C.S.; Kaslow, R.A.; Variakojis, D.; Bream, J.H.; Rinaldo, C.R.J.C.E.; et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS–associated non-hodgkin B-Cell lymphoma. Cancer Epidemiol. Prev. Biomark. 2011, 20, 1303–1314. [Google Scholar] [CrossRef]
- Masood, R.; Zhang, Y.; Bond, M.W.; Scadden, D.T.; Moudgil, T.; Law, R.E.; Kaplan, M.H.; Jung, B.; Espina, B.M.; Lunardi-Iskandar, Y.; et al. Interleukin-10 is an autocrine growth factor for acquired immunodeficiency syndrome-related B-cell lymphoma. Blood 1995, 85, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Yokote, T.; Hirata, Y.; Akioka, T.; Miyoshi, T.; Hiraoka, N.; Iwaki, K.; Takayama, A.; Nishiwaki, U.; Masuda, Y.; et al. TNF-α expression in tumor cells as a novel prognostic marker for diffuse large B-cell lymphoma, not otherwise specified. Am. J. Surg. Pathol. 2014, 38, 228–234. [Google Scholar] [CrossRef]
- Landgren, O.; Goedert, J.J.; Rabkin, C.S.; Wilson, W.H.; Dunleavy, K.; Kyle, R.A.; Katzmann, J.A.; Rajkumar, S.V.; Engels, E.A. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J. Clin. Oncol. 2010, 28, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhou, X.; Li, B.; Xiao, X.; Yan, S.; Shi, D. FOXP1 expression and its clinicopathologic significance in nodal and extranodal diffuse large B-cell lymphoma. Ann. Hematol. 2011, 90, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, M.; Facciolà, A.; Taibi, R.; Pellicanò, G.F.; Nunnari, G.; Venanzi Rullo, E. The treatment of Kaposi’s sarcoma: Present and future options, a review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7488–7497. [Google Scholar] [CrossRef]
- Pereira, P.F.; Cuzzi, T.; Galhardo, M.C. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An. Bras. Dermatol. 2013, 88, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Dezube, B.J.; Pinkus, G.S.; Tahan, S.R. Histological characterization of regression in acquired immunodeficiency syndrome-related Kaposi’s sarcoma. J. Cutan. Pathol. 2004, 31, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, W.; Wong, K.O.; Wong, C.S.; Dinkel, J.E.; Ben-Dor, D.; Chan, J.K. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am. J. Clin. Pathol. 2004, 121, 335–342. [Google Scholar] [CrossRef]
- Horenstein, M.G.; Cesarman, E.; Wang, X.; Linkov, I.; Prieto, V.G.; Louie, D.C. Cyclin D1 and retinoblastoma protein expression in Kaposi’s sarcoma. J. Cutan. Pathol. 1997, 24, 585–589. [Google Scholar] [CrossRef]
- Hong, A.; Davies, S.; Stevens, G.; Lee, C.S. Cyclin D1 overexpression in AIDS-related and classic Kaposi sarcoma. Appl. Immunohistochem. Mol. Morphol. 2004, 12, 26–30. [Google Scholar] [CrossRef]
- Moses, A.V.; Jarvis, M.A.; Raggo, C.; Bell, Y.C.; Ruhl, R.; Luukkonen, B.G.; Griffith, D.J.; Wait, C.L.; Druker, B.J.; Heinrich, M.C.; et al. Kaposi’s sarcoma-associated herpesvirus-induced upregulation of the c-kit proto-oncogene, as identified by gene expression profiling, is essential for the transformation of endothelial cells. J. Virol. 2002, 76, 8383–8399. [Google Scholar] [CrossRef]
- Cai, X.; Lu, S.; Zhang, Z.; Gonzalez, C.M.; Damania, B.; Cullen, B.R. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5570–5575. [Google Scholar] [CrossRef]
- Sakakibara, S.; Pise-Masison, C.A.; Brady, J.N.; Tosato, G. Gene regulation and functional alterations induced by Kaposi’s sarcoma-associated herpesvirus-encoded ORFK13/vFLIP in endothelial cells. J. Virol. 2009, 83, 2140–2153. [Google Scholar] [CrossRef]
- Ballon, G.; Chen, K.; Perez, R.; Tam, W.; Cesarman, E. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice. J. Clin. Investig. 2011, 121, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.M.; Biddolph, S.; Lucas, S.B.; Howells, D.D.; Picton, S.; McGee, J.O.; Silva, I.; Uhlmann, V.; Luttich, K.; O’Leary, J.J. Cyclin D1 expression and HHV8 in Kaposi sarcoma. J. Clin. Pathol. 1999, 52, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Koopal, S.; Furuhjelm, J.H.; Järviluoma, A.; Jäämaa, S.; Pyakurel, P.; Pussinen, C.; Wirzenius, M.; Biberfeld, P.; Alitalo, K.; Laiho, M.; et al. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 2007, 3, 1348–1360. [Google Scholar] [CrossRef]
- Friborg, J., Jr.; Kong, W.; Hottiger, M.O.; Nabel, G.J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 1999, 402, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Robertson, E.S. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J. Virol. 2006, 80, 697–709. [Google Scholar] [CrossRef]
- Kahn, H.J.; Bailey, D.; Marks, A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod. Pathol. 2002, 15, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Igari, T.; Shimbo, T.; Sekine, K.; Akiyama, J.; Hamada, Y.; Yazaki, H.; Ohmagari, N.; Teruya, K.; Oka, S.; et al. Diagnostic value of endothelial markers and HHV-8 staining in gastrointestinal Kaposi sarcoma and its difference in endoscopic tumor staging. World J. Gastroenterol. 2013, 19, 3608–3614. [Google Scholar] [CrossRef]
- Rosado, F.G.; Itani, D.M.; Coffin, C.M.; Cates, J.M. Utility of immunohistochemical staining with FLI1, D2-40, CD31, and CD34 in the diagnosis of acquired immunodeficiency syndrome-related and non-acquired immunodeficiency syndrome-related Kaposi sarcoma. Arch. Pathol. Lab. Med. 2012, 136, 301–304. [Google Scholar] [CrossRef]
- Russell Jones, R.; Orchard, G.; Zelger, B.; Wilson Jones, E. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J. Clin. Pathol. 1995, 48, 1011–1016. [Google Scholar] [CrossRef]
- Aoki, Y.; Yarchoan, R.; Wyvill, K.; Okamoto, S.; Little, R.F.; Tosato, G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood 2001, 97, 2173–2176. [Google Scholar] [CrossRef]
- Guo, Z.; Gillam, E.M.; Ohmori, S.; Tukey, R.H.; Guengerich, F.P. Expression of modified human cytochrome P450 1A1 in Escherichia coli: Effects of 5′ substitution, stabilization, purification, spectral characterization, and catalytic properties. Arch. Biochem. Biophys. 1994, 312, 436–446. [Google Scholar] [CrossRef]
- Faris, M.; Ensoli, B.; Kokot, N.; Nel, A.E. Inflammatory cytokines induce the expression of basic fibroblast growth factor (bFGF) isoforms required for the growth of Kaposi’s sarcoma and endothelial cells through the activation of AP-1 response elements in the bFGF promoter. AIDS 1998, 12, 19–27. [Google Scholar] [CrossRef]
- Simonart, T.; Van Vooren, J.P. Interleukin-1 beta increases the BCL-2/BAX ratio in Kaposi’s sarcoma cells. Cytokine 2002, 19, 259–266. [Google Scholar] [CrossRef]
- Cai, J.; Gill, P.S.; Masood, R.; Chandrasoma, P.; Jung, B.; Law, R.E.; Radka, S.F. Oncostatin-M is an autocrine growth factor in Kaposi’s sarcoma. Am. J. Pathol. 1994, 145, 74–79. [Google Scholar] [PubMed]
- Amaral, M.C.; Miles, S.; Kumar, G.; Nel, A.E. Oncostatin-M stimulates tyrosine protein phosphorylation in parallel with the activation of p42MAPK/ERK-2 in Kaposi’s cells. Evidence that this pathway is important in Kaposi cell growth. J. Clin. Investig. 1993, 92, 848–857. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Mbulaiteye, S.M.; Engels, E.A. Underestimation of relative risks by standardized incidence ratios for AIDS-related cancers. Ann. Epidemiol. 2008, 18, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xu, L.; Yang, R.; Meng, Y.; Qiu, L. Ki-67 and P16 proteins in cervical cancer and precancerous lesions of young women and the diagnostic value for cervical cancer and precancerous lesions. Oncol. Lett. 2019, 18, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Schim van der Loeff, M.F.; Mooij, S.H.; Richel, O.; de Vries, H.J.; Prins, J.M. HPV and anal cancer in HIV-infected individuals: A review. Curr. HIV/AIDS Rep. 2014, 11, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K. Utility of proliferation-associated marker MIB-1 in evaluating lesions of the uterine cervix. Adv. Anat. Pathol. 1999, 6, 177–185. [Google Scholar] [CrossRef]
- Murphy, N.; Ring, M.; Heffron, C.C.; King, B.; Killalea, A.G.; Hughes, C.; Martin, C.M.; McGuinness, E.; Sheils, O.; O’Leary, J.J. p16INK4A, CDC6, and MCM5: Predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J. Clin. Pathol. 2005, 58, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Chokchaichamnankit, D.; Watcharatanyatip, K.; Subhasitanont, P.; Weeraphan, C.; Keeratichamroen, S.; Sritana, N.; Kantathavorn, N.; Diskul-Na-Ayudthaya, P.; Saharat, K.; Chantaraamporn, J.; et al. Urinary biomarkers for the diagnosis of cervical cancer by quantitative label-free mass spectrometry analysis. Oncol. Lett. 2019, 17, 5453–5468. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Masuda, M.; Kai, K.; Nakao, Y.; Kawaguchi, A.; Yokoyama, M.; Aishima, S. Decreased cytokeratin 7 expression correlates with the progression of cervical squamous cell carcinoma and poor patient outcomes. J. Obstet. Gynaecol. Res. 2019, 45, 2228–2236. [Google Scholar] [CrossRef]
- Kanthiya, K.; Khunnarong, J.; Tangjitgamol, S.; Puripat, N.; Tanvanich, S. Expression of the p16 and Ki67 in Cervical Squamous Intraepithelial Lesions and Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 3201–3206. [Google Scholar]
- Duk, J.M.; Groenier, K.H.; de Bruijn, H.W.; Hollema, H.; ten Hoor, K.A.; van der Zee, A.G.; Aalders, J.G. Pretreatment serum squamous cell carcinoma antigen: A newly identified prognostic factor in early-stage cervical carcinoma. J. Clin. Oncol. 1996, 14, 111–118. [Google Scholar] [CrossRef]
- Mitildzans, A.; Arechvo, A.; Rezeberga, D.; Isajevs, S. Expression of p63, p53 and Ki-67 in Patients with Cervical Intraepithelial Neoplasia. Turk Patoloji Derg. 2017, 33, 9–16. [Google Scholar] [CrossRef]
- Hessol, N.A.; Martínez-Maza, O.; Levine, A.M.; Morris, A.; Margolick, J.B.; Cohen, M.H.; Jacobson, L.P.; Seaberg, E.C. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS 2015, 29, 1183–1193. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.-H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A. The immune landscape of cancer. Immunity 2019, 48, 812–830. [Google Scholar] [CrossRef]
- Gopal, S.; Achenbach, C.J.; Yanik, E.L.; Dittmer, D.P.; Eron, J.J.; Engels, E.A. Moving forward in HIV-associated cancer. J. Clin. Oncol. 2014, 32, 876. [Google Scholar] [CrossRef] [PubMed][Green Version]
| HIV-Associated KS Biomarkers | Changes in HIV-KS | References |
|---|---|---|
| HHV8/LANA-1 | Elevated | [1,114,115,116] |
| Cyclin D1 | Elevated | [114,117,118] |
| bcl2 | Elevated | [96,97,99] |
| c-kit | Elevated | [98,119] |
| K12 | Elevated | [25,120] |
| K13/vFLIP | Elevated | [25,121,122] |
| vCyclin | Elevated | [97,123,124] |
| P53 | Suppressed | [125,126] |
| pRb | Suppressed | [25,117] |
| D2–40 | Elevated | [35,115,127] |
| CD31 | Elevated | [116,128] |
| CD34 | Elevated | [128,129,130] |
| FLI1 | Elevated | [1,129] |
| vIL-6 | Elevated | [25,131,132] |
| Tat | Elevated | [92] |
| bFGF | Elevated | [133] |
| TNF-α | Elevated | [1,133] |
| IL-1 | Elevated | [134] |
| Oncostatin M | Elevated | [133,135,136] |
| Biomarker | Normal | CIN1 | CIN2 | CIN3 | I | II | III | IV | Ref |
|---|---|---|---|---|---|---|---|---|---|
| MiB-1 | 10%> | >10% | - | - | >71% | >I | >II,I | High | [140] |
| CDC6 | 90% | 38% | - | 24% | - | - | 70% | - | [141] |
| CD44 | - | - | - | - | High | <I | <I,II | <I,II,III | [142] |
| CK7 | Positive | - | - | - | - | - | negative | Negative | [143] |
| hMSH2 | - | - | - | - | - | - | 54.3% | - | [71] |
| Ki-67 | 20%> | 22.6% | 82.1% | 88.6% | - | - | 100% | - | [144] |
| p16 | >10% | 10.4% | 84.5% | 90.5% | - | - | 91.3% | - | [144] |
| SSC-Ag | - | - | - | - | 24% | 53% | 75% | 90% | [145] |
| MCM3 | negative | 4–6% | - | - | - | - | 18% | - | [141] |
| p63 | 100% | 100% | - | - | 30% | - | - | 10% | [146] |
| p53 | <10% | 10% | 50% | 71% | - | - | - | - | [146] |
| Biomarker | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Ki67 | 95.2 | 86.7 |
| P16 | 85.4 | 94.6 |
| Ki67 + p16 | 94.8 | 93.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, Z.; Mbele, M.; Makhafola, T.J.; Hull, R.; Marima, R. HIV-Associated Cancer Biomarkers: A Requirement for Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 8127. https://doi.org/10.3390/ijms22158127
Dlamini Z, Mbele M, Makhafola TJ, Hull R, Marima R. HIV-Associated Cancer Biomarkers: A Requirement for Early Diagnosis. International Journal of Molecular Sciences. 2021; 22(15):8127. https://doi.org/10.3390/ijms22158127
Chicago/Turabian StyleDlamini, Zodwa, Mzwandile Mbele, Tshepiso J. Makhafola, Rodney Hull, and Rahaba Marima. 2021. "HIV-Associated Cancer Biomarkers: A Requirement for Early Diagnosis" International Journal of Molecular Sciences 22, no. 15: 8127. https://doi.org/10.3390/ijms22158127
APA StyleDlamini, Z., Mbele, M., Makhafola, T. J., Hull, R., & Marima, R. (2021). HIV-Associated Cancer Biomarkers: A Requirement for Early Diagnosis. International Journal of Molecular Sciences, 22(15), 8127. https://doi.org/10.3390/ijms22158127






